Abstract
Patients with acute myocardial infarction (AMI) complicated by heart failure with preserved ejection fraction (HFpEF) are likely to have more adverse cardiovascular events and higher mortality. The purpose of this study was to examine the predictors and outcomes in AMI patients complicated by HFpEF.
We examined the demographics, clinical data, and clinical outcomes in 405 consecutive subjects who firstly presented with AMI after undergoing emergency percutaneous coronary intervention from January 2013 to June 2016.
Three hundred twenty patients and eighty-five patients were classified into the nonheart failure (non-HF) group and HFpEF group, respectively. Patients with HFpEF had higher prevalence of prior hypertension, had higher levels of biomarkers, and had a larger left atrial diameter with a nondilated left ventricle were more likely to develop multivessel disease-vessels and had infarction-related artery located in left anterior descending artery than patients without HF. Moreover, patients with HFpEF had a higher probability of developing the in-hospital incident cardiovascular complications and death than non-HF patients.
Two routine biomarkers, levels of hypersensitive C-reactive protein and N-terminal-pro brain natriuretic peptide, and number of diseased-vessels were independent predictors for in-hospital HFpEF incidence in AMI patients with preserved LVEF. AMI patients with HFpEF had a higher probability of in-hospital cardiovascular outcomes and mortality.
Keywords: acute myocardial infarction, cardiovascular event, heart failure with preserved ejection fraction, predictor, risk factor
1. Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is increasingly being recognized as a major public health problem accountable for nearly 50% of all HF cases. The 2-year mortality is up to 26.6% in Asians complicating HFpEF.[1] Acute myocardial infarction (AMI) is one of the main causes of morbidity and in-hospital mortality worldwide, although the increasing use of early myocardial reperfusion and medical treatments have contributed significantly to the decline in mortality.[2–4] In AMI patients, the presence of HFpEF might worsen the prognosis, and the incident adverse events increase due to the therapeutic limitations.[3,4] Timely evaluation of HFpEF would be useful for risk stratification in patients after an attack of AMI.
Previous studies have identified several risk factors for the development of post-AMI HF, such as advancing age, coexistence of previous coronary disease, infarction size, multivessel coronary disease, reperfusion efficiency, and other concomitant medical conditions[5,6]; however, these studies did not differentiate between HFpEF and HF with reduced ejection fraction, or classified them by the in-hospital Killip score at admission.[7] Data pertaining to the prevalence of HFpEF in AMI patients are highly limited especially in Asia. Several clinical studies on post-AMI diastolic dysfunction have established the diagnosis of HFpEF using the clinical HF symptoms; few clinical studies have focused on the predictive factors and prognosis of patients with HFpEF following AMI, especially after primary percutaneous coronary intervention (PCI). Further, homologous left ventricular ejection fraction (LVEF) cut-off points have also been used to establish a diagnosis of HFpEF.[8–10] Notably, it has been demonstrated by another study that the prevalence for HFpEF in AMI patients has remained stable despite the declining of prevalence HF with reduced LVEF in post-AMI patients.[11] Therefore, we aimed to early identify HFpEF patients in patients after AMI with normal LVEF value, and evaluate the predictors, prognosis of patients with HFpEF after AMI by analyzing clinical characteristics, biomarkers, angiographic course, medical treatments, and clinical outcomes.
2. Materials and methods
2.1. Study population
The procedures in this study complied with the Declaration of the Helsinki, and the study protocol was approved by the Ethics Committee of the local institution (The First Affiliated Hospital of Soochow University). Our study was registered at ClinicalTrials.gov with clinicalTrials.gov ID NCT03351179. In this single-center observational study, we retrospectively evaluated 705 consecutive patients with first incident AMI treated with primary PCI in the cardiac intensive care unit of our hospital from January 2013 to June 2016. The diagnosis of AMI was based on the presence of a typical chest pain history, electrocardiographic changes, an increase in the serum cardiac biomarkers, and results of coronary angiography.[12,13] The definition of HFpEF was established in accordance with the latest published guidelines.[14] HFpEF diagnosis was established in AMI patients with typical symptoms or signs of HF, LVEF ≥ 0.5 which is measured with M-mode method, or other evidences of diastolic dysfunction to diagnose HFpEF of echocardiography measurements, but not accompanied by any evidence of reduced systolic function, as evaluated using echocardiography. The HFpEF diagnosis was also referred to the Killip grade and N-terminal-pro brain natriuretic peptide (NT-proBNP) level. Exclusion criteria were severe inflammatory diseases, valvular heart disease, noncardiac cause symptoms, serious hepatic and renal failure, congenital cardiomyopathy, and pericardial diseases.
