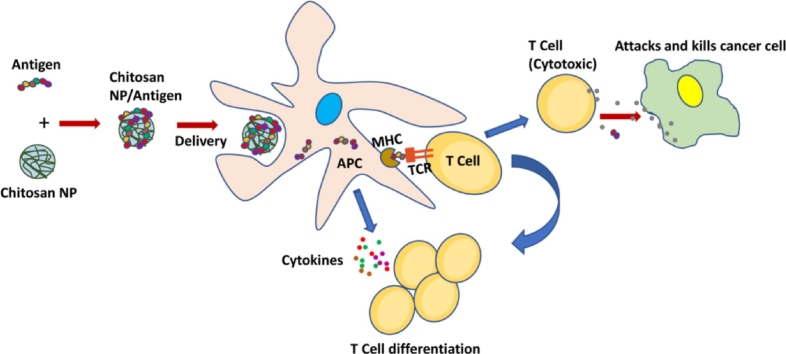
Graphical abstract
Keywords: Chitosan nanoparticles, HCC, Inflammatory markers, Apoptotic markers
Abstract
Nanotechnology is a promising era of medicine for developing targeted drug delivery system. Chitosan nanoparticles (CNPs) have attracted increasing attention for their wide applications as anticancer drugs. This article is concerned with the therapeutic index of chitosan nanoparticles against diethyl nitrosamine (DEN) induced hepatocellular carcinoma (HCC). HCC was induced in rats via repeated DEN administration in a dose of 200 mg/kg BW IP, 2 weeks later rats received (2 ml/kg BW) CCl4 orally for 2 months followed by daily treatment with chitosan nanoparticles in an oral dose of 12 mg/kg for 1 month. Then the gene expression of glycogen synthase kinase-3 (GSK-3), (c-FOS), nuclear factor kappa-B (NFκB) and tumor necrosis factor- α (TNF-α) were reported in rats sera and the correlation between GSK-3, C-Fos, NFƘB and TNF-α and liver tumorigenesis was investigated. The results elucidated that DEN significantly increased serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Marked increments in serum malondialdehyde (MDA) and nitric oxide (NOx) levels along with a slight reduction of glutathione (GSH) level were evidenced in HCC. Liver injury triggered an inflammatory response by enhancing the mRNA gene expression of NFκB and TNF-α. DEN effectively activated apoptotic markers GSK-3 and c-FOS. Oral administration of CNPs alleviated the oxidative, inflammatory and apoptotic hazards induced via DEN. The histopathological examination reinforced these results. The present study highlights the anti-inflammatory and anti-apoptotic potentials of CNPs against DEN-induced HCC.
1. Introduction
HCC is potentially one of the leading causes of death worldwide. Nowadays treatment strategies for cancer include radiation and chemotherapy [1].
Conventional chemotherapy lack selectivity and can inflect damage also on healthy tissue [2].
In order to surpass adverse effects of chemotherapies, nanotechnology became urgent in tumor targeted drug delivery with harmless effect to normal cells [2]. Nanoparticles with dimensions 1–100 nm are attractive in cancer therapy pointed to their small size and stability [3,4]
Chitosan nanoparticles (CNPs) chitin derivative is a widely utilized biopolymer in food and pharmaceutics. It can enhance immunity and is utilized as anticancer. CNPs positive charge elucidates higher affinity for negatively charged biological membranes [4].
The GSK-3 regulates glycogen synthesis. GSK-3 functions in a wide range of cellular processes. GSK-3 was implicated in many human pathologies including: Cancer [5].
GSK-3 regulates the activity of other transcription factors including NF-κB. GSK-3 modifies the activity of other transcription factors frequently implicated in cancer including: activation protein 1 (AP-1); (C-Fos and C-Jun). GSK-3 can phosphorylate NF-κB [5].
HCC, express c-Fos, as pointed out by Karin et al, [6]. Transcription factors such as NF-κB and AP-1 play an important role in HCC development [6].
TNF-α induces specific signaling pathways in hepatocytes leading to activation of transcription factor NF-κB that promotes survival [7,8].
An important role in the prevention of hepatocyte death is exercised by NF-κB transcription factors, which upon activation can protect hepatocytes from apoptosis induced by TNF-α [9,10].
