Abstract
Lipoxin receptor (ALX)/N-formyl peptide receptor (FPR)-2 is a G-protein–coupled receptor that has multiple binding partners, including the endogenous lipid mediators resolvin D1, lipoxin A4, and the Ca2+-dependent phospholipid-binding protein annexin A1. Previous studies have demonstrated that resolvin D1 activates ALX/Fpr2 to resolve salivary gland inflammation in the NOD/ShiLtJ mouse model of Sjögren syndrome. Moreover, mice lacking the ALX/Fpr2 display an exacerbated salivary gland inflammation in response to lipopolysaccharide. Additionally, activation of ALX/Fpr2 has been shown to be important for regulating antibody production in B cells. These previous studies indicate that ALX/Fpr2 promotes resolution of salivary gland inflammation while modulating adaptive immunity, suggesting the need for investigation of the role of ALX/Fpr2 in regulating antibody production and secretory function in mouse salivary glands. Our results indicate that aging female knockout mice lacking ALX/Fpr2 display a significant reduction in saliva flow rates and weight loss, an increased expression of autoimmune-associated genes, an up-regulation of autoantibody production, and increased CD20-positive B-cell population. Although not all effects were noted among the male knockout mice, the results nonetheless indicate that ALX/Fpr2 is clearly involved in the adaptive immunity and secretory function in salivary glands, with further investigation warranted to determine the cause(s) of these between-sex differences.
Resolution of inflammation is an actively regulated process mediated in part by a family of lipid-based, specialized, pro-resolving mediators that include resolvins, protectins, and lipoxins, and their aspirin-triggered forms.1, 2, 3, 4, 5, 6, 7, 8, 9 Specifically, these specialized pro-resolving mediators limit inflammation in response to infection, injury, and/or environmental challenges while promoting tissue repair.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Our previous studies have demonstrated that resolvin D1 prevents tumor necrosis factor α–mediated inflammation and leads to enhancement of epithelial integrity in rat parotid Par-C10 cells.24, 25, 26 Moreover, resolvin D1 was noted to block caspase-3 activation, thereby promoting cell survival in mouse submandibular gland (SMG) cells,27 and also to prevent Sjögren syndrome (SS)-like symptom progression in NOD/ShiLtJ mice.25, 28 Furthermore, mice lacking lipoxin receptor (ALX)/N-formyl peptide receptor (Fpr)-2 displayed an exacerbated salivary gland inflammatory response to lipopolysaccharide, leading in turn to salivary gland hypofunction.29 Studies by others have further demonstrated that the activation of ALX/FPR2 within human B cells inhibits antibody production and proliferation, leading to a decrease in antibody production in vivo.30 These studies have demonstrated that ALX/Fpr2 promotes resolution of salivary gland inflammation and regulates antibody production and secretory function in mouse salivary glands.
Materials and Methods
Animals
Wild-type (WT) C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME); Alx/Fpr2−/− (knockout; KO) mice were donated by Dr. Mauro Perretti (William Harvey Research Institute, Barts and The London School of Medicine, London, UK). Groups of male and female mice at 12 weeks of age were genotyped, as described previously.31 Mice were then anesthetized with 100 mg/kg ketamine and 5 mg/kg xylazine and euthanized by abdominal exsanguination, with the SMGs removed for the assays described in Quantitative PCR, Enzyme-Linked Immunosorbent Assay, Immunofluorescence, and Western Blot Analysis. The animal protocol (14-006007) was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Utah (Salt Lake City, UT), in compliance with the US Animal Welfare Act (7 USC 2131 et seq). Finally, all mouse experiments were performed at the animal care facility of the University of Utah, in accordance with approved guidelines.
Quantitative PCR
Fresh isolated SMG was immersed in RNA-stabilization solution (InvitroGen, Carlsbad, CA) at 4°C overnight. On the following day, total RNA was extracted using the RNAeasy Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Total RNA was then reverse-transcribed into cDNA with the iScript Reverse Transcription Kit (Bio-Rad Laboratories, Segrate, Italy). Additionally, total cDNA was used as a template for quantitative PCR, as previously described,29 with primers stocked at 10 μmol/L (Table 1). Finally, relative fold changes of gene expression were normalized using β-actin and results plotted and analyzed using Prism software version 6.05 (GraphPad Software Inc., San Diego, CA).
Table 1.
Primer Sequences Used in This Study
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| Actb | 5′-GTAACAATGCCATGTTCAAT-3′ | 5′-CTCCATCGTGGGCCGCTCTAG-3′ |
| Baff | 5′-CAGCGACACGCCGACTATAC-3′ | 5′-CCTCCAAGGCATTTCCTCTTTT-3′ |
| Cxcr5 | 5′-TCCTGTAGGGGAATCTCCGT-3′ | 5′-ACTAACCCTGGACATGGGC-3′ |
| Cxcl13 | 5′-GGCCACGGTATTCTGGAAGC-3′ | 5′-GGGCGTAACTTGAATCCGATCTA-3′ |
Saliva Collection
Mice were anesthetized with ketamine/xylazine (100 mg/kg ketamine and 5 mg/kg xylazine) and injected with pilocarpine HCl/phosphate-buffered saline (Sigma-Aldrich, St. Louis, MO) at 10 mg/kg i.p. to stimulate saliva secretion. Similar to previous studies,29, 32 total saliva was then collected and immediately placed on ice in the presence of a protease inhibitor cocktail (Sigma-Aldrich) for 15 minutes, with the volume of saliva measured by pipettes (Eppendorf AG, Hamburg, Germany) and results analyzed and plotted by Prism.
