Abstract
Background
Involuntary memories are a hallmark symptom of posttraumatic stress disorder (PTSD), but studies of the neural basis of involuntary memory retrieval in posttraumatic stress disorder (PTSD) are sparse. The study of the neural correlates of involuntary memories of stressful events in PTSD focuses on the voluntary retrieval of memories that are sometimes recalled as intrusive involuntary memories, not on involuntary retrieval while being scanned. Involuntary memory retrieval in controls has been shown to elicit activity in the parahippocampal gyrus, precuneus, inferior parietal cortex, and posterior midline regions. However, it is unknown whether involuntary memories are supported by the same mechanisms in PTSD. Because previous work has shown that both behavioral and neural responsivity is slowed in PTSD, we examined the spatiotemporal dynamics of the neural activity underlying negative and neutral involuntary memory retrieval.
Methods
Twenty-one individuals with PTSD and 21 non-PTSD, trauma-exposed controls performed an involuntary memory task, while undergoing a functional magnetic resonance imaging scan. Environmental sounds served as cues for well-associated pictures of negative and neutral scenes. We used a finite impulse response model to analyze temporal differences between groups in neural responses.
Results
Compared with controls, participants with PTSD reported more involuntary memories, which were more emotional and more vivid, but which activated a similar network of regions. However, compared to controls, individuals with PTSD showed delayed neural responsivity in this network and increased vmPFC/ACC activity for negative > neutral stimuli.
Conclusions
The similarity between PTSD and controls in neural substrates underlying involuntary memories suggests that, unlike voluntary memories, involuntary memories elicit similar activity in regions critical for memory retrieval. Further, the delayed neural responsivity for involuntary memories in PTSD suggests that factors affecting cognition in PTSD, like increased fatigue, or avoidance behaviors could do so by delaying activity in regions necessary for cognitive processing. Finally, compared to neutral memories, negative involuntary memories elicit hyperactivity in the vmPFC, whereas the vmPFC is typically shown to be hypoactive in PTSD during voluntary memory retrieval. These patterns suggest that considering both the temporal dynamics of cognitive processes as well as involuntary cognitive processes would improve existing neurobiological models of PTSD.
Abbreviations: PTSD, posttraumatic stress disorder; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; IPC, inferior parietal cortex; vmPFC, ventromedial prefrontal cortex; MTL, medial temporal lobes; IAPS, International Affective Picture System; SPGR, spoiled gradient recalled; TR, repetition time; TE, echo time; TI, inverse recovery time; SPM, Statistical Parametric Mapping; FIR, finite impulse response; FDR, false detection rate; FWE, family-wise error
Keywords: Posttraumatic stress disorder (PTSD), Involuntary memory, Functional magnetic resonance imaging (fMRI), Finite impulse response (FIR), Memory network, Ventromedial prefrontal cortex (vmPFC)
Highlights
-
•
We conducted the first fMRI study of involuntary memory (IM) retrieval in PTSD.
-
•
Activity in IM regions was similar in PTSD and control groups but delayed in PTSD.
-
•
Activity in the vmPFC was higher in PTSD > controls for negative > neutral IMs.
-
•
People with PTSD had more IMs than controls.
-
•
Models of PTSD would benefit from studies of the timing of neural responsivity.
1. Introduction
Involuntary episodic memories are explicit memories of past events that can be described by the rememberer, but are not preceded by a retrieval attempt (Berntsen, 1996). Involuntary memories are assumed to be critical for maintaining a sense of continuity of self across time and for giving fast access to memories that may have functional relevance in novel situations (Rasmussen and Berntsen, 2009). They occur at least as frequently as their voluntary counterparts (Rasmussen and Berntsen, 2011; Rubin and Berntsen, 2009), and they are viewed as a basic mode of remembering, operating on the same system as voluntary memories (Berntsen, 2010).
Involuntary memories of a traumatic event are a hallmark symptom of posttraumatic stress disorder (PTSD; American Psychiatric Association, 2013), with intrusive memories being present in almost 90% of individuals with PTSD (Roszell et al., 1991). Past research indicates the frequency of these intrusive memories—and especially associated responses such as negative reinterpretations, a strong sense of reliving, and maladaptive emotional reactions to the memories—correlates highly with PTSD severity (e.g., Michael et al., 2005). Little is known about the neural underpinnings of the retrieval of involuntary memory. This paucity of research stands in marked contrast with the fact that in clinical disorders, such as PTSD, involuntary memories often become disruptive and distressing. Despite their centrality to the disorder, the basic cognitive and neural mechanisms underlying both emotional and non-emotional involuntary memories in PTSD are not well understood.
We were able to identify four studies that specifically addressed the neural basis of intrusive involuntary memories of stressful events. Two of these studies used a prospective design with healthy individuals to examine whether neural activity during the encoding of stressful stimuli would predict the reporting of involuntary intrusive memories of this material during a subsequent diary study. Both reported increased activity in limbic regions, including the amygdala, anterior cingulate cortex (ACC), striatum, thalamus, and parahippocampal gyrus, with such increased activity during the encoding of stressful films subsequently reported as flashbacks (Bourne et al., 2013), and during encoding in individuals reporting a high level of intrusions (Battaglini et al., 2016). Neither of these studies examined neural activation during retrieval.
Whalley et al. (2013) had PTSD patients and controls provide a narrative account of their trauma or most distressing event before the scan. During the scan they conducted a recognition task in which they were asked to identify keywords culled from their own trauma narrative versus keywords derived from other participants' narratives. Outside the scanner, the participants subsequently identified which of the keywords had led to flashback experiences during the scan and which led to ordinary episodic memories. Whalley et al. (2013) found that flashbacks versus episodic recollections were associated with increased neural activity in the sensory and motor areas. However, since a voluntary autobiographical recognition task generated both types of memories, the neural activity did not target involuntary retrieval per se.
Clark et al. (2016) examined involuntary retrieval of emotional film clips in healthy participants while they were at rest. The participants indicated that they had an involuntary memory from the film clip by pressing a button. In contrast to other work examining the neural underpinnings of involuntary memories in healthy individuals (e.g., Hall et al., 2014), Clark et al. (2016) found that involuntary retrieval elicited activity in frontal regions. This finding may reflect that the memory recording necessarily involved a source monitoring judgment to decide whether a memory ‘popping into mind’ matched what had been seen in the film clip or not, since only involuntary memories related to the film clips should be reported. Thus, the frontal activity may be due to making this judgment, which might involve a voluntary search process and related cognitive control processes. We designed our task to address this issue by using retrospective report to determine when involuntary memories occur (Hall et al., 2014). We also expand on this work by including individuals with PTSD and comparing negative to neutral involuntary memories.
Outside the clinical literature, a few studies examined brain activity during involuntary memory retrieval. Hall et al. (2014) compared voluntary and involuntary memories of scenes cued by environmental sounds to mimic the kinds of cues and memories that often occur during everyday life (Berntsen et al., 2013; Rubin et al., 2008; Schlagman and Kvavilashvili, 2008). Both voluntary and involuntary memories elicited activity in regions typically found to be active during visual voluntary memory retrieval, including the parahippocampal gyrus, posterior midline regions like the posterior cingulate cortex (PCC) and retrosplenial cortex, precuneus, and inferior parietal cortex (IPC). However, involuntary memory retrieval did not elicit activity in frontal cortices, likely reflecting the lack of effort involved in such retrieval (Hall et al., 2014).
To our knowledge, no studies addressing the neural underpinnings of involuntary memories in PTSD have used a methodology that does not include the voluntary retrieval of a memory during the scan either to rate directly or to compare with a memory that has come involuntarily. The goal of the present study was to fill this gap in the literature. We wanted to examine the neural activations associated with involuntary memory processing in PTSD in general and not specifically for traumatic events. Developing a deeper understanding of such general processing deficits is relevant because, among other things, diagnostic inclusion criteria for PTSD include a general enhancement of negative affect that is not limited to trauma-relevant material. In particular, this includes difficulty experiencing positive affect, decreased interest in activities, and overly negative thoughts or assumptions about oneself or the world (American Psychiatric Association, 2013).
Involuntary memories in PTSD compared to non-PTSD participants, irrespective of their trauma-relevance, involve greater emotionality and are more central to the life story (Berntsen et al., 2015; Rubin et al., 2008, Rubin et al., 2011). Also, there is evidence of broad emotion regulation deficits in PTSD beyond traumatic material. Individuals with PTSD have abnormal responses to non-trauma-related negative stimuli, including increased self-reported emotion (Amdur et al., 2000; Orsillo et al., 2004; Wolf et al., 2009) and enhanced neural activity to non-trauma-related stimuli (Offringa et al., 2013; Rauch et al., 2000; Shin et al., 2005). Our focus on the broad emotion processing deficit in PTSD is consistent with an increasing emphasis in the PTSD literature on dysfunctional emotion regulation processes across a range of contexts, and not simply in response to the traumatic event per se (e.g., Bonanno et al., 2004; Brohawn et al., 2010; Orcutt et al., 2014; Shin et al., 2005). Because of this aim we used the same paradigm as Hall et al. (2014, described in detail below), and developed in our previous work (Berntsen et al., 2013; Staugaard and Berntsen, 2014), but varied the emotionality of the scenes.