The aim of the present study is designed to investigate the potential effect of CNPs on modulating the signaling pathways of GSK-3, c-FOS, NFƘB and TNF-α in diethyl nitrosamine induced hepatocellular carcinoma.
2. Materials and methods
2.1. Chemicals
CNPs (particle size 40 nm) [Nanostreams-Egypt; Lot#NS0115] and diethyl nitrosamine was purchased from Sigma-Aldrich Co (St. Louis, MO, USA). Diagnostic kits utilized were obtained from Randox Company (UK). Primers used in real time- PCR analysis were purchased from Qia Gene (USA). All other chemicals are of highest analytical grade.
The size distribution of the NPs in the suspension (hydrodynamic size) and the zeta potential were analyzed with a Brookhaven 90 Plus particle size analyzer. Scanning electron microscopy (SEM) was used to evaluate the size of CNPs.
2.2. Animals
30 Male albino Westar rats, weight ranging 170–200 g m, from the animal house of National Research Center (Dokki, Giza, Egypt) were utilized in this study. Animals were housed in cages and kept at standardized conditions (22 ± 5 °C, 55 ± 5% humidity, and 12 h light/dark cycle). They were allowed free access to water and ad libitum.
All procedures regarding animal care and treatments adhered strictly to the ethical procedures approved by Animal Care and Use Committee of National Research Center, and complied with the Guide for Care and Use of Laboratory published by the US National Institute of Health.
2.3. Experimental design
1 week post acclimatization, animals were randomly divided into three groups (each of 10 animals):
Group1: Animals received saline and served as a normal control group.
Group 2: DEN - intoxicated animals that received repeated DEN doses of 200 mg/kg BW IP, 2 weeks later rats received (2 ml/kg BW) CCl4 orally for 2 months [11].
Group 3: DEN - intoxicated animals that received repeated DEN administration in a dose of 200 mg/kg BW IP, 2 weeks later rats received (2 ml/kg BW) CCl4 orally for 2 months followed by daily treatment with chitosan nanoparticles in an oral dose of 500 mg/kg for 1 month [4].
Note: Diethyl nitrosamine was dissolved in saline while CCl4 was dissolved in olive oil in a ratio 9:1.
2.4. Blood sampling and liver tissue preparation
At the end of the experimental period, rats were weighed, slightly anesthetized and blood samples were collected from the sublingual vein. Sera were separated by centrifugation at 4,000 rpm for 10 min and were kept at − 80 °C for subsequent estimation of biochemical parameters.
Animals were sacrificed by cervical dislocation and liver tissue was separated and kept in 10% formaldehyde, for histopathological examination.
2.5. Measured parameters
2.5.1. Histopathological examination
Deparaffinized sections of liver tissues 4 μm were stained with hematoxylin and eosin (H&E) and examined under light microscope as a confirmatory analysis for HCC incidence [12].
2.5.2. Serum liver function and antioxidants
Serum alanine and aspartate aminotransferases (ALT & AST) activities were estimated spectrophotometrically using commercially available kits provided from Randox Company [13]. Serum malondialdehyde (MDA) was measured using kit provided by Randox Company according to the manufacturer’s instructions [14]. Serum total Nitrite/Nitrate (NOx) was measured according to the method of Miranda et al. [15], using kit provided by Randox Company. Serum glutathione (GSH) level was estimated using kit provided by Randox company [16].
2.5.3. Quantitative RT-PCR analysis
Total RNA was isolated using Tripure Isolation Reagent (Roche) according to the manufacturer’s instructions. Complementary DNA (cDNA) was generated using Superscript Choice systems (Life Technologies, Breda, Netherlands) according to the manufacturer’s instructions. To assess the mRNA expression of GSK-3, C-Fos, NF-κB and TNF-α, quantitative real-time PCR was performed using SYBR green PCR Master Mix (Applied Biosystems, CA, USA) as described by the manufacturer. Reaction volume of 25 μl, 5 μl of cDNA were added to 12.5 μl of 2x SYBR green Master Mix and 200 ng of each primer. The primers sequences are described in Table 1.The temperature was: 94◦C for 3 min, 94 °C for 20 s, 58 °C for 20 s and 72 °C for 10 s for 40 cycles [17].
