Abstract
Arsenic (As) is a widely existing metalloid in the biosphere. Drinking water contamination by arsenic is a major route of human exposure, either by natural means or through industrial pollution. Numerous evidence form earlier reports suggest that arsenic exposure causes cerebral neurodegeneration which initiates behavioral disturbances concomitant to psychiatric disorders. Also, mood disorders in humans as well as in animals correlate with arsenic exposure; the present study is carried out to implore the neuroprotective potential of thymoquinone (TQ) in arsenic-stressed rats. TQ is an active component of Nigella sativa (Kalonji) seed oil. Arsenic exposure in the form of sodium arsenate (10 mg/kg/day; p.o) caused neurobehavioral deficits as evidenced by changes in locomotion and exploratory behavior in open-field and elevated plus maze tasks. Alongside this, arsenate also elevated hippocampal oxidative stress parameters like lipid peroxidation (TBARS) and protein carbonyl formation with a decrease in superoxide dismutase (SOD) and reduced glutathione (GSH) content. Genotoxicity assessment by Comet assay also showed prominent levels of DNA damage. Furthermore, arsenic also elevated hippocampal cytokine levels, TNF-α and INF-γ. However, TQ supplementation (2.5 and 5 mg/kg/day, p.o) preceded three days before arsenic administration, significantly attenuated arsenic-associated anxiogenic changes which majorly attributed to its antioxidant and anxiolytic potential. Also, TQ pre-treated rats expressed positive shifts in the hippocampal oxidative stress and cytokine levels with decreased DNA fragmentation. Thus, this study concludes that TQ might serve as a strong therapeutic agent for management of anxiety and depressive outcomes of arsenic intoxication.
Keywords: Toxicology, Neuroscience
1. Introduction
Arsenic positions first on the US government's Priority List of Hazardous Substances, given its lethality and its common occurrence in nature (Chou and De Rosa, 2003). Arsenic is a metalloid that is ranked first in a list of 20 hazardous substances by the Agency for Toxic Substances and Disease Registry and the United States Environmental Protection Agency (ATSDR, 2017).
It has been for the most part acknowledged that arsenic exposure in nature is causing a huge worldwide medical issue. In addition to causing health concerns, Arsenic in nature also disturbs ecological balance and leads to costly cleanup operations burdening our economy. Arsenic exposure and its related lethality is a more major issue in developing nations because of inadequate municipal consumable water supply, and consequently, various populations opted for sinking tube wells which get contaminated by geogenic arsenic (Gebel, 2000).
The lethality and assimilation of arsenic are subject to its chemical state. Studies conducted on humans and animals reveal that more than 90% of the ingested dosage of inorganic form of arsenic (trivalent or pentavalent) gets absorbed from the gastrointestinal tract. Severe levels of Arsenic poisoning can bring about acute encephalopathy (cerebral edema), including peripheral neuropathy (motor dysfunction, long Axon Wallerian degeneration, paresthesia), cognitive impairment and delirium, convulsions, paralysis, coma, and death (Morton and Caron, 1989). A headache, lethargy, hallucinations, seizures can also occur. Peripheral neuropathy resembling Gullian-Barre-Strohl syndrome (LGBS) (ascending flaccid paralysis) is common (Jha et al., 2002). First to appear are the sensory symptoms like 'pins and needles' or electric stun like a pain in lower extremities, which is often followed by numbness and motor dysfunction. Polyneuropathy was additionally detailed by Guha Mazumder et al. (1998) in Indian people exposed to increased levels of arsenic in drinking water.
Sub chronic poisoning causes focal sensory system shortfalls like hearing loss, vision problems, irregular EEGs, mental impediment, epilepsy. Arsenite has been reported to cause neural tube defects more effectively than arsenate (Chaineau et al., 1990). Neurological defects (like peripheral nerve dysfunction) showed by anomalous nerve conduction velocity are common symptoms in smelter laborers exposed to arsenic (Blom et al., 1985).
Nigella sativa L. (NS) is a yearly blooming plant local to various districts of southern Europe and Asia. The blossoms are light blue and white in shading with little dark seeds. In conventional pharmaceutical, its seed is known for their awesome restorative esteem and broadly utilized as a part of the Middle East and the Far East to treat illnesses. Thymoquinone (TQ; 2-isopropyl-5- methyl-1, 4-benzoquinone) is the prime component of the essential oil obtained from Nigella sativa seeds. A few reports are showing that TQ has antioxidative, mitigating, antitumor, antiulcerogenic, chemopreventive, and neuroprotective properties (Ashraf et al., 2011; Nagi et al., 2010). The defensive impact of TQ is ascribed to its free radical scavenging action (Badary et al., 2003). The capability of TQ to ensure the survival of dopaminergic neurons in cell culture against MPP+ and rotenone cytotoxicity is a recent finding (Radad et al., 2009).
Our aim in this study was to investigate the neurotoxicological outcomes of inorganic arsenic exposure in rats. The dose range of arsenic selected at 10 mg As/kg/day is higher than the usual human exposure level, although it may be relevant to unexpectedly high exposures from drinking water which may occur accidentally or from workplace settings (OSHA, 2004). Rats are reportedly less sensitive to inorganic forms of arsenic as compared to humans, due to differences in kinetics and metabolism. In rats, a higher proportion of inorganic arsenic will be bound to the erythrocytes resulting in less free arsenic level in plasma and body fluids. Further, the methylation rate which determines the detoxification of arsenic in rat liver is almost 10 times higher than that in human liver (Styblo et al., 1999). In light of the favorable impacts associated with TQ administration, the present study endeavored to explore the adequacy of TQ against arsenic-related learning and memory impairment and oxidative stress associated hippocampal toxicity in male Wistar rats.
2. Materials and methods
2.1. Chemicals and reagents
Thymoquinone, corn oil and sodium arsenate were purchased from Sigma–Aldrich Co. (St. Louis, MO). Reduced glutathione (GSH), 5,5-dithiobis-2-nitrobenzoic acid (DTNB), thiobarbituric acid (TBA), trichloroacetic acid (TCA), epinephrine, agarose low EEO and all routine reagents were purchased from Merck India Pvt. Ltd. CBA kits for TNFα (Cat no. 558309) and INF-γ (Cat no. 558305) were purchased from BD Biosciences, USA.
2.2. Animals
Male Wistar rats (body weight 200 ± 25 g) were used for the current study. Animals were procured from the Central Animal House facility of Jawaharlal Nehru Medical College (JNMC), Aligarh Muslim University and housed in cages maintained at temperature (25 ± 1 °C) and humidity (60 ± 10%) along with proper ventilation and illumination (a 12-h dark and 12-h light cycle). Rats were provided with ad libitum water and standard animal feed procured from Ashirwad Industries, Chandigarh. All experiments were approved by Institutional Animal Ethics Committee, J.N Medical College, Aligarh Muslim University as per Committee for the Purpose of Control and Supervision of Experiments on Animals [CPCSEA guidelines (Registration no. 401/RO/C/2001/CPCSEA), Government of India].