Voluntary retrieval of scenes, such as those we used here, is commonly used in studies of involuntary memories in healthy individuals (Berntsen, 2009; Berntsen et al., 2013). They elicit activity in a well-characterized network of regions including the parahippocampal gyrus, the inferior and superior parietal cortices, precuneus, posterior cingulate cortex, and dorsomedial and dorsolateral prefrontal cortex (for reviews, see Kim, 2013; Spaniol et al., 2009). In previous work, we showed activity in a subset of these regions for involuntary memory retrieval (Hall et al., 2014): the parahippocampal gyrus, thought to underlie successful retrieval, making contributions to scene and context memory (Ranganath and Ritchey, 2014), the inferior parietal cortex, thought to be involved in bottom-up (i.e., non-goal-driven, or source-driven) attention to memory (Cabeza et al., 2008; Hutchinson et al., 2009) and posterior midline regions like the posterior cingulate cortex, a key region in the default mode network, which has been associated with self-referential thought (Buckner and Carroll, 2007; Greicius et al., 2003; Gusnard et al., 2001; Wicker et al., 2003) and the precuneus, which has been associated with visual imagery (Cavanna and Trimble, 2006). There may be an additional anterior/posterior dissociation within the inferior parietal cortex, with anterior regions being involved in attention reorienting and posterior regions being associated with the default mode network (Hutchinson et al., 2009). Other regions not found to be active during involuntary memory retrieval (Hall et al., 2014) are regions thought to be involved in top-down processes that are less likely to be invoked when retrieval is involuntary. The dorsolateral PFC is thought to be involved in memory search and maintenance (Svoboda et al., 2006). The superior parietal cortex underlies top-down, goal-driven attention to memory (Cabeza et al., 2008; Hutchinson et al., 2014). The differential and common activity in the superior and inferior parietal cortices, respectively, in voluntary and involuntary retrieval illustrates the dissociation in top-down versus bottom-up cognitive processes used during these two types of retrieval. These top-down processes allow for the maintenance of retrieval goals and may be more engaged during low-confidence memory retrieval in an attempt to retrieve additional details of a weak memory, whereas bottom-up processes are particularly engaged when the memory is salient (Cabeza et al., 2008). Though these processes are thought to exist in two dimensions, they interact. Bottom-up processes can occur during voluntary memories and top-down processes can occur during involuntary memories.
To examine the neural activations associated with involuntary memory processing in PTSD generally, we did not intentionally select scenes that were trauma-relevant for each participant. Critically, participants did not indicate when they had an involuntary memory during the task because doing so would likely invoke top-down processes that would not typically occur during naturalistic involuntary remembering (Berntsen, 2009) and would produce neural activity common to voluntary retrieval tasks. We also examined the specific time course for the neural activations, following up on previous work. Because retrieval in our task and in autobiographical memory can take seconds, we used fMRI to study the time course of retrieval, which allowed us to track the time course of neural processes (Daselaar et al., 2008; Hall et al., 2014). Investigating differences in the time course of neural processes is critical in PTSD because previous work indicates such differences in neural activity during memory retrieval (St Jacques et al., 2011a) and in response time during reflexive, involuntary processing in PTSD (Clark et al., 2003; Veltmeyer et al., 2005). Below, we present whole-brain results but with a theoretical focus on what we refer to as the involuntary memory network, derived from previous work on non-emotional involuntary memories, which includes the MTL, the precuneus, IPC, and posterior midline regions, as well as on emotion-processing regions consisting of the amygdala and the vmPFC.
2. Materials & methods
2.1. Participants
Fifty-seven participants (35 women, mean age = 21.9 years, range = 18–37 years) completed the scan session. Participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board and the Duke University Medical Center Institutional Review Board. All participants had an A1 trauma in which they were exposed to death, threatened death, actual or threatened serious injury, or actual or threatened sexual violence (American Psychiatric Association, 2013). After excluding participants who did not meet inclusion criteria (see Supplemental materials) there were 21 participants in the PTSD group (15 women, mean age = 21.5 years, range = 18–31 years) and 21 in the trauma-exposed control group (14 women, mean age = 22.1 years, range = 18–37 years). Additional demographic and clinical data are shown in Table 1 and described in the Supplemental materials.
Table 1.
Participant characteristics.
| Demographics | PTSD mean (SD) | Control mean (SD) | p |
|---|---|---|---|
| Age | 21.48 ( 3.34) | 22.10 ( 4.75) | 0.640 |
| CAPS | 54.29 (15.88) | 11.29 (10.78) | <0.001 |
| BDI | 13.55 ( 9.10) | 3.33 ( 3.69) | <0.001 |
| CES | 25.60 ( 5.23) | 15.00 ( 8.77) | <0.001 |
| PCL-S | 32.48 (12.42) | 8.67 ( 6.17) | <0.001 |
| TLEQ | 6.14 ( 3.53) | 5.19 ( 4.48) | 0.474 |
| Type of trauma exposure | PTSD total (n) | Control total (n) |
|---|---|---|
| Sexual assault | 6 | 5 |
| Physical assault | 4 | 2 |
| Injury, illness, or accident | 4 | 6 |
| Family/friend injury or death | 7 | 6 |
| Natural disaster | 0 | 2 |
2.2. Materials
The stimuli included 55 scenes and 95 environmental sounds (e.g., woman sneezing, lawn mower starting). We chose these stimuli because scene memories are typical of autobiographical memories and sounds are common cues for such memories (Berntsen et al., 2013). We obtained the images from the International Affective Picture System (Lang et al., 2008) and used normative IAPS valence ratings. We considered ratings from 1.0–3.5 to be negative, 3.6–5.5 to be neutral, and 5.6–9.0 to be positive. We only included negative and neutral scenes and did not choose images to be related to a specific trauma. The negative pictures depicted poverty, death/illness, violence, gore, filth, an accident, and hate groups. The neutral pictures depicted animals sometimes looked threatening, daily life activities, objects, and locations, military people and equipment, modes of transportation, locations, and portraits of people. While we did not intentionally choose scenes to be relevant to any individual participant's index trauma, some images may have been relevant to particular traumas. We did not obtain trauma-relevance ratings from the participants; however, a rater judged the trauma-relevance of each image for each trauma and found no more than nine images to be relevant to any participant's index trauma.
2.3. Experimental design and procedure
The experimental design and procedure is nearly identical to that described in a previous publication in healthy subjects (Hall et al., 2014). Here we report a short summary of the experimental design and procedures modified for the goals of the current study. For a full description, see the Supplemental materials and Fig. 1.
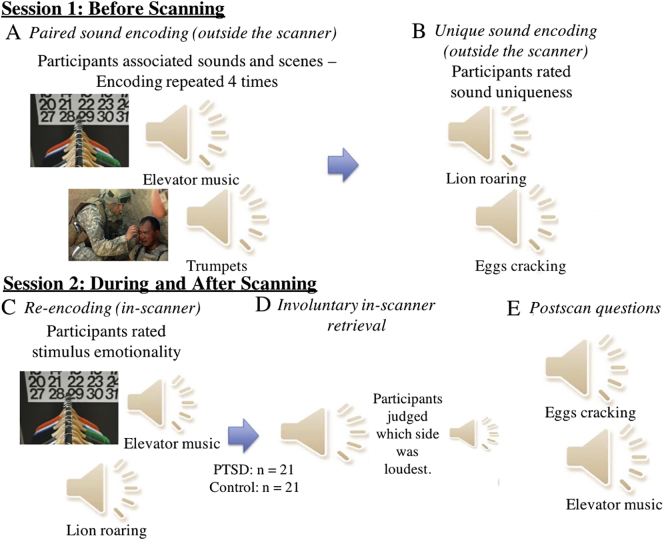
Fig. 1.
Behavioral paradigm. (A) In session 1, during prescan encoding, participants heard a sound paired with a picture and were asked to type a sentence linking the sound and picture. (B) During another prescan encoding session, participants heard unpaired sounds and rated the uniqueness of the sound. (C) In session 2, participants performed a re-encoding session in the scanner, whereupon they were presented with previous stimuli and asked to rate on a 7-point scale how emotional the stimuli were. Participants were instructed that ‘stimuli’ referred to either the sound-picture pair for paired sounds or sounds alone for unpaired sounds. (D) Participants subsequently heard both paired and unpaired sounds and were asked to judge where the sound was located (left or right). (E) After the scan, all participants heard all sounds and were asked questions about their experiences in the scanner (see text for post-scan questions details).
Two days before MRI scanning, participants encoded the 55 paired and 40 unpaired sounds. To ensure strong memory associations, there were four encoding runs, during which participants were presented with the sound-picture pairs and wrote a sentence or a short story linking the sound and the picture. Two days after encoding, they participated in an fMRI session during which they re-encoded the paired and unpaired sounds and then completed the critical sound localization/memory recall runs. During the re-encoding runs, each sound-picture pair and unpaired sound was presented. Participants rated on a 7-point scale how emotional the stimuli were. During the recall runs, the 55 paired and 40 unpaired sounds (randomly intermixed) were presented, panned 15° to either the left or the right using specialized audio software (Audacity, audacity.sourceforge.net). Participants indicated the side on which the sound was louder. We presented each sound for 4 s, followed by a 1.5-second response period, during which the screen was blank and participants completed the sound localization judgment. A fixation period followed (jittered with a mean of 4 s, range: 1.5 s–10.4 s). At the beginning of the run, we instructed participants to wait until after the sound had ended to respond. Immediately following the scanning session, participants completed a post-scan questionnaire to assess their memory for the scenes. Participants heard all 95 sounds. After the presentation of each sound, participants reported: (1) whether they had remembered a picture when the sound was played during the sound localization task (yes/no), (2) how hard they tried to perform the sound localization task correctly (1 = did not try at all, 7 = tried very hard), (3) how hard they tried to recall a picture during the scan (1 = did not try at all, 7 = tried very hard), and (4) how vivid the memory of the picture was during the scan (1 = not at all vivid, 7 = very vivid). Lastly, we presented the 55 paired sounds a final time and participants provided a description of the picture originally paired with the sound. Two independent judges rated these descriptions for accuracy. In the case of disagreement, a third judge broke the tie. As described below, only paired sounds that elicited a memory without effort and unpaired sounds that did not elicit a memory and had no retrieval effort were analyzed.