Table 1.
primers sequence used in RT-PCR analysis.
| Gene | sequence |
|---|---|
| GSK-3β forward | 5’-GGAACTCCAACAAGGGAGCA- 3’ |
| GSK-3β Reverse | 5’-TTCGGGGTCGGAAGACCTT A-3’ |
| c-FOS forward | 5′- GGGACAGCCTTTCCTACTACC-3′ |
| c-FOS Reverse | 5′- GATCTGCGCAAAAGTCCTGT-3 |
| TNF-α forward | 5'-CCAGACCCTCACACTCAGATCA-3' |
| TNF-α Reverse | 5'-TCCGCTTGGTGGTTTGCTA-3' |
| NFƘB forward | 5'- CATGAAGAGAAGACACTGACCATGGAAA- 3' |
| NF ƘB Reverse | 3'-TGGATAGAGGCTAAGTGT AGACACG-5' |
2.6. Statistical analysis
Statistical analysis was performed using Instat-3 computer program (Graph pad software Inc, San Diego, CA, USA). One way analysis of variance (ANOVA) by SPSS 12 program followed by Post HOC test to determine the variance within different groups was performed. Data were expressed as means ± SEM. The level of significance was set at p < 0.05 using Tukey ҆s test.
3. Results
3.1. Characterization studies
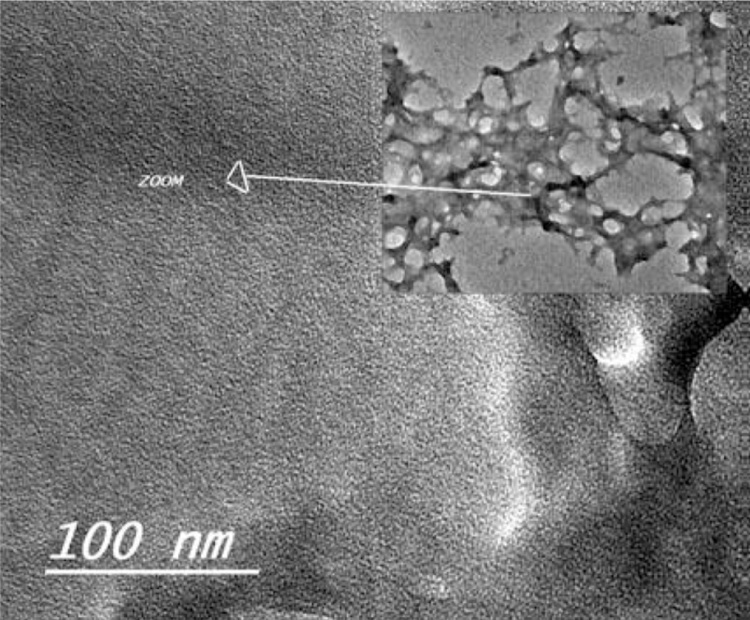
CNPs had a mean hydrodynamic diameter potential of 43.58 ± 1.5 nm and zeta potential of +9 to + 20 mV. The average size reported by TEM was 40 ± 12 nm, the remaining particles showed no agglomerates. Sample Code: NS0115. Particle size 40 nm s; with fine nanostructure of few nanometers particles. Concentration: 30 mg/ml equivalent to 3% wt/Vol. Dispersion Medium: water. CNPs posses narrow particle size distribution without agglomeration. Fig. 1 showed TEM image of CNPs.
Fig. 1.
TEM of chitosan nanoparticles.
3.2. Biochemical findings
3.2.1. Inhibition of DEN -induced liver injury
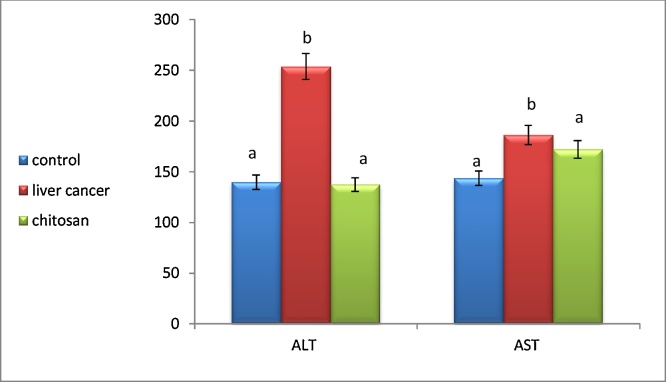
Our results showed that, DEN intoxication significantly increased serum ALT and AST levels by 110% and 50%, respectively as compared to the control values. On the other hand, CNPs groups reduced the levels of liver enzymes comparatively to that of DEN intoxicated group, implying the possible protective effects of CNPs on hepatocytes as presented in Fig. 2.