2.3. Experimental design
Rats divided into four groups with 6 animals in each group randomly: Group 1: Vehicle only (Control), Group 2: Arsenic in the form of sodium arsenate, 10 mg/kg/day; through gavage for 8 days (As only), Group 3: TQ (2.5 mg/kg/day; oral gavage) pretreatment for 3 consecutive days followed by administration with Arsenic up to 11th day (As + TQ2.5), Group 4: TQ (5 mg/kg/day; oral gavage) pretreatment for 3 consecutive days followed by administration with Arsenic up to 11th day (As + TQ5).
As neurological implications of arsenic are known, and many chronic and sub-chronic exposures at a much higher dose as this have already been reported (Gora et al., 2014; Kassab and El-Hennamy, 2017); we used this exposure regimen of 11 days to mimic sub-acute exposure which might occur accidentally. Dosing regime was planned to have same endpoints and animals were sacrificed on the 12th day. TQ and As dose schedules were based on the pilot studies and previously published reports (Bhattacharya and Haldar, 2012; Das et al., 2010; García-Chávez et al., 2006; Hosseinzadeh et al., 2007; Li et al., 2015; Rodríguez et al., 2005).
2.4. Analysis of anxiolytic effect of thymoquinone on arsenic induced behavioral alterations
Behavioral tests were done on the 12th day of study. The behavioral trials were performed between 9.00 a.m. to 4.00 p.m. The tests were implemented and evaluated by a researcher blind to the experimental groups and dosing regimen.
2.4.1. Open field test
Open field arena (60 × 60 cm), with black floor was divided into 15 × 15 cm squares, fenced by continuous 30-cm-high walls made of black wooden board. In the test, squares adjoining to the wall represent a secure area named ‘field periphery,’ whilst other squares represent an exposed field or ‘area center.’ The test was commenced by placing a single rat in the center of arena and was allowed to move freely for 5 minutes. Behavioral pattern was constantly videotaped and scored using ANY-maze, V4.3 (Steolting IL, USA). The maze was carefully cleaned with 70% ethanol after every trial.
2.4.2. Elevated plus-maze
Elevated plus-maze (EPM) comprised of two open arms and closed arms (30 × 5 cm) each, enclosed in 15 cm-high walls and central platform (5 × 5 cm). The apparatus was raised 45 cm above the ground and the test begun by placing the rat on central platform, facing one of the open arms. Each session lasted 3 minutes. Rat's behavior was continuously videotaped and scored using ANY-maze, V4.3 (Steolting IL, USA). The maze was carefully cleaned with 70% ethanol after each test.
2.5. Biochemical and enzymatic assays
2.5.1. Tissue preparation for biochemical analysis
After the completion of dosing regime, animals were anesthetized by using 50 mg/kg pento-barbital and sacrificed by cervical dislocation. For general clinical observations, blood was collected through direct heart puncture. Brains were dissected and rinsed in ice-cold saline prior to harvesting hippocampus. Post-harvest, hippocampi were immediately homogenized (5% w/v) in Potter-Elvehjem PTFE pestle and glass tube homogenizer (GW124, Himedia labs) in Tris-HCl buffer (50 mM, pH 7.4). Resulting homogenate was centrifuged at 1500 g, and the supernatant (S1) was collected. A small portion of the S1 fraction was used in LPO estimation. Moreover, remaining homogenate was centrifuged at for 20 min on 10,000 g at 4 °C to prepare S9 fraction, which was used for the assessment of GSH, PC, and SOD.
2.5.2. Assay of TBARS level as lipid peroxidation (LPO) marker
LPO levels were assessed as per the procedure described by Mihara and Uchiyama (1978) with minor modifications. Detection of LPO in tissues relies on estimation of thiobarbituric acid reactive species (TBARS), which is mostly malondialdehyde. Briefly, the reaction mixture included 0.25 ml sample, 10 mM BHT, 3 ml ortho-phosphoric acid (1% w/v) and 1 ml TBA (0.67%). The resulting concoction was then incubated at 95 °C for 45 min. The absorbance of the supernatant was measured at 535 nm. LPO was determined as n moles of TBA-reactive substances (TBARS) formed/h/g of tissue using 1.56′105 M−1 cm−1 as molar extinction coefficient.
2.5.3. Measurement of protein carbonyl (PC)
Oxidative protein damage was assessed by the method explained by Floor and Wetzel (1998) with slight modifications. Quantification of carbonyl fractions was based on their reaction with 2′, 4′-dinitrophenyl hydrazine (DNPH). Quickly, proteins were precipitated by the addition of 40% trichloroacetic acid (TCA). Content of carbonyl moieties were measured spectrophotometrically at 340 nm. Results were presented as nmol of DNPH combined per mg protein based on the molar extinction coefficient of 22,000 M−1 cm−1.
2.5.4. Measurement of reduced glutathione (GSH)
The GSH content was analysed by using the method of Jollow et al. (1974) as revised by Zafeer et al. (2012). Reaction was based on the reactivity of thiol group (-SH) present in GSH reacts with DTNB to form TNB. Absorbance of samples was measured at 412 nm spectrophotometrically. Results were presented as nmoles of DTNB conjugated per mg protein.
2.5.5. Measurement of Superoxide Dismutase (SOD) activity
SOD activity was measured according to the method described by Misra and Fridovich (1972). The method relies on the ability of SOD to prevent autooxidation of epinephrine at alkaline pH. Briefly, the reaction mixture contains 50 mM glycine buffer (pH 10.4), 0.2 ml S9 fraction and epinephrine. Activity of SOD was analysed at 480 nm and the results were expressed as nmol of (−) epinephrine protected from oxidation per minute per milligram protein using a molar extinction coefficient of 4,020 M−1 cm−1.
2.5.6. Estimation of protein content
The protein content in the sample was determined using the method of Lowry et al. (1951) using BSA as standard.
2.6. Preparation of single cell suspension for comet assay
Freshly isolated hippocampi were kept in cold Hibernate A® medium (supplemented by B27® serum free supplement and Glutamax-I). Single cell suspension was obtained mechanically by trituration using a pipette and was passed through 70 μm cell strainer and then centrifuged at 1500 RPM for 10 min at 4 °C, supernatant was discarded, and pellet was again gently suspended in complete Hibernate A® media.
2.6.1. Assessment of DNA fragmentation by comet assay
Comet assay technique was used for the assessment of DNA fragmentation as described by Singh et al. (1988) with some modifications. Single cell suspensions were mixed with 0.5 % low melting point agarose (LMPA) and overlaid on slides pre-coated with 1% normal melting agarose (NMA). A sealing coat of 1 % LMPA was placed over cells to seal them. Slides were submerged overnight in lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Trizma base, 0.2 mM NaOH, 1% Triton X-100 and DMSO; pH 10) at 4 °C followed by an alkali unwinding solution (300 mM NaOH, 1 mM EDTA, pH > 13) for 20 minutes. Electrophoresis was done at 25 V, 300 mA in TBE buffer for 45 min. Dried slides were stained with propidium iodide (1X). Photographs were captured at 40X using Nikon Eclipse Ci-L fluorescence microscope. Comets were selected and analyzed with Cometscore TM software (version 1.5, TriTek Corporation, Sumerduck) in a single blind manner. The amount of DNA damage was assessed by tail length and percent DNA in the tail.