2.4. Image acquisition and preprocessing
Imaging was conducted on a 3 T GE Signa Excite MRI scanner (GE Healthcare, Waukesha, WI) with an eight-channel head coil. Following a localizer scan was a T1-weighted structural image, (96 contiguous slices acquired with a high-resolution, 3D fast inverse recovery-prepared spoiled gradient recalled (SPGR) sequence, with a repetition time (TR) = 3.22 ms, echo time (TE) = 8.2 ms, inversion recovery time (TI) = 450 ms, 1 mm slice thickness, and parallel imaging with a selection factor of 2). During the task, T2*- weighted echo-planar, functional images were acquired using a spiral-in sequence with SENSE imaging (34 contiguous slices, TR = 2000 ms, TE = 30 ms, FOV = 240 mm, 3.8 mm slice thickness with no gaps, flip angle = 70°, voxel size = 3.75 × 3.75 × 3.8 mm, 64 × 64 matrix, SENSE factor = 2). Preprocessing and analyses of functional imaging data were conducted with Statistical Parametric Mapping software (SPM12; Wellcome Department of Cognitive Neurology, London, UK), along with locally developed Matlab (Mathworks, Natick, MA) scripts. We discarded the first three volumes of each run, including the first trial of each run, for scanner stability. We also excluded the discarded trials for each run from behavioral analyses. Images were corrected for slice-timing and head motion, spatially normalized to the Montreal Neurological Institute template using a 12-parameter affine model, and then spatially smoothed with an 8-mm Gaussian kernel. A high-pass filter was included in every model to correct for scanner drift.
2.5. fMRI data analysis
We created two general linear models: a Memory model comparing paired and unpaired sound trials and an Emotion model comparing high and low emotional valence trials. In the Memory model, the first-level model included regressors for paired sound trials in which there was a reported memory and a low effort rating (1 or 2 out of 7), and unpaired sound trials for which there was no reported memory and a low effort rating. We split these trials according to sound lateralization (paired left, paired right, unpaired left, and unpaired right) to account for this additional variance. We collapsed the sound lateralization factor at the second level. In both models, we included all high-effort trials, paired sound trials that did not elicit a memory, and unpaired sound trials that did elicit a memory as trials of no interest, as well as regressors accounting for motion and run-level effects.
In the Emotion model, at the first level, we separately included regressors for high and low emotion paired sounds (both with low-effort and that elicited a memory). We only used paired sounds in this model because unpaired sounds did not have a paired picture from which IAPS ratings could be used to classify it as high or low emotion. Therefore, we included unpaired sounds as trials of no interest.
We initially identified activations using a finite impulse response (FIR) model (Goutte et al., 2000; Henson et al., 2001), which we preferred over a standard canonical hemodynamic response model to better understand the temporal dynamics of involuntary memory retrieval. We initially modeled seven TRs, or 14 s, starting at the beginning of the trial, not adjusted for the hemodynamic lag, but included only the 3rd to 6th TRs in analyses. The 1st and 2nd TRs were not analyzed to account for the hemodynamic lag (Buxton et al., 2004) as activity occurring before this could not reliably be attributed to the presentation of the sound at the beginning of each trial. The 7th TR was not analyzed because it corresponded to the beginning of the subsequent trial. The 3rd and 4th TRs corresponded to the time period during which peak activity associated with the sound would be expected to occur, accounting for hemodynamic lag. The 5th and the 6th TRs corresponded to the time period during which involuntary memories could continue to be formed.
Additionally, exploratory analyses revealed that activity was similar in the 3rd and 4th TRs and in the 5th and the 6th TRs. Therefore, to maintain a consistent length of time represented by each time point we collapsed these pairs of TRs to create an early and a late time point. The late time point included the 1.5 s response period and 2.5 s of the jitter. The late time point was not contaminated by the cognitive processes involved in the subsequent trial because the hemodynamic response for the subsequent trial does not peak until approximately 6 s after that trial begins. We report whole-brain results at a significance threshold of p < 0.05 using a false discovery rate (FDR) correction and a cluster correction of p < 0.05, using a family-wise error (FWE) correction.
3. Results
3.1. Behavioral results
3.1.1. Memory model: paired > unpaired
We report behavioral results for paired > unpaired sounds in Table 2. PTSD participants reported more involuntary memories (those retrieved with low effort) than controls, and paired sounds elicited more involuntary memories than unpaired sounds. Additionally, focusing only on trials included in the fMRI analyses (paired sounds that elicited a memory and unpaired sounds that did not), an ANOVA revealed higher emotion ratings (gathered during encoding) and vividness (gathered during the post-scan retrieval run) for paired sounds relative to unpaired sounds (see Table 2).
Table 2.
Memory model behavioral results. Note, the number of trials modeled, shown in parantheses in the % recalled column, were not included in tests of behavioral differences.
| % recalled |
Emotion |
Vividness |
Reaction time |
|||||
|---|---|---|---|---|---|---|---|---|
| Avg. | SD | Avg. | SD | Avg. | SD | Avg. | SD | |
| Paired sounds | ||||||||
| Control | 56.19% (30.00 trials modeled) | 24.24% (12.89) | 4.09 | 1.20 | 4.55 | 1.4 | 689.22 | 231.53 |
| PTSD | 68.84% (36.95 trials modeled) | 22.52% (12.11) | 4.24 | 0.68 | 5.32 | 1.3 | 713.16 | 138.59 |
| Unpaired sounds | ||||||||
| Control | 4.08% (36.43 trials modeled) | 5.18% (4.34) | 2.51 | 1.18 | 1.10 | 0.40 | 751.06 | 223.20 |
| PTSD | 6.66% (31.67 trials modeled) | 5.41% (9.60) | 2.47 | 0.77 | 1.06 | 0.22 | 765.60 | 157.02 |
| ANOVA | F | p | F | p | F | p | F | p |
| Main effect of group | 4.23 | <0.05⁎ | 0.05 | >0.05 | 2.79 | >0.05 | 0.32 | >0.05 |
| Main effect of pairing | 238.29 | <0.001⁎⁎ | 60.23 | <0.001⁎⁎ | 309.54 | <0.001⁎⁎ | 0.68 | >0.05 |
| Interaction | 1.85 | >0.05 | 0.20 | >0.05 | 3.41 | >0.05 | 0.25 | >0.05 |
p < 0.05.
p < 0.001.
3.1.2. Emotion model: high > low emotion
We report behavioral analyses for high > low emotion sound-picture pairs in Table 3. Notably, the PTSD group had more involuntary memories to both high and low emotion trials than controls. There was no effect of emotion on the number of memories recalled.
Table 3.
Emotion model behavioral results. Note, the number of trials modeled, shown in parantheses in the % Recalled column, were not included in tests of behavioral differences.
| % recalled |
Emotion |
Vividness |
Reaction time |
|||||
|---|---|---|---|---|---|---|---|---|
| Avg. | SD | Avg. | SD | Avg. | SD | Avg. | SD | |
| High emotion | ||||||||
| Control | 55.40% (19.05 trials modeled) | 24.77% (8.46) | 4.77 | 1.34 | 4.53 | 1.41 | 723.63 | 235.07 |
| PTSD | 70.12% (24.29 trials modeled) | 23.52% (8.19) | 4.85 | 0.88 | 5.35 | 1.27 | 707.70 | 151.46 |
| Low emotion | ||||||||
| Control | 57.64% (11.95 trials modeled) | 26.02% (4.94) | 2.82 | 1.09 | 4.49 | 1.55 | 709.77 | 289.52 |
| PTSD | 66.48% (12.67 trials modeled) | 22.51% (4.29) | 2.38 | 0.82 | 5.04 | 1.47 | 677.40 | 150.83 |
| ANOVA | F | p | F | p | F | p | F | p |
| Main effect of group | 4.95 | <0.05⁎ | 0.07 | >0.05 | 5.55 | <0.05⁎ | 0.76 | >0.05 |
| Main effect of emotion | 0.02 | >0.05 | 69.36 | <0.001⁎⁎ | 0.69 | >0.05 | 0.01 | >0.05 |
| Interaction | 0.02 | >0.05 | 0.29 | >0.05 | 0.07 | >0.05 | 0.00 | >0.05 |
p < 0.05.
p < 0.001.
There were no reaction time differences for either model. Behavioral performance on the localization task for both models is reported in the Supplemental materials.
3.1.3. Post-scan recall
We quantified the number of pictures that were accurately recalled voluntarily after the scan. Technical error resulted in missing data for one PTSD participant. There was an effect of Group (F(1, 82) = 5.80, p < 0.05), with the PTSD group accurately recalling a greater percentage of the pictures (PTSD mean: 0.92, control mean: 0.85), an effect of Emotion (F(1, 82) = 4.03, p < 0.05), with a greater percentage of low emotion pictures being accurately recalled (low emotion mean: 0.92, high emotion mean: 0.86), but no Group x Emotion interaction (F(1, 82) = 0.06, p > 0.05).
3.2. Neuroimaging results
Because we could not be certain when the involuntary memories would occur, we probed neural activity at two time points: an early time point including the 4 s when the sound played, and a late time point including the 4 s after the sound stopped. We first discuss the results of a Memory model in which the neural underpinnings of involuntary memories can be probed in both groups. The Memory model is a 2 (Group) × 2 (Time) model. Then we discuss an Emotion model in which we contrast high and low emotion trials. Since all results shown are for the negative > neutral trials, this model is also a 2 (Group) × 2 (Time) design.