Fig. 2.
Effect of chitosan nanoparticles on serum ALT and AST in DEN induced hepatocellular carcinoma. Data are expressed as means ± SEM (n = 10). p <0.05 is considered significant. Groups having the same letter are not significantly different from each other, while those having different letters are significantly different.
3.2.2. Modulation of oxidative stress biomarkers
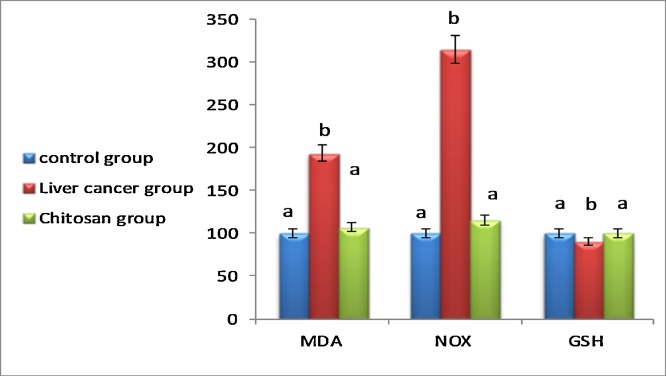
DEN intoxication revealed oxidative stress evidenced by a slight lowering in serum GSH along with an increment of MDA and Nox levels as compared to the control (Fig. 3). Administration of CNPs slightly elevated GSH values as compared to animals intoxicated with DEN alone. It is worthy to note that CNPs exhibited a pronounced effect in this regard. Meanwhile, the MDA value was elevated reaching 200.2% as compared to DEN intoxicated group. Obviously, NOx was also decreased reaching 190.8% as compared to DEN intoxicated group displaying thus a pronounced antioxidant effect of CNPs.
Fig. 3.
Effect of chitosan nanoparticles on serum MDA, NOx induced hepatocellular carcinoma. Data are expressed as means ± SEM (n = 10). p <0.05 is considered significant. Groups having the same letter are not significantly different from each other, while those having different letters are significantly different.
3.2.3. Impact of CNPs on NFκB expression and inflammatory cytokines
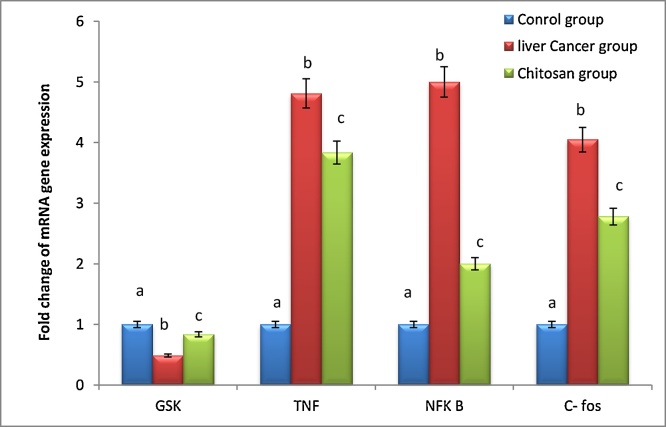
Fig. 4 diduced that DEN intoxication caused a significant up- regulation in the gene expression of NFκB by almost 5-fold as compared to the control value. Nevertheless, a significant down- regulation was apparent in rats treated with CNPs.
Fig. 4.
Effect of chitosan nanoparticles on mRNA gene expression of GSK-3, TNF-α, NFƘB and C-Fos following DEN induced hepato-cellular carcinoma. GAPHD was used as an internal control for calculating mRNA fold changes. Data are expressed as means ± SEM (n = 10). P-value <0.05 is considered significant. Groups having the same letter are not significantly different from each other, while those having different letters are significantly different from each other.
As demonstrated in Fig. 4, mRNA level of TNF-α was significantly increased in DEN group, significantly this level was reduced post CNPs treatment as compared to DEN intoxicated group.