2.7. Flow cytometric bead assay (CBA) for TNFα and IFNγ
Proinflammatory cytokines, TNFα and IFNγ, were analyzed in hippocampal tissue lysates and quantified as per instructions provided with the CBA flex kit (BD Biosciences, U.S.A). The acquisition was done on LSR-II Flow Cytometer at BD FACS Academy, Jamia Hamdard, New Delhi, India and analysis was done using FCAP ARRAY software version 3.0 (BD Biosciences, U.S.A.). The levels of TNFα and IFNγ were quantified as pg/ml of tissue lysate.
2.8. Statistical analysis
Results are presented as Mean ± standard error (SE). Data analysis was done using one-way analysis of variance (ANOVA) and Tukey's as a post hoc test. Values of p ≤ 0.05 were considered significant. All the statistical analysis were made using GraphPad Prism7 (GraphPad Soft. Inc., SD, CA).
3. Results
3.1. Anxiolytic effect of thymoquinone against arsenic induced changes in OFT and EPM
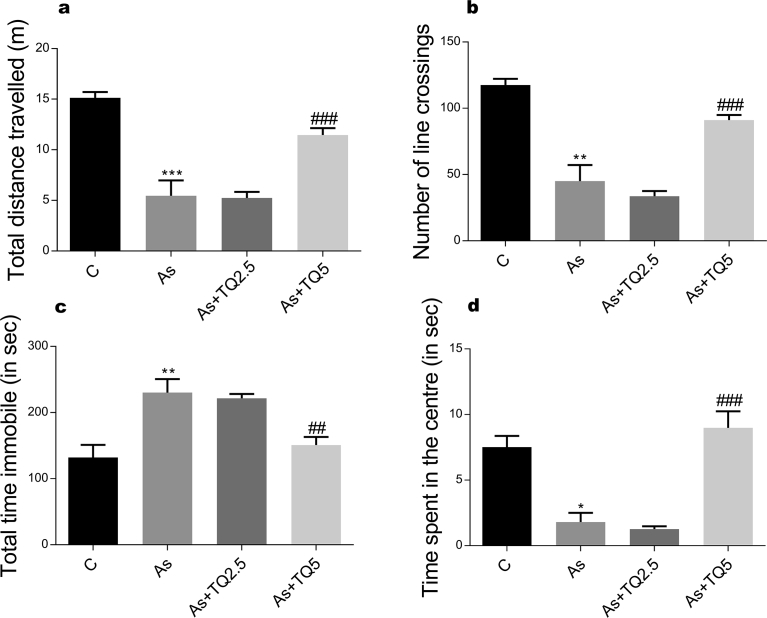
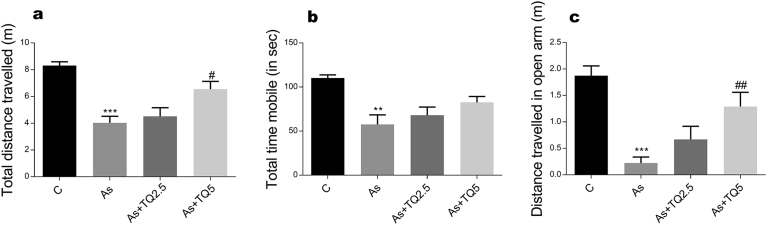
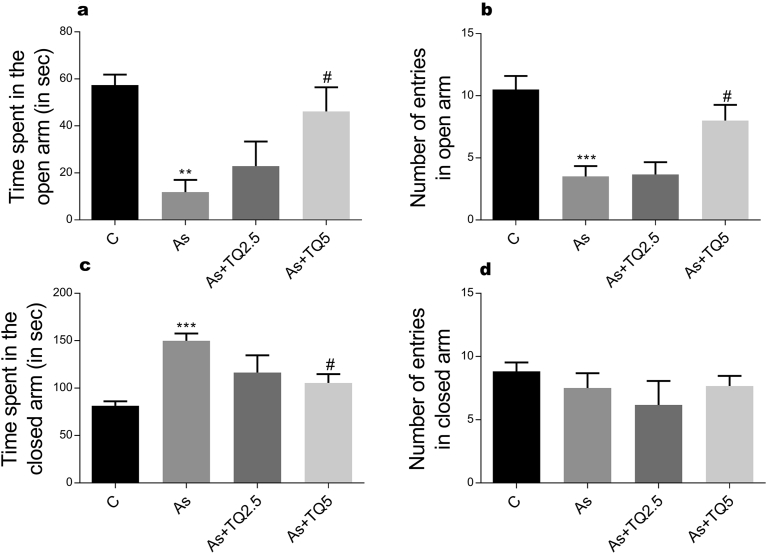
Our study shows that arsenic intoxication induces anxious behavior in rats. This is evident from decrease in the total distance travelled in both OFT and EPM; significantly less time spent in the centre of arena in comparison to the peripheral area; increase in freezing episodes, freezing time in OFT and decrease in entries and time spent in the open arms of EPM in contrast to the animals in the control group (Figs. 1, 2, and 3). Anxiolytic effect of thymoquinone was much obvious on pretreatment as its supplementation has significantly increased the total exploratory behavior by enhancing total distance traveled in OFT and EPM along with an increase in the time spent and the number of entries in the open arm of EPM, which depends heavily on thigmotaxis.
Fig. 1.
The effects of arsenic (10 mg/kg/day) and TQ (2.5, 5 mg/kg/day) in the open field test (n = 6 in each group) with each graph representing (a) Total distance traveled, (a) Total mobile time, (b) Number of line crossings, (c) Total immobile time, and (d) Time spent in the central zone. Values are shown as Mean ± SEM. *, ** and *** indicate p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001 versus Control while ## and ### indicate p ≤ 0.01 and p ≤ 0.001 versus As-treatment respectively.
Fig. 2.
The effects of arsenic (10 mg/kg/day) and TQ (2.5, 5 mg/kg/day) on EPM test on the 12th day of exposure. Graphs showing: (a) Total distance traveled, (b) Total mobile time, and (c) Distance traveled in the open arm. Values are expressed as Mean ± SEM. **, *** indicate p ≤ 0.01 and p ≤ 0.001 versus Control while #,## indicates p ≤ 0.05 and p ≤ 0.01 versus As-treatment respectively.
Fig. 3.
The effects of arsenic (10 mg/kg/day) and TQ (2.5, 5 mg/kg/day) on EPM test on the 12th day of exposure. Graphs showing: (a) time spent in the open arm, (b) Number of entries in open arm (c) time spent in the closed arm, and (d) Number of entries in closed arm were evaluated during 3-minute test period. Values are expressed as Mean ± SEM. *, ** and *** indicate p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001 versus Control while # and ## indicate p < 0.05 and p < 0.01 versus As-treatment respectively.