3.2.1. Memory model: paired sounds > unpaired sounds
3.2.1.1. Group × time interaction
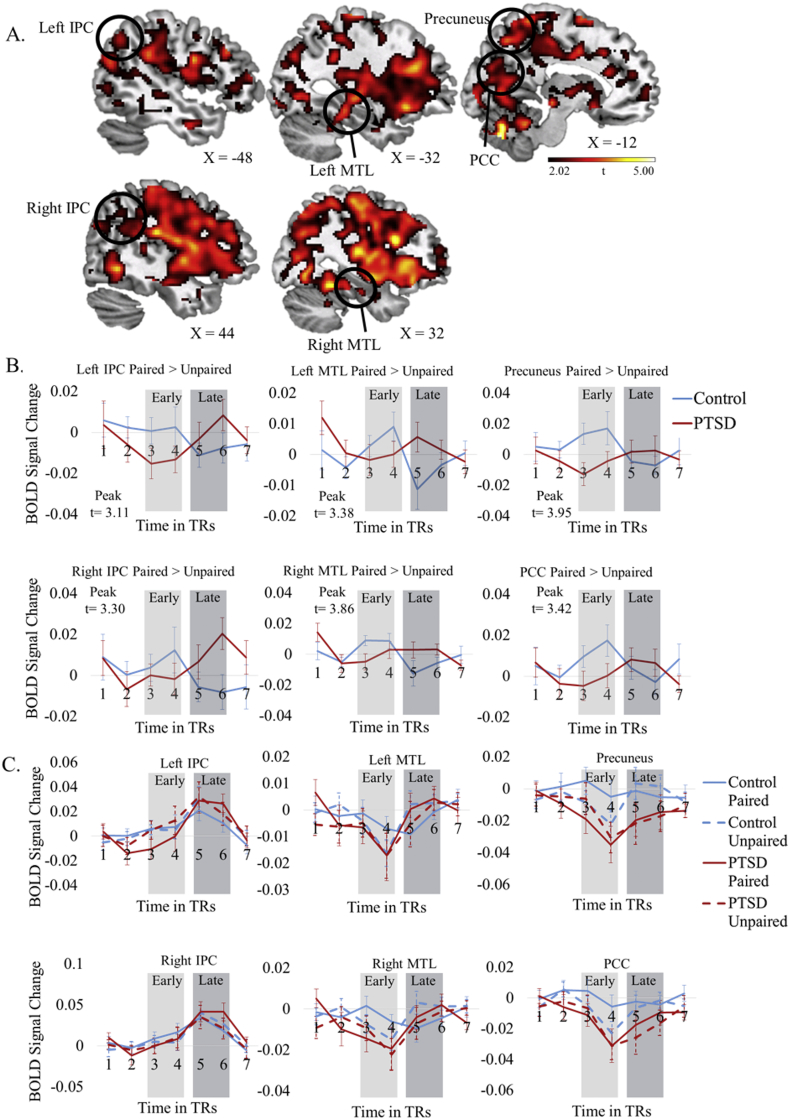
Results from the Group × Time interaction revealed timing differences in a similar set of regions for controls (early > late) and PTSD (late > early): bilateral parahippocampal gyrus, precuneus, IPC, and PCC exhibited delayed activity in the PTSD group compared to the control group (Fig. 2, Table 4). There was no activity for the reverse contrast (control late > early, PTSD early > late).
Fig. 2.
Memory model. Group (PTSD, Control) × Time (Early, Late) interaction. All activity is significant at p < 0.05, FDR-corrected, cluster corrected to p < 0.05, FWE-corrected. A. Regions of interest are highlighted. B. Time courses plotting paired > unpaired differences at each TR for regions of interest shown above. Each TR is 2 s. The first, second, and seventh TRs were not included in any analyses and are included for illustrative purposes only. Activity relating to the early and late time points is indicated with light and dark gray boxes over the respective time points. These graphs indicate that the paired > unpaired sound difference is greater for the control group than the PTSD group during the early time point but that this difference is greater for the PTSD group than the control group in the late time point. C. Time courses for paired and unpaired sounds are plotted separately. The data are available on neurovault: https://neurovault.org/collections/3400/.
Table 4.
Memory model. Top peaks from the Group × Time interaction. All results are significant at p < 0.05, FDR corrected for multiple comparisons, cluster corrected at p < 0.05, FWE-corrected. Note: peaks from the ROIs were not among the top peaks in this table but are shown in Fig. 2.
| Region | Cluster size | Side | x | y | z | t-Score |
|---|---|---|---|---|---|---|
| Control early ≥ late, PTSD late ≥ early | ||||||
| Orbitofrontal cortex | 73,321 | Right | 26 | 22 | −16 | 5.04 |
| Cerebellum | Left | −10 | −72 | −36 | 5.01 | |
| Insula | Right | 38 | −2 | −2 | 4.67 | |
| Claustrum | Right | 30 | −20 | 14 | 4.52 | |
| Fusiform gyrus | Right | 30 | −48 | −18 | 4.49 | |
| Lentiform nucleus | Right | 20 | −12 | 4 | 4.35 | |
| Postcentral gyrus | Right | 40 | −30 | 54 | 4.27 | |
| Orbitofrontal cortex | Left | −20 | 26 | −16 | 4.24 | |
| Precentral gyrus | Right | 34 | −16 | 56 | 4.21 | |
| Cuneus | Left | −16 | −94 | 0 | 4.19 | |
| Mid cingulate cortex | Right | 6 | −8 | 44 | 4.17 | |
| vlPFC (Inferior frontal gyrus) | Right | 38 | 24 | −2 | 4.16 | |
| Anterior cingulate cortex | Right | 18 | 28 | 30 | 4.15 | |
| Inferior parietal cortex | Right | 58 | −30 | 28 | 4.14 | |
| Lentiform nucleus | Right | 16 | 10 | −8 | 4.13 | |
| Control late ≥ early, PTSD early ≥ late | ||||||
| None | ||||||
3.2.1.1.1. Group × time interaction follow-up analyses
To further explore the change in neural activity between the early and the late time points in the control and PTSD groups, we test the observation that our results may be due to a difference between groups at one time window but not the other. We first calculated the average BOLD signal estimates for each time point for each of the six regions shown in Fig. 2. We then subtracted the early and late BOLD signal estimates to determine the change in activity between the early minus the late time points in the control group and the late minus the early time points in the PTSD group. Finally, we calculated the ratio of the change in activity between time points. The closer the ratio is to one, the more similar the change in activity between time points. The average ratio across all six regions is 0.659, suggesting that the change in activity between the early and late time points was similar across groups. See Table S1 for more details.
Additionally, we examined the paired > unpaired sound contrast (i.e. sounds that elicited a memory > sounds that did not elicit a memory) during the early time point for the control group combined with the late time point for the PTSD group. This analysis revealed whether activity at the early time point for the control group and the late time point for the PTSD group was greater than zero. There was significant activity in posterior midline regions and inferior parietal cortex. When the threshold was raised slightly to p < 0.055, FDR corrected for multiple comparisons, our a priori hypothesized region, the parahippocampal gyrus, was also active (see Fig. S1 and Table S2). There was no activity for the control group late combined with the PTSD group early. This supports our claim that involuntary memories elicit activity in a similar network of regions at different times for the two groups.
3.2.1.2. Main effect of group
There was no significant main effect of group, suggesting that the regions associated with memory retrieval are similar between groups. To test for similarities within the memory network between groups, we conducted two additional analyses. First, we extracted estimates of activity in these regions for both groups in both time points and ran t-tests to compare the groups. We compared control early to PTSD late and compared PTSD early to control late since the Group x Time analysis suggested that the memory network was similar between groups but peaked at different times. There were no differences in activity between the early time point in the control group and the late time point in the PTSD group in any region and the absolute value of the t-scores was lower than one for five out of the six regions of interest. Turning to differences between the late time point in the control group and the early time point in the PTSD group, there were no differences in activity and t-scores were lower than 1.2 for the all regions (see Table 5). This suggests that memory network activity is similar between groups but with different peak times.
Table 5.
A between-group, between-time point comparison of activity associated with involuntary memory retrieval. The regions are shown in Fig. 2. There is similar activity between the early time point in the control group and the late time point in the PTSD group in all regions. There is also similar activity between the late time point in the control group and the early time point in the PTSD group in all regions. This suggests that a similar neural network underlies involuntary memory retrieval in PTSD and controls with the difference being the time that it peaks.
| Control early, PTSD late |
Control late, PTSD early |
|||
|---|---|---|---|---|
| t-Score | p | t-Score | p | |
| All regions | 0.31 | >0.75 | −0.05 | >0.95 |
| Left MTL | 0.48 | >0.60 | −1.07 | >0.25 |
| Right MTL | 1.07 | >0.25 | −1.17 | >0.20 |
| Precuneus | 0.69 | >0.45 | −0.11 | >0.90 |
| Left IPC | −0.10 | >0.90 | 0.56 | >0.55 |
| Right IPC | −0.51 | >0.60 | −0.70 | >0.45 |
| PCC | 0.76 | >0.45 | 0.36 | >0.70 |
All df = 40 except in the comparison between control early and PTSD late, the df = 39.89.
Second, we lowered the threshold for the test of the Main Effects of Group to determine whether there were differences between groups at a slightly more lenient threshold. There was no activity for control > PTSD at a threshold as high as p < 0.45, FDR corrected for multiple comparisons. Even raising the threshold as high as p < 0.65 only revealed a 16-voxel cluster in the postcentral gyrus. There was no activity for PTSD > control at a threshold as high as p < 0.95. The lack of activity in regions thought to be involved in memory retrieval even at these extremely lenient thresholds supports our claim that activity in these regions is similar between groups but with peaks at different times.
3.2.1.3. Main effect of time
There was no significant main effect of time.
3.2.2. Emotion model: high versus low emotion
Having established that high and low emotion involuntary memories elicit activity in regions that we predicted, but at different times for the control and PTSD groups, we turn to examining the differences between high and low emotion involuntary memories.
3.2.2.1. Comparing high > low emotion involuntary memories across group and time: group × time interaction
There was no significant Group × Time interaction.
