Abstract
In patients with a functionally univentricular heart, the Fontan strategy achieves separation of the systemic and pulmonary circulation and reduction of ventricular volume overload. Contemporary modifications of surgical techniques have significantly improved survival. However, the resulting Fontan physiology is associated with high morbidity. In this review, we discuss the state of the art of the Fontan strategy by assessing survival and risk factors for mortality. Complications of the Fontan circulation, such as cardiac arrhythmia, thromboembolism, and protein-losing enteropathy, are discussed. Common surgical and catheter-based interventions following Fontan completion are outlined. We describe functional status measurements such as quality of life and developmental outcomes in the contemporary Fontan patient. The current role of drug therapy in the Fontan patient is explored. Furthermore, we assess the current use and outcomes of mechanical circulatory support in the Fontan circulation and novel surgical innovations. Despite large improvements in outcomes for contemporary Fontan patients, a large burden of disease exists in this patient population. Continued efforts to improve outcomes are warranted. Several remaining challenges in the Fontan field are outlined.
Keywords: Fontan Procedure, Total cavopulmonary connection, Congenital heart defects, Single ventricle, Pediatrics, Mortality, Morbidity, Re-interventions
Introduction
Functionally univentricular congenital heart disease (CHD), in which only one ventricle is fully developed, poses a complex clinical problem. Estimates of the incidence of this disease entity range from 0.08 to 0.4 per 1,000 births 1– 3. Functionally univentricular CHD entails different morphological diagnoses, the most common of which are hypoplastic left heart syndrome (HLHS) (25 to 67% of functionally univentricular hearts), tricuspid atresia (15 to 24%), and double inlet left ventricle (14 to 18%) 1, 3– 5. It is estimated that currently there are about 22,000 patients in Europe and about 50,000 in the US 6. Recent advancements in prenatal screening have increased the rates of prenatal diagnosis and possibly termination of pregnancy in patients with univentricular hearts 1, 7. Despite the low incidence, improvements in treatment have reduced the mortality to the point where a large number of patients survive into adulthood.
Palliation can be achieved with the Fontan strategy. A series of operations is performed to palliate the adverse effects of a univentricular heart. The Fontan strategy refers to the landmark surgery for tricuspid atresia by Fontan and Baudet 8. In the “early days” of this procedure, it was attempted to replace the function of the right ventricle with the right atrium by connecting the right atrium to the pulmonary artery. Although short-term results were unprecedented, this strategy caused dilation of the right atrium, resulting in arrhythmia and thromboembolism due to sluggish blood flow 9. Modifications of this surgery are referred to as atriopulmonary connections (APCs). In a later era, de Leval et al. found that atrial contractions did not contribute significant power to the APC circuit and proposed the intra-atrial lateral tunnel (ILT), a transatrial connection using an intra-atrial baffle connecting the inferior caval vein to the pulmonary artery in a more energetically favorable manner 10. Currently, most centers employ an extracardiac conduit (ECC), a prosthetic conduit that bypasses the atrium completely. Both ILT and ECC are referred to as total cavopulmonary connection (TCPC) Fontan modifications.
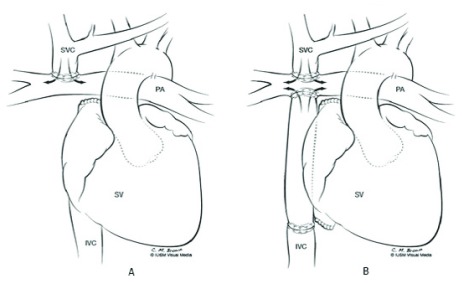
A Fontan circuit was originally created in a single surgical setting. This resulted in relatively high mortality. A staged TCPC, in which a series of operations is performed at different ages, is the current standard of care. These operations are tailored to the individual anatomy of the patient. First, the single ventricle (SV) needs to be connected to the aorta, which may require extensive surgery, such as the Norwood procedure for HLHS. At about 3 to 6 months of age, a partial cavopulmonary connection (PCPC), connecting the superior caval vein to the pulmonary artery (that is, bidirectional Glenn procedure), is performed. Completion of the TCPC is usually performed between 18 months and 4 years of age 4. The connections and circulatory pattern after these operations are illustrated in Figure 1.
Figure 1.
Illustration of the anatomic relationships following partial cavopulmonary connection ( A) and total cavopulmonary connection ( B) palliation. IVC, inferior vena cava; PA, pulmonary artery; SV, single ventricle; SVC, superior vena cava. This figure has been reproduced with permission from Kerlo et al. and Springer Nature 11.
These patients require a lifetime of highly specialized care and significant health-care resources. In Oceania, the mean hospital costs across all stages of palliation are about $200,000 per patient 12. Following Fontan palliation, hospital admission rates for patients are eight times higher than for the general population 13, and both length of stay and hospital costs are higher compared with those of other CHDs, including tetralogy of Fallot 14– 16.
Physiology
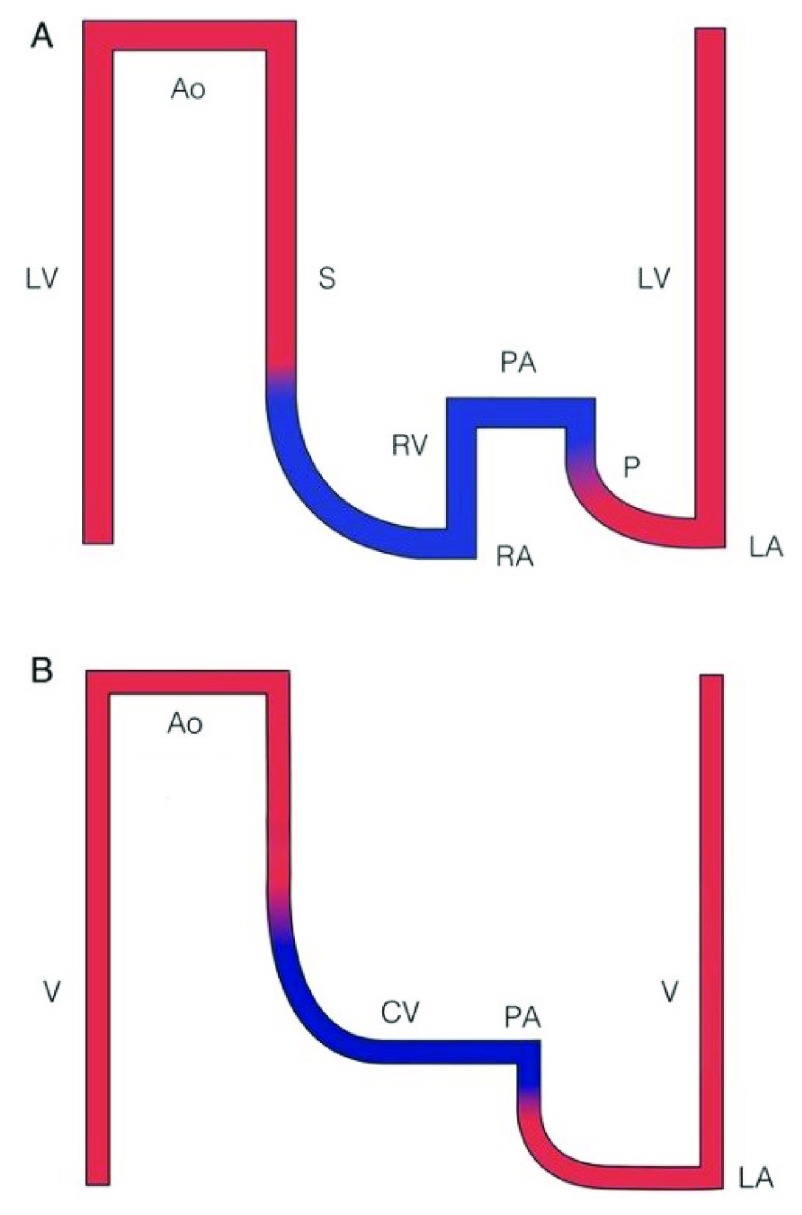
In unpalliated univentricular CHD, cyanosis occurs because of mixing of saturated and unsaturated blood in the heart. The SV is also exposed to volume overload as it drains both systemic and pulmonary venous return at the same time. The Fontan strategy reduces volume overload and restores normoxemia. Following PCPC surgery, some volume unloading of the SV is achieved. Following TCPC, the volume load of the SV is further reduced 17. Furthermore, after TCPC, the systemic and pulmonary blood flows are connected in series rather than parallel as before this strategy has been deployed. This comes at the expense of the lack of a ventricle supplying energy to the pulmonary circulation. This is illustrated in Figure 2. The SV provides the energy needed to attain blood flow through the systemic as well as the pulmonary vascular bed and is subjected to increased afterload. After TCPC, central venous pressures are higher than normal. Pulsatility in the pulmonary artery is mostly lost and there is preload insufficiency of the SV. This highly abnormal circulation is called the Fontan circulation. The resulting physiology has been referred to as a “Fontan paradox”, where systemic venous pressure is high in the presence of relative pulmonary artery hypotension 18. This might augment lymphatic outflow and impede lymphatic inflow from the thoracic duct. Several complications of the Fontan strategy have been linked to abnormalities in lymphatic drainage. Because of the multiple inherent hemodynamic challenges of the Fontan circulation, it is generally considered a palliative procedure 19.
Figure 2.
Scheme of pressures in the normal circulation ( A) and the Fontan circulation ( B). This scheme illustrates the effects of the lack of a prepulmonary pump in the Fontan physiology. Red represents oxygenated blood and blue represents deoxygenated blood. Ao, aorta; CV, caval veins; LA, left atrium; LV, left ventricle; P, pulmonary circulation; PA, pulmonary artery; RA, right atrium; RV, right ventricle; S, systemic circulation; V, single ventricle. This figure has been reproduced with permission from Gewillig and Brown and the British Medical Journal Publishing Group Ltd 20.
The aim of this article is to provide an overview of current outcomes, treatment options, and remaining challenges to improve outlook for patients with univentricular heart disease.
State of the art
Overall survival
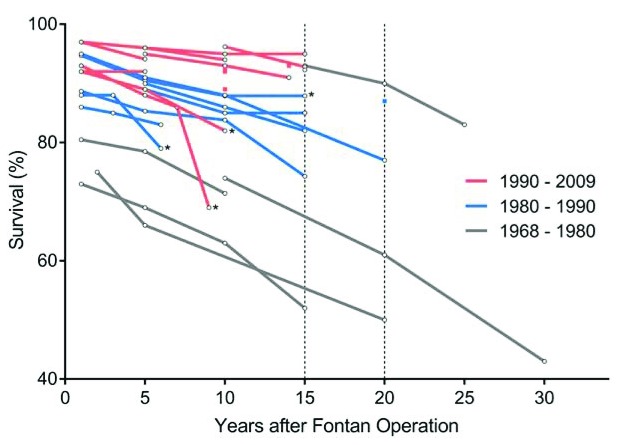
Survival following the Fontan procedure has increased dramatically in the past few decades. We will discuss data from recently published reports of large cohorts with long follow-up intervals. An overview of studies assessing survival is presented in Figure 3, obtained from Kverneland et al. 21.
Figure 3. Survival following Fontan completion.
Each line represents a study assessing survival at multiple time points and is colored by surgical era. Dots represent Kaplan-Meier survival estimates. Studies marked with an asterisk show survival curves for death, transplant, or Fontan revision; others show survival curves for only death. This figure has been reproduced with permission from Kverneland et al. and John Wiley and Sons 21.
In a recent study from Oceania, perioperative mortality decreased from 8% between 1975 and 1990 to 1% in 2001–2010 22. In this cohort, early Fontan takedown occurred in 2% of patients. Ten-year survival among patients discharged with a Fontan circulation was 89% following APC and 97% for both ECC and ILT 23. Survival at 25 years was 76%. This group was composed only of APC patients.
In a retrospective study from the Mayo Clinic of 40 years and 1,052 Fontan patients, overall survival rates were 74% at 10 years, 61% at 20 years, and 43% at 30 years 24. Survival was significantly higher in later surgical eras, and 10-year survival was 95% for patients operated on after 2001. Interestingly, patients operated on with the ECC technique showed better overall survival over ILT. It should be taken into account that patients with cardiac defects with worse prognosis were more frequently operated on using the ILT technique.
A Danish national registry described outcomes for SV patients from 1977 to 2009 1. Fifty percent of patients died before Fontan completion. Overall survival improved in later birth eras. Five-year survival for any univentricular CHD increased from 22% in 1977–1989 to 51% in 2000–2009.
One should note that follow-up studies after Fontan procedures reflect results of an earlier surgical era and do not necessarily represent the outlook of Fontan patients undergoing surgery in the current era.
Determinants of survival
Several factors have been associated with survival after the Fontan procedure. Of preoperative factors, male gender 23 and specific CHD diagnosis, most strikingly for HLHS 24, have been associated with worse long-term survival. Perioperative risk factors for mortality include APC type procedure, earlier surgical era, older age at procedure, concomitant valve replacement, and prolonged postoperative pleural effusion 25. Postoperative factors that affect survival include elevated central venous pressure and lower arterial saturation 26; imaging-derived parameters such as lower global longitudinal strain on echocardiography and higher end-diastolic volume measured by magnetic resonance imaging (MRI) 25; peak heart rate and peak oxygen uptake during cardiopulmonary exercise testing 25, 27, 28; and serum levels of sodium, creatinine, and brain natriuretic peptide 25, 29, 30.
Cardiac complications
Although survival following Fontan completion has increased over time, the Fontan strategy has been associated with important morbidity, most likely related to extensive surgical procedures and the highly abnormal circulatory state after these procedures. In recent cohorts, event-free survival has ranged from 59 to 81% at 15 years 23, 31.
The most commonly reported event is cardiac arrhythmia. Supraventricular tachycardia (SVT) contributes significantly to late mortality in Fontan patients 32. SVT has been associated with Fontan type; the highest risk is for APC type, followed by ILT, and the lowest is for ECC 23, 33. Results may have improved for the ILT technique after introduction of the prosthetic modification 34. The incidence of SVT increases during follow-up, and after 20 years of follow-up, 10 to 60% of patients have experienced some form of SVT 34, 35.
Failure of the Fontan circulation can occur even in long-standing uncomplicated Fontan circulations. The definition of Fontan failure varies but generally includes excessive constitutional limitations of the Fontan physiology, abnormal parameters of hemodynamic function, poor functional status, or the presence of Fontan sequelae 36. Fontan failure occurs in 2 to 13% of patients, depending on definitions and follow-up period 36. A 56% 25-year freedom of Fontan failure for APC connections has been reported 23.
Extracardiac complications
Abnormalities of coagulation. Blood flow can be slow in the Fontan circuit because of the absence of a prepulmonary pump. This promotes coagulation. Furthermore, coagulation factor abnormalities have been described in the SV patient, even before PCPC palliation 37. Thromboembolic events are more common following APC surgery than TCPC 37. The prevalence of thrombi is particularly high in those who develop atrial arrhythmias 38. Up to 65% of thrombi are detected within the first year following TCPC (early thromboembolic events) 39. Early thromboembolic events probably relate to perioperative factors, such as extracorporeal membrane oxygenation (ECMO) use and altered hemodynamics. Beyond 10 years after Fontan completion, incidence of late thromboembolism steadily increases 39. Reports of the incidence of thromboembolism are complicated by different definitions and methods of detection. Studies report incidences of late thromboembolism of between 1 and 25% 31, 40. Silent thrombi are detected with transesophageal echocardiography in up to 33% of patients 41. Thromboembolic events have been reported to account for 8% of Fontan deaths 42.
Liver function abnormalities, cirrhosis, and even hepatocellular carcinoma have been reported following Fontan surgery 43. The elevated systemic venous pressures encountered in the Fontan circulation lead to chronic venous congestion in the liver. However, the exact mechanism for Fontan-associated liver disease remains unknown, as does how best to monitor progression 44, 45. The American College of Cardiology recently provided a position statement on the subject, advising laboratory and imaging screening at least every 3–5 years in children and at least every 1–3 years in adult Fontan patients 46. Preventive strategies need to be developed.
Protein-losing enteropathy (PLE) is a devastating complication of the Fontan circulation in which loss of protein in the gastrointestinal tract occurs, leading to low albumin levels, edema, pleural effusions, and ascites. Incidences of between 3 and 29% have been reported 24, 31, 35, 47. It is thought to be caused, in part, by impairments in the lymphatic drainage. Hepatoduodenal lymphatic connections exist in some patients as a normal anatomic variant, which might induce competition in lymphatic flow in the presence of elevated venous pressure, diverting lymphatic flow to the gastrointestinal tract.
Plastic bronchitis is a serious pulmonary complication of the Fontan circulation in which large gelatinous casts are formed in the airways. It is thought to be related to abnormal lymphatic drainage directly into the airways, resulting in cast formation. Reported incidences range from 0.5 to 4% 48– 50.
Chronic kidney disease is estimated to be present in up to 50% of adult Fontan patients and is associated with adverse outcomes 30, 51. With the increasing availability of cystatin C-determined glomerular filtration rate (GFR), a muscle mass-independent estimate, the prevalence of clinically significant renal dysfunction appears to be lower 52. GFR estimates based on serum creatinine concentrations probably overestimate renal function in Fontan patients 51.
Psychological, psychiatric, and cognitive defects have been described in Fontan patients. Cerebral MRI has shown morphological differences in some cerebral structures of Fontan patients compared with those of healthy controls 53, 54. Interestingly, the pituitary gland, supplied by a portal venous system similar to the liver, appears to be enlarged following Fontan surgery 55. The relevance of this possible congestion on the endocrine system remains unclear.
Re-interventions
Many patients require additional surgical and catheter-based interventions following Fontan completion. Twenty-year freedom of re-operation following TCPC procedures in recent eras ranges from 86 to 92% 56. In older cohorts, higher re-operation rates have been reported 24, 57, 58. The most common surgical re-intervention procedures, in order of incidence, are pacemaker implantation in 9 to 23% of patients 23, 24, Fontan revision or conversion in 3 to 18% of patients 9, 24, 59, and atrioventricular (AV) valve repair in 1 to 14% of patients 24, 59, 60.
Fontan conversion, from APC to TCPC, can improve functional status and exercise tolerance in the failing Fontan circulation 61. AV valve repair after Fontan completion is considered in patients with moderate to severe regurgitation, but survival following successful repair remains inferior to that of patients without prior AV regurgitation 62, 63.
For several reasons, re-interventions by catheter may be required. A fenestration in the atrial tunnel, inducing a right-to-left atrial shunt, can be created during surgery to decrease systemic venous pressure, increase ventricular preload, and improve cardiac output at the cost of lower arterial saturation. This fenestration sometimes closes spontaneously or can be closed via catheter at a later time 64.
Hemodynamically significant obstruction in the Fontan pathway may occur, most commonly in the left pulmonary artery 57, 65. In the absence of a prepulmonary pump, this can severely affect the Fontan circulation, and obstructions are routinely dilated or stented.
Systemic to pulmonary venous collaterals can produce a right-to-left shunting which often worsens in time. Coiling of these collaterals is routinely performed in some centers, although no survival benefit has been demonstrated 66.
Aortopulmonary collaterals are common in the Fontan circulation. They increase pulmonary blood flow but induce a volume overload on the SV and might increase pulmonary artery pressure, limiting flow from the caval veins 65. During exercise, aortopulmonary flow increases, possibly augmenting loading conditions of the ventricle 67. A large aortopulmonary collateral burden has been associated with worse short-term outcomes 68. No clear consensus regarding the long-term effects and management of these collaterals exists 65.
Catheter ablation of an arrhythmogenic substrate is common. Long-term success rates vary between 15 and 72% 69, 70.
The reported incidence of catheter-based interventions (that is, excluding diagnostic cardiac catheterization without intervention) varies heavily; 3 to 65% of patients require at least one additional catheter intervention following Fontan completion 31, 56, 57, 59, 71. The most common catheter interventions are fenestration closure (10 to 64% of patients with a fenestration require catheter-based fenestration closing 56, 57, 59, 72), occlusion of veno-venous or aortopulmonary collaterals (incidence of 10 to 20%) 57, 59, and stenting and dilation of (all types of) obstructions in the Fontan pathway (incidence of 6 to 19%) 57, 59, 73.
Other outcomes and functional status
Most studies report a diminished quality of life in Fontan patients compared with healthy controls 74– 78. Physical and emotional functioning are the most severely affected domains 74, 79. Low perceived health status can lead to unnecessary restrictions in daily life. Furthermore, increased rates of developmental disorders and lower intelligence scores have been reported in the Fontan population 53, 76.
Fontan patients have a moderately decreased exercise capacity compared with healthy controls 80. Mean peak oxygen uptake ranges from 61 to 74% of predicted values 80– 82. A small fraction of Fontan patients have normal exercise capacity 83. Exercise capacity in the Fontan patient has been shown to decrease over time 81, 82. Exercise capacity is predictive of hospital admissions, quality of life, and late mortality 25, 28, 79, 82. Exercise training can be done successfully in Fontan patients and can improve quality of life, functional class, and health perception in a short-term follow-up 84– 87. Whether exercise training has a role in optimizing long-term outcome is currently not clear 84, 86– 89. Resistance training can be used to increase muscle mass. In the Fontan patient, this could augment peripheral venous return, augment ventricular preload, and improve cardiac output 90. Similarly, a benefit of inspiratory muscle training has been demonstrated 91.
Despite high morbidity and suboptimal outcomes, most patients with a well-functioning Fontan circulation manage to lead fulfilling lives, are employed, may attain academic achievements, can participate in sports, and are able to successfully carry pregnancy to term 92– 94.
Assessment techniques
Fontan patients are routinely assessed for health and functional status. Diagnostic cardiac catheterization has been standard practice in the pre-TCPC evaluation, as it provides excellent anatomic and necessary hemodynamic information regarding the pulmonary artery pressure, pulmonary vascular resistance, and end-diastolic SV pressure 95. There is recent interest in omitting cardiac catheterization in the pre-TCPC assessment for low-risk SV patients 95– 97. No consensus regarding this policy has been reached, as long-term outcomes are currently unavailable. Catheter-based interventions are discussed above.
Cardiac magnetic resonance imaging (CMR) is routinely performed during follow-up after TCPC, particularly to assess ventricular size and function and to quantify large vessel flow, including the amount of collateral flow 98– 102. Death and (being listed for) heart transplantation have been associated with higher end-diastolic volume index (EDVi) (>125 mL/m 2) as assessed with CMR in adolescents with a Fontan circulation 103, 104. Combined with a computational fluid dynamics approach, CMR might provide very useful information on the Fontan circulation and can aid in the evaluation of modifications in treatment strategies 104, 105.
Echocardiographic strain measurements have been shown to predict survival in the Fontan population and predict length of hospital stay following TCPC 104, 106. Assessment of ventriculo-arterial (VA) coupling may be another important parameter, as it is independent of the (often impaired) ventricular load. VA coupling has been shown to be suboptimal in some Fontan populations 107. Currently, VA coupling has not been associated with long-term outcomes in the Fontan population.
Lymphangiography could play an important role in the management of Fontan complications with a suspected lymphatic pathogenesis, such as PLE and plastic bronchitis. In patients with PLE or plastic bronchitis, increased diameters of major lymphatic vessels have been noted 108. Abnormal lymphatic depositions in the lungs and liver have been visualized in patients with plastic bronchitis and PLE, respectively 109, 110.
Medical therapy
Anticoagulation, in the form of anti-platelet drugs or vitamin K antagonists (VKAs), is commonly indicated considering the increased risk for thromboembolic events, as discussed above in the “Extracardiac complications” section 111. A meta-analysis by Alsaied et al. showed that both acetylsalicylic acid and VKA were equally effective in preventing thromboembolic complications 112. However, if international normalized ratio (INR) is not properly controlled, outcomes on VKA are worse compared with acetylsalicylic acid 113. Novel oral anticoagulants (NOACs) do not require frequent monitoring and have mostly outperformed VKA in the adult population. Thirty-day outcomes following NOAC initiation show no major adverse events in the adult CHD population 114. However, no NOAC agent currently has US Food and Drug Administration approval for use in children.
Medical prevention of circulatory failure in the Fontan circulation
Various medications have been assessed in the management of Fontan failure. No studies have shown benefit of angiotensin-converting enzyme (ACE) inhibitor therapy on survival, ventricular function, or cardiopulmonary exercise outcomes 115, 116.
Vasodilator drugs have been used to lower pulmonary vascular resistance 117, 118. Sildenafil has increased ventricular function, exercise capacity, and New York Heart Association (NYHA) status after 6 weeks of follow-up 119, 120. The effects of bosentan, an endothelin antagonist, in the Fontan population have varied 121– 126. No long-term survival benefit of vasodilator therapy has yet been demonstrated 119.
Mechanical circulatory support for the failing Fontan circulation
The failing Fontan circulation can be supported by mechanical assist devices. Despite increasing use and the development of novel devices specifically for the pediatric and CHD population, experience in this population is still limited 127. Mechanical support devices are mostly used as a bridge to transplant in the failing Fontan 128. Recent reports showed a 60% 12-month survival in 48 Fontan patients with a ventricular assist device, proving viability of longer mechanical circulatory support 129, 130. A total biventricular artificial heart, the SynCardia, has been used to bridge a failing Fontan patient to transplant 131. A registry of mechanical circulatory support specifically for SV patients has been initiated 132. Currently, mechanical circulatory support in Fontan patients is associated with worse survival compared with mechanical circulatory support patients with a biventricular circulation 133– 135.
Cardiac transplantation
Cardiac transplantation is the only treatment that truly corrects Fontan physiology, and it is employed in the failing Fontan circulation. In large cohorts, 1.6 to 3.6% of patients ultimately underwent cardiac transplant 23, 24. Survival following cardiac transplantation in Fontan patients is generally worse compared with other types of CHD 136, 137. Five-year survival ranges from 60 to 67% 136– 138.
Surgical innovations
Continual efforts are made to improve the surgical techniques used in Fontan surgery. Recently, a Y-shaped graft was proposed for the connection of the inferior vena cava to the left and right pulmonary artery 139. Theoretically, this graft is more energetically favorable and provides better distribution of hepatic blood flow between the left and right pulmonary artery, distributing “hepatic factors” that may prevent the formation of intrapulmonary collaterals more equally. Worse energetic performance and pulmonary flow distributions in comparison with ECC connections have been noticed in practice 140, 141.
Fontan completion without cardiopulmonary bypass, particularly with the ECC technique, is an attractive option. However, experience is still limited and reported rates of conduit replacement and outcomes following off-pump procedures differ across centers 142– 145.
Less-invasive surgical approaches such as lateral thoracotomy have been described in this population 146. Hybrid procedures, which combine transcatheter and surgical approaches, have been implemented in the initial management of HLHS 147. Long-term outcomes are favorable, and some centers have adopted this hybrid approach as the standard for selected patient populations 148.
Remaining challenges
A contemporary Fontan strategy uses either the ILT or the ECC modification. Two large meta-analyses have recently compared surgical strategies and found no differences in early or late mortality and Fontan takedown between ECC and ILT 33, 149. Theoretical advantages of both techniques have been discussed extensively in the literature 150, 151. Further research should assess contemporary differences in outcomes between modifications and help guide the preferred procedure for future Fontan patients. This may include alternative concepts, like the Y-graft or combinations with external energy supply (pumps).
Remodeling of the SV, which is exposed to volume overload at birth and is volume-deprived following the TCPC procedure, is not very well understood. A better understanding of mechanisms of remodeling during these stages and the interaction of ventricular size and function with the Fontan baffle function, pulmonary circulation, atrial function, and VA interaction is required to find better means to preserve cardiac function. The search for new targets for drugs that may help to preserve cardiac and circulatory function continues.
Some controversy regarding the timing of TCPC surgery exists. Proponents of early TCPC argue that a prolonged period of volume overload leads to adverse cardiac remodeling and reduced cardiac function 152. Others argue that the Fontan circulation inherently leads to complications and that surgery should be delayed to reduce the amount of time in Fontan physiology 153. Other factors to be considered are the techniques used; ILT allows TCPC at lower body weight than ECC since small-sized conduits (<18 mm) need to be avoided 152, 154. Studies assessing the optimal timing of ECC procedures are currently being performed.
The effect of systemic to pulmonary venous and aortopulmonary collaterals on the Fontan circulation remains poorly understood. These collaterals could provide some benefit in patients with a suboptimal Fontan circuit. How these collaterals develop and why some patients seem more prone to this development remain to be determined. Increasing our understanding of the role of collaterals could help guide the selection of patients who will benefit from intervention. This requires well-designed (multicenter) studies. Several treatment modalities of PLE have been described in small series, including catheter-based strategies of both blood and lymphatic vessels and surgical re-implantation of the innominate vein into the common atrium 155– 159. More comprehensive analysis is needed to determine the efficacy and safety of these procedures.
Drug therapy has been shown to be able to decrease pulmonary vascular resistance in the short-term, making this a promising therapy for the Fontan patient. However, currently, no long-term benefit has been demonstrated. The role of drug therapy in the Fontan circulation needs to be studied more extensively.
These questions require answers to make better-informed decisions in the management of these challenging patients, who have some of the most severe kinds of CHD. We have an opportunity to help this growing patient population not just to survive but also to thrive and live full and satisfying lives.
Conclusions
The modern Fontan strategy has significantly transformed outcomes for patients with univentricular CHD. This has led to a large and growing population of Fontan patients surviving into adulthood. However, morbidity remains high and increases as this population ages and grows in proportion. Efforts to reduce morbidity and improve quality of life in these patients are ongoing. These efforts are focused on improving surgical techniques, developing novel diagnostic and therapeutic tools, and increasing our understanding of the highly abnormal Fontan physiology.
Abbreviations
APC, atriopulmonary connection; AV, atrioventricular; CHD, congenital heart disease; CMR, cardiac magnetic resonance (imaging); ECC, extracardiac conduit; GFR, glomerular filtration rate; HLHS, hypoplastic left heart syndrome; ILT, intra-atrial lateral tunnel; MRI, magnetic resonance imaging; NOAC, novel oral anticoagulant; PCPC, partial cavopulmonary connection; PLE, protein-losing enteropathy; SV, single ventricle; SVT, supraventricular tachycardia; TCPC, total cavopulmonary connection; VA, ventricular-arterial; VKA, vitamin K antagonist.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Amy Throckmorton, BioCirc Research Laboratory, School of Biomedical Engineering, Science and Health Systems, Drexel University, Philadelphia, Pennsylvania, USA
Gruschen R. Veldtman, Adolescent and Adult Congenital Heart Disease Program, Cincinnati Children's Hospital Medical Centre, Cincinnati, Ohio, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Idorn L, Olsen M, Jensen AS, et al. : Univentricular hearts in Denmark 1977 to 2009: incidence and survival. Int J Cardiol. 2013;167(4):1311–6. 10.1016/j.ijcard.2012.03.182 [DOI] [PubMed] [Google Scholar]
- 2. Moons P, Sluysmans T, De Wolf D, et al. : Congenital heart disease in 111 225 births in Belgium: birth prevalence, treatment and survival in the 21st century. Acta Paediatr. 2009;98(3):472–7. 10.1111/j.1651-2227.2008.01152.x [DOI] [PubMed] [Google Scholar]
- 3. Schwedler G, Lindinger A, Lange PE, et al. : Frequency and spectrum of congenital heart defects among live births in Germany: a study of the Competence Network for Congenital Heart Defects. Clin Res Cardiol. 2011;100(12):1111–7. 10.1007/s00392-011-0355-7 [DOI] [PubMed] [Google Scholar]
- 4. Khairy P, Poirier N, Mercier LA: Univentricular heart. Circulation. 2007;115(6):800–12. 10.1161/CIRCULATIONAHA.105.592378 [DOI] [PubMed] [Google Scholar]
- 5. Coats L, O'Connor S, Wren C, et al. : The single-ventricle patient population: a current and future concern a population-based study in the North of England. Heart. 2014;100(17):1348–53. 10.1136/heartjnl-2013-305336 [DOI] [PubMed] [Google Scholar]
- 6. Beghetti M: Pulmonary vasodilators in Fontan Patients. EUROGUCH 2017; Lausanne2017. Reference Source [Google Scholar]
- 7. van Velzen CL, Ket JCF, van de Ven PM, et al. : Systematic review and meta-analysis of the performance of second-trimester screening for prenatal detection of congenital heart defects. Int J Gynaecol Obstet. 2018;140(2):137–45. 10.1002/ijgo.12373 [DOI] [PubMed] [Google Scholar]
- 8. Fontan F, Baudet E: Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–8. 10.1136/thx.26.3.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poh CL, Zannino D, Weintraub RG, et al. : Three decades later: The fate of the population of patients who underwent the Atriopulmonary Fontan procedure. Int J Cardiol. 2017;231:99–104. 10.1016/j.ijcard.2017.01.057 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. de Leval MR, Kilner P, Gewillig M, et al. : Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96(5):682–95. [PubMed] [Google Scholar]
- 11. Kerlo AEM, Delorme YT, Xu D, et al. : Experimental characterization of powered Fontan hemodynamics in an idealized total cavopulmonary connection model. Exp Fluids. 2013;54:1581 10.1007/s00348-013-1581-8 [DOI] [Google Scholar]
- 12. Huang L, Dalziel KM, Schilling C, et al. : Hospital costs and cost implications of co-morbid conditions for patients with single ventricle in the period through to Fontan completion. Int J Cardiol. 2017;240:178–82. 10.1016/j.ijcard.2017.04.056 [DOI] [PubMed] [Google Scholar]
- 13. Cedars A, Benjamin L, Vyhmeister R, et al. : Contemporary Hospitalization Rate Among Adults With Complex Congenital Heart Disease. World J Pediatr Congenit Heart Surg. 2016;7(3):334–43. 10.1177/2150135116639541 [DOI] [PubMed] [Google Scholar]
- 14. Tabtabai S, DeFaria Yeh D, Stefanescu A, et al. : National Trends in Hospitalizations for Patients With Single-Ventricle Anatomy. Am J Cardiol. 2015;116(5):773–8. 10.1016/j.amjcard.2015.05.053 [DOI] [PubMed] [Google Scholar]
- 15. Collins RT, 2nd, Fram RY, Tang X, et al. : Hospital utilization in adults with single ventricle congenital heart disease and cardiac arrhythmias. J Cardiovasc Electrophysiol. 2014;25(2):179–86. 10.1111/jce.12294 [DOI] [PubMed] [Google Scholar]
- 16. Huang L, Schilling C, Dalziel KM, et al. : Hospital Inpatient Costs for Single Ventricle Patients Surviving the Fontan Procedure. Am J Cardiol. 2017;120(3):467–72. 10.1016/j.amjcard.2017.04.049 [DOI] [PubMed] [Google Scholar]
- 17. Gewillig M: The Fontan circulation. Heart. 2005;91(6):839–46. 10.1136/hrt.2004.051789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rychik J: The Relentless Effects of the Fontan Paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016;19(1):37–43. 10.1053/j.pcsu.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 19. Fontan F, Kirklin JW, Fernandez G, et al. : Outcome after a "perfect" Fontan operation. Circulation. 1990;81(5):1520–36. 10.1161/01.CIR.81.5.1520 [DOI] [PubMed] [Google Scholar]
- 20. Gewillig M, Brown SC: The Fontan circulation after 45 years: update in physiology. Heart. 2016;102(14):1081–6. 10.1136/heartjnl-2015-307467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kverneland LS, Kramer P, Ovroutski S: Five decades of the Fontan operation: A systematic review of international reports on outcomes after univentricular palliation. Congenit Heart Dis. 2018;13(2):181–93. 10.1111/chd.12570 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Iyengar AJ, Winlaw DS, Galati JC, et al. : Trends in Fontan surgery and risk factors for early adverse outcomes after Fontan surgery: the Australia and New Zealand Fontan Registry experience. J Thorac Cardiovasc Surg. 2014;148(2):566–75. 10.1016/j.jtcvs.2013.09.074 [DOI] [PubMed] [Google Scholar]
- 23. d'Udekem Y, Iyengar AJ, Galati JC, et al. : Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation. 2014;130(11 Suppl 1):S32–8. 10.1161/CIRCULATIONAHA.113.007764 [DOI] [PubMed] [Google Scholar]
- 24. Pundi KN, Johnson JN, Dearani JA, et al. : 40-Year Follow-Up After the Fontan Operation: Long-Term Outcomes of 1,052 Patients. J Am Coll Cardiol. 2015;66(15):1700–10. 10.1016/j.jacc.2015.07.065 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Alsaied T, Bokma JP, Engel ME, et al. : Factors associated with long-term mortality after Fontan procedures: a systematic review. Heart. 2017;103(2):104–10. 10.1136/heartjnl-2016-310108 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Ohuchi H, Yasuda K, Miyazaki A, et al. : Comparison of prognostic variables in children and adults with Fontan circulation. Int J Cardiol. 2014;173(2):277–83. 10.1016/j.ijcard.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 27. Fernandes SM, Alexander ME, Graham DA, et al. : Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit Heart Dis. 2011;6(4):294–303. 10.1111/j.1747-0803.2011.00500.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Diller GP, Giardini A, Dimopoulos K, et al. : Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31(24):3073–83. 10.1093/eurheartj/ehq356 [DOI] [PubMed] [Google Scholar]
- 29. Assenza GE, Graham DA, Landzberg MJ, et al. : MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. 2013;99(7):491–6. 10.1136/heartjnl-2012-303347 [DOI] [PubMed] [Google Scholar]
- 30. Dimopoulos K, Diller GP, Koltsida E, et al. : Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117(18):2320–8. 10.1161/CIRCULATIONAHA.107.734921 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Nakano T, Kado H, Tatewaki H, et al. : Results of extracardiac conduit total cavopulmonary connection in 500 patients. Eur J Cardiothorac Surg. 2015;48(6):825–32; discussion 832. 10.1093/ejcts/ezv072 [DOI] [PubMed] [Google Scholar]
- 32. Giannakoulas G, Dimopoulos K, Yuksel S, et al. : Atrial tachyarrhythmias late after Fontan operation are related to increase in mortality and hospitalization. Int J Cardiol. 2012;157(2):221–6. 10.1016/j.ijcard.2010.12.049 [DOI] [PubMed] [Google Scholar]
- 33. Lin Z, Ge H, Xue J, et al. : Comparison of extracardiac conduit and lateral tunnel for functional single-ventricle patients: A meta-analysis. Congenit Heart Dis. 2017;12(6):711–20. 10.1111/chd.12503 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Bossers SS, Duppen N, Kapusta L, et al. : Comprehensive rhythm evaluation in a large contemporary Fontan population. Eur J Cardiothorac Surg. 2015;48(6):833–40; discussion 840–1. 10.1093/ejcts/ezu548 [DOI] [PubMed] [Google Scholar]
- 35. Atz AM, Zak V, Mahony L, et al. : Longitudinal Outcomes of Patients With Single Ventricle After the Fontan Procedure. J Am Coll Cardiol. 2017;69(22):2735–44. 10.1016/j.jacc.2017.03.582 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Deal BJ, Jacobs ML: Management of the failing Fontan circulation. Heart. 2012;98(14):1098–104. 10.1136/heartjnl-2011-301133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Viswanathan S: Thromboembolism and anticoagulation after Fontan surgery. Ann Pediatr Cardiol. 2016;9(3):236–40. 10.4103/0974-2069.189109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Egbe AC, Connolly HM, McLeod CJ, et al. : Thrombotic and Embolic Complications Associated With Atrial Arrhythmia After Fontan Operation: Role of Prophylactic Therapy. J Am Coll Cardiol. 2016;68(12):1312–9. 10.1016/j.jacc.2016.06.056 [DOI] [PubMed] [Google Scholar]
- 39. Seipelt RG, Franke A, Vazquez-Jimenez JF, et al. : Thromboembolic complications after Fontan procedures: comparison of different therapeutic approaches. Ann Thorac Surg. 2002;74(2):556–62. 10.1016/S0003-4975(02)03677-9 [DOI] [PubMed] [Google Scholar]
- 40. Egbe AC, Connolly HM, Niaz T, et al. : Prevalence and outcome of thrombotic and embolic complications in adults after Fontan operation. Am Heart J. 2017;183:10–7. 10.1016/j.ahj.2016.09.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Balling G, Vogt M, Kaemmerer H, et al. : Intracardiac thrombus formation after the Fontan operation. J Thorac Cardiovasc Surg. 2000;119(4 Pt 1):745–52. 10.1016/S0022-5223(00)70010-9 [DOI] [PubMed] [Google Scholar]
- 42. Khairy P, Fernandes SM, Mayer JE, et al. : Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117(1):85–92. 10.1161/CIRCULATIONAHA.107.738559 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Ghaferi AA, Hutchins GM: Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129(6):1348–52. 10.1016/j.jtcvs.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 44. Greenway SC, Crossland DS, Hudson M, et al. : Fontan-associated liver disease: Implications for heart transplantation. J Heart Lung Transplant. 2016;35(1):26–33. 10.1016/j.healun.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 45. Hilscher MB, Johnson JN, Cetta F, et al. : Surveillance for liver complications after the Fontan procedure. Congenit Heart Dis. 2017;12(2):124–32. 10.1111/chd.12446 [DOI] [PubMed] [Google Scholar]
- 46. Daniels CJ, Bradley EA, Landzberg MJ, et al. : Fontan-Associated Liver Disease: Proceedings from the American College of Cardiology Stakeholders Meeting, October 1 to 2, 2015, Washington DC. J Am Coll Cardiol. 2017;70(25):3173–94. 10.1016/j.jacc.2017.10.045 [DOI] [PubMed] [Google Scholar]
- 47. Johnson JN, Driscoll DJ, O'Leary PW: Protein-losing enteropathy and the Fontan operation. Nutr Clin Pract. 2012;27(3):375–84. 10.1177/0884533612444532 [DOI] [PubMed] [Google Scholar]
- 48. Schwartz I, McCracken CE, Petit CJ, et al. : Late outcomes after the Fontan procedure in patients with single ventricle: a meta-analysis. Heart. 2018; pii: heartjnl-2017-312807. 10.1136/heartjnl-2017-312807 [DOI] [PubMed] [Google Scholar]
- 49. Atz AM, Zak V, Mahony L, et al. : Survival data and predictors of functional outcome an average of 15 years after the Fontan procedure: the pediatric heart network Fontan cohort. Congenit Heart Dis. 2015;10(1):E30–42. 10.1111/chd.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caruthers RL, Kempa M, Loo A, et al. : Demographic characteristics and estimated prevalence of Fontan-associated plastic bronchitis. Pediatr Cardiol. 2013;34(2):256–61. 10.1007/s00246-012-0430-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee D, Levin A, Kiess M, et al. : Chronic kidney damage in the adult Fontan population. Int J Cardiol. 2018;257:62–6. 10.1016/j.ijcard.2017.11.118 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Opotowsky AR, Baraona FR, Mc Causland FR, et al. : Estimated glomerular filtration rate and urine biomarkers in patients with single-ventricle Fontan circulation. Heart. 2017;103(6):434–42. 10.1136/heartjnl-2016-309729 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Singh S, Kumar R, Roy B, et al. : Regional brain gray matter changes in adolescents with single ventricle heart disease. Neurosci Lett. 2018;665:156–62. 10.1016/j.neulet.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Pike NA, Roy B, Gupta R, et al. : Brain abnormalities in cognition, anxiety, and depression regulatory regions in adolescents with single ventricle heart disease. J Neurosci Res. 2018;96(6):1104–18. 10.1002/jnr.24215 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Muneuchi J, Nagatomo Y, Okada S, et al. : Increased Pituitary Volumes in Children after Fontan Operation: Congestion in the Other Portal Circulation. J Pediatr. 2018;193:249–51. 10.1016/j.jpeds.2017.09.065 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Ono M, Boethig D, Goerler H, et al. : Clinical outcome of patients 20 years after Fontan operation--effect of fenestration on late morbidity. Eur J Cardiothorac Surg. 2006;30(6):923–9. 10.1016/j.ejcts.2006.08.025 [DOI] [PubMed] [Google Scholar]
- 57. Hannan RL, Zabinsky JA, Salvaggio JL, et al. : The Fontan operation: the pursuit of associated lesions and cumulative trauma. Pediatr Cardiol. 2011;32(6):778–84. 10.1007/s00246-011-9973-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Palumbo T, Sluysmans T, Rubay JE, et al. : Long-term outcome and anaesthetic management for non-cardiac surgery after Fontan palliation: a single-centre retrospective analysis. Cardiol Young. 2015;25(6):1148–54. 10.1017/S1047951114001814 [DOI] [PubMed] [Google Scholar]
- 59. Downing TE, Allen KY, Goldberg DJ, et al. : Surgical and Catheter-Based Reinterventions Are Common in Long-Term Survivors of the Fontan Operation. Circ Cardiovasc Interv. 2017;10(9): pii: e004924. 10.1161/CIRCINTERVENTIONS.116.004924 [DOI] [PubMed] [Google Scholar]
- 60. Honjo O, Atlin CR, Mertens L, et al. : Atrioventricular valve repair in patients with functional single-ventricle physiology: impact of ventricular and valve function and morphology on survival and reintervention. J Thorac Cardiovasc Surg. 2011;142(2):326–35.e2. 10.1016/j.jtcvs.2010.11.060 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Mavroudis C, Deal BJ: Fontan Conversion: Literature Review and Lessons Learned Over 20 Years. World J Pediatr Congenit Heart Surg. 2016;7(2):192–8. 10.1177/2150135115623960 [DOI] [PubMed] [Google Scholar]
- 62. Warnes CA, Williams RG, Bashore TM, et al. : ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation. 2008;118(23):2395–451. 10.1161/CIRCULATIONAHA.108.190811 [DOI] [PubMed] [Google Scholar]
- 63. Honjo O, Mertens L, van Arsdell GS: Atrioventricular valve repair in patients with single-ventricle physiology: mechanisms, techniques of repair, and clinical outcomes. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2011;14(1):75–84. 10.1053/j.pcsu.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 64. Bridges ND, Lock JE, Castaneda AR: Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation. 1990;82(5):1681–9. 10.1161/01.CIR.82.5.1681 [DOI] [PubMed] [Google Scholar]
- 65. Kreutzer J, Graziano JN, Stapleton G, et al. : Late catheter interventions in hypoplastic left heart syndrome. Cardiol Young. 2011;21Suppl 2:65–76. 10.1017/S1047951111001612 [DOI] [PubMed] [Google Scholar]
- 66. Poterucha JT, Johnson JN, Taggart NW, et al. : Embolization of Veno-venous Collaterals after the Fontan Operation Is Associated with Decreased Survival. Congenit Heart Dis. 2015;10(5):E230–6. 10.1111/chd.12276 [DOI] [PubMed] [Google Scholar]
- 67. Schmitt B, Steendijk P, Ovroutski S, et al. : Pulmonary vascular resistance, collateral flow, and ventricular function in patients with a Fontan circulation at rest and during dobutamine stress. Circ Cardiovasc Imaging. 2010;3(5):623–31. 10.1161/CIRCIMAGING.109.931592 [DOI] [PubMed] [Google Scholar]
- 68. Odenwald T, Quail MA, Giardini A, et al. : Systemic to pulmonary collateral blood flow influences early outcomes following the