2.2. Data collection
We collected data pertaining to demographic characteristics (age and sex), previous history, and risk factors of AMI (hypertension, hyperlipidemia, diabetes mellitus, smoking, and stroke), biomarkers, echocardiographic measurements, clinical characteristics, medical procedures and treatments, as well as in-hospital complications. Laboratory biomarkers, including white blood cells (WBCs), neutrophils, hemoglobin, red blood cell distribution width, hematocrit, platelet distribution width (PDW), platelet count, total bilirubin (TB), alanine aminotransferase (ALT), aspartate transaminase (AST), total protein, albumin, hypersensitive C-reactive protein (Hs-CRP), uric acid, serum creatinine (Scr), glucose, triglyceride, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A, apolipoprotein B, lipoprotein (a), and NT-proBNP, were measured during first 24 h. Peak levels of MB isoenzyme of creatine kinase (CK-MB) and cardiac troponin I were measured. Clinical characteristics included heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Echocardiographic data comprised the left atrium diameter (LAd), left ventricular end-systolic dimensions (LVESd), and left ventricular end-diastolic dimensions (LVEDd) on admission. Each enrolled subject was undergoing PCI therapy and appropriated prescriptions. The associated coronary pathological changes, medical therapy, in-hospital complications (severe sinus bradycardia or sinus arrest, acute attack of atrial fibrillation/flutter, malignant ventricular tachycardia/fibrillation [VT/VF], and acute attack of severe HF), length of hospital stay, and death evaluated in the current study. Finally, the variables related to incident HFpEF in AMI patients were analyzed using univariate logistic regression analyses and multivariate logistic regression analyses to identify the independent predictors.
2.3. Statistical analyses
All data were analyzed using the SPSS software package (version 17.0, SPSS, Chicago, IL). Continuous variables are presented as mean ± standard deviation or median (range) values and were compared using the independent-sample t test (data with normal distribution) or Mann–Whitney U test (data with non-normal distribution). Categorical variables are expressed by numbers (percentage) of patients in each group and then were analyzed using the chi-squared or Fisher exact test. The independent predictors for HFpEF in AMI patients were identified by conducting stepwise multivariate logistic regression analyses of various variables confirmed using univariate logistic regression analyses. A receiver operating characteristic (ROC) curve was constructed to assess the predicted probability based on the logistic model. P < .05 (2-sided) was considered statistically significant.
3. Results
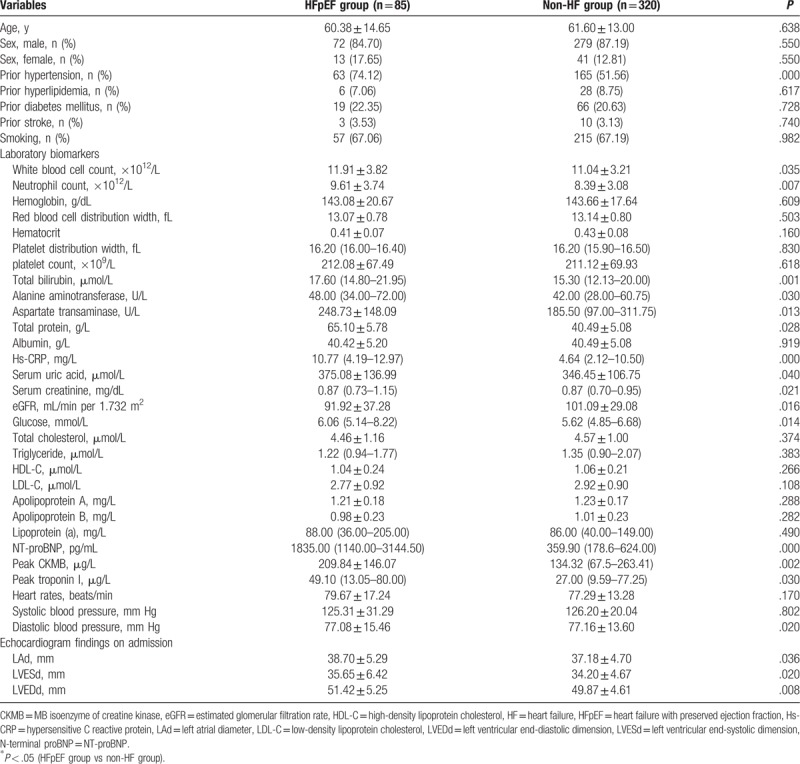