3.2.2.2. Comparing high > low emotion involuntary memories across group and time: main effect of group
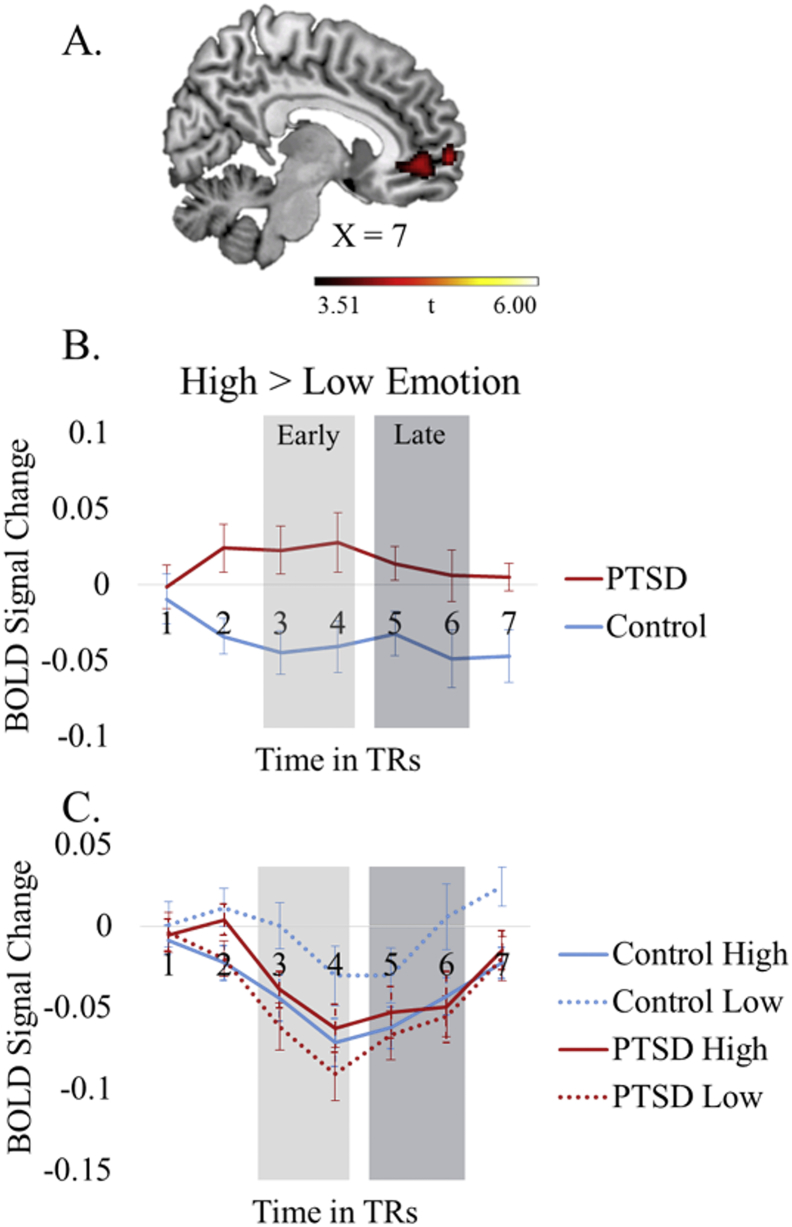
Across both time points, the PTSD group exhibited greater activity in vmPFC and ACC. (Fig. 3, Table 6).
Fig. 3.
Emotion model. Main effect of Group (PTSD versus Control). All activity is significant at p < 0.05, FDR corrected, cluster-corrected at p < 0.05, FWE corrected. A. Activity in the mPFC/ACC. Activity in this region is greater for high > low emotion stimuli in the PTSD group compared to the control group. B. Time courses plotting high > low emotion differences at each TR. Each TR is 2 s. The first, second, and seventh TRs were not included in any analyses and are included for illustrative purposes only. Activity relating to the early and late time points is indicated with light and dark gray boxes over the respective time points. This graph indicates that the high > low emotion difference is greater for the PTSD group than the control group across both time points. C. Time courses for high and low emotion stimuli plotted separately.
Table 6.
Emotion model. Main effect of Group (PTSD versus Control). All results are significant at p < 0.05, FDR corrected for multiple comparisons, cluster corrected at p < 0.05, FWE-corrected.
| Region | Cluster size | Side | x | y | z | t-Score |
|---|---|---|---|---|---|---|
| PTSD ≥ control | ||||||
| Anterior cingulate cortex | 646 | Left | −8 | 30 | −4 | 4.80 |
| Ventromedial prefrontal cortex | Right | 8 | 62 | −2 | 4.58 | |
| Control ≥ PTSD | ||||||
| None | ||||||
3.2.2.3. Comparing high > low emotion involuntary memories across group and time: main effect of time
There was no significant main effect of Time.
4. Discussion
We introduce three novel findings on the neural basis of emotional and neutral involuntary memories in PTSD. First, a similar network of regions is active during involuntary memory retrieval for PTSD and controls. Second, compared to controls, individuals with PTSD showed delayed neural responsivity in regions typically associated with memory retrieval (Kim, 2013; Spaniol et al., 2009) and previously shown to be associated with involuntary memory retrieval in healthy adults (Hall et al., 2014). Third, compared to controls, individuals with PTSD showed increased vmPFC/ACC activity for high > low emotion stimuli.
4.1. Finding 1: similarities in the memory network between PTSD and controls
Involuntary memories elicited similar activity between controls and PTSD in regions involved in the memory network, including the MTL, precuneus, PCC, and IPC. This is in contrast with neurobiological models of PTSD based on voluntary memory retrieval that emphasize dysfunctional activity in the medial temporal lobes and related memory circuits (Carrion et al., 2010; Geuze et al., 2008; Rauch et al., 2006; Werner et al., 2009). Also, in contrast with the voluntary memory literature, we show that the PTSD group had more involuntary memories than the control group. This is consistent with work showing a positive correlation between the frequency of involuntary memories and PTSD symptoms (Berntsen et al., 2015) whereas voluntary memory retrieval, as measured by neuropsychological testing, is impaired in PTSD (Samuelson, 2011) and may even be a risk factor for PTSD (Vasterling et al., 2018). One reason for these deficits could be that individuals with PTSD have decreased executive control functioning (Hayes et al., 2011; Johnsen and Asbjørnsen, 2009; for reviews, see Aupperle et al., 2012; Samuelson, 2011). Since involuntary memory retrieval requires little, if any, executive control, unlike voluntary memory retrieval, these results suggest that memory network integrity and behavioral performance may partially depend on whether retrieval requires cognitive control.
4.2. Finding 2: delayed activity in the memory network in PTSD
Analyses indicated a lag in activity in the memory network in PTSD compared with controls. This timing effect is extremely strong, with over 70,000 voxels in 15 peaks showing earlier activity in the control group compared to no significant activity peaking earlier in the PTSD group. This effect has negligible odds of occurring by chance. Moreover, the activity includes locations we predicted would be active during involuntary memory retrieval based on the existing literature. These findings align with both behavioral work showing delayed response times in PTSD during voluntary memory retrieval (Werner et al., 2009) and neuroimaging work showing slow neural responsivity in PTSD in similar regions during voluntary memory retrieval (St Jacques et al., 2011a). Our results indicate that this processing delay is not limited to effortful retrieval.
The time of peak activity in the control group is consistent with other work showing that neural activity in response to involuntary recall in healthy individuals peaks up to 6 s after the memory (Clark et al., 2016). However, other work comparing the retrieval of memories that had previously been recalled as flashbacks in PTSD showed that compared with control participants, PTSD showed decreased posterior midline and parahippocampal activity (Whalley et al., 2013). Though at first glance this appears to be inconsistent with our results, it highlights the necessity of investigating the difference in the timing of the neural response to such memories. In the current study, when activity was compared between groups in the early time point, there appeared to be hypoactivity in the controls and hyperactivity in the PTSD group in similar regions. It was not until later activity was examined that activity in these regions was revealed in the PTSD group. This suggests that hypoactivity found in Whalley et al. (2013) and other studies (Carrion et al., 2010; Geuze et al., 2008; Rauch et al., 2006; Werner et al., 2009) could be reexamined in light of these results.
We offer four possible explanations for this delayed activity in the memory network in PTSD. The first explanation is that involuntary memories take longer to construct in PTSD. The involuntary memories measured here were more vivid in PTSD than controls. This finding is consistent with other work showing highly vivid intrusive memories in PTSD (Berntsen et al., 2003; Michael et al., 2005) that decrease in vividness after therapy (Hackmann et al., 2004). Memories become more specific and likely more vivid as more time passes to construct the memory (Daselaar et al., 2008; Haque and Conway, 2001). It is possible that more time is needed to construct highly vivid memories in PTSD. A second possible explanation is that the slowed processing speed is driven by factors that affect both effortful and non-effortful cognitive processes. Not only do PTSD patients have slowed reaction times to tasks that require cognitive control (Barrett et al., 1996; Vasterling et al., 2002; for a review, see Aupperle et al., 2012) but they have slower reaction times during reflexive attention tasks (Clark et al., 2003; Veltmeyer et al., 2005), which may reflect a generalized cognitive slowing. This could be caused by increased fatigue or decreased cognitive capacity. PTSD participants in our study did not have slowed reaction times to the localization task. This is likely because participants were given 4 s to respond and speeded responding was not emphasized in the instructions. Other work has shown that when speed is not emphasized, reaction time is not slowed in PTSD (e.g. Dickie et al., 2008; St Jacques et al., 2011a). Third, this processing delay is the result of increased cognitive demand caused by coping with internal psychological distress or monitoring the environment for threats (Jennings, 1986). Fourth is that people with PTSD try to avoid having memories. Using the think/no-think paradigm (Anderson and Green, 2001) activity during memory retrieval can be modulated by instructing participants to not think of a memory (Depue et al., 2007; Detre et al., 2013). One of the required symptoms of PTSD is avoidance (American Psychiatric Association, 2013), which involves trying to avoid having emotional memories or to suppress the emotional response. People with PTSD avoid thinking about their trauma (Berntsen et al., 2003; Moulds et al., 2007) and avoid emotionally processing memories (Williams and Moulds, 2007). In contrast, people without PTSD are less likely to suppress or distract themselves after an involuntary memory (Reynolds and Brewin, 1998). However, suppression is generally unsuccessful in PTSD (Catarino et al., 2015), so it is possible that the attempt at suppression only delays the onset of the memory. Future work controlling for these factors and investigating different types of involuntary memories, like trauma-specific memories, will shed light on this issue.
