Abstract
Investigational tobacco products, specifically variable nicotine content research cigarettes (SPECTRUM), are available through the National Institute of Drug Abuse Drug Supply Program. Randomized controlled trials using research cigarettes are intended to support tobacco regulatory science research. The current paper provides an in-depth look into managing research cigarettes for two multi-site clinical trials and the design of a computer-based Cigarette Management System (CMS). The paper provides guidance intended for any investigator using similar products on the operating procedures under Good Clinical Practice standards and describes features of the CMS. The CMS and procedures described have been field tested for the past three years and have dispensed over 160,000 cigarette packs to participants. The CMS can accommodate a range of practical issues with real-world study implementation making it a robust application that is scalable to any study.
1. Background
Cigarette smoking is the leading cause of preventable disease and death in the United States [1,2]. In 2009, the Family Smoking Prevention and Tobacco Control Act (FSPTCA) gave the U. S. Food and Drug Administration (FDA) jurisdiction over the regulation of all tobacco products, including their nicotine content [3]. The FSPTCA gives the FDA authority under Section 907 of the Federal Food, Drug, and Cosmetic Act (FDCA) to require tobacco product standards for nicotine and other harmful ingredients to protect public health. Given that nicotine is the primary addictive ingredient in cigarettes [4], it has been proposed that reducing the nicotine in cigarettes to levels that are not addicting could prevent future death and disease from cigarettes and protect future generations from ever becoming addicted to cigarettes [4].
Reduced nicotine product standards can only be implemented if they are shown through empirical evidence to reduce addiction and in turn protect public health. To facilitate an evidence base to support the public health benefit of cigarettes with reduced nicotine, SPECTRUM research cigarettes (22nd Century Group, Inc.) are provided to research investigators through an investigational tobacco product (ITP) application process by the National Institute on Drug Abuse (NIDA) Drug Supply Program and FDA. Currently, twenty-three different cigarette types with varying nicotine/tar yields are available in menthol and non-menthol flavors. The physical properties of these cigarettes are described elsewhere [5].
The use of SPECTRUM cigarettes in research has progressed from single laboratory sessions to multi-center scale clinical trials [[6], [7], [8]]. As part of the Penn State University Tobacco Center of Regulatory Science (TCORS), two large-scale double-blind randomized controlled trials are currently being conducted using SPECTRUM research cigarettes with usual nicotine content [UNC] (e.g. similar to commercial cigarettes) and progressively reduced nicotine content [RNC] [9,10]. The management of research cigarettes for large scale, multi-center trials requires the consideration of unique trial design elements and logistical issues. In order to effectively and efficiently manage a large, blinded cigarette inventory, a computer-based Cigarette Management System (CMS) was developed and the details of its application are provided here. The aims of this paper are to outline the CMS system and to describe operating procedures for studies using SPECTRUM cigarettes. This paper also reviews considerations for managing cigarette inventory outside of the CMS.
2. Design/methods
2.1. Study protocol
The study protocols described herein are two multi-site, two-arm, 34-week, parallel-group, double-blind randomized controlled trials of reduced nicotine content cigarettes in smokers of lower socioeconomic status (N = 280) and with mood and/or anxiety disorders (N = 200). More information about each of the study protocols is published elsewhere [9,10]. The study sites include the Pennsylvania State University College of Medicine, Hershey, Pennsylvania, Milken School of Public Health, George Washington University, Washington, D.C., and Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Both studies consist of four phases over 34 weeks (Table 2). At the first two baseline phases, participants smoke their usual brand of cigarettes for one week and then switch to the UNC SPECTRUM (11 mg nicotine/cigarette) research cigarettes for two weeks. During the double-blind experimental phase, participants are randomized to smoke either the UNC SPECTRUM research cigarettes or RNC SPECTRUM research cigarettes with nicotine strengths tapered from ∼7.4 to ∼0.2 mg/cigarette over 18 weeks. During the treatment choice and final phase of the study, all participants are offered the choice to either (a) quit smoking with counseling and nicotine replacement therapy, (b) continue smoking the research cigarettes, or (c) return to their usual brand cigarettes, and all are followed for a further 12 weeks. To monitor research cigarette usage, a daily cigarette log was filled out every day by the participant. Participants and study staff are blind to the randomization assignment. Participants complete biological measures (blood pressure, pulse, height, weight, blood and urine collection, exhaled carbon monoxide), questionnaires, and procedures throughout the trial which are intended to collect, measure, and communicate study endpoints.