The entire study population consisted of 705 first incident AMI patients undergoing primary PCI, 279 of these patients were excluded owing to an LVEF value <0.5. The remaining 426 patients were included and divided into the HFpEF and non-HF groups; of these, 15 patients of the non-HF group and 6 patients of the HFpEF group were excluded because of death on the operating-table, incomplete data, or other exclusion criteria. Finally, the study was conducted on 320 patients in the non-HF group and 85 patients in the HFpEF group. First, the clinical characteristics, biomarkers, and echocardiological data of the 2 groups were compared and had been summarized in Table 1. AMI patients with HFpEF had higher prevalence of prior history of hypertension, markedly increased levels of WBCs, neutrophils, TB, ALT, AST, total protein, Hs-CRP, Scr, glucose, NT-proBNP, peak CK-MB value, and peak troponin I, and had significant decreased level of DBP than those without HF. Moreover, patients with AMI and HFpEF had larger LAd, LVESd, and LVEDd than those without HF. There were some nonsignificant differences with regard to age; the sex distribution; previous history except prior hypertension; hemoglobin level; red blood cell distribution width; hematocrit; PDW; platelet count; level of albumin, uric acid, TG, TC, HDL-C, LDL-C, apolipoprotein A, apolipoprotein B, and lipoprotein (a); HR; and SBP between the 2 groups.
Table 1.
Baseline characteristics, biomarkers, and echocardiological measurements of study subjects.
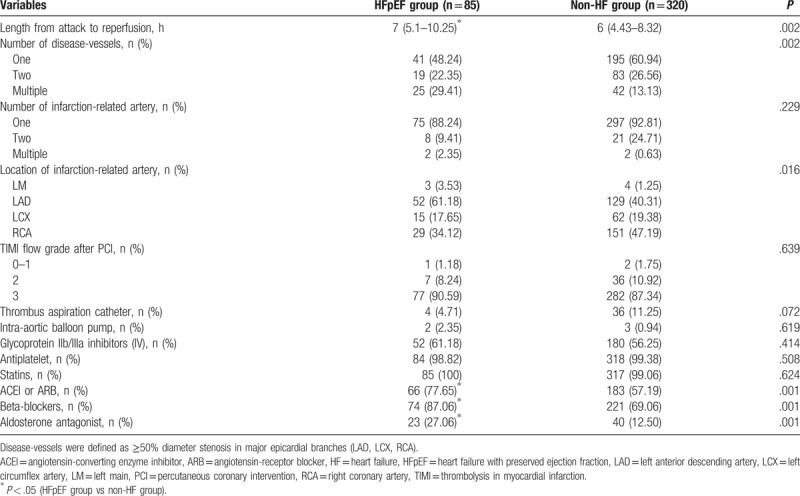
Thereafter, further comparisons of the angiographic findings and medical procedures were conducted between the 2 groups (Table 2). A longer length of attack to reperfusion was found in AMI patients with HFpEF than the other group. There was considerable difference in the relative number of disease-vessels and distribution in the location of infarction-related artery (IRA) between the groups. Significantly higher number of patients in the HFpEF had multiple disease-vessels (25 [29.41%] vs 42 [13.13%]), and a notable increased proportion of coronary IRA located in left anterior descending artery (LAD) (52 [61.18%] vs 129 [40.31%]) than those in the non-HF group, while a significantly lower number of these patients had single disease-vessel (41 [48.24%] vs 195 [60.94%]) and IRA located in right coronary artery (29 [34.12%) vs 151[47.19%]) (Table 2). Moreover, AMI patients with HFpEF were more likely to receive angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, beta-blockers, aldosterone antagonists than those without HF; by contrast, no significant difference was observed with respect to the adherence to therapeutic intravenous injection of glycoprotein IIb/IIIa inhibitors and oral usage of antiplatelet, statins between the groups. In addition to the above parameters, relative number of 2 disease-vessels; relative number of 1, 2, multiple IRA; relative number of IRA located in the left main (LM) or left circumflex artery (LCX); relative number of thrombolysis in myocardial infarction flow grade after PCI; and the utilization rate of thrombus aspiration catheter and intra-aortic balloon pump showed no statistical differences between the 2 groups.