3.2. General clinical observations
Control: RBC's are spherical and fully saturated with hemoglobin; WBC's (TLC∼18,000 cells/mm3, DLC-N15, E1,L18,M3); platelets are seen in clumps (∼600 × 103/mm3).
Arsenic: RBC's show distorted shape with occasional macrocytes and frequent echinocytes seen. Few tear drop cells are also seen. Haemoglobinization is normal. WBC's (TLC∼21,000 cells/mm3, DLC-N10, E6,L84,M0); platelets are seen in clumps (∼500 × 103/mm3).
As + TQ2.5: Few spherical RBC's mixed with echinocytes, macrocytes and tear drop cells are seen. Haemoglobinization is normal. WBC's (TLC∼19,000 cells/mm3, DLC-N12, E6,L82,M0); platelets are seen in clumps (∼650 × 103/mm3).
As + TQ5: larger number of normal appearing RBC's are seen with frequent spherocytes mixed with echinocytes and macrocytes. Haemoglobinization is normal. WBC's (TLC∼20,000 cells/mm3, DLC-N10, E2,L88,M0); platelets are seen in clumps (∼650 × 103/mm3).
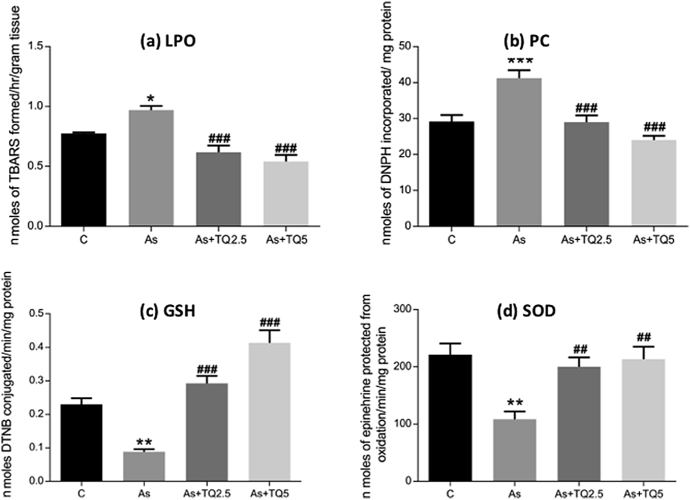
3.3. Effect of thymoquinone on arsenic-mediated ROS generation
Oxidative stress biomarkers such TBARS assay, PC, SOD activity and GSH content were investigated in the hippocampus of rats. Arsenic intoxication has significantly increased lipid and protein oxidation levels (Fig. 4a, b) along with a decrease in the GSH content and SOD activity (Fig. 4c, d). Pretreatment with thymoquinone significantly restored GSH content and SOD activity in a dose-dependent manner and caused a simultaneous decrease in the lipid and protein oxidation products (Fig. 4).
Fig. 4.
Graphs showing effect of Arsenic (10 mg/kg/day) and TQ (2.5, 5 mg/kg/day) on (a) LPO, (b) PC content, (c) GSH, (d) SOD activity in the hippocampus of rat. Each value represents mean ± SE (n = 6). Significant differences are indicated by Mean ± SEM. *, ** and *** indicate p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001 versus Control while ## and ### indicate p ≤ 0.01 and p ≤ 0.001 versus As- treatment respectively.
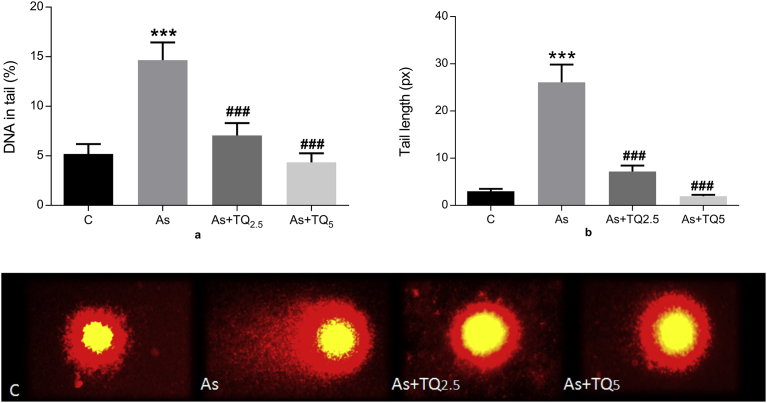
3.4. Effect of thymoquinone against arsenic-induced DNA damage
Single cell gel electrophoresis based comet assay analysis was performed for the assessment of genotoxic DNA damage. The length of comet tail as a result of DNA fragmentation was used as a measure of genotoxicity. Arsenic exposure caused a remarkable amount of DNA rupture in the hippocampal neurons, while thymoquinone pretreatment was able to mitigate the damage associated with it as shown in Fig. 5.
Fig. 5.
Graphs showing effect of Arsenic (10 mg/kg/day) and TQ (2.5, 5 mg/kg/day) on DNA damage in rat brain hippocampi cells as visualized by comet assay pictures, 40X. The values were derived from comet assay and expressed as Mean ± SE (n = 50). DNA content is estimated by (a) tail length and (b) percent DNA in the tail. Significant differences are shown as ***p ≤ 0.001 in comparison to Control and ###p ≤ 0.001 when compared to As-treatment respectively.
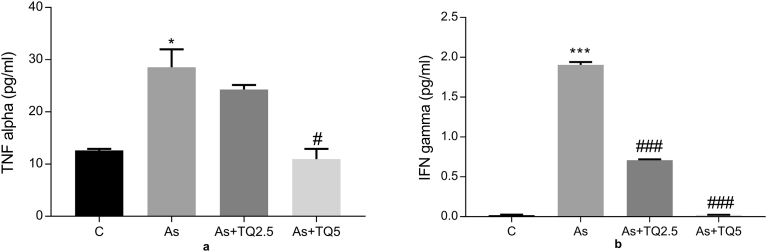
3.5. Effect of thymoquinone and arsenic on bead-based assay of pro-inflammatory cytokines-TNFα and IFNγ
Proinflammatory cytokine, TNF-α level in tissue supernatant of rat hippocampi was significantly higher *p ≤ 0.05 in the arsenic exposed group compared with control, while it declined dose dependently in the thymoquinone treated animals (Fig. 6a). Moreover, IFN-γ level showed a remarkable increase (***p ≤ 0.001) in the arsenic exposed group, which is a significant proinflammatory marker in arsenic neuropathies. Furthermore, treatment groups demonstrated dose-dependent decrease (###p ≤ 0.001) as shown in Fig. 6b.
Fig. 6.
Graphs showing effect of Arsenic (10 mg/kg/day) and TQ (2.5, 5 mg/kg/day) on (a) The level of TNFα in the hippocampal lysate of rat brain using CBA kit; results are expressed as protein levels of TNFα in pg/ml, and (b) The level of INFγ in the hippocampal lysate of rat brain using CBA kit; results are expressed as protein levels of INFγ in pg/ml. Significant differences are shown as ***p ≤ 0.001 in comparison to Control while #p ≤ 0.05 and ###p ≤ 0.001 when compared to As-treatment respectively.