Serum Collection
Mice were anesthetized with ketamine/xylazine (100 mg/kg ketamine and 5 mg/kg xylazine) and euthanized, and whole blood was drawn using cardiac micropuncture. Then, blood was immediately transferred to heparinized tubes and centrifuged at 17,949 × g for 10 minutes, followed by serum collection and storage at −80°C.
Enzyme-Linked Immunosorbent Assay
Three different enzyme-linked immunosorbent assay kits were used to detect total IgG, IgA, and IgM (all from MyBioSource, San Diego, CA). Total immunoglobulin quantification in serum was performed in triplicate using serial dilutions and standard curves according to the manufacturer. Data were expressed in micrograms per microliter, and optical densities at both 450 and 540 nm were measured using a microplate reader (Gen5 version 3.0; BioTek Instruments, Winooski, VT).
Immunofluorescence
SMGs were removed and fixed in 4% paraformaldehyde and processed using routine paraffin-embedding procedures, as previously described.29 Seven micron–thick paraffin-embedded mouse SMG sections from each group were then deparaffinized with xylene and rehydrated with serial ethanol solutions (100%, 70%, and 50%). Next, sections were incubated in sodium citrate buffer (10 mmol/L sodium citrate, 0.05% Tween 20, pH 6.0) at 95°C for 30 minutes and permeabilized with 0.1% Triton X-100/phosphate-buffered saline at room temperature for another 45 minutes. Sections were then blocked in 5% rabbit serum and incubated at 4°C with the following primary antibodies or serum: anti–mouse E-cadherin at 1:250 dilution (Abcam, Cambridge, MA), anti–B lymphocyte activating factor (BAFF) at 1:250 dilution (Abcam), anti-CD20 (Abcam) at 1:200 dilution, and serum from each group at 1: 250 dilution overnight. Samples were incubated for 1 hour with either anti-mouse Alexa Fluor 488 secondary antibody at 1:500 dilution (InvitroGen), anti-rabbit Alexa Fluor 488 secondary antibody at 1:500 dilution, or anti-rat Alexa Fluor 568 secondary antibody at 1:500 dilution, as appropriate. TO-PRO-3 Iodide (InvitroGen) was then used as a counterstain for DNA at 1:1000 dilution. Finally, specimens were analyzed using a confocal microscope (LSM 700; Carl Zeiss, Oberkochen, Germany) at ×63 magnification, with a total depth of 7 μm acquired for each sample and total projection visualized in the xy planes. Quantification of CD20-positive cells within SMGs from each group was performed using ImageJ software version 1.8.0 (NIH, Bethesda, MD; http://imagej.nih.gov.ij) and verified with manual counting from 12 random fields (0.35 mm2 each) at ×20 magnification. The CD20-positive cells within SMG was expressed as number per square millimeter.
Western Blot Analysis
Total proteins from freshly dissected mouse SMG were obtained using radioimmunoprecipitation assay buffer containing a cocktail of protease inhibitors (Sigma-Aldrich). Lyzed samples were then centrifuged at 17,949 × g for 10 minutes to remove cellular debris, and protein concentration was determined with a bicinchoninic acid protein determination kit (Pierce, Rockford, IL). Next, samples were separated using a gradient 4% to 15% SDS-PAGE gel (Bio-Rad) and transferred to a nitrocellulose membrane (Bio-Rad). Subsequently, membranes were blocked in 1% bovine serum albumin and incubated overnight at 4°C with the following primary antibodies: rabbit anti–β-actin (Abcam) at 1:2500 dilution, donkey anti-BAFF at 1:1000 dilution (Abcam), rabbit anti–chemokine (C-X-C motif) receptor (CXCR)-5 (Abcam) at 1:1000 dilution, rabbit anti-CD20 (Abcam) at 1:1000 dilution, and rabbit anti–CXC ligand (CXCL)-13 (Abcam) at 1:1000 dilution. After initial incubation, membranes were further incubated using either peroxidase-linked goat anti-rabbit IgG or goat anti-donkey antibodies (Cell Signaling Technology, Beverly, MA) at 1:5000 dilution at 4°C overnight, as appropriate. The membranes were then treated with a Clarity detection reagent (Bio-Rad), and signals were visualized using a ChemiDoc MP imager (Bio-Rad). Next, band intensities were quantified using Image Lab software version 4.1 (Bio-Rad), with β-actin used for protein normalization. Finally, relative protein expression levels were presented as the ratio of BAFF to actin, and results were analyzed and plotted using Prism.
Statistical Analysis
Data are expressed as the means ± SD of the results from three or more experiments, with P values of <0.05 considered significant and calculated from a two-tailed t-test or a two-way analysis of variance with Prism, as needed.