3.2.4. Impact of CNPs on apoptosis
As demonstrated in Fig. 4, DEN intoxication produced a significant up regulation in C- FOS and a significant down regulation on GSK-3 gene expression amounted to 4 and 0.5 folds respectively, as compared to the control value.
CNPs administration counteracted these changes by inducing a significant down regulation in C-FOS activity that reached 1.5 folds, as compared to DEN group. Additionally, CNPs significantly elevated GSK-3 level by 0.5 folds as compared to DEN group.
3.2.5. Histopathology of liver in animal experimental model
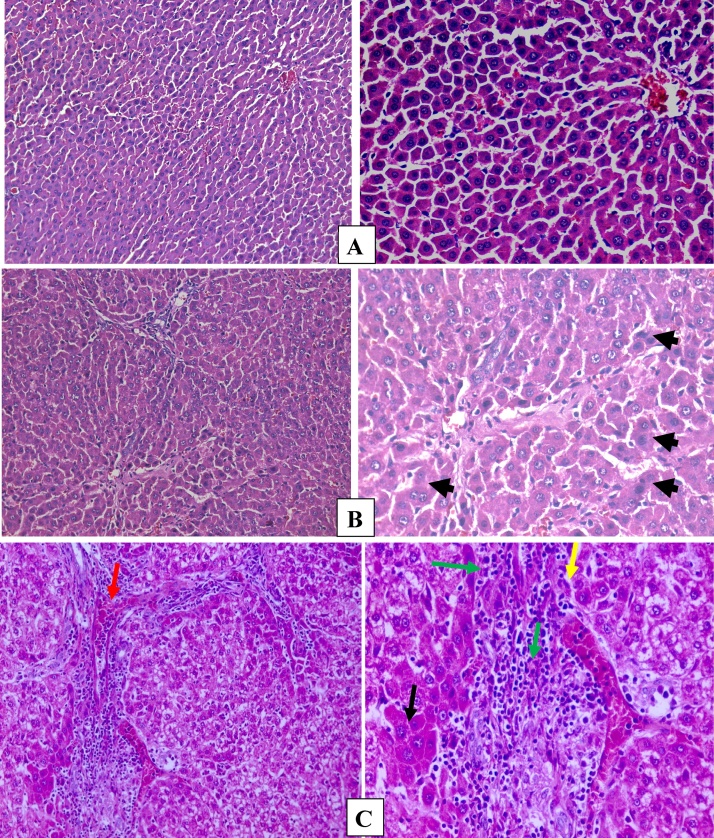
Fig. 5 showed control group with normal hepatocytes (A). On the other side, DEN intoxicated group displayed malignant hepatocytes showing large polyhedral cells with eosinophilic cytoplasm with enlarged nuclei arranged in cords and acinar pattern (B). However, CNPs groups showed regeneration of many of degenerated hepatocytes with some cellular infiltration. Finally, apparently normal liver architecture was seen in the group receiving the CNPs regimen (C).
Fig. 5.
(A): Control group showing normal hepatocytes, preserved architecture and liver cells arranged in thin plates (B) DEN group showing, malignant hepatocytes showing large polyhedral cells with esophilic cytoplasm and enlarged nuclei arranged in cords and acinar pattern (arrows). (C) Chitosan group, showed hepatic tissue with loss architecture, hepatocytes arranged in thick plates (black arrow) and sinusoids (yellow arrow), portal tracts contain one bile duct, one artery and one vein (red arrow), infiltration of hepatocytes in portal tracts and in between hepatocytes (green arrow) (H&E, x200, x400).
4. Discussion
Hepatocellular carcinoma (HCC), is the most propagated liver cancer worldwide and fifth most common cause of mortality [7,1].
DEN, induce disturbance in DNA replication and is used as hepatocarcinogen in animal models [8]. DEN is metabolized to active ethyl radical that interacts with DNA causing mutation, leading to carcinogenesis [18].
In the current study, the therapeutic assessment of CNPs on DEN-CCl4 induced HCC was reported in addition to the crosstalk between the signaling pathways partially related to its action.
The present study revealed that DEN-CCl4induced a significant elevation in liver function enzymes including ALT and AST activities as compared to the control value. Meanwhile, treatment with CNPs significantly modulated this elevation.