Table 2.
In-hospital angiographic characteristics and other medical procedures.
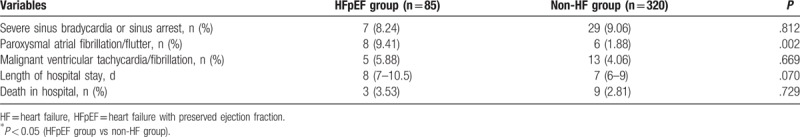
Data regarding the incidence of cardiovascular complications and prognosis are presented in Table 3. We found that the incidence of paroxysmal atrial fibrillation/flutter was significantly higher in AMI patients with HFpEF than in those without HF. In addition, we found that higher probability of the incidence of severe sinus bradycardia or sinus arrest, malignant VT/VF, length of hospital stay, and number of death in hospital in HFpEF group, though no significant difference, compared to patients in the non-HF group in this study.
Table 3.
In-hospital adverse events as a function of acute myocardial infarction in HFpEF group and non-HF group.
In the univariate logistic regression analyses, levels of WBC counts; neutrophil counts; TB; AST; Hs-CRP; Scr; NT-proBNP; peak troponin I; LAd; LVESd; LVEDd; number of disease-vessels; and patients with prior hypertension predicted the incidence of HFpEF in the AMI patients (Table 4). The stepwise multivariate logistic regression analysis adjusted for these variables demonstrated that Hs-CRP (odds ratio [OR]: 1.248; 95% confidence interval [CI]: 1.031–1.511; P = .023), NT-proBNP (OR: 1.005; 95% CI: 1.003–1.008; P = .000), and number of disease-vessels (OR: 4.108; 95% CI: 1.595–10.578; P = .003) were independently correlated with the occurrence of HFpEF in AMI patients (Table 4). The area of the ROC curve of the NT-proBNP, Hs-CRP, and number of disease-vessels were 0.959 (95% CI: 0.934–0.983, P = .000), 0.705 (95% CI: 0.630–0.779 P = .000), and 0.589 (95% CI: 0.506–0.673, P = .039), respectively, suggesting the predictive effect on the risk of HFpEF in AMI patients (Fig. 1).
Table 4.
Univariate and multivariate logistic regression analyses for predictors of hospitalized HFpEF in post-AMI patients.
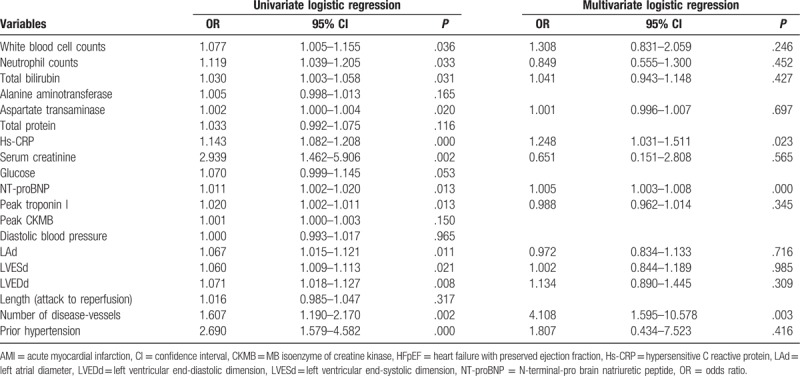
Figure 1.