Results
Female Alx/Fpr2−/− Mice Display a Reduction in Saliva Flow Rates and Weight Loss
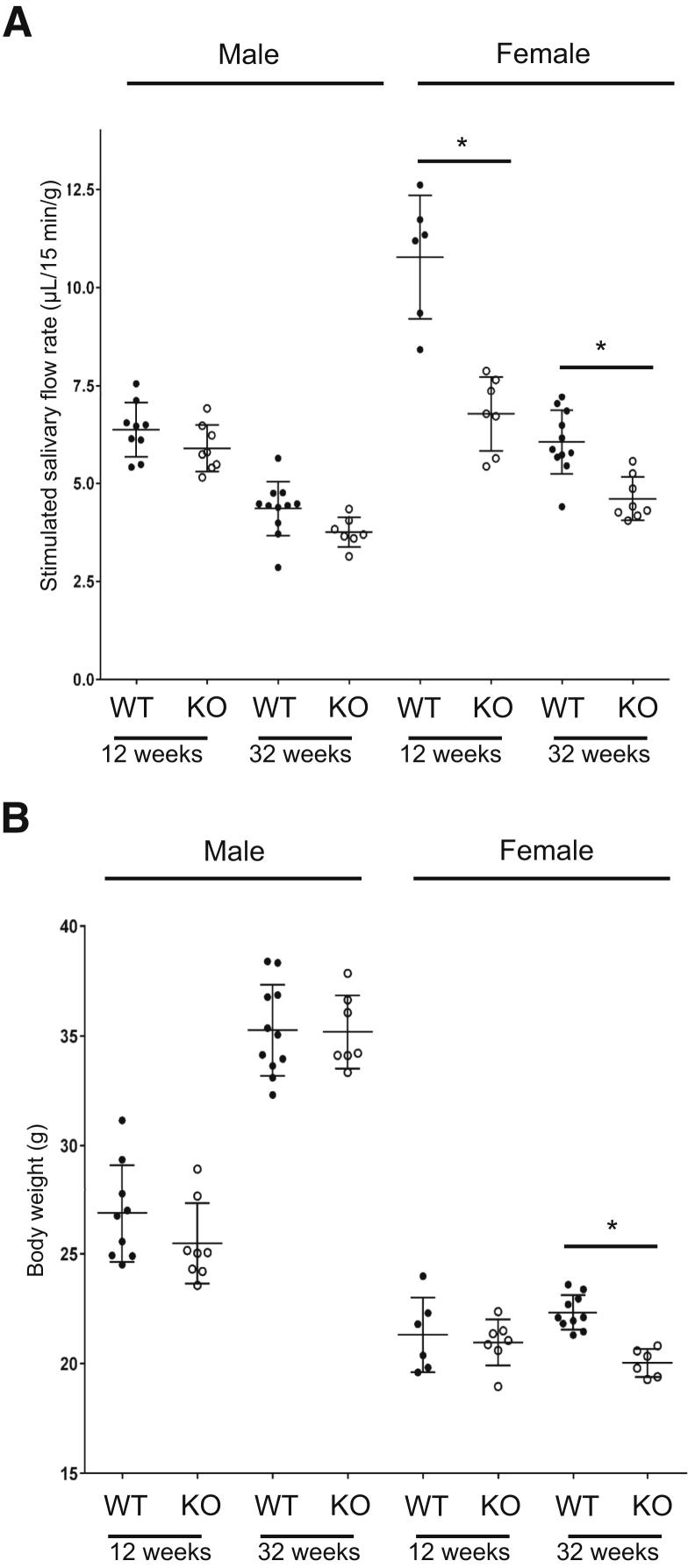
To determine the impact of ALX/Fpr2 on salivary gland function, saliva flow rates were measured in response to pilocarpine stimulation in WT and KO mice, with results indicating that the 12-week-old female KO mice displayed a significant reduction in saliva flow rates as compared to WT mice (Figure 1A). In contrast, 12-week-old male WT mice did not show a reduction in saliva flow rate as compared to WT mice (Figure 1A). Interestingly, saliva flow rates were further reduced in female KO mice with age (ie, 32 weeks) as compared to WT mice (Figure 1A); however, this effect was observed in neither KO nor WT male mice (Figure 1A). Moreover, a significant weight loss was observed in female KO mice at 32 weeks as compared to WT mice (Figure 1B). In contrast, male KO mice did not display significant changes in weight loss as compared to WT mice at either 12 or 32 weeks. Note that previous studies have demonstrated that body weight is a useful parameter for corroborating the presence of hyposalivation.33 Together, these results indicate a reduction in salivary flow rates among females alone, with a positive correlation between reduction in saliva flow rates and body weight.
Figure 1.
Saliva flow rates are altered in female but not in male knockout (KO) mice. A: Saliva flow rate was measured in both female and male wild-type (WT; closed circles) and KO (open circles) mice at 12 and 32 weeks of age. Specifically, mice were anesthetized, stimulated with pilocarpine HCl/phosphate-buffered saline at 10 mg/kg i.p. Then, saliva was collected for 15 minutes. B: Body weight of mice in each group were measured at both 12 and 32 weeks of age. Data are expressed as means ± SEM. n = ≥6 mice. ∗P < 0.05.