Table 2.
Study design and cigarette type for each cigarette treatment group.
| Phase |
Baseline Phase 1 |
Baseline Phase 2 |
Randomized Double-Blind Phase 3 |
Treatment Choice Phase 4 |
||||
|---|---|---|---|---|---|---|---|---|
| Week(s) | 1 | 2 | 3 | 3 | 3 | 3 | 6 | 12 |
| UNC Cigarette Group | Own Brand | Usual Nicotine Research Cigarettes | Usual Nicotine Research Cigarettes | Usual Nicotine Research Cigarettes | Usual Nicotine Research Cigarettes | Usual Nicotine Research Cigarettes | Usual Nicotine Research Cigarettes | Variable |
| SPECTRUM Tobacco Product Master File Codes (non-menthol/menthol)a | NRC600/NRC601 | NRC600/NRC601 | NRC600/NRC601 | NRC600/NRC601 | NRC600/NRC601 | NRC600/NRC601 | ||
| RNC Cigarette Group | Own Brand | Usual Nicotine Research Cigarettes | Reduced Nicotine Step 1 | Reduced Nicotine Step 2 | Reduced Nicotine Step 3 | Reduced Nicotine Step 4 | Reduced Nicotine Step 5 | Variable |
| SPECTRUM Tobacco Product Master File Codes (non-menthol/menthol)a | NRC600/NRC601 | NRC500/NRC501 | NRC400/NRC401 | NRC300/NRC301 | NRC200/NRC201 | NRC102/NRC103 | ||
Abbreviations: RNC reduced nicotine content, UNC usual nicotine content.
Tobacco product master file codes designate the cigarette flavor and nicotine content and are used when ordering cigarettes from the NIDA Drug Supply Program.
Study staff includes both blinded and unblinded individuals. Unblinded research staffs that manage inventory across all sites and maintain the CMS are affiliated with the Penn State Health Investigation Drug Service Pharmacy. The unblinded research staff includes Registered Pharmacists and Pharmacy Technicians. The blinded research staff performs the daily interactions with the participants and data collection for the study.
2.2. SPECTRUM research cigarettes
SPECTRUM cigarettes are manufactured by the 22nd Century Group, Inc. (Clarence, N.Y.) and are made available to research investigators via the NIDA Drug Supply Program (NOT-DA-14-004). Cigarettes are shipped from the Research Triangle Institute (RTI) International in Research Triangle Park, North Carolina. Cigarette packs are packaged in cartons containing 10 packs and 20 cigarettes in each pack. Each pack is labelled with a Surgeon General's Warning and a “for research purposes only” indication. Packs and cartons are labelled with a blue bar for non-menthol and a green bar for menthol. Individual cigarettes do not contain any additional labeling.
2.3. Applying for research cigarettes
Research investigators who wish to acquire research cigarettes must compile a request package to the NIDA Drug Supply Program containing grant and investigator information, the study protocol, and the quantity and types of research cigarettes being requested to obtain the cigarettes. For clinical research involving human subjects, the request package must also include an ethics committee review approval letter, a Data Safety and Monitoring Plan, and an Investigational Tobacco Product (ITP) application and response letter from the FDA. An ITP is defined as: “a new or modified risk tobacco product that is not legally marketed; or a tobacco product that is required to comply with a tobacco product standard and that does not conform in all respects to the applicable tobacco product standard and is intended for investigational use.” An ITP application is reviewed by the FDA and includes a detailed study protocol and plans for the protection of subjects. Once a project has been approved, requests for inventory needs are made to NIDA and approved by the NIH program officer and the appropriate division at NIDA. Cigarettes are then shipped from RTI.