Liver injury was confirmed histopathologically by the appearance of malignant hepatocytes showing large polyhedral cells with eosinophilic cytoplasm with enlarged nuclei arranged in cords and acinar pattern in DEN-CCl4 group. Increased liver enzymes in DEN intoxicated group was pointed to cellular leakage and loss of functional integrity of liver cell membranes [19].
In this context, the present study elucidated that the IP administration of DEN-CCl4caused a significant increment in the serum levels of MDA and NOx along with a significant reduction in GSH levels. Meanwhile, treatment with CNPs significantly modulated these effects.
In harmony, DEN is metabolized to active ethyl radical that interacts with DNA causing mutation, leading to carcinogenesis. DEN cause oxidative stress and cellular injury due to enhancing the formation of free radicals [18,20].
Antioxidant effect of CNPs plays a vital role in human health. Previous researchers, concluded that CNPs has a direct antioxidant activity by lowering oxidative stress also it inhibited MDA elevation and glutathione depletion via activation of Nrf2. CNPs significantly suppressed NO, iNOS, and NF-κB [22,23].
GSK-3 catalyzes the transfer of a phosphate group from ATP to target substrates [24]. The phosphorylation process regulates various biological activities, including cell signaling, apoptosis and intracellular communication. GSK-3 plays a crucial role in cancer progression [25]. It may act as a tumor suppressor in some tumor types, meanwhile, it is associated with tumor progression in other tumor types by stabilizing the β-catenin complex [5].
Arouna et al. [21], reported that the over expression of c-Fos level was correlated with higher nuclear levels of inactive GSK-3β.
c-Fos is an important member of AP-1 involved in proliferation and apoptosis [26].
The present study revealed that DEN-CCl4 induced a significant upregulation in the apoptotic marker C-Fos along with a significant down regulation in GSK-3 gene expression as compared to the control value. Meanwhile, treatment with CNPs significantly modulated these deviations.
According to Bakiri and Wagner [8], c-Fos is expressed, in HCC. c-Fos contributes to premalignant transformation of hepatocytes. DEN experimental HCC model; demonstrate that c-Fos is essential for hepatocyte transformation and HCC development [27,28] which may be contributed to the stabilization of Cyclin D1 [[29], [30], [31]].
Meanwhile, CNPs can act on tumor cells and interfere with cell metabolism and cause cell apoptosis [32]. Positively charged CNPs have neutralizing effects on the tumor cells [30].
Oxidative stress could also trigger the activation of NF-κB, which displays a crucial role in the inflammatory cascade by initiating the gene transcription of many proinflammatory cytokines including TNF-α, IL-1β, IL-6, COX-2 and iNOS [33,34].
The present study revealed that DEN-CCl4 induced a significant elevation in the gene expression of inflammatory markers NFƘB and TNF-α as compared to the control values. Meanwhile, treatment with CNPs significantly reduced their levels.
NFκB is activated via oxidative stress, which then triggers inflammatory cascade by initiating the gene transcription of many proinflammatory cytokines TNF-α, IL-6, cycloxygenase (COX-2) and intrinsic nitric oxide synthase (iNOS) [33,34]
NF-κB elucidates survival of mutated hepatocytes, leading to malignancy and cancer development [35].
Ma et al. [7], verified that CNPs could significantly decrease the gene expression of NF-κB by blocking the degradation of inhibitory kappa B alpha (IκB-α) protein and translocation of NF-κB from cytoplasm to the nucleus [7].
CNPs, significantly inhibit proinflammatory cytokines TNF-α, IL-1 MIF and IL-8 by inhibiting NF-κB, TLR-4 and c-fos activation [36].
CNPs, significantly inhibit proinflammatory cytokines TNF-α and IL-8 through blockade of mitogen activated protein kinase (MAPK) and phosphoinistol kinase (PI3K/Akt) signaling pathways and suppressing the activation of NFκB [7].
5. Conclusion
Chitosan NPs, elucidated a significantly modulatory effect on proinflammatory cytokines, oxidative stress and apoptotic markers and it may be recommended as a promising cancer therapy.
Funding
This research was financially supported by internal projects, reference number: 11010314, National Research Centre, Dokki, Giza, Egypt, P.O: 12662.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgment
Is directed to prof. Dr. Olfat Ali Hammam, professor of pathology Theodor Bilharz research institute for histopathological aid.