Female Alx/Fpr2−/− Mice Display Altered Antibody Production
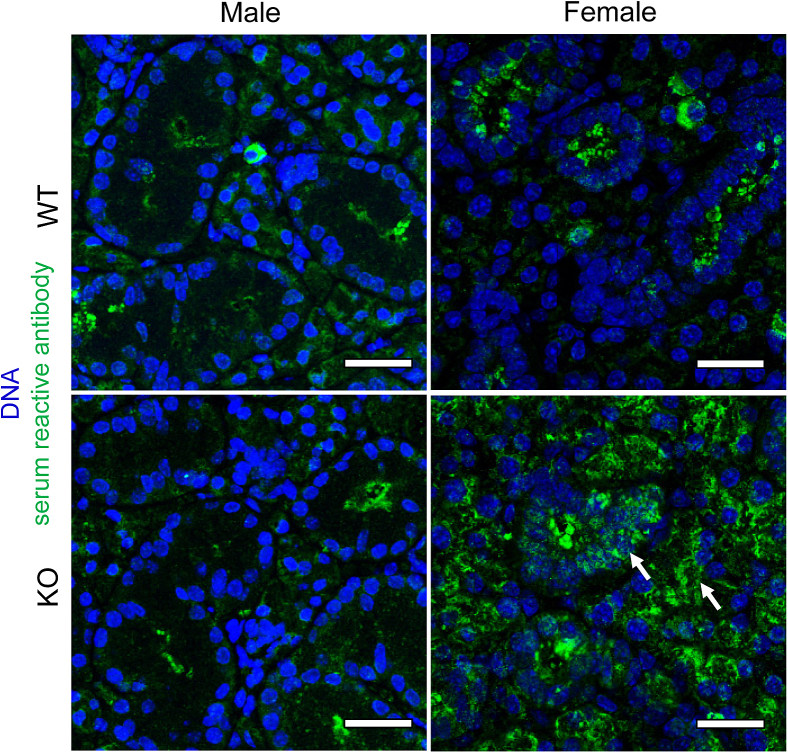
Given that ALX/FPR2 regulates antibody production in both human and mouse memory B cells,30 studies were warranted to determine whether the reduction in saliva secretion in female KO mice was attributable to a comparable mechanism (eg, overproduction of antibodies occurs in salivary gland epithelial cells and thus leads to dysfunction of salivary epithelium). To test this hypothesis, healthy SMG tissue sections were incubated with serum from both KO and WT mice, as described in Materials and Methods. Antibodies were then detected using an anti-mouse IgG secondary antibody, with results indicating that incubation of healthy mouse SMG tissue sections with serum from female KO mice at 12 weeks leads to the detection of serum-reactive antibodies (Figure 2). Specifically, a Green fluorescence signal was detected in the cytoplasm of SMG cells (Figure 2), whereas incubation of healthy mouse SMG tissue sections with serum from WT control mice and male KO mice did not generate such reactivity (Figure 2).
Figure 2.
Serum from female knockout (KO) mice contains serum reactive antibodies against healthy submandibular gland (SMG) tissue. Five-micron paraffin-embedded SMG sections from both sexes of healthy wild-type (WT) mice were obtained and processed for immunofluorescence as described in Materials and Methods. Serum from each group (WT male, KO male, WT female, and KO female) was applied to healthy SMG sections, and positive reactivity of antibodies was detected by confocal microscopy. Note the presence of serum reactive antibodies (green fluorescent signal) in female KO mouse SMG section. TO-PRO-3 Iodide (InvitroGen, Carlsbad, CA) was used to distinguish DNA (blue). Scale bars = 50 μm.
Alx/Fpr2−/− Mice Display Altered Immunoglobulin Production
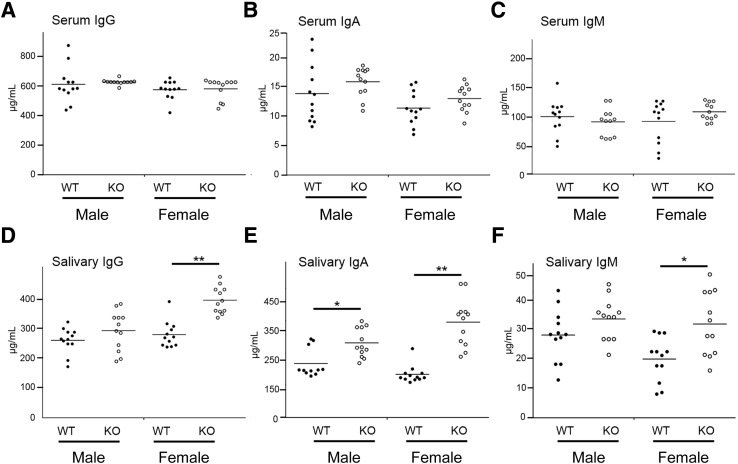
Elevated serum and saliva levels of immunoglobulins are observed in a variety of local and systemic oral diseases34, 35; however, the contribution of ALX/FPR2 to these imbalances is unknown. Therefore, expression levels of IgA, IgM, and IgG were measured in KO mice, both in serum and saliva, with WT mice as controls. Results indicated no elevations in serum (Figure 3, A–C) in all mice tested; however, significant elevations were observed in saliva in KO females across all three immunoglobulins (ie, IgA, IgG, and IgM) (Figure 3, D–F) and in KO males for one of them (ie, IgA) (Figure 3E). These results suggest that ALX/Fpr2 is involved in the pathogenesis of oral diseases, with further investigation needed to learn more about its role and to determine the cause(s) for the sex differences observed. Together, these findings suggest that ALX/FPR2 regulates autoantibody production in salivary glands in a sex-dependent manner.
Figure 3.
Immunoglobulin levels are altered in female but not in male knockout (KO) mice. Saliva and serum were collected from both female and male wild-type (WT) (closed circles) and KO (open circles) mice at 12 weeks of age. Expression levels of serum IgG (A), IgA (B), and IgM (C), and salivary IgG (D), IgA (E), and IgM (F) were measured by enzyme-linked immunosorbent assay. Data are expressed as means ± SEM. n = 12 mice per group. ∗P < 0.05, ∗∗P < 0.01.