2.4. Storage of research cigarettes
Recommended storing conditions from RTI are −20 °C within 72 h of shipment receipt. Each carton is bagged in cellophane bags to prevent moisture damage and stored in a freezer at Penn State. Freezer areas are separate locked areas away from participants and blinded study staff. This space is continually occupied by unblinded study staff only. Non-menthol cigarettes should be kept in separate freezers from menthol cigarettes to prevent menthol contamination from menthol fumes. The storage facility at Penn State is continuously monitored remotely via the AmegaView Temperature Monitoring System (Mesa Laboratories, Inc.). Room temperature set points are 15 °C–30 °C and freezer temperature set points are −10 °C and −30 °C. Phone calls and pager notifications to pharmacy staff are sent immediately when temperature settings are breached until someone acknowledges the alarm. When temperatures are out of range they are recorded every 15 min until the area is back within the acceptable range. The CMS manager and pharmacist have options to temporarily suppress the alarm when large shipments are added or moved from the freezers. The temperature probes are calibrated yearly and certificates are maintained within the AmegaView system. All logs are available for auditing purposes online.
Whenever freezer space is not available the cigarette cartons are kept at room temperature. The room temperature is monitored as delineated above or temperature and humidity logs are routinely taken and kept on paper record. The cartons stored at room temperature are affixed with ‘DO NOT USE after’ expiration labels dated 1 year from the date the carton was pulled from the freezer storage.
2.5. Overview of CMS design
The CMS was created to inventory, dispense, and manage research cigarettes for the two multi-site trials described above. The Department of Public Health Sciences (DPHS) at the Pennsylvania State University College of Medicine served as the application development team for the CMS. The team consists of developers and database specialists that have experience in designing, developing, and maintaining customized research applications. Once the study project requirements were identified, the development team customized a functional web-based application. The CMS was developed using the Adobe ColdFusion programming language in order for all study sites to enter data via a web browser into Oracle, a remote state-of-the-art relational database management system. This configuration streamlines updates by which all changes by programmers and system users made in the CMS occur in real time, limiting the interruption to study implementation.
The CMS database is physically located within the DPHS servers and provides optimal database technology for ensuring data integrity, security, and manageability. DPHS maintains detailed specifications and testing plans to validate the functionality of each application. Furthermore, a comprehensive risk analysis is completed prior to development of an application to proactively identify and correct security vulnerabilities. Before deploying an application to production, Penn State University's Office of Information Security conducts a vulnerability scan and any risk findings are remediated. Once deployed, the applications and databases are proactively monitored using the customized scripts and error notifications, enterprise monitoring tools, and vulnerability scans.
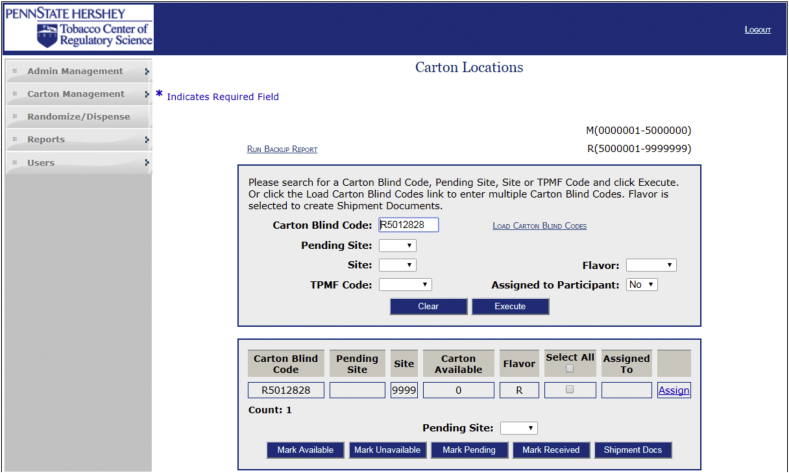
Based on the different facets of the management of the research cigarettes, multiple modules were designed for the CMS application -- Cigarette Carton Management, Randomization and Dispensation, Reports, Administrative Management, and Users. The Carton Management module allows the administrator to add cigarette cartons into inventory using a carton blind code number after they are received from RTI. Cartons can be moved within the system into a study site's inventory for dispensing when shipments are received by the site. The carton blind code can be queried to trace a carton location (e.g. site/storage location) and confirm a carton's status (e.g. dispensed to a participant/available at the site) (Fig. 2). Once a participant is enrolled into the study, the Randomization and Dispensation module allows the user to enter a participant into the system and dispense cigarettes at study visits. Upon entering a participant into the CMS, they are assigned a cigarette treatment group within the system determined by a preloaded randomization assignment table. The Reports module is useful in assisting the CMS administrator in managing and projecting the inventory needs at the study sites by cigarette strength and flavor. The Administrative Management module allows the CMS administrator to terminate participants no longer enrolled and change the cigarette inflation value (Inflation value discussed on page 15) as cigarette usage fluctuates. Finally, the Users module allows the CMS administrator to add/change and remove users and set user level access within the system. The role constraints are limited based on appropriateness. For example, the study coordinator role can randomize participants, dispense cartons, and terminate participants but does not have access to any unblinded level information. All actions performed by a user are logged to provide an audit trail.