4. Discussion
In the present study, negative affective behaviors along with oxidative stress, DNA fragmentation, and inflammation were observed in the hippocampal region of rat brain following administration of arsenic (10 mg/kg/day; p.o.). The current dose regime was selected according to previous reports as mentioned earlier to analyze the neurobehavioral and neurotoxicological outcomes of this toxicity with its protection with the active component (TQ) of a commonly used flavoring agent, N. sativa seeds. The protective efficacy of dual doses of TQ (2.5 and 5 mg/kg/day) were also assessed in relation to these parameters. In experimental models, acute exposure orally causes gastrointestinal and neurological effects. Oral LD50 for inorganic salts of arsenic ranges from about 10 to 90 mg/kg. Life term exposures of low doses of arsenic may result in improper functioning of CNS but these cannot be speculated until such studies are done. Earlier studies on chronic inorganic arsenic exposure in rodents revealed that it causes behavioral (BonakdarYazdi et al., 2017; Jing et al., 2012), morphological (Ríos et al., 2009), physiological, (Arslan-Acaroz et al., 2018) and neurochemical (Yadav et al., 2011) effects. Recent studies have also supported the neurodevelopmental toxicity associated with arsenic exposure (Aung et al., 2016; Sidhu et al., 2006). Similar doses of inorganic arsenic have also shown toxic effect on organs like liver and kidney that reported increased generation of reactive oxygen species and histopathological changes (Bali et al., 2016; Noman et al., 2015).
OFT and EPM are highly cited behavioral paradigms used in the assessment of anxiety (Anseloni and Brandão, 1997; Li et al., 2017; Pellow et al., 1985; Prut and Belzung, 2003). Rodents show aversion from open and illuminated spaces along with an innate drive to explore any new environment or perceived aggressive stimulus. The conflict between these two drives showcases the level of anxiety. Arsenic treated rats displayed a remarkable increase in the time spent in the closed arm and immobility time in EPM. Also, an increase in anxiety, the rats will show a preference for edges of arena or avoidance to enter the central zone of OFT as found in Arsenic conditioned rats. Such behavioral profile proposes that Arsenic encouraged anxiety-like behavior in rats. Our results are corroborating with the findings of Umezu et al. (2012) and Chang et al. (2015) in mice. Interestingly, thymoquinone supplementation showed a significant anxiolytic effect in arsenic conditioned rats as evidenced by an increase in the distance traveled and time spent in exploring the open arm in EPM along with an increase in some line crossings in OFT. Also, a significant increase in total distance traveled in both tests was found which shows enhancement in exploratory behavior. Mechanistic studies associated with arsenic-induced toxicity have established that it alters learning and memory in behavioral assessments and influences multiple neurobiological processes including neurogenesis and cholinergic, glutamatergic, and monoaminergic signaling pathways. Recent studies involving animal models have revealed potent alterations in hippocampal function, morphology, and signaling leading to altered cognitive behavior after arsenic exposure (Luo et al., 2009; Sharma and Sharma, 2013; Yadav et al., 2009). While the exposure paradigms and concentrations of arsenic have been highly varied, the overall conclusions have been congruent between studies. Overall working with animal models has demonstrated that arsenic induces deficits in multiple learning paradigms, particularly those relying on proper hippocampal function. Identifying the hippocampus as a sensitive area for arsenic's effects does not necessarily define a molecular target. In the hippocampus, the mossy fiber terminals contain substantial amounts of zinc. Thus, the sensitivity of the hippocampus may be due to the effect of arsenic on zinc either via displacement or substitution. But till date, no studies investigating this mechanism have been published.
Mood and anxiety are considered as pro-oxidant factors and can contribute to oxidative burden (Morimoto et al., 2008; Tsuboi et al., 2004). These findings are in accord with earlier studies that have reported that behavioral abnormality in rats treated with arsenic is linked to enhanced oxidative stress in the brain (Kadeyala et al., 2013; Prakash and Kumar, 2016; Prakash et al., 2015). The discrepancy in between pro-oxidant and antioxidant ratio leads to the generation of ROS significant oxidative stress. Arsenic exposure increases TBARS level (LPO) in the hippocampus (Ashok et al., 2015; Kumar et al., 2013), which may culminate into neuronal damage as it causes oxidative degradation of the cellular macromolecules leading to neurodegeneration (Alizadeh-Ghodsi et al., 2016; Cholanians et al., 2016; Escudero-Lourdes, 2016). A significant increase in the level of LPO was observed that correlates with weak antioxidant defense mechanism or inactivation of antioxidant enzymes such as SOD (Muthumani and Miltonprabu, 2015; Prakash and Kumar, 2016; Prakash et al., 2015). Furthermore, we have explored the probable role of arsenic in provoking protein oxidation and observed a marked increase in PC formation. There are presumptions that excess reactive species causes oxidation of proteins as they govern most biological functions in cells and their oxidation can contribute to diverse functional consequences (Wang et al., 2009). The protective effects of thymoquinone against membrane and protein damage elicited by oxidative stress may be a result of its free radical scavenging properties. Restoration of SOD on thymoquinone preconditioning suggests depletion in ROS as it has depressant effects on antioxidant defense mechanisms. Thymoquinone pre-administration notably reduced the level of TBARS, PC formation and increased SOD activity in arsenic exposed rats and it appears to reduce overall toxic environment in the cell by its free radical scavenging mechanism.
Increased ROS and depleted levels of GSH following arsenic exposure demonstrate an image in which energy metabolism and antioxidant system endure negative transformations. Depletion of cytosolic GSH concomitantly leads to mitochondrial dysfunction because mitochondria cannot synthesize GSH itself (Griffith and Meister, 1985). Cytosolic GSH is continuously cycled in and out of mitochondria as it is a major antioxidant and detoxifying agent (Lash, 2006; Lee and Yu, 2016).
Our comet assay results show elongated tails in the arsenic treated group (Fig. 5). Also, percent DNA in the tail was considerably high suggesting genotoxic damage in the form of DNA strand breaks. This damage can be attributed to ROS as it may cause DNA base modifications and strand fractures. About 20 base modifications, such as thymine glycol, 5-hydroxy methyl uracil, 8-hydroxylamine, and 7-methyl-8-hydroxyguanosine, have been reported (Cadet and Wagner, 2013). Supplementation with 2.5 mg/kg/day and 5 mg/kg/day thymoquinone dose dependently reduced comet tail lengths, thereby, indicating lesser genotoxic damage by scavenging free radicals.