Female Alx/Fpr2−/− Mice Show Increased Gene and Protein Levels of B-Cell Regulation Markers and Increased CD20-Positive B cells in SMG
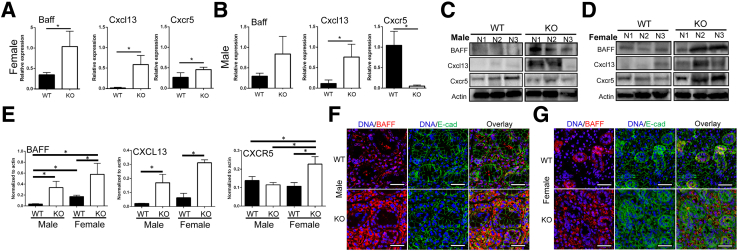
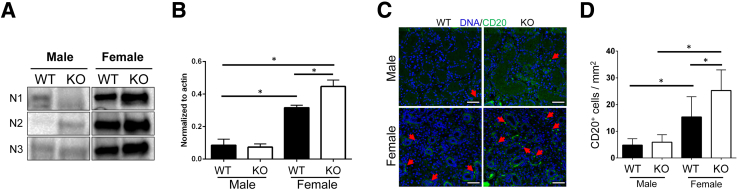
To determine whether the gene expression levels involved with B-cell maturation are present in SMG, the following were studied: i) BAFF, ii) CXCL13, and iii) CXCR5. Gene levels of these three factors (ie, Baff, Cxcr5, and Cxcl13) were found to be up-regulated in female KO mice (Figure 4A), whereas male KO mice showed up-regulation in only Cxcl13 (Figure 4B). Moreover, the expression patterns of BAFF and CXCL13 were studied to confirm their up-regulation at the protein levels using Western blot analysis. Protein levels of BAFF and CXCL13 were found to be up-regulated in both female and male KO mice (Figure 4, C–E). Moreover, the expression level of CXCR5 showed a significant increase in female KO mice, and this observation was not noticed in male KO mice (Figure 4, C–E). In addition, immunofluorescence localization of BAFF revealed that BAFF was overexpressed by salivary epithelium (Figure 4, F and G) in both sexes of KO mice, which is consistent with gene and protein expression profiles (Figures 4 and 5). To determine the impact of ALX/Fpr2 in B-cell activation and overproduction of associated proteins, expression levels and localization of the B-cell marker CD20 were measured. CD20 was overexpressed in female KO mice but not in male KO mice or other groups (Figure 5, A and B). Moreover, female KO mice showed a significant increase in CD20-positive B cells as compared to male KO mice (Figure 5, C and D). Overall, these results suggest that ALX/FPR2 regulates B-cell maturation and differentiation in a sex-dependent manner.
Figure 4.
Up-regulation of Sjögren syndrome–related genes and proteins occurs in female but not in male knockout (KO) mice. A and B: Submandibular glands (SMGs) were collected from male (A) and female (B) wild-type (WT) and KO mice at 12 weeks of age and total RNA were obtained and reverse-transcribed into cDNA. Then quantitative PCR reactions were performed on 96-well plates according to the manufacturer's instructions. Relative gene expression of Baff, Cxcl13, and Cxcr5 were normalized using β-actin. C–E: Protein lysates were prepared from male (C) and female (D) WT and KO mouse SMG. B lymphocyte activating factor (BAFF) and chemokine (C-X-C motif) ligand (CXCL)-13 and receptor (CXCR)-5 expression were detected by Western blot analysis, as described in Materials and Methods, using β-actin as normalization control (representative image shown); data are shown in E. F and G: Male (F) and female (G) SMG sections were subjected to immunofluorescence using rabbit anti-BAFF and mouse anti–E-cadherin (E-cad) antibodies followed by Alexa Fluor 488–conjugated goat anti-rabbit IgG antibody (green), Alexa Fluor 568–conjugated goat anti-mouse IgG antibody (red), and TO-PRO-3 Iodide (InvitroGen, Carlsbad, CA) nuclear stain (blue). xy Images were obtained and analyzed using a confocal microscope (model 510; Carl Zeiss, Oberkochen, Germany). Images were taken at the widest point of the xy plane. Data are expressed as means ± SEM. n = ≥6 mice (A), n = ≥3 experiments (E). ∗P < 0.05. Scale bars = 50 μm.
Figure 5.
Overexpression of CD20 and increased CD20-positive B cells in female knockout (KO) mice. A and B: Protein lysates were prepared from male and female wild-type (WT) and KO mouse submandibular gland. CD20 expression was detected by Western analysis as described in Materials and Methods using β-actin as normalization control (representative image shown); data are shown in B. C: Five-micron representative sections from each group were stained with CD20 antibody and counterstained with TO-PRO-3 Iodide (InvitroGen, Carlsbad, CA). Arrows indicate CD20-positive cells within SMG. D: ImageJ (NIH, Bethesda, MD; http://imagej.nih.gov.ij) quantification of CD20-positive cells from 12 representative fields at ×20 magnification per animal. Data are expressed as means ± SEM. n ≥ 3 experiments (B); n = 3 mice per group (D). ∗P < 0.05. Scale bars = 50 μm.
Discussion
Our previous studies demonstrated that ALX/FPR2 plays a significant role in salivary gland innate immunity, as mice that were lacking this receptor and treated with lipopolysaccharide developed exacerbated inflammation in the submandibular glands.29 Following on this, the current study demonstrates that ALX/Fpr2 also regulates adaptive immunity in salivary glands. Specifically, increased levels of antibody production and secretory dysfunction were noted in the absence of the receptor (Figures 3 and 4). Our studies are consistent with previous work30 demonstrating a link between resolution signals and adaptive immunity, as regulation of antibody production is crucial to prevent unwanted inflammation. Particularly, activation of ALX/FPR2 with lipoxin A4 decreased IgM and IgG production in human B cells via down-regulation of NF-κB p65 nuclear translocation.30 Moreover, lipoxin A4 inhibited human memory B-cell antibody production and proliferation and decreased antigen-specific antibody production in an ovoalbumin immunization mouse model.30 Lastly, ALX/Fpr2 has been shown to decrease IgG levels in joints from arthritic-like mice,36 further supporting the role of this receptor in immunoglobulin production.