Fig. 2.
A screen capture of the search capability of the CMS to be able to track cartons by their carton blind code number. In addition cartons can be searched by study site, nicotine strength, flavor, and PID.
2.6. Blinding of the research cigarettes
Once the research cigarettes arrive at Penn State, the blinding of the research cigarettes is completed by unblinded research staff only in a controlled setting. To begin the blinding process, a label sheet is generated using a random number generator and a bar code generator for each carton (Fig. 1). The random number generator produces the carton blind code number that contains an ‘M’ or ‘R’ for menthol or regular flavor followed by a 7 digit number string. The blind code numbers are printed on sticker adhesives and are physically attached to the front of the carton and each cigarette pack within the carton. All the manufacturer's labeling is removed from the cartons. The carton blind code number along with the corresponding nicotine content, RTI log number, and batch number of the carton is entered into the CMS. The link between the blind code number and the nicotine content of each carton is housed in the CMS to allow the system to dispense appropriate cartons to participants in a blinded fashion. Each carton is packaged with a sheet of 10 additional labels of the same blind code labels to be affixed to the individual packs within the carton (if needed). After the labeling has been done, each individual pack undergoes a quality control check by a Registered Pharmacist who verifies the blind code label corresponds with the correct nicotine strength code, RTI log number, and batch number and verifies the entry into the CMS. This verification process includes reviewing the accuracy of the labels attached and any discernible damage to the pack or cartons including the quality of the printed label. In cases when there is a damaged pack, the pack in question along with the entire carton is removed from the CMS and is no longer available for dispensing to research participants. After the pharmacist, a secondary step is initiated by a different individual (independent secondary verification) where the quality check is verified. Paper records of the shipping documentation, blind code labels, RTI log numbers, and how they are linked are kept for auditing purposes.
Fig. 1.
The carton blind code number and the bar code (label on the left) are generated using a random number generator and a bar code generator. The bar code facilitates quick and accurate entry into the CMS. The right label goes on each of the 10 individual cigarette packs in a carton. The sides of each carton have the blind code and bar code label on them and the front has same information as the individual packs within the carton. The CMS administrator fills out the participant identifier number (PID) portion when the carton is dispensed.
2.7. Entering cigarette inventory into the CMS
When the cartons have been blinded and the quality control checks are complete, the cigarettes are entered into the CMS using the CMS Carton Management Module. All cartons are initially added to the CMS as part of an overall inventory (example site 9999). The cigarettes are then moved into site-specific inventories (example site 1888 or site 1889) within the CMS (based on the storage capacities and needs of the individual site) and shipped to the sites for dispensing to research participants. Once the cartons are physically received by the site the CMS administrator changes the status of the cartons within the CMS to make them available for dispensing to the participants.
2.8. Entering participants into the CMS
Eligible and consented participants are asked at the first visit to fill out a daily cigarette log while smoking their usual brand of cigarettes, during the first baseline phase, which is used to evaluate their cigarette consumption. This method is considered to provide more accurate information than asking participants to estimate cigarettes smoked per day from memory, but the validity depends on the extent to which logs are filled out accurately. When the participant returns one week later for the first dispensing visit, the participant's identifier number (PID), average cigarettes per day (CPD) from the participant's daily cigarette log, and preferred cigarette flavor is entered into the CMS using the CMS Randomization and Dispensation Module. Once the participant's information has been entered at the first dispensing visit, this information remains for the participant's entire study enrollment period. In addition, the participant is assigned into a cigarette treatment group upon their first entry into the CMS using a preloaded randomization assignment table generated beforehand by a statistician.