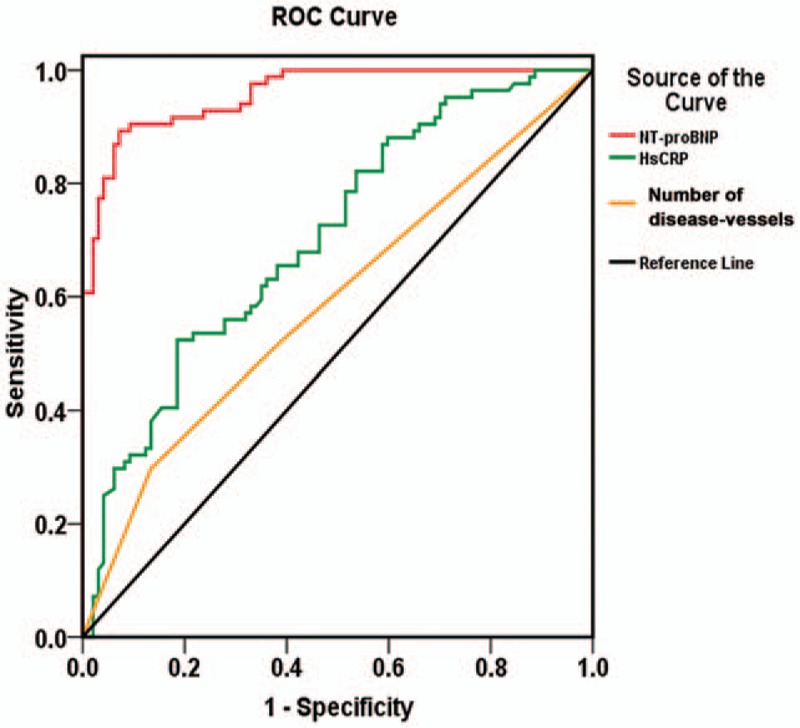
Receiver operating characteristic (ROC) curve of NT-proBNP, Hs-CRP, and number of disease-vessels for predicting the incidence of HFpEF in patients with acute myocardial infarction. Hs-CRP = hypersensitive C-reactive protein, NT-proBNP = N-terminal-pro brain natriuretic peptide.
4. Discussion
In the present study, AMI patients with HFpEF had features of higher prevalence of prior hypertension; higher levels of WBC counts, neutrophils counts, TB, ALT, AST, total protein, Hs-CRP, Scr, blood glucose, NT-proBNP, peak CK-MB, and troponin I; and larger LAd, LVESd, and LVEDd than those without HF. In addition, AMI patients with HFpEF had longer length of attack to reperfusion, were more likely to have multiple diseased-vessels and IRA located in the LAD than AMI patients without HF. Moreover, AMI patients with HFpEF had higher in-hospital paroxysmal atrial fibrillation/flutter than non-HF patients. It is noteworthy that in the present study, the Hs-CRP, NT-proBNP, and number of diseased-vessels were important independent predictors of the HFpEF course in AMI patients after PCI who have preserved LVEF. Moreover, AMI patients with HFpEF had higher incidence of paroxysmal atrial fibrillation/flutter and higher probability of risk in other cardiovascular outcomes and death by comparison with patients in the non-HF group.
The occurrence of HFpEF in post-AMI patients is a complex process. On one hand, ischemic and necrotic myocardium promotes the process of cardiac systolic and/or diastolic dysfunction. On the other hand, a stunned or hibernating myocardium in surrounding the surviving cardiomyocytes can present transient impairment of contraction and relaxation,[15] especially affecting the diastolic function because ventricular diastole is an active process that requires consuming oxygen and glucose.[16] Several studies have investigated the potential risk factors for incident HFpEF in AMI patients. Some investigators have reported that laboratory examinations also provide information regarding incident and prognostic HFpEF in AMI patients. High CRP levels and increased WBC counts and neutrophils counts are considered to be strongly and independently associated with the development of HF in atherosclerosis and have related to adverse effects in the myocardium.[17–19] Acute hyperglycemia is frequently observed as transient increases in response to stressful conditions and critical illnesses, and appears to be an independent predictor of in-hospital outcomes in AMI.[20,21] Liver abnormalities can be assessed by measuring the levels of parameters such as bilirubin, ALT, and AST. Accurate identification of liver abnormalities cannot only reflect nonhepatic diseases, and influence diagnostic as well as therapeutic processes, but also indicate the infarct size and thrombus burden, and enable the prediction of prognosis and mortality in AMI patients and in HF patients.[21–26] Prior studies had also suggested that elevations in the CK-MB and troponin I levels were widely accepted as indicators of myocardial necrosis and as risk factors for the development of a fulminant course, and were useful in the diagnosis, risk stratification, guiding treatments, and provide prognostic information in patients with AMI.[12,13,27] Consistent with these findings, AMI patients presenting with HFpEF in our study had higher levels of inflammatory indicators (WBC counts, neutrophils counts, and Hs-CRP), elevated level of blood glucose, liver biomarkers (TB, ALT, and AST), and myocardial necrosis indicators (CK-MB, troponin I) than non-HF patients. In addition, echocardiographic parameters such as a nondilated left ventricle and an increased left atrial size could aid the diagnosis and assessment of left ventricular diastolic dysfunction.[14] The occurrence of HFpEF in AMI patients is significantly influenced by the coronary artery findings. In the present study, patients with HFpEF in AMI patients are more likely to have multiple disease-vessels and IRA located in LAD, and therefore the revascularization of disease-vessel and infarction-related LAD might be plays a protective role for the restoration of cardiac function.[28]