The increase in hippocampal TNF-α is in agreement with studies in which hyperproduction of TNF-α occurs by acute and chronic stress paradigms (Cosen-Binker et al., 2004) or to agents which stimulate stress-like symptoms (Kim et al., 2011). This finding is of particular interest for the pathophysiology of depression as high levels of proinflammatory cytokines, like TNF-α have been found in depressed patients (Felger and Lotrich, 2013). Likewise, arsenic-treated rats showed depressive behavior in both OFT and EPM tests (Figs. 1 and 3). Moreover, investigational immune stimulation associated with an increase in TNF-α activity in humans as well as in rodents, induced depression-like symptoms such as behavioral, emotional and cognitive disturbances (Dantzer et al., 2008). Disproportionate TNF-α levels, as a consequence of any injury, have an inhibitory effect on glutamate transporters, resulting in amplified glutamate concentration in the CNS parenchyma. In this perspective, even a small increase in TNF-α levels induced Ca2+ permeable-AMPA and NMDA receptors trafficking to become toxic for neurons (Olmos and Lladó, 2014). Furthermore, Monteiro et al. (2016) reported that absence of IFNγ promotes hippocampal plasticity and enhances cognitive performance by producing an IFNγ knockout mice. Fig. 6 shows that TQ administration dose-dependently mitigated the levels of TNF-α and IFN-γ (#p ≤ 0.05 and ###p ≤ 0.001 respectively with the higher dose) as suggested by previous reports (Bargi et al., 2017; El Gazzar et al., 2006; Keyhanmanesh et al., 2010; Umar et al., 2015).
There are reports that suggest N. sativa exerts its neuroprotective effect particularly due to improvement of antioxidant defenses and reduction in oxidative damage. It is also suggested that N. sativa and its active components has interactions with the GABA, opioid and NO system (Khader and Eckl, 2014). Most interestingly, there are reports which suggest TQ inhibited the transcription of NF-κB through reducing its promoter activity, either through inhibiting the NF-κB signalling pathway or inhibiting its transcription (Asgharzadeh et al., 2017; Sethi et al., 2008). However, the details of the mechanism by which TQ downregulates NF-κB promoter activity and regulate inflammation are still unclear.
In a nutshell, our results are strongly suggestive of the protective role of thymoquinone against arsenic-related changes in behavior by modulating TNFα and IFNγ levels. The potent anti-inflammatory effect of TQ on these inflammatory mediators is promising for its potential as a preventive and therapeutic strategy in arsenic related neurological symptoms. With this study, we conclude that thymoquinone at these doses could serve as a suitable nutraceutical for populations residing in arsenic hotspots due to its strong free radical scavenging property.
Declarations
Author contribution statement
Fakiha Firdaus: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mohd Faraz Zafeer: Performed the experiments; Analyzed and interpreted the data.
Masood Ahmad, Mohammad Afzal: Contributed reagents, materials, analysis tools or data.
Funding statement
Fakiha Firdaus was supported by the Department of Science and Technology of the Government of India (INSPIRE Fellowship Programme, IF-120626).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We deeply acknowledge BD-FACS Academy, Jamia Hamdard for conducting flow cytometric analysis of our samples and to Dr. Pradeep Kumar Rai for his expertise in analyzing results. We are also thankful to Dr. Fakhrul Islam (College of Pharmacy, Jazan University, KSA) for guiding us in identification and isolation of hippocampus.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) 2017. Priority List of Hazardous Substances. Retrieved from: http://www.atsdr.cdc.gov/spl/index.html. [Google Scholar]
- Alizadeh-Ghodsi M., Zavvari A., Ebrahimi-Kalan A., Shiri-Shahsavar M.R., Yousefi B. The hypothetical roles of arsenic in multiple sclerosis by induction of inflammation and aggregation of tau protein: a commentary. Nutr. Neurosci. 2016;4:1–5. doi: 10.1080/1028415X.2016.1239399. [DOI] [PubMed] [Google Scholar]
- Anseloni V.C.Z., Brandão M.L. Ethopharmacological analysis of behaviour of rats using variations of the elevate plus-maze. Behav. Pharmacol. 1997;8:533–540. doi: 10.1097/00008877-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Arslan-Acaroz D., Zemheri F., Demirel H.H., Kucukkurt I., Ince S., Eryavuz A. In vivo assessment of polydatin, a natural polyphenol compound, on arsenic-induced free radical overproduction, gene expression, and genotoxicity. Environ. Sci. Pollut. Res. Int. 2018;25(3):2614–2622. doi: 10.1007/s11356-017-0391-6. [DOI] [PubMed] [Google Scholar]
- Asgharzadeh F., Bargi R., Beheshti F., Hosseini M., Farzadnia M., Khazaei M. Thymoquinone restores liver fibrosis and improves oxidative stress status in a lipopolysaccharide-induced inflammation model in rats. Avicenna J. Phytomed. 2017;7(6):502–510. PMID: 29299433. [PMC free article] [PubMed] [Google Scholar]
- Ashok A., Rai N.K., Tripathi S., Bandyopadhyay S. Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress- dependent neuroinflammation in young rats. Toxicol. Sci. 2015;143(1):64–80. doi: 10.1093/toxsci/kfu208. [DOI] [PubMed] [Google Scholar]
- Ashraf S.S., Rao M.V., Kaneez F.S., Qadri S., Al-Marzouqi A.H., Chandranath I.S., Adem A. Nigella sativa extract as a potent antioxidant for petrochemical-induced oxidative stress. J. Chromatogr. Sci. 2011;49(4):321–326. doi: 10.1093/chrsci/49.4.321. [DOI] [PubMed] [Google Scholar]
- Aung K.H., Kyi-Tha-Thu C., Sano K. Prenatal exposure to arsenic impairs behavioral flexibility and cortical structure in mice. Front. Neurosci. 2016;10:137. doi: 10.3389/fnins.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badary O.A., Taha R.A., Gamal el-Din A.M., Abdel-Wahab M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem. Toxicol. 2003;26(2):87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- Bali İ., Bilir B., Emir S. The effects of melatonin on liver functions in arsenic-induced liver damage. Turkish J. Surg. 2016;32(4):233–237. doi: 10.5152/UCD.2015.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargi R., Asgharzadeh F., Beheshti F., Hosseini M., Sadeghnia H.R., Khazaei M. The effects of thymoquinone on hippocampal cytokine level, brain oxidative stress status and memory deficits induced by lipopolysaccharide in rats. Cytokine. 2017;96:173–184. doi: 10.1016/j.cyto.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Haldar P.K. Ameliorative effect Trichosanthes dioica root against experimentally induced arsenic toxicity in male albino rats. Environ. Toxicol. Pharmacol. 2012;33(3):394–402. doi: 10.1016/j.etap.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Blom S., Lagerkvist B., Linderholm H., Arsenic exposure to smelter workers Clinical and neurophysiological studies. Scand. J. Work Environ. Health. 1985;11(4):265–269. doi: 10.5271/sjweh.2227. [DOI] [PubMed] [Google Scholar]