2.9. Dispensing packs to participants
To calculate the number of packs to dispense to a participant, the following calculation is used within the CMS: Maximum number of days in the visit window * Baseline CPD * 150% (inflation value)/20. The number of packs is rounded up to the nearest whole pack. Table 1 shows examples of the number of cigarettes packs dispensed by baseline CPD and visit number. At each visit the CMS identifies the cigarette strength to be dispensed to the research participant by using the randomization assignment and visit number (Table 2). In the end, the CMS assigns a list of carton blind code numbers to be removed from storage and dispensed to the participant. The cellophane bag is removed and labels containing the PID and visit number are placed on each pack in the assigned carton. If less than 10 packs are assigned from a carton, the unassigned packs are destroyed at the time of dispensation. The dispensing record may be printed after every assignment is made for the participant's file.
Table 1.
Calculation table used by the CMS to determine the number of cigarette packs needed based on visit number, number of days in the visit window, and the cigarettes smoked per day by the participant. Number of packs is rounded up to the nearest whole number. Number of packs dispensed based on visit number and days in each visit window.
| Visit number | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|
| Number of days in visit windowa | 14 | 21 | 21 | 21 | 21 | 21 | 21 | 28 | 56 |
| Baseline cigarettes per day | Number of packs dispensed | ||||||||
| 5 | 6 | 8 | 8 | 8 | 8 | 8 | 8 | 11 | 21 |
| 6 | 7 | 10 | 10 | 10 | 10 | 10 | 10 | 13 | 26 |
| 7 | 8 | 12 | 12 | 12 | 12 | 12 | 12 | 15 | 30 |
| 8 | 9 | 13 | 13 | 13 | 13 | 13 | 13 | 17 | 34 |
| 9 | 10 | 15 | 15 | 15 | 15 | 15 | 15 | 19 | 38 |
| 10 | 11 | 16 | 16 | 16 | 16 | 16 | 16 | 21 | 42 |
Number of days in visit window represents the number of days until the next visit.
An inflation value of 150% was incorporated into the CMS to cover rescheduling of the study visits or changes in the amount smoked from baseline. Fluctuations in cigarettes smoked per day was anticipated due to inaccuracies in reported baseline CPD, the acceptability of the research cigarettes, or increases in cigarette use due to possible compensatory smoking of reduced nicotine content cigarettes. The CMS was built with the capability to allow changes to the cigarette inflation rate or un-assign cigarette cartons if the number of packs to dispense to a participant needed to be increased or decreased.
Once the cigarettes are ready for delivery to the participant, a second person must initial and date that (s)he has verified the carton blind code numbers from the CMS match the carton and packs being dispensed before the cigarettes leave the supply storage area. This is an additional quality control measure which helps prevent incorrect strengths of cigarettes being dispensed erroneously. The CMS also has mechanisms for performing accountability. The CMS administrator enters the PID and visit number and then has to manually enter the actual number of cartons and packs given in the fields shown beside the carton number listed in the system. If there is any deviation between the numbers that were originally assigned by the CMS and the number of packs actually dispensed, a message flashes on the screen alerting the user that the numbers do not equal and a comment is required if there are discrepancies.
2.10. Destruction of cigarette packs
When the cigarette packs are delivered to the participant appointment, the study coordinator will collect any and all packs that were previously dispensed for destruction and disposal by the CMS administrator as defined by pharmacy protocol. Specifically, based on recommendations from RTI, research cigarette packs are discarded in biological waste containers that are autoclaved before being disposed as medical waste ensuring that it cannot be retrieved and used for unintended purposes. Destroying unused packs is due in part to the Good Clinical Practices followed in the pharmaceutical industry where any medication once dispensed is never returned into stock inventory.
2.11. Tracking and management of inventory
Another feature of the CMS is its ability to track cigarette cartons in real time. Any carton blind code that has ever entered the inventory can be queried by the CMS to identify its location, availability, and dispensation record (Fig. 2). This functionality is extremely useful for accountability records and audit purposes.