It is noteworthy that level of Hs-CRP, NT-proBNP, and number of diseased-vessels have been proposed as independent predictors of HFpEF incidence in AMI patients; this was examined using multivariate logistic regression analyses. The development and progression of HFpEF involve multiple aspects. Considerable evidence exists to indicate that CRP is increased in response to the pathophysiological processes and can impair endothelial cells and stimulate cytokine production.[29] The increased CRP levels in AMI patients may reflect the magnitude of the response to ischemic myocardial injury and myocardial necrosis, the severity of myocytes loss, ventricular remodeling, and prognosis in patients with AMI or cardiac failure.[30–34] Previous studies had demonstrated that NT-proBNP level could predict adverse cardiovascular outcomes, cardiovascular morbidity and mortality in patients with ventricular dysfunction, as natriuretic peptides are secreted from the ventricle in response to wall stress, adverse hemodynamic alterations, and vascular dysfunction. Importantly, by examining the independent association of risk factors with HFpEF occurrence in AMI patients, our results had been noted that elevated NT-proBNP level predicts incident in-hospital HFpEF strongly in patients after AMI.[35–38] Beyond above the routine use of above biomarkers for predictive purposes about incident HFpEF in AMI patients with preserved LVEF, number of disease-vessels could be recommended as an another independent contributor to HFpEF incidence in AMI patients in this study. Coronary characteristics of multiple disease-vessels indicate a large atherosclerotic burden, and are closely related to micro-embolization in the IRA that can potentially diminish myocardial perfusion and impair microcirculation.[30,39] Furthermore, patients with multiple disease-vessels disease may have previous chronic myocardial ischemic injury, adverse ventricular remodeling, and poor tolerance to AMI injury.
5. Study limitations
Our study has certain limitations. First, this was a retrospective single-center observational study with a relatively small sample size. Second, the diagnosis of HFpEF is challenging. The study used LVEF ≥ 50% as the threshold for diagnosing HFpEF; however, some patients did not have a completely normal EF accompanied by the absence of any symptoms and signs of reduced systolic function. These patients were not diagnosed with HFpEF in the current study. Third, echocardiographic measurements (such as E/A ratio and E/e′ ratio) related to diastolic function upon admission was available. Finally, the exact score of the severity of coronary lesion stenosis was not analyzed.
6. Conclusions
This study showed that older age; higher WBC and neutrophil counts; elevated levels of TB, ALT, AST, Hs-CRP, blood glucose, CK-MB, and troponin I; LAd; multiple diseased-vessels; and LAD-IRA are potential risk factors of incident HFpEF in first incident AMI patients with preserved LVEF who were undergoing primary PCI. Moreover, patients with HFpEF after AMI had a probably higher risk of in-hospital mortality and clinical complications. The Hs-CRP, NT-proBNP, and number of diseased-vessels were independent risk factors associated with HFpEF in first incident AMI patients. These findings can help clinicians perform prevention treatments for high-risk HFpEF incidence in patients with preserved LVEF who had higher levels of Hs-CRP and NT-proBNP, and multivessel of diseased-vessels.
Acknowledgments
The authors thank Dr Liu (the First Affiliated Hospital of Soochow University, Suzhou, China) for providing language modification and suggestion.
Author contributions
Conceptualization: Jialiang Xu.
Data curation: Mingzhu Xu, Tingbo Jiang.
Formal analysis: Mingzhu Xu, Jialiang Xu, Tingbo Jiang.
Investigation: Xiangjun Yang, Tingbo Jiang.
Project administration: Xiangjun Yang.