4.3. Finding 3: hyperactivity in vmPFC/ACC
There was also increased vmPFC/ACC activity in PTSD compared to controls for high versus low emotion involuntary memories. Fear regulation models have posited that the vmPFC/ACC modulates fear extinction and emotion regulation by downregulating activity in the amygdala (Koenigs and Grafman, 2009; Rauch et al., 2006); the vmPFC/ACC may be involved in regulating other emotions as well (Bush et al., 2000; Etkin et al., 2011). In PTSD, the vmPFC/ACC is typically hypoactive, putatively leading to a hyperresponsive amygdala (Shin et al., 2006). This has also been shown in voluntary recall of traumatic events and emotional scenes (Frewen et al., 2008; Lanius et al., 2007; Protopopescu et al., 2005; Shin et al., 1997, Shin et al., 2004; Whalley et al., 2009) but it is not clear whether this hypoactivity could be expected to generalize to involuntary memories. While there is no previous work on the neural basis of involuntary memories in PTSD to inform this question, involuntary memories do share traits with studied phenomena, like responses to unexpected stimuli. Models discussing this vmPFC/ACC hypoactivity are based on task paradigms in which the emotional items are the focal point (Bremner et al., 1999; Britton et al., 2005; Hou et al., 2007; Lanius et al., 2001, Lanius et al., 2003; Mazza et al., 2013; Phan et al., 2006; Shin et al., 1999, Shin et al., 2004, Shin et al., 2005; Williams et al., 2006), and are thus expected. However, investigating responses to unexpected stimuli is critical because many key symptoms revolve around such responses (American Psychiatric Association, 2013). In fact, unexpected stimuli appear to elicit different neural responses than do expected stimuli. For example, stimuli presented quickly, below the threshold of conscious detection, elicit heightened vmPFC/ACC activity in PTSD compared to controls (Bryant et al., 2008a, Bryant et al., 2008b, Bryant et al., 2010), as do emotional stimuli presented as distractors (Bruce et al., 2013; Fani et al., 2012; Hayes et al., 2009; Kim et al., 2008, but see Shin et al., 2007). Our results suggest that the unexpected nature of involuntary memories may elicit hyperactivity not predicted by prevailing PTSD models. Hyperactivity in these contexts may reflect the generation of nonconscious fear signals that reorient attention to threatening stimuli (Bryant et al., 2008b; Liddell et al., 2005).
Consistent with the idea that vmPFC/ACC hypoactivity is not a canonical PTSD correlate, as implied by prevailing PTSD models, is a lesion study showing that vmPFC lesions decrease the likelihood of developing PTSD (Koenigs and Grafman, 2009). If vmPFC/ACC hypoactivity results in increased amygdala activity, then a lesion here should cause increased emotional responsivity and increased PTSD symptomology. The role of the vmPFC is multifaceted. It has been shown to be involved in self-referential thought (Motzkin et al., 2015; St Jacques et al., 2011b), and the retrieval of contextual details and initiating subsequent action based on those details (Kveraga et al., 2011; Panichello et al., 2012). Understanding the circumstances under which there is hypo- versus hyperactivity here and integrating what is known about the role of this region in other cognitive processes is vital to understanding emotion dysfunction in PTSD.
It can also be noted that the control group had greater activity in the vmPFC/ACC for the neutral stimuli than the negative. It is possible that this relates to attention reorienting (Pessoa et al., 2002) or safety signaling (Ahs et al., 2015) in controls but not PTSD, but these speculative ideas warrant direct investigation in future work.
4.4. Limitations
This study has several limitations. First, we used the post-scan self-report of memory retrieval and effort to classify trials as successful, low effort memory trials or not. We classified trials as successful based on participants' self-report of memory retrieval and effort. Though the use of self-report to classify trials may lead to the misclassification of some trials, there is both behavioral and neural evidence that this paradigm does elicit involuntary memories with high frequency. When unique cues are used to cue memories of unique scenes, similar to the cues and scenes used in this paradigm, and participants report the retrieval of involuntary memories as they occur in real time, almost 60% of the cues elicit involuntary memories (Berntsen et al., 2013). This rate is similar to the frequency with which involuntary memories are elicited in our control group, shown above. Additionally, involuntary memories occur more frequently in PTSD than in controls (Rubin et al., 2011), similar to the pattern that we find. Finally, the similarity in neural activity for voluntary and involuntary memory retrieval (Hall et al., 2014) suggests that the use of post-hoc self-report to classify trials is reasonably accurate.
Second, our claim that there is similar activity between groups in regions critical for memory retrieval, but at different times, is derived from the Group x Time interaction effect in the Memory Model, and the lack of a Main Effect of Group. To ensure that the similar activity did not rely solely on a null result, we conducted four additional analyses to make four additional claims. In the first analysis, we focused on the six key regions of interest for memory retrieval. We conducted t-tests comparing activity between the early time point in the control group and the late time point in the PTSD group, as well as between the late time point in the control group and the early time point in the PTSD group. The t-scores were all smaller than 1.5 and for 9 of the 12 they were smaller than one. In the second analysis, we showed that there was no Main Effect of Group even at extremely lenient thresholds. In the third analysis, we showed that there was significant activity in these critical memory regions for the control group early and the PTSD group late, and that there was no activity for the control group late and the PTSD group early. This shows that activity in these regions was not driven solely by the difference between the early and late time points, but that the activity in both groups is significantly greater than zero. In the fourth analysis, we compared the change in activity between the early and late time points in the six regions of interest between groups and showed that it was similar between groups for most regions. Together, this set of supplemental analyses lend significant support to our claim that the activity in this involuntary memory network is similar between groups but that it peaks at different times between groups.
Third, encoding processes are sometimes disrupted in PTSD (Samuelson, 2011), suggesting that the neural differences we find may be due to differences in encoding. However, impaired encoding should result in impaired voluntary retrieval. The voluntary post-scan recall scores were similar between groups, suggesting that in our paradigm, encoding was preserved in PTSD. This could be due to having several encoding sessions.
Fourth, we did not directly measure the relatedness of the stimuli to our participants' trauma. However, using trauma descriptions, we determined the relatedness of each picture to each individual's trauma and found that very few pictures were trauma-relevant for anyone. Therefore, we do not think this issue will have a large impact on results. Again, it should be underscored that the aim of the study was to examine the neural underpinning of involuntary memory processing generally in PTSD, and not specifically in relation to personally traumatic material as this overgeneralization to less traumatic situations is what produces many of the symptoms and dysfunctions of the disorder. However, since the present study was not designed to examine neural activity underlying trauma-related involuntary memories in PTSD, it is therefore not clear whether the findings of delayed activity in the memory network in PTSD will generalize to involuntary, intrusive memories of real-life traumatic events. This is an important question for future research to address.
Fifth, we used a relatively high-functioning population; all participants were students or were in the work force. It is possible that people with more debilitating PTSD symptoms could have different neural responses to involuntary memories.
4.5. Conclusions
Understanding the neural mechanisms underlying emotional involuntary memories can pave the way toward the development of a treatment that could alleviate this key symptom of PTSD and can also add to the understanding of the basic emotional processing deficits seen in PTSD. The importance of involuntary memories to PTSD is widely recognized with work seeking to understand the mechanisms underlying the encoding of such memories (Clark et al., 2014, Clark et al., 2016). However, the current study fills a gap in understanding the mechanisms underlying their retrieval. We show that existing models of PTSD do not predict spatiotemporal neural patterning of involuntary memories particularly well. Given that many of the symptoms of PTSD revolve around responses to the unexpected (involuntary memories, startle responses, hypervigilance toward unknown threats), our innovative approach opens up additional lines of inquiry that could be key to understanding the fundamental mechanisms underlying these symptoms.
Acknowledgments
Acknowledgments
The authors wish to thank the Danish National Research Foundation (DNRF89) and the National Institute of Mental Health (grant R01 MH066079) for funding.
Financial disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.05.009.
Appendix A. Supplementary data
Supplemental Table S1
Supplemental Figure S1
Supplemental Table S2
References
- Ahs F., Kragel P.A., Zielinski D.J., Brady R., LaBar K.S. Medial prefrontal pathways for the contextual regulation of extinguished fear in humans. NeuroImage. 2015;122:262–271. doi: 10.1016/j.neuroimage.2015.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdur R.L., Larsen R., Liberzon I. Emotional processing in combat-related posttraumatic stress disorder: a comparison with traumatized and normal controls. J. Anxiety Disord. 2000;14:219–238. doi: 10.1016/s0887-6185(99)00035-3. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, D.C.: 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. [Google Scholar]
- Anderson M.C., Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D.H., Green M.L., Morris R., Giles W.H., Croft J.B. Cognitive functioning and posttraumatic stress disorder. Am. J. Psychiatry. 1996;153:1492–1494. doi: 10.1176/ajp.153.11.1492. [DOI] [PubMed] [Google Scholar]
- Battaglini E., Liddell B., Das P., Malhi G., Felmingham K., Bryant R.A. Intrusive memories of distressing information: an fMRI study. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0140871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen D. Involuntary autobiographical memories. Appl. Cogn. Psychol. 1996;10:435–454. [Google Scholar]
- Berntsen D. Cambridge University Press; New York: 2009. Involuntary Autobiographical Memories: An Introduction to the Unbidden past. [Google Scholar]
- Berntsen D. The unbidden past: involuntary autobiographical memories as a basic mode of remembering. Curr. Dir. Psychol. Sci. 2010;19:138–142. [Google Scholar]
- Berntsen D., Willert M., Rubin D.C. Splintered memories or vivid landmarks? Qualities and organization of traumatic memories with and without PTSD. Appl. Cogn. Psychol. 2003;17:675–693. [Google Scholar]
- Berntsen D., Staugaard S.R., Sorensen L.M. Why am I remembering this now? Predicting the occurrence of involuntary (spontaneous) episodic memories. J. Exp. Psychol.-Gen. 2013;142:426–444. doi: 10.1037/a0029128. [DOI] [PubMed] [Google Scholar]
- Berntsen D., Rubin D.C., Salgado S. The frequency of involuntary autobiographical memories and future thoughts in relation to daydreaming, emotional distress, and age. Conscious. Cogn. 2015;36:352–372. doi: 10.1016/j.concog.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno G.A., Papa A., Lalande K., Westphal M., Coifman K. The importance of being flexible - the ability to both enhance and suppress emotional expression predicts long-term adjustment. Psychol. Sci. 2004;15:482–487. doi: 10.1111/j.0956-7976.2004.00705.x. [DOI] [PubMed] [Google Scholar]
- Bourne C., Mackay C.E., Holmes E.A. The neural basis of flashback formation: the impact of viewing trauma. Psychol. Med. 2013;43:1521–1532. doi: 10.1017/S0033291712002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Staib L.H., Kaloupek D., Southwick S.M., Soufer R., Charney D.S. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol. Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J.C., Phan K.L., Taylor S.F., Fig L.M., Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol. Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Brohawn K.H., Offringa R., Pfaff D.L., Hughes K.C., Shin L.M. The neural correlates of emotional memory in posttraumatic stress disorder. Biol. Psychiatry Neuroplast. Anxiety Disord. 2010;68:1023–1030. doi: 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Bruce S.E., Buchholz K.R., Brown W.J., Yan L., Durbin A., Sheline Y.I. Altered emotional interference processing in the amygdala and insula in women with post-traumatic stress disorder. Neuroimage-Clin. 2013;2:43–49. doi: 10.1016/j.nicl.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Felmingham K., Kemp A., Das P., Hughes G., Peduto A., Williams L. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol. Med. 2008;38:555–561. doi: 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- Bryant R.A., Kemp A.H., Felmingham K.L., Liddell B., Olivieri G., Peduto A., Gordon E., Williams L.M. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum. Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Kemp A.H., Felmingham K., Liddell B.J., Olivieri G., Peduto A., Gordan E., Williams L.M. Simulating emotional responses in posttraumatic stress disorder: an fMRI study. Psychol. Inj. Law. 2010;3:111–117. [Google Scholar]
- Buckner R.L., Carroll D.C. Self-projection and the brain. Trends Cogn. Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Buxton R.B., Uludağ K., Dubowitz D.J., Liu T.T. Modeling the hemodynamic response to brain activation. NeuroImage Math. Brain Imaging. 2004;23:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion V.G., Haas B.W., Garrett A., Song S., Reiss A.L. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an fMRI study. J. Pediatr. Psychol. 2010;35:559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino A., Kupper C.S., Werner-Seidler A., Dalgleish T., Anderson M.C. Failing to forget: inhibitory-control deficits compromise memory suppression in posttraumatic stress disorder. Psychol. Sci. 2015;26:604–616. doi: 10.1177/0956797615569889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Clark C.R., Mcfarlane A., Morris P., Weber D., Sonkkilla C., Shaw M., Marcina J., Tochon-Danguy H., Egan G. 2003. Cerebral Function in Posttraumatic Stress Disorder during Verbal Working Memory Updating: A Positron Emission Tomography Study. [DOI] [PubMed] [Google Scholar]
- Clark I.A., Niehaus K.E., Duff E.P., Di Simplicio M.C., Clifford G.D., Smith S.M., Mackay C.E., Woolrich M.W., Holmes E.A. First steps in using machine learning on fMRI data to predict intrusive memories of traumatic film footage. Behav. Res. Ther. 2014;62:37–46. doi: 10.1016/j.brat.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I.A., Holmes E.A., Woolrich M.W., Mackay C.E. Intrusive memories to traumatic footage: the neural basis of their encoding and involuntary recall. Psychol. Med. 2016;46:505–518. doi: 10.1017/S0033291715002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar S.M., Rice H.J., Greenberg D.L., Cabeza R., LaBar K.S., Rubin D.C. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb. Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Depue B.E., Curran T., Banich M.T. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- Detre G.J., Natarajan A., Gershman S.J., Norman K.A. Moderate levels of activation lead to forgetting in the think/no-think paradigm. Neuropsychologia. 2013;51:2371–2388. doi: 10.1016/j.neuropsychologia.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie E.W., Brunet A., Akerib V., Armony J.L. An fMRI investigation of memory encoding in PTSD: influence of symptom severity. Neuropsychologia. 2008;46:1522–1531. doi: 10.1016/j.neuropsychologia.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., Tone E.B., Phifer J., Norrholm S.D., Bradley B., Ressler K.J., Kamkwalala A., Jovanovic T. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med. 2012;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P., Lane R.D., Neufeld R.W.J., Densmore M., Stevens T., Lanius R. Neural correlates of levels of emotional awareness during trauma script-imagery in posttraumatic stress disorder. Psychosom. Med. 2008;70:27–31. doi: 10.1097/PSY.0b013e31815f66d4. [DOI] [PubMed] [Google Scholar]
- Geuze E., Vermetten E., Ruf M., de Kloet C.S., Westenberg H.G.M. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. J. Psychiatr. Res. 2008;42:659–669. doi: 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Goutte C., Nielsen F.A., Hansen L.K. Modeling the haemodynamic response in fMRI using smooth FIR filters. IEEE Trans. Med. Imaging. 2000;19:1188–1201. doi: 10.1109/42.897811. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackmann A., Ehlers A., Speckens A., Clark D.M. Characteristics and content of intrusive memories in PTSD and their changes with treatment. J. Trauma. Stress. 2004;17:231–240. doi: 10.1023/B:JOTS.0000029266.88369.fd. [DOI] [PubMed] [Google Scholar]
- Hall S.A., Rubin D.C., Miles A., Davis S.W., Wing E.A., Cabeza R., Berntsen D. The neural basis of involuntary episodic memories. J. Cogn. Neurosci. 2014;26 doi: 10.1162/jocn_a_00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S., Conway M.A. Sampling the process of autobiographical memory construction. Eur. J. Cogn. Psychol. 2001;13:529–547. [Google Scholar]
- Hayes J.P., LaBar K.S., Petty C.M., McCarthy G., Morey R.A. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res. Neuroimaging. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., LaBar K.S., McCarthy G., Selgrade E., Nasser J., Dolcos F., workgroup, V.M.-A.M., Morey R.A. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J. Psychiatr. Res. 2011;45:660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R., Rugg M.D., Friston K.J. The choice of basis functions in event-related fMRI. NeuroImage. 2001;13:S149-S149. [Google Scholar]
- Hou C.L., Liu J., Wang K., Li L.J., Liang M., He Z., Liu Y., Zhang Y., Li W.H., Jiang T.Z. Brain responses to symptom provocation and trauma-related short-term memory recall in coal mining accident survivors with acute severe PTSD. Brain Res. 2007;1144:165–174. doi: 10.1016/j.brainres.2007.01.089. [DOI] [PubMed] [Google Scholar]
- Hutchinson J.B., Uncapher M.R., Wagner A.D. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn. Mem. 2009;16:343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J.B., Uncapher M.R., Weiner K.S., Bressler D.W., Silver M.A., Preston A.R., Wagner A.D. Functional heterogeneity in posterior parietal cortex across attention and episodic memory retrieval. Cereb. Cortex N. Y. N. 2014;1991(24):49–66. doi: 10.1093/cercor/bhs278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J.R. Bodily changes during attention. In: Coles M.G.H., Donchin E., Porges S.W., editors. Psychophysiology: Systems, Processes and Applications. Elsevier; Amsterdam: 1986. [Google Scholar]
- Johnsen G.E., Asbjørnsen A.E. Verbal learning and memory impairments in posttraumatic stress disorder: the role of encoding strategies. Psychiatry Res. 2009;165:68–77. doi: 10.1016/j.psychres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Kim H. Differential neural activity in the recognition of old versus new events: an activation likelihood estimation meta-analysis. Hum. Brain Mapp. 2013;34:814–836. doi: 10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Chey J., Chung A., Bae S., Khang H., Ham B., Yoon S.J., Jeong D.U., Lyoo I.K. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. J. Psychiatr. Res. 2008;42:268–277. doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Koenigs M., Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kveraga K., Ghuman A.S., Kassam K.S., Aminoff E.A., Hamalainen M.S., Chaumon M., Bar M. Early onset of neural synchronization in the contextual associations network. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3389–3394. doi: 10.1073/pnas.1013760108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual (Technical Report B-3) [Google Scholar]
- Lanius R.A., Williamson P.C., Densmore M., Boksman K., Gupta M.A., Neufeld R.W., Gati J.S., Menon R.S. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am. J. Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Hopper J., Densmore M., Boksman K., Gupta M.A., Neufeld R.W.J., Gati J.S., Menon R.S. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol. Psychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Frewen P.A., Girotti M., Neufeld R.W.J., Stevens T.K., Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res. Neuroimaging. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Liddell B.J., Brown K.J., Kemp A.H., Barton M.J., Das P., Peduto A., Gordon E., Williams L.M. A direct brainstem-amygdala-cortical “alarm” system for subliminal signals of fear. NeuroImage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Mazza M., Tempesta D., Pino M.C., Catalucci A., Gallucci M., Ferrara M. Regional cerebral changes and functional connectivity during the observation of negative emotional stimuli in subjects with post-traumatic stress disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263:575–583. doi: 10.1007/s00406-013-0394-3. [DOI] [PubMed] [Google Scholar]
- Michael T., Ehlers A., Halligan S.L., Clark D.M. Unwanted memories of assault: what intrusion characteristics are associated with PTSD? Behav. Res. Ther. 2005;43:613–628. doi: 10.1016/j.brat.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry. 2015;77:276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulds M.L., Kandris E., Starr S., Wong A.C.M. The relationship between rumination, avoidance and depression in a non-clinical sample. Behav. Res. Ther. 2007;45:251–261. doi: 10.1016/j.brat.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Offringa R., Handwerger Brohawn K., Staples L.K., Dubois S.J., Hughes K.C., Pfaff D.L., Vanelzakker M.B., Davis F.C., Shin L.M. Diminished rostral anterior cingulate cortex activation during trauma-unrelated emotional interference in PTSD. Biol. Mood Anxiety Disord. 2013;3:10. doi: 10.1186/2045-5380-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcutt H.K., Bonanno G.A., Hannan S.M., Miron L.R. Prospective trajectories of posttraumatic stress in college women following a campus mass shooting. J. Trauma. Stress. 2014;27:249–256. doi: 10.1002/jts.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsillo S.M., Batten S.V., Plumb J.C., Luterek J.A., Roessner B.M. An experimental study of emotional responding in women with posttraumatic stress disorder related to interpersonal violence. J. Trauma. Stress. 2004;17:241–248. doi: 10.1023/B:JOTS.0000029267.61240.94. [DOI] [PubMed] [Google Scholar]
- Panichello M.F., Cheung O.S., Bar M. Predictive feedback and conscious visual experience. Front. Psychol. 2012;3:620. doi: 10.3389/fpsyg.2012.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., McKenna M., Gutierrez E., Ungerleider L.G. Neural processing of emotional faces requires attention. Proc. Natl. Acad. Sci. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Britton J.C., Taylor S.F., Fig L.M., Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Protopopescu X., Pan H., Tuescher O., Cloitre M., Goldstein M., Engelien W., Epstein J., Yang Y., Gorman J., LeDoux J., Silbersweig D., Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol. Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Ranganath C., Ritchey M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 2014;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Rasmussen A.S., Berntsen D. The possible functions of involuntary autobiographical memories. Appl. Cogn. Psychol. 2009;23:1137–1152. [Google Scholar]
- Rasmussen A.S., Berntsen D. The unpredictable past: spontaneous autobiographical memories outnumber autobiographical memories retrieved strategically. Conscious. Cogn. 2011;20:1842–1846. doi: 10.1016/j.concog.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Whalen P.J., Shin L.M., McInerney S.C., Macklin M.L., Lasko N.B., Orr S.P., Pitman R.K. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol. Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Shin L.M., Phelps E.A. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research - past, present, and future. Biol. Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Reynolds M., Brewin C.R. Intrusive cognitions, coping strategies and emotional responses in depression, post-traumatic stress disorder and a non-clinical population. Behav. Res. Ther. 1998;36:135–147. doi: 10.1016/s0005-7967(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Roszell D.K., Mcfall M.E., Malas K.L. Frequency of symptoms and concurrent psychiatric-disorder in Vietnam veterans with chronic Ptsd. Hosp. Community Psychiatry. 1991;42:293–296. doi: 10.1176/ps.42.3.293. [DOI] [PubMed] [Google Scholar]
- Rubin D.C., Berntsen D. The frequency of voluntary and involuntary autobiographical memories across the life span. Mem. Cogn. 2009;37:679–688. doi: 10.3758/37.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D.C., Boals A., Berntsen D. Memory in posttraumatic stress disorder: properties of voluntary and involuntary, traumatic and nontraumatic autobiographical memories in people with and without posttraumatic stress disorder symptoms. J. Exp. Psychol. Gen. 2008;137:591–614. doi: 10.1037/a0013165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D.C., Dennis M.F., Beckham J.C. Autobiographical memory for stressful events: the role of autobiographical memory in posttraumatic stress disorder. Conscious. Cogn. 2011;20:840–856. doi: 10.1016/j.concog.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson K.W. Post-traumatic stress disorder and declarative memory functioning: a review. Dialogues Clin. Neurosci. 2011;13:346–351. doi: 10.31887/DCNS.2011.13.2/ksamuelson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagman S., Kvavilashvili L. Involuntary autobiographical memories in and outside the laboratory: how different are they from voluntary autobiographical memories? Mem. Cogn. 2008;36:920–932. doi: 10.3758/mc.36.5.920. [DOI] [PubMed] [Google Scholar]
- Shin L.M., McNally R.J., Kosslyn S.M., Thompson W.L., Rauch S.L., Alpert N.M., Metzger L.J., Lasko N.B., Orr S.P., Pitman R.K. A positron emission tomographic study of symptom provocation in PTSD. Psychobiol. Posttrauma. Stress Disord. 1997;821:521–523. doi: 10.1111/j.1749-6632.1997.tb48320.x. [DOI] [PubMed] [Google Scholar]
- Shin L.M., McNally R.J., Kosslyn S.M., Thompson W.L., Rauch S.L., Alpert N.M., Metzger L.J., Lasko N.B., Orr S.P., Pitman R.K. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am. J. Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Orr S.P., Carson M.A., Rauch S.L., Macklin M.L., Lasko N.B., Peters P.M., Metzger L.J., Dougherty D.D., Cannistraro P.A., Alpert N.M., Fischman A.J., Pitman R.K. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch. Gen. Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Wright C.I., Cannistraro P.A., Wedig M.M., McMullin K., Martis B., Macklin M.L., Lasko N.B., Cavanagh S.R., Krangel T.S., Orr S.P., Pitman R.K., Whalen P.J., Rauch S.L. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Rauch S.L., Pitman R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Psychobiol. Posttrauma. Stress Disord. Decade Prog. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Bush G., Whalen P.J., Handwerger K., Cannistraro P.A., Wright C.L., Mattis B., Macklin M.L., Lasko N.B., Orr S.P., Pitman R.K., Rauch S.L. Dorsal anterior cingulate function in posttraumatic stress disorder. J. Trauma. Stress. 2007;20:701–712. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- Spaniol J., Davidson P.S.R., Kim A.S.N., Han H., Moscovitch M., Grady C.L. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- St Jacques P.L., Botzung A., Miles A., Rubin D.C. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J. Psychiatr. Res. 2011;45:630–637. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques P.L., Conway M.A., Lowder M.W., Cabeza R. Watching my mind unfold versus yours: an fMRI study using a novel camera technology to examine neural differences in self-projection of self versus other perspectives. J. Cogn. Neurosci. 2011;23:1275–1284. doi: 10.1162/jocn.2010.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staugaard S.R., Berntsen D. Involuntary memories of emotional scenes: the effects of cue discriminability and emotion over time. J. Exp. Psychol.-Gen. 2014;143:1939–1957. doi: 10.1037/a0037185. [DOI] [PubMed] [Google Scholar]
- Svoboda E., McKinnon M.C., Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling J.J., Duke L.M., Brailey K., Constans J.I., Allain A.N., Stuker P.B. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Aslan M., Lee L.O., Proctor S.P., Ko J., Jacob S., Concato J. Longitudinal associations among posttraumatic stress disorder symptoms, traumatic brain injury, and neurocognitive functioning in army soldiers deployed to the Iraq war. J. Int. Neuropsychol. Soc. 2018;24:311–323. doi: 10.1017/S1355617717001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltmeyer M.D., Clark C.R., Mcfarlane A., Felmingham K., Bryant R., Gordon E. Integrative assessment of brain and cognitive function in post-traumatic stress disorder. J. Integr. Neurosci. 2005;4:145–159. doi: 10.1142/s0219635205000719. [DOI] [PubMed] [Google Scholar]
- Werner N.S., Meindl T., Engel R.R., Rosner R., Riedel M., Reiser M., Fast K. Hippocampal function during associative learning in patients with posttraumatic stress disorder. J. Psychiatr. Res. 2009;43:309–318. doi: 10.1016/j.jpsychires.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Whalley M.G., Rugg M.D., Smith A.P.R., Dolan R.J., Brewin C.R. Incidental retrieval of emotional contexts in post-traumatic stress disorder and depression: an fMRI study. Brain Cogn. 2009;69:98–107. doi: 10.1016/j.bandc.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley M.G., Kroes M.C.W., Huntley Z., Rugg M.D., Davis S.W., Brewin C.R. An fMRI investigation of posttraumatic flashbacks. Brain Cogn. 2013;81:151–159. doi: 10.1016/j.bandc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B., Ruby P., Royet J.-P., Fonlupt P. A relation between rest and the self in the brain? Brain Res. Brain Res. Rev. 2003;43:224–230. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Williams A.D., Moulds M.L. Cognitive avoidance of intrusive memories: recall vantage perspective and associations with depression. Behav. Res. Ther. 2007;45:1141–1153. doi: 10.1016/j.brat.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Williams L.M., Kemp A.H., Felmingham K., Barton M., Olivieri G., Peduto A., Gordon E., Bryant R.A. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Wolf E.J., Miller M.W., McKinney A.E. Emotional processing in PTSD heightened negative emotionality to unpleasant photographic stimuli. J. Nerv. Ment. Dis. 2009;197:419–426. doi: 10.1097/NMD.0b013e3181a61c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1
Supplemental Figure S1
Supplemental Table S2