The CMS administrator is responsible for calculating the inventory needs of all the remote sites and shipping supplies to them in a timely manner, accounting for enrollment rates, storage capacity, and shipping transit times. These complex calculations are made easier with the help of the CMS reports that can quickly project the cartons needed by cigarette strength and flavor. The CMS is able to generate past randomization reports as well as future randomization reports for a particular participant, in addition to site specific usage and inventory reports using the CMS Reports Module.
2.12. Replenishment ordering of research cigarettes
As mentioned, the CMS administrator must take into account considerations such as the limitations of storage space and delivery time when orders are placed with NIDA and RTI for SPECTRUM cigarettes. NIDA may then take up to four weeks to review the order request prior to authorizing the supply chain distribution at RTI to fulfill the order. RTI will forward Order and Assurance forms to the requestor for completion prior to processing the shipment. It can take up to two weeks for RTI to process the order once the forms are returned to them. Shipments include a NIDA Drug Inventory and Control System Shipping Document which must be signed and returned to RTI within 48 h of shipment receipt. This document includes the quantities of cartons shipped specifying the cigarette strengths, bar code numbers, and lot identifiers. Shipments also include data sheets that should be retained for reference on cigarette specifications. Cigarettes are shipped overnight from RTI usually on Thursdays.
Updated projections should be sent to the NIDA Drug Supply Program to notify them of changes to the initial inventory request based on trends, current usage, and anticipated future needs. Our initial inventory request used the assumptions of a 50/50 menthol to non-menthol flavor preference and an average of 20 CPD smoked for each participant, but as enrollment began the actual type and quantity of cigarettes smoked per day differed from initial estimates. The CMS is useful in calculating the projection updates based on actual research cigarettes smoked per day and menthol/non-menthol flavor ratio from the currently enrolled sample. Additional factors that affected cigarette inventory requests in our trials were the amount of waste from unopened packs and whether participants chose to make a quit attempt or continue to smoke research cigarettes in the Treatment Choice Phase of the study. These practical factors all lead to constraints on the availability of cigarettes through the NIDA Drug Supply Program.
2.13. Interstate shipping
For investigators shipping research cigarettes to sites in different states, shipment and receipt of cigarettes may require legal licenses and waivers depending on state and federal laws pertaining to the Jenkins Act. The United States Postal Service will not mail tobacco or smokeless tobacco as per 18 U.S. Code § 1716E-Tobacco products as nonmailable (with some exceptions). We obtained a tobacco product license through the Pennsylvania Department of Revenue which is a license required to receive or distribute cigarettes. A waiver from the imposition of excise taxes for the purposes of receipt and distribution of research cigarettes was also obtained. Evidence of this documentation may be required by commercial shippers (e.g. United Postal Service/Federal Express) for both receipt and return of unused cigarette product to the principal site depending on the state. The shipment of research cigarettes between two institutions is facilitated by a Materials Transfer Agreement signed by the authorized individuals at each institution. When shipping supplies to remote sites it is desirable to avoid shipments near the end of the week or weekends in case of interruptions in transportation as well as lack of study staff to receive shipments.
2.14. Training
Our protocol treats cigarettes as investigational drugs and therefore, the same high standard of quality control and training is expected. Standard operating procedure manuals for the CMS and dispensing process were developed between the application development team and the TCORS pharmacy staff for referral and training. Users were trained on how to access the system and correctly document cigarette dispensing activities based on their roles in the study. Additional training was done for troubleshooting problems from the remote study sites, such as handling randomization and dispensation in the event the CMS is inaccessible. In addition to written manuals, hands-on training was done using mock participant dispensing and a separate CMS for training purposes. All members of the TCORS pharmacy team have completed the CITI training in Good Clinical Practices and Human Subjects Protection which provides basic research training. Documentation of study training is filed along with the regulatory documents for each project.
3. Discussion
This manuscript was written to provide a guide for investigators using investigational tobacco products in clinical research studies in tobacco regulatory science. Research involving tobacco regulatory science and investigational tobacco products is expected to continue with funded research opportunities available to investigators. Some of the key recommendations outlined in this paper cover the details of designing a computer-based management system, proper shipping and documentation, the importance of quality control when managing a blinded cigarette inventory, and to diligently monitor inventory by taking into account dispensing trends and shipping timelines.