- BonakdarYazdi B., Khodagholi F., Shaerzadeh F., Sharifzadeh A., Ahmadi R., Sanati M., Mehdizadeh H., Payandehmehr B., Vali L., Jahromi M.M., Taghizadeh G., Sharifzadeh M. The effect of arsenite on spatial learning: involvement of autophagy and apoptosis. Eur. J. Pharmacol. 2017;796:54–61. doi: 10.1016/j.ejphar.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Cadet J., Wagner J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013;5(2) doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaineau E., Binet S., Pol D., Chatellier G., Meininger V. Embryotoxic effects of sodium arsenite and sodium arsenate on mouse embryos in culture. Teratology. 1990;41(1):105–112. doi: 10.1002/tera.1420410111. [DOI] [PubMed] [Google Scholar]
- Chang C.Y., Guo H.R., Tsai W.C., Yang K.L., Lin L.C., Cheng T.J., Chuu J.J. Subchronic arsenic exposure induces anxiety-like behaviors in normal mice and enhances depression-like behaviors in the chemically induced mouse model of depression. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/159015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C.H., De Rosa C.T. Case studies–arsenic. Int. J. Hyg. Environ. Health. 2003;206(4–5):381–386. doi: 10.1078/1438-4639-00234. [DOI] [PubMed] [Google Scholar]
- Cholanians A.B., Phan A.V., Ditzel E.J., Camenisch T.D., Lau S.S., Monks T.J. From the cover: arsenic induces accumulation of α-synuclein: implications for synucleinopathies and neurodegeneration. Toxicol. Sci. 2016;153(2):271–281. doi: 10.1093/toxsci/kfw117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosen-Binker L.I., Binker M.G., Negri G., Tiscornia O. Influence of stress in acute pancreatitis and correlation with a stress-induced gastric ulcer. Pancreatology. 2004;4:470–484. doi: 10.1159/000079956. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A.K., Sahu R., Dua T.K., Bag S., Gangopadhyay M., Sinha M.K., Dewanjee S. Arsenic-induced myocardial injury: protective role of Corchorus olitorius leaves. Food Chem. Toxicol. 2010;48(5):1210–1217. doi: 10.1016/j.fct.2010.02.012. [DOI] [PubMed] [Google Scholar]
- El Gazzar M., El Mezayen R., Marecki J.C., Nicolls M.R., Canastar A., Dreskin S.C. Anti- inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int. Immunopharmcol. 2006;6(7):1135–1142. doi: 10.1016/j.intimp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Escudero-Lourdes C. Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: role of oxidative stress and inflammatory responses. Neurotoxicology. 2016;53:223–235. doi: 10.1016/j.neuro.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Felger J.C., Lotrich F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floor E., Wetzel M.G. Increased protein oxidation in human substantia nigra parscompacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 1998;70(1):268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- García-Chávez E., Jiménez I., Segura B., Del Razo L.M. Lipid oxidative damage and distribution of inorganic arsenic and its metabolites in the rat nervous system after arsenite exposure: influence of alpha tocopherol supplementation. Neurotoxicology. 2006;27(6):1024–1031. doi: 10.1016/j.neuro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gebel T. Confounding variables in the environmental toxicology of arsenic. Toxicology. 2000;144(1–3):155–162. doi: 10.1016/s0300-483x(99)00202-4. [DOI] [PubMed] [Google Scholar]
- Gora R.H., Baxla S.L., Kerketta P., Patnaik S., Roy B.K. Hepatoprotective activity of Tephrosia purpurea against arsenic induced toxicity in rats. Indian J. Pharmacol. 2014;46(2):197–200. doi: 10.4103/0253-7613.129317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O.W., Meister A. Origin and turnover of mitochondrial glutathione. Proc. Natl. Acad. Sci. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha Mazumder D.N., Haque R., Ghosh N., De B.K., Santra A., Chakraborty D., Smith A.H. Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int. J. Epidemiol. 1998;27(5):871–877. doi: 10.1093/ije/27.5.871. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Parvardeh S., Asl M.N., Sadeghnia H.R., Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia- reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Jha S., Dhanuka A.K., Singh M.N. Arsenic poisoning in a family. Neurol. India. 2002;50(3):364–365. Available from: http://www.neurologyindia.com/text.asp?2002/50/3/364/1423. [PubMed] [Google Scholar]
- Jing J., Zheng G., Liu M., Shen X., Zhao F., Wang J., Zhang J., Huang G., Dai P., Chen Y., Chen J., Luo W. Changes in the synaptic structure of hippocampal neurons and impairment of spatial memory in a rat model caused by chronic arsenite exposure. Neurotoxicology. 2012;33(5):1230–1238. doi: 10.1016/j.neuro.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Jollow D.J., Mitchell J.R., Zampaglione N., Gillette J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Kadeyala P.K., Sannadi S., Gottipolu R.R. Alterations in apoptotic caspases and antioxidant enzymes in arsenic exposed rat brain regions: reversal effect of essential metals and a chelating agent. Environ. Toxicol. Pharmacol. 2013;36(3):1150–1166. doi: 10.1016/j.etap.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Kassab R.B., El-Hennamy R.E.D. The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rat. Egypt. J. Basic Appl. Sci. 2017;4(3):160–167. [Google Scholar]
- Keyhanmanesh R., Boskabady M.H., Khamneh S., Doostar Y. Effect of thymoquinone on the lung pathology and cytokine levels of ovalbumin-sensitized Guinea pigs. Pharmacol. Rep. 2010;62(5):910–916. doi: 10.1016/s1734-1140(10)70351-0. [DOI] [PubMed] [Google Scholar]
- Khader M., Eckl P.M. Thymoquinone: an emerging natural drug with a wide range of medical applications. Iran. J. Basic Med. Sci. 2014;17(12):950–957. [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Kim K., Ahn J.H., Chang H.K. Increased expression of tumor necrosis factor-alpha in the rat hippocampus after acute homocysteine administration. J. Epilepsy Res. 2011;1(1):6–12. doi: 10.14581/jer.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.M., Flora S.J., Reddy G.R. Monoisoamyl 2,3-dimercaptosuccinic acid attenuates arsenic induced toxicity: behavioral and neurochemical approach. Environ. Toxicol. Pharmacol. 2013;36(1):231–242. doi: 10.1016/j.etap.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Lash L.H. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Yu H.S. Role of mitochondria, ROS, and DNA damage in arsenic induced carcinogenesis. Front. Biosci. 2016;1(8):312–320. doi: 10.2741/s465. [DOI] [PubMed] [Google Scholar]
- Li M.M., Jiang Z.E., Song L.Y., Quan Z.S., Yu H.L. Antidepressant and anxiolytic-like behavioral effects of erucamide, a bioactive fatty acid amide, involving the hypothalamus- pituitary-adrenal axis in mice. Neurosci. Lett. 2017;640:6–12. doi: 10.1016/j.neulet.2016.12.072. [DOI] [PubMed] [Google Scholar]