Software: Mingzhu Xu, Tingbo Jiang.
Supervision: Xiangjun Yang, Tingbo Jiang.
Writing – original draft: Mingzhu Xu, Tingbo Jiang.
Writing – review and editing: Lihua Yan, Xiangjun Yang.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AMI = acute myocardial infarction, AST = aspartate transaminase, CI = confidence interval, CK-MB = MB isoenzyme of creatine kinase, DBP = diastolic blood pressure, HDL-C = high-density lipoprotein cholesterol, HF = heart failure, HFpEF = heart failure with preserved ejection fraction, HR = heart rate, Hs-CRP = hypersensitive C-reactive protein, IRA = infarction-related artery, LAD = left anterior descending artery, LAd = left atrium diameter, LCX = left circumflex artery, LDL-C = low-density lipoprotein cholesterol, LM = left main, LVEDd = left ventricular end-diastolic dimensions, LVEF = left ventricular ejection fraction, LVESd = left ventricular end-systolic dimensions, NT-proBNP = N-terminal-pro brain natriuretic peptide, OR = odds ratio, PCI = percutaneous coronary intervention, PDW = platelet distribution width, ROC = receiver operating characteristic, SBP = systolic blood pressure, Scr = serum creatinine, TB = total bilirubin, VT/VF = ventricular tachycardia/fibrillation, WBC = white blood cell.
The authors were grateful to the support from the National Natural Science Foundation of China (81170174). Those responsible for funding had no influence over the study design, data collection and analyses, manuscript preparation, and decision to publish.
The authors have no conflicts of interest to disclose.
References
- [1].Yap J, Sim D, Lim CP, et al. Predictors of two-year mortality in Asian patients with heart failure and preserved ejection fraction. Int J Cardiol 2015;183:33–8. [DOI] [PubMed] [Google Scholar]
- [2].Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol 2010;56:254–63. [DOI] [PubMed] [Google Scholar]
- [3].Yoon HJ, Kim KH, Kim JY, et al. Impaired diastolic recovery after acute myocardial infarction as a predictor of adverse events. J Cardiovasc Ultrasound 2015;23:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nijland F, Kamp O, Karreman AJ, et al. Prognostic implications of restrictive left ventricular filling in acute myocardial infarction: a serial Doppler echocardiographic study. J Am Coll Cardiol 1997;30:1618–24. [DOI] [PubMed] [Google Scholar]
- [5].Myftiu S, Bara P, Sharka I, et al. Heart failure predictors in a group of patients with myocardial infarction. Open Access Maced J Med Sci 2016;4:435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spencer FA, Meyer TE, Gore JM, et al. Heterogeneity in the management and outcomes of patients with acute myocardial infarction complicated by heart failure: the National Registry of Myocardial Infarction. Circulation 2002;105:2605–10. [DOI] [PubMed] [Google Scholar]
- [7].Steg PG, Dabbous OH, Feldman LJ, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation 2004;109:494–9. [DOI] [PubMed] [Google Scholar]
- [8].Bennett KM, Hernandez AF, Chen AY, et al. Heart failure with preserved left ventricular systolic function among patients with non-ST-segment elevation acute coronary syndromes. Am J Cardiol 2007;99:1351–6. [DOI] [PubMed] [Google Scholar]
- [9].Poulsen SH, Jensen SE, Gotzsche O, et al. Evaluation and prognostic significance of left ventricular diastolic function assessed by Doppler echocardiography in the early phase of a first acute myocardial infarction. Eur Heart J 1997;18:1882–9. [DOI] [PubMed] [Google Scholar]
- [10].Shah RV, Holmes D, Anderson M, et al. Risk of heart failure complication during hospitalization for acute myocardial infarction in a contemporary population: insights from the National Cardiovascular Data ACTION Registry. Circ Heart Fail 2012;5:693–702. [DOI] [PubMed] [Google Scholar]
- [11].Gerber Y, Weston SA, Berardi C, et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol 2013;178:1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hamm CW, Bassand JP, Agewall S, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- [13].Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012;60:645–81. [DOI] [PubMed] [Google Scholar]
- [14].McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2012;14:803–69. [DOI] [PubMed] [Google Scholar]