Contributor Information
Mai O. Kadry, Email: mo.Kadri@nrc.sci.eg.
Rehab M. Abdel-Megeed, Email: ra.abdel-megeed@nrc.sci.eg.
Emad El-Meliegy, Email: el-meliegy@nrc.sci.eg.
Abdel-Hamid Z. Abdel-Hamid, Email: az.abdel-hamid@nrc.sci.eg.
References
- 1.Erkki R., Sangeeta N., Bhatia J., Michael R., Sailor Targeting of drugs and nanoparticles to tumors. Arch. 2010;188(6):759. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiao Y., Wickett N.J., Ayyampalayam S., Chanderbali A.S., Landherr L., Ralph P.E., Tomsho L.P., Hu Y., Liang H., Soltis P.S., Soltis D.E., Clifton S.W., Schlarbaum S.E., Schuster S.C., Ma H., Leebens-Mack J., dePamphilis C.W. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;5(473):97–100. doi: 10.1038/nature09916. (7345) [DOI] [PubMed] [Google Scholar]
- 3.Wilms L.C., Boots A.W., de Boer V.C., Maas L.M., Pachen D.M., Gottschalk R.W. Impact of multiple genetic polymorphisms on effects of a 4-week blueberry juice intervention on ex vivo induced lymphocytic DNA damage in human volunteers. Carcinogenesis. 2007;28(8):1800–1806. doi: 10.1093/carcin/bgm145. [DOI] [PubMed] [Google Scholar]
- 4.Wang J.J., Zhao W.Z., Ren Z.X., Tian X., Guang L.Z., Xiao R.Z., Wang S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011;6:765–774. doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao C., Holscher C., Liu Y., Li L. GSK3: a key target for the development of novel treatments for type 2 diabetes mellitus and Alzheimer disease. Rev. Neurosci. 2012;23:1–11. doi: 10.1515/rns.2011.061. [DOI] [PubMed] [Google Scholar]
- 6.Karin M., Lin A. NF-kappa B at the crossroads of life and death. Nat. Immunol. 2002;3(3):221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 7.Ma L., Shen C.A., Gao L., Li D.W., Shang Y.R., Yin K., Zhao D.X., Cheng W.F., Quan D.Q. Anti-inflammatory activity of chitosan nanoparticles carrying NF-κB/p65 antisense oligonucleotide in RAW264.7 macropghage stimulated by lipopolysaccharide. Colloids Surf. B Biointerfaces. 2016;1(142):297–306. doi: 10.1016/j.colsurfb.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Bakiri L., Wagner E.F. Mouse models for liver cancer. Mol. Oncol. 2013;7(2):206–223. doi: 10.1016/j.molonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papa S., Zazzeroni F., Bubici C., Jayawardena S., Alvarez K., Matsuda S., Nguyen D.U., Pham C.G., Nelsbach A.H., Melis T. Gadd45β mediates the NF-κB suppression of JNK signalling by targeting MKK7/JNKK2. Nat. Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- 10.Wullaert A., van Loo G., Heyninck K., Beyaert R. Hepatic tumor necrosis factor signaling and nuclear factor-kB: effects on liver homeostasis and beyond. Endocr. Rev. 2007;28:365–386. doi: 10.1210/er.2006-0031. [DOI] [PubMed] [Google Scholar]
- 11.Hassan S.K., Aaria M.M., Eshak M.G., Abd El H.F., Razik M.B. Abd El Fattah. Therapeutic and chemopreventive effects of nano curcumin against diethylnitrosamine induced hepatocellular carcinoma in rats. Int. J. Pharm. Sci. 2014;3(6):54–62. [Google Scholar]
- 12.Bancroft J.D., Stevens A. 4th ed. Churchill Livingstone; London: 1996. Theory and Practice of Histological Techniques; p. 163. [Google Scholar]
- 13.Reitman S., Frankle S. A colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Miranda K.M., Espey M.G., Wink D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 16.Moron M.S., J Depierre, Mannervik B. Levels of glutathione, glutathione reductase andglutathione-S-transferase activities in rat lung and liver. Biochem. Biophys. Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 17.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Nermin A.H., Sadik S.A., EL-Maraghy Manal F., Ismail Diethylnitrosamine-induced hepatocarcinogenesis in rats: possible chemoprevention by blueberries. AJBR. 2008;2(3):81–87. [Google Scholar]