- Li J., Duan X., Dong D., Zhang Y., Li W., Zhao L., Nie H., Sun G., Li B. Hepatic and nephric NRF2 pathway up-regulation, an early antioxidant response, in acute arsenic-exposed mice. Int. J. Environ. Res. Public Health. 2015;12(10):12628–12642. doi: 10.3390/ijerph121012628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Luo J.H., Qiu Z.Q., Shu W.Q., Zhang Y.Y., Zhang L., Chen J.A. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol. Lett. 2009;184(2):121–125. doi: 10.1016/j.toxlet.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- Monteiro S., Ferreira F.M., Pinto V., Roque S., Morais M., de Sá-Calçada D., Mota C., Correia- Neves M., Cerqueira J.J. Absence of IFNγ promotes hippocampal plasticity and enhances cognitive performance. Transl. Psychiatry. 2016;6:e707. doi: 10.1038/tp.2015.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K., Morikawa M., Kimura H., Ishii N., Takamata A., Hara Y., Uji M., Yoshida K. Mental stress induces sustained elevation of blood pressure and lipid peroxidation in postmenopausal women. Life Sci. 2008;82(1–2):99–107. doi: 10.1016/j.lfs.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Morton W.E., Caron G.A. Encephalopathy: an uncommon manifestation of workplace arsenic poisoning? Am. J. Ind. Med. 1989;15(1):1–5. doi: 10.1002/ajim.4700150102. [DOI] [PubMed] [Google Scholar]
- Muthumani M., Miltonprabu S. Ameliorative efficacy of tetrahydrocurcumin against arsenic induced oxidative damage, dyslipidemia and hepatic mitochondrial toxicity in rats. Chem. Biol. Interact. 2015;235:95–105. doi: 10.1016/j.cbi.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Nagi M.N., Almakki H.A., Sayed-Ahmed M.M., Al-Bekairi A.M. Thymoquinone supplementation reverses acetaminophen-induced oxidative stress, nitric oxide production and energy decline in mice liver. Food Chem. Toxicol. 2010;48(8–9):2361–2365. doi: 10.1016/j.fct.2010.05.072. [DOI] [PubMed] [Google Scholar]
- Noman A.S.M., Dilruba S., Mohanto N.C. Arsenic-induced histological alterations in various organs of mice. J. Cytol. Histol. 2015;6(3):323. doi: 10.4172/2157-7099.1000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos G., Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHA . US Department of Labor, Occupational Safety and Health Administration; 2004. Safety and Health Topics: Arsenic. Available at http://www.osha.gov/SLTC/arsenic/index.html. [Google Scholar]
- Pellow S., Chopin P., File S.E., Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Prakash C., Kumar V. Arsenic-induced mitochondrial oxidative damage is mediated by decreased PGC-1α expression and its downstream targets in rat brain. Chem. Biol. Interact. 2016;256:228–235. doi: 10.1016/j.cbi.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Prakash C., Soni M., Kumar V. Biochemical and molecular alterations following arsenic-induced oxidative stress and mitochondrial dysfunction in rat brain. Biol. Trace Elem. Res. 2015;167(1):121–129. doi: 10.1007/s12011-015-0284-9. [DOI] [PubMed] [Google Scholar]
- Prut L., Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety like behaviors: a review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Radad K., Moldzio R., Taha M., Rausch W.D. Thymoquinone protects dopaminergic neurons against MPP+ and rotenone. Phytother Res. 2009;23:696–700. doi: 10.1002/ptr.2708. [DOI] [PubMed] [Google Scholar]
- Ríos R., Zarazúa S., Santoyo M.E., Sepúlveda-Saavedra J., Romero-Díaz V., Jiménez V., Pérez-Severiano F., Vidal-Cantú G., Delgado J.M., Jiménez-Capdeville M.E. Decreased nitric oxide markers and morphological changes in the brain of arsenic-exposed rats. Toxicology. 2009;261(1–2):68–75. doi: 10.1016/j.tox.2009.04.055. [DOI] [PubMed] [Google Scholar]
- Rodríguez V.M., Del Razo L.M., Limón-Pacheco J.H., Giordano M., Sánchez-Peña L.C., Uribe-Querol E., Gutiérrez-Ospina G., Gonsebatt M.E. Glutathione reductase inhibition and methylated arsenic distribution in Cd1 mice brain and liver. Toxicol. Sci. 2005;84(1):157–166. doi: 10.1093/toxsci/kfi057. [DOI] [PubMed] [Google Scholar]
- Sethi G., Ahn K.S., Aggarwal B.B. Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol. Cancer. Res. 2008;6(6):1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- Sharma B., Sharma P.M. Arsenic toxicity induced endothelial dysfunction and dementia: pharmacological interdiction by histone deacetylase and inducible nitric oxide synthase inhibitors. Toxicol. Appl. Pharmacol. 2013;273(1):180–188. doi: 10.1016/j.taap.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Sidhu J.S., Ponce R.A., Vredevoogd M.A., Yu X., Gribble E., Hong S.W., Schneider E., Faustman E.M. Cell cycle inhibition by sodium arsenite in primary embryonic rat midbrain neuroepithelial cells. Toxicol. Sci. 2006;89(2):475–484. doi: 10.1093/toxsci/kfj032. [DOI] [PubMed] [Google Scholar]
- Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Styblo M., Del Razo L.M., LeCluise E.L., Hamilton G.A., Wang C., Cullen W.R., Thomas D.J. Metabolism of arsenic in primary cultures of human and rat hepatocytes. Chem. Res. Toxicol. 1999;12:560–565. doi: 10.1021/tx990050l. [DOI] [PubMed] [Google Scholar]
- Tsuboi H., Shimoi K., Kinae N., Oguni I., Hori R., Kobayashi F. Depressive symptoms are independently correlated with lipid peroxidation in a female population: comparison with vitamins and carotenoids. J. Psychosom. Res. 2004;56(1):53–58. doi: 10.1016/S0022-3999(03)00567-1. [DOI] [PubMed] [Google Scholar]
- Umar S., Hedaya O., Singh A.K., Ahmed S. Thymoquinone inhibits TNF-α-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation. Toxicol. Appl. Pharmacol. 2015;287(3):299–305. doi: 10.1016/j.taap.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu T., Nakamiya K., Kita K., Ochi T., Shibata Y., Morita M. Diphenylarsinic acid produces behavioral effects in mice relevant to symptoms observed in citizens who ingested polluted well water. Neurotoxicol. Teratol. 2012;34(1):143–145. doi: 10.1016/j.ntt.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li S., Piao F., Hong Y., Liu P., Zhao Y. Arsenic down-regulates the expression of Camk4, an important gene related to cerebellar LTD in mice. Neurotoxicol. Teratol. 2009;31(5):318–322. doi: 10.1016/j.ntt.2009.04.064. [DOI] [PubMed] [Google Scholar]
- Yadav R.S., Chandravanshi L.P., Shukla R.K., Sankhwar M.L., Ansari R.W., Shukla P.K., Pant A.B., Khanna V.K. Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. Neurotoxicology. 2011;32(6):760–768. doi: 10.1016/j.neuro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Yadav R.S., Sankhwar M.L., Shukla R.K., Chandra R., Pant A.B., Islam F., Khanna V.K. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol. Appl. Pharmacol. 2009;240(3):367–376. doi: 10.1016/j.taap.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Zafeer M.F., Waseem M., Chaudhary S., Parvez S. Cadmium-induced hepatotoxicity and its abrogation by thymoquinone. J. Biochem. Mol. Toxicol. 2012;26(5):199–205. doi: 10.1002/jbt.21402. [DOI] [PubMed] [Google Scholar]