- [15].Braunwald E, Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation 1982;66:1146–9. [DOI] [PubMed] [Google Scholar]
- [16].Solomon SD, Glynn RJ, Greaves S, et al. Recovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early after load reducing therapy study. Ann Intern Med 2001;134:451–8. [DOI] [PubMed] [Google Scholar]
- [17].Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bekwelem W, Lutsey PL, Loehr LR, et al. White blood cell count, C-reactive protein and incident heart failure in the atherosclerosis risk in communities (ARIC) study. Ann Epidemiol 2011;21:739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res 1999;43:860–78. [DOI] [PubMed] [Google Scholar]
- [20].Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation 2008;117:1018–27. [DOI] [PubMed] [Google Scholar]
- [21].Jang HJ, Oh PC, Moon J, et al. Prognostic impact of combined dysglycemia and hypoxic liver injury on admission in patients with ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention (from the INTERSTELLAR Cohort). Am J Cardiol 2017;119:1179–85. [DOI] [PubMed] [Google Scholar]
- [22].van Deursen VM, Edwards C, Cotter G, et al. Liver function, in-hospital, and post-discharge clinical outcome in patients with acute heart failure-results from the relaxin for the treatment of patients with acute heart failure study. J Card Fail 2014;20:407–13. [DOI] [PubMed] [Google Scholar]
- [23].Lazzeri C, Valente S, Tarquini R, et al. Prognostic values of admission transaminases in ST-elevation myocardial infarction submitted to primary angioplasty. Med Sci Monit 2010;16:R567–74. [PubMed] [Google Scholar]
- [24].Gul M, Uyarel H, Ergelen M, et al. Prognostic value of total bilirubin in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Am J Cardiol 2013;111:166–71. [DOI] [PubMed] [Google Scholar]
- [25].Hamur H, Duman H, Bakirci EM, et al. Bilirubin levels and thrombus burden in patients with ST-segment elevation myocardial infarction. Angiology 2016;67:565–70. [DOI] [PubMed] [Google Scholar]
- [26].Huang FY, Peng Y, Huang BT, et al. The correlation between serum total bilirubin and outcomes in patients with different subtypes of coronary artery disease. Clin Chim Acta 2017;465:101–5. [DOI] [PubMed] [Google Scholar]
- [27].Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). G Ital Cardiol 2016;17:831–72. [DOI] [PubMed] [Google Scholar]
- [28].Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2014;35:2541–619.25173339 [Google Scholar]
- [29].Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 2000;102:2165–8. [DOI] [PubMed] [Google Scholar]
- [30].Yamada DM, Topol EJ. Importance of microembolization and inflammation in atherosclerotic heart disease. Am Heart J 2000;140(suppl):S90–102. [DOI] [PubMed] [Google Scholar]
- [31].Berton G, Cordiano R, Palmieri R, et al. C-reactive protein in acute myocardial infarction: association with heart failure. Am Heart J 2003;145:1094–101. [DOI] [PubMed] [Google Scholar]
- [32].Suleiman M, Aronson D, Reisner SA, et al. Admission C-reactive protein levels and 30-day mortality in patients with acute myocardial infarction. Am J Med 2003;115:695–701. [DOI] [PubMed] [Google Scholar]
- [33].Anand IS, Latini R, Florea VG, et al. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation 2005;112:1428–34. [DOI] [PubMed] [Google Scholar]
- [34].Suleiman M, Khatib R, Agmon Y, et al. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J Am Coll Cardiol 2006;47:962–8. [DOI] [PubMed] [Google Scholar]
- [35].Bibbins-Domingo K, Gupta R, Na B, et al. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. Jama 2007;297:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kistorp C, Raymond I, Pedersen F, et al. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. Jama 2005;293:1609–16. [DOI] [PubMed] [Google Scholar]
- [37].Ahluwalia A, MacAllister RJ, Hobbs AJ. Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res Cardiol 2004;99:83–9. [DOI] [PubMed] [Google Scholar]
- [38].D’ Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther 2004;101:113–29. [DOI] [PubMed] [Google Scholar]
- [39].De Luca G, Gibson M, Cutlip D, et al. Impact of multivessel disease on myocardial perfusion and survival among patients undergoing primary percutaneous coronary intervention with glycoprotein IIb/IIIa inhibitors. Arch Cardiovasc Dis 2013;106:155–61. [DOI] [PubMed] [Google Scholar]