T cells have long been known to infiltrate salivary glands with SS, which in turn leads to the production of inflammatory cytokines and subsequent salivary gland dysfunction.37, 38, 39 However, less prevalent but highly reactive B cells are coming to be understood to be equally if not more important in T cell infiltration. Specifically in SS, B cells are hyperreactive and result in excessive production of immunoglobulins and antibodies, thereby altering salivary gland epithelial integrity.40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Moreover, B cells have been shown to control T-cell differentiation and proliferation, resulting in chronic inflammation of salivary glands with SS.38, 39, 51 Lastly, B cells are involved in the formation of ectopic germinal centers (ie, nonlymphoid collections of mature B lymphocytes) observed in the labial glands of SS patients, which are strongly associated with an increased risk for lymphoma development.52, 53, 54, 55
Although the specific mechanisms by which ALX/FPR2 regulates B-cell functioning in these studies are not well understood, the following may help to explain how loss of this receptor causes B-cell overproduction in salivary glands. One possibility is that loss of ALX/FPR2 may lead to an increase in BAFF expression within salivary epithelium, thereby causing sustained BAFF receptor, AKT, and p38 activation, all of which are signaling pathways that promote B-cell survival and maturation.56 Alternatively, loss of ALX/FPR2 may cause a BAFF-mediated up-regulation in CXCL13, the receptor for CXCR5, thereby causing chemotaxis of memory B cells (ie, CD20-positive) and overproduction of antibodies in salivary glands as has been observed previously in SS.42, 43, 57 Finally, the issue of sex differences as they relate to the expression of ALX/FPR2 is complex and worthy of further investigation; particularly, surgical or medical (Lupron) (REF) castration experiments in female KO mice and/or male KO mice to determine whether estrogen and/or testosterone co-regulates with ALX/FPR2 saliva flow rates.58 However, the fact that the reduction of saliva secretion effect is observed only in female mice does not take away from the significance of this finding, given that the vast majority of SS cases (approximately 90% or more) are found in women.
Acknowledgments
We thank Dr. Mauro Perretti (William Harvey Research Institute, Barts and The London School of Medicine, London, UK) for sharing ALX/FPR2−/− mice.
C.-S.W. and O.J.B. designed the experiments, acquired data, and wrote the manuscript; C.-S.W. performed all experiments; O.J.B. revised the manuscript and approved the final draft.
Footnotes
Supported by NIH-National Institute of Dental and Craniofacial Research grants R01DE021697, R01DE021697S1, R56DE02169707A1, and R01 DE02297107 (O.J.B).
Disclosures: None declared.
References
- 1.Bannenberg G., Serhan C.N. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basil M.C., Levy B.D. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredman G., Serhan C.N. Specialized pro-resolving mediators: wiring the circuitry of effector immune and tissue homeostasis. Endod Top. 2011;24:39–58. [Google Scholar]
- 4.Buckley C.D., Gilroy D.W., Serhan C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariel A., Serhan C.N. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Ariel A., Chiang N., Arita M., Petasis N.A., Serhan C.N. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-α secretion from human T cells. J Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 7.Serhan C.N. Novel chemical mediators in the resolution of inflammation: resolvins and protectins. Anesthesiol Clin. 2006;24:341–364. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N.A., Serhan C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bannenberg G., Arita M., Serhan C.N. Endogenous receptor agonists: resolving inflammation. ScientificWorldJournal. 2007;7:1440–1462. doi: 10.1100/tsw.2007.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogerio A.P., Haworth O., Croze R., Oh S.F., Uddin M., Carlo T., Pfeffer M.A., Priluck R., Serhan C.N., Levy B.D. Resolvin D1 and its aspirin-triggered epimer AT-RvD1 promote the resolution of allergic airways responses. J Immunol. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Oliveira J.R., Favarin D.C., Tanaka S.C., Balarin M.A., Teixeira D.N., Levy B.D., Rogério Ade P. AT-RvD1 modulates CCL-2 and CXCL-8 production and NF-κB, STAT-6, SOCS1, and SOCS3 expression on bronchial epithelial cells stimulated with IL-4. Biomed Res Int. 2015;2015:178369. doi: 10.1155/2015/178369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dondoni L., Scarparo R.K., Kantarci A., Van Dyke T.E., Figueiredo J.A., Batista E.L. Effect of the pro-resolution lipid mediator Resolvin E1 (RvE1) on pulp tissues exposed to the oral environment. Int Endod J. 2014;47:827–834. doi: 10.1111/iej.12224. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z.Z., Zhang L., Liu T., Park J.Y., Berta T., Yang R., Serhan C.N., Ji R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dmitrieva N., Suess G., Shirley R. Resolvins RvD1 and 17(R)-RvD1 alleviate signs of inflammation in a rat model of endometriosis. Fertil Steril. 2014;102:1191–1196. doi: 10.1016/j.fertnstert.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Nelson J.W., Leigh N.J., Mellas R.E., McCall A.D., Aguirre A., Baker O.J. ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am J Physiol Cell Physiol. 2014;306:C178–C185. doi: 10.1152/ajpcell.00284.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortina M.S., Bazan H.E. Docosahexaenoic acid, protectins and dry eye. Curr Opin Clin Nutr Metab Care. 2011 Mar;14:132–137. doi: 10.1097/MCO.0b013e328342bb1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Börgeson E., McGillicuddy F.C., Harford K.A., Corrigan N., Higgins D.F., Maderna P., Roche H.M., Godson C. Lipoxin A4 attenuates adipose inflammation. FASEB J. 2012;26:4287–4294. doi: 10.1096/fj.12-208249. [DOI] [PubMed] [Google Scholar]