While we have highlighted and discussed many features of the CMS that make it a robust inventory management tool, there are real-world implementation elements that should be taken into consideration in any study protocol. The CMS is a dynamic system in that it allows the administrator flexibility to manually enter changes to account for exceptions or contingencies. For example, a study site may not have access to freezers to store the cigarettes. In this case the cigarettes supplied to them from the primary site at Penn State come with expiration dates (usually 1 year from receipt or from the day it was pulled from the freezers for shipping to the remote site). Since the carton blind codes are randomly assigned it is possible that a particular carton could expire before it is dispensed. Under such a scenario the CMS administrator is able to make certain cartons temporarily unavailable, forcing the CMS to dispense older stock. In addition, the CMS allows the administrator to manually dispense additional cigarette packs to a participant to hold them until their next visit if they run out of cigarettes or misplace them. Lastly, after each dispensation, the CMS re-calculates the cartons available and generates a backup randomization report across all sites. This unblinded report is sent to an email alias that is accessible to unblinded staff only and contains carton blind codes by nicotine strength and flavor that are available in the system. The back-up randomization report can be used by the unblinded CMS administrator to make manual assignments if the CMS is unavailable during a participant visit either due to planned maintenance outage, internet connectivity issues, or any other unforeseen failure. If manual assignments are made, the unblinded CMS administrator must manually enter this information into the CMS when it becomes available.
Some limitations with implementing a system like this are the expertise and the time available to the research investigator when building the system. Secondly, the cost of building a system should be incorporated into the cost of running the trial up front to ensure the resources are available. Finally, our CMS tracked cartons versus individual packs and in some respects tracking packs would have provided more accuracy with usage.
Over 160,000 SPECTRUM research cigarette packs have been dispensed by the CMS in our trials over the past three years. The CMS provided ease in managing inventory, quality control, and accountability across sites that would have otherwise been expensive and time consuming to achieve. The robustness of the system accommodated changes in cigarette supply issues and real-world research implementation issues within the trials. For research investigators looking to implement a similar inventory management system the benefits can increase the quality and productivity of their research.
Funding
Supported by the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration under Award Number P50 DA036107 and U54 DA031659. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. Participants at the Penn State University site are seen at the Clinical Research Center supported by the Penn State Clinical & Translational Research Institute, Pennsylvania State University CTSA, NIH/NCATS Grant Number UL1 TR000127 and TR002014.
References
- 1.U.S. Department of Health and Human Services . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. The Health Consequences of Smoking: 50 Years of Progress: a Report of the Surgeon General. [Google Scholar]
- 2.Jamal A.K.B., Neff L.J., Whitmill J., Babb S.D., Graffunder C.M. Current cigarette smoking among adults — United States, 2005–2015. MMWR Morb. Mortal. Wkly. Rep. 2016;65:1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 3.The Family Smoking Prevention and Tobacco Control Act: Hearing before the Subcommittee on Health of the Committee on Energy and Commerce, House of Representatives, One Hundred Tenth Congress, First Session, on H.R. 1108. US GPO: For sale by the Supt. of Docs; Washington, DC: 2007. [Google Scholar]
- 4.Benowitz N.L., Henningfield J.E. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N. Engl. J. Med. 1994;331(2):123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 5.Richter P. Characterization of SPECTRUM variable nicotine research cigarettes. Tob Regul Sci. 2016;2(2):94–105. doi: 10.18001/TRS.2.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donny E.C. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 2015;373(14):1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatsukami D.K. Dose-response effects of spectrum research cigarettes. Nicotine Tob. Res. 2013;15(6):1113–1121. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins S.T. Response to varying the nicotine content of cigarettes in vulnerable populations: an initial experimental examination of acute effects. Psychopharmacology (Berlin) 2017;234(1):89–98. doi: 10.1007/s00213-016-4438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krebs N.M. Reduced nicotine content cigarettes in smokers of low socioeconomic status: study protocol for a randomized control trial. Trials. 2017;18(1):300. doi: 10.1186/s13063-017-2038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen S. A two-site, two-arm, 34-week, double-blind, parallel-group, randomized controlled trial of reduced nicotine cigarettes in smokers with mood and/or anxiety disorders: trial design and protocol. 2017;17 doi: 10.1186/s12889-016-3946-4. [DOI] [PMC free article] [PubMed] [Google Scholar]