- 19.Heidelbaugh J.J., Sherbondy M. Cirrhosis and chronic liver failure. Part II: complications and treatment. Am. Fam. Phys. 2006;74:767–776. [PubMed] [Google Scholar]
- 20.Asuku O., Atawodi S.E., Onyike E. Antioxidant, hepatoprotective and ameliorative effects of methanolicextract of leaves of grewiamollisjusson carbon tetrachloride-treated albino rats. J. Med. Food. 2011;15(1):83–88. doi: 10.1089/jmf.2010.0285. [DOI] [PubMed] [Google Scholar]
- 21.Aruna U., Rajalakshmi R., Indira M.Y., Vinesha V., Sushma M., Vandana K.R., Vijay K.N. Role of chitosan nanoparticles induced cancer therapy. Int. J. Innov. Pharmac. Res. 2013;4(3):318–324. [Google Scholar]
- 22.Garry K., Riga, Azenes, Riga, Latvia The potential of chitosan and its derivatives in prevention and treatment of age-related diseases. Mar. Drugs. 2015;13:2158–2182. doi: 10.3390/md13042158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi W., Akoh C.C., Fischer J., Krewer G. Effects of phenolic compounds in blueberries and muscadine grapes on HepG2 cell viability and apoptosis. Food Res. Int. 2006;39(5):628–638. [Google Scholar]
- 24.Woodgett J.R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton T.M., Pedraza-Alva G., Deng B., Wood C.D., Aronshtam A., Clements J.L., Sabio G., Davis R.J., Matthews D.E., Doble B., Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min L., Ji Y., Bakiri L., Qiu Z., Cen J., Chen X., Chen L., Scheuch H., Zheng H., Qin L. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat. Cell Biol. 2012;14:1203–1211. doi: 10.1038/ncb2590. [DOI] [PubMed] [Google Scholar]
- 27.Fan Q., He M., Deng X., Wu W.K., Zhao L., Tang J., Wen G., Sun X., Li Y. Derepression of c-Fos caused by microRNA-139 down-regulation contributes to the metastasis of human hepatocellular carcinoma. Cell Biochem. Funct. 2013;31:319–324. doi: 10.1002/cbf.2902. [DOI] [PubMed] [Google Scholar]
- 28.Eferl R., Ricci R., Kenner L., Zenz R., David J.P., Rath M., Wagner E.F. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 29.Wang H., Brown J., Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine. 2011;53:130–140. doi: 10.1016/j.cyto.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma P., Liu H.T., Wei P., Xu Q.S., Bai X.F., Du Y.G., Yu C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydr. Polym. 2011;84:1391–1398. [Google Scholar]
- 31.Meryem G., Kahina T.A., Agnès L., Laurence M., Alain M., Dominique B., Fanny D. c-Fos overexpression increases the proliferation of human hepatocytes by stabilizing nuclear Cyclin D1. World J. Gastroenterol. 2008;7:14(41):6339–6346. doi: 10.3748/wjg.14.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngo D.H., Kim S.K. Antioxidant effects of chitin, chitosan and their derivatives. In: Kim S.-K., editor. In Marine Carbohydrates: Fundamentals and Applications, Part B. Elsevier Inc.; Oxford, UK: 2014. pp. 15–31. [Google Scholar]
- 33.Salvatore P., Concetta B., Francesca Z., Guido F. Mechanisms of liver disease: the crosstalk between the NF-κB and JNK pathways. Biol. Chem. 2009;390(10):965–976. doi: 10.1515/BC.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wullaert A., Heyninck K., Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kB and JNK activation in hepatocytes. Biochem. Pharmacol. 2006;72:1090–1101. doi: 10.1016/j.bcp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Dutta J., Fan Y., Gupta N., Fan G., Gélinas C. Current insights into the regulation of programmed cell death by NF-kB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 36.Jue T., Yinglei X., Jianqin X., Yun L., Yueqin C. Chitosan nanoparticles reduce LPS-induced inflammatory reaction via inhibition of NF-κB pathway in Caco-2 cells. Int. J. Biol. Macromol. 2016;86:848–856. doi: 10.1016/j.ijbiomac.2016.02.015. [DOI] [PubMed] [Google Scholar]