- 18.Levy B.D., Lukacs N.W., Berlin A.A., Schmidt B., Guilford W.J., Serhan C.N., Parkinson J.F. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007;21:3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozinovski S., Uddin M., Vlahos R., Thompson M., McQualter J.L., Merritt A.-S., Wark P.A., Hutchinson A., Irving L.B., Levy B.D. Serum amyloid A opposes lipoxin A4 to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Paiva C.S., Schwartz C.E., Gjorstrup P., Pflugfelder S.C. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea. 2012;31:1299–1303. doi: 10.1097/ICO.0b013e31823f789e. [DOI] [PubMed] [Google Scholar]
- 21.Hodges R.R., Li D., Shatos M.A., Serhan C.N., Dartt D.A. Lipoxin A4 counter-regulates histamine-stimulated glycoconjugate secretion in conjunctival goblet cells. Sci Rep. 2016;6:36124. doi: 10.1038/srep36124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasturk H., Abdallah R., Kantarci A., Nguyen D., Giordano N., Hamilton J., Van Dyke T.E. Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35:1123–1133. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasturk H., Kantarci A., Ohira T., Arita M., Ebrahimi N., Chiang N., Petasis N.A., Levy B.D., Serhan C.N., Van Dyke T.E. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 2005;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 24.Baker O.J., Camden J.M., Redman R.S., Jones J.E., Seye C.I., Erb L., Weisman G.A. Proinflammatory cytokines tumor necrosis factor-α and interferon-γ alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol. 2008;295:C1191–C1201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Easley J.T., Maruyama C.L.M., Wang C.-S., Baker O.J. AT-RvD1 combined with DEX is highly effective in treating TNF-α-mediated disruption of the salivary gland epithelium. Physiol Rep. 2016;4:e12990. doi: 10.14814/phy2.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odusanwo O., Chinthamani S., McCall A., Duffey M.E., Baker O.J. Resolvin D1 prevents TNF- -mediated disruption of salivary epithelial formation. AJP Cell Physiol. 2012;302:C1331–C1345. doi: 10.1152/ajpcell.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leigh N., Nelson J., Mellas R., Aguirre A., Baker O. Expression of resolvin D1 biosynthetic pathways in salivary epithelium. J Dent Res. 2014;93:300–305. doi: 10.1177/0022034513519108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C.-S., Maruyama C.L., Easley J.T., Trump B.G., Baker O.J. AT-RvD1 promotes resolution of inflammation in NOD/ShiLtJ mice. Sci Rep. 2017;7:45525. doi: 10.1038/srep45525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C.-S., Wee Y., Yang C.-H., Melvin J.E., Baker O.J. ALX/FPR2 modulates anti-inflammatory responses in mouse submandibular gland. Sci Rep. 2016;6:24244. doi: 10.1038/srep24244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramon S., Bancos S., Serhan C.N., Phipps R.P. Lipoxin A4 modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. Eur J Immunol. 2014;44:357–369. doi: 10.1002/eji.201343316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dufton N., Hannon R., Brancaleone V., Dalli J., Patel H.B., Gray M., D'Acquisto F., Buckingham J.C., Perretti M., Flower R.J. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voigt A., Esfandiary L., Nguyen C.Q. Sexual dimorphism in an animal model of Sjögren's syndrome: a potential role for Th17 cells. Biol Open. 2015;4:1410–1419. doi: 10.1242/bio.013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam K., Maruyama C.L., Wang C.-S., Trump B.G., Lei P., Andreadis S.T., Baker O.J. Laminin-111-derived peptide conjugated fibrin hydrogel restores salivary gland function. PLoS One. 2017;12:e0187069. doi: 10.1371/journal.pone.0187069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sistig S., Vucicevic-Boras V., Lukac J., Kusic Z. Salivary IgA and IgG subclasses in oral mucosal diseases. Oral Dis. 2002;8:282–286. doi: 10.1034/j.1601-0825.2002.20844.x. [DOI] [PubMed] [Google Scholar]
- 35.Brandtzaeg P. Secretory immunity with special reference to the oral cavity. J Oral Microbiol. 2013;5:20401. doi: 10.3402/jom.v5i0.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L., Zhang X., Wu P., Li H., Jin S., Zhou X., Li Y., Ye D., Chen B., Wan J. BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis. Inflamm Res. 2008;57:157–162. doi: 10.1007/s00011-007-7141-z. [DOI] [PubMed] [Google Scholar]
- 37.Adamson T.C., Fox R., Frisman D., Howell F. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983;130:203–208. [PubMed] [Google Scholar]
- 38.Robinson C.P., Cornelius J., Bounous D.I., Yamamoto H., Humphreys-Beher M.G., Peck A.B. Infiltrating lymphocyte populations and cytokine production in the salivary and lacrimal glands of autoimmune NOD mice. Lacrimal Gland Tear Film Dry Eyes Syndr 2. In: Sullivan D.A., editor. Plenum Press; New York: 1998. pp. 493–497. [DOI] [PubMed] [Google Scholar]
- 39.Cuello C., Palladinetti P., Tedla N., Di Girolamo N., Lloyd A.R., McCluskey P.J., Wakefield D. Chemokine expression and leucocyte infiltration in Sjogren's syndrome. Br J Rheumatol. 1998;37:779–783. doi: 10.1093/rheumatology/37.7.779. [DOI] [PubMed] [Google Scholar]
- 40.Chan E.K., Andrade L.E. Antinuclear antibodies in Sjogren's syndrome. Rheum Dis Clin N Am. 1992;18:551–570. [PubMed] [Google Scholar]
- 41.Cheema G.S., Roschke V., Hilbert D.M., Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 42.Mackay F., Mackay C.R. The role of BAFF in B-cell maturation, T-cell activation and autoimmunity. Trends Immunol. 2002;23:113–115. doi: 10.1016/s1471-4906(01)02159-7. [DOI] [PubMed] [Google Scholar]
- 43.Salomonsson S., Larsson P., Tengnér P., Mellquist E., Hjelmström P., Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren's syndrome. Scand J Immunol. 2002;55:336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 44.Gordon T.P., Bolstad A.I., Rischmueller M., Jonsson R., Waterman S.A. Autoantibodies in primary Sjogren's syndrome: new insights into mechanisms of autoantibody diversification and disease pathogenesis. Autoimmunity. 2001;34:123–132. doi: 10.3109/08916930109001960. [DOI] [PubMed] [Google Scholar]
- 45.Daridon C., Devauchelle V., Hutin P., Le Berre R., Martins-Carvalho C., Bendaoud B., Dueymes M., Saraux A., Youinou P., Pers J.O. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum. 2007;56:1134–1144. doi: 10.1002/art.22458. [DOI] [PubMed] [Google Scholar]
- 46.Tsuboi H., Nakamura Y., Iizuka M., Matsuo N., Matsumoto I., Sumida T. Generation and functional analysis of monoclonal antibodies against the second extracellular loop of human M3 muscarinic acetylcholine receptor. Mod Rheumatol. 2012;22:264–271. doi: 10.1007/s10165-011-0514-8. [DOI] [PubMed] [Google Scholar]
- 47.He J., Qiang L., Ding Y., Wei P., Li Y.N., Hua H., Li Z.G. The role of muscarinic acetylcholine receptor type 3 polypeptide (M3RP205-220) antibody in the saliva of patients with primary Sjogren's syndrome. Clin Exp Rheumatol. 2012;30:322–326. [PubMed] [Google Scholar]
- 48.Shen L., Suresh L., Lindemann M., Xuan J., Kowal P., Malyavantham K., Ambrus J.L. Novel autoantibodies in Sjogren's syndrome. Clin Immunol Orlando Fla. 2012;145:251–255. doi: 10.1016/j.clim.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Gumpel J.M., Hobbs J.R. Serum immune globulins in Sjögren's syndrome. Ann Rheum Dis. 1970;29:681–683. doi: 10.1136/ard.29.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levy Y., Dueymes M., Pennec Y.L., Shoenfeld Y., Youinou P. IgA in Sjogren's syndrome. Clin Exp Rheumatol. 1994;12:543–551. [PubMed] [Google Scholar]
- 51.Jamin C., Morva A., Lemoine S., Daridon C., de Mendoza A.R., Youinou P. Regulatory B lymphocytes in humans: a potential role in autoimmunity. Arthritis Rheum. 2008;58:1900–1906. doi: 10.1002/art.23487. [DOI] [PubMed] [Google Scholar]
- 52.Theander E., Vasaitis L., Baecklund E., Nordmark G., Warfvinge G., Liedholm R., Brokstad K., Jonsson R., Jonsson M.V. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjogren's syndrome. Ann Rheum Dis. 2011;70:1363–1368. doi: 10.1136/ard.2010.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee K.-E., Kang J.-H., Yim Y.-R., Kim J.-E., Lee J.-W., Wen L., Park D.-J., Kim T.-J., Park Y.-W., Yoon K.C., Lee J.S., Lee S.-S. The significance of ectopic germinal centers in the minor salivary gland of patients with Sjögren's syndrome. J Korean Med Sci. 2016;31:190–195. doi: 10.3346/jkms.2016.31.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bombardieri M., Barone F., Lucchesi D., Nayar S., van den Berg W.B., Proctor G., Buckley C.D., Pitzalis C. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol. 2012;189:3767–3776. doi: 10.4049/jimmunol.1201216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenspan J.S., Daniels T.E., Talal N., Sylvester R.A. The histopathology of Sjogren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–229. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- 56.Zheng N., Wang D., Ming H., Zhang H., Yu X. BAFF promotes proliferation of human mesangial cells through interaction with. BMC Nephrol. 2015;16:72. doi: 10.1186/s12882-015-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin L., Yu D., Li X., Yu N., Li X., Wang Y., Wang Y. CD4(+)CXCR5(+) follicular helper T cells in salivary gland promote B cells maturation in patients with primary Sjogren's syndrome. Int J Clin Exp Pathol. 2014;7:1988–1996. [PMC free article] [PubMed] [Google Scholar]
- 58.Mitani Y., Rao P.H., Maity S.N., Lee Y.C., Ferrarotto R., Post J.C., Licitra L., Lippman S.M., Kies M.S., Weber R.S., Caulin C. Alterations associated with androgen receptor gene activation in salivary duct carcinoma of both sexes: potential therapeutic ramifications. Clin Cancer Res. 2014;20:6570–6581. doi: 10.1158/1078-0432.CCR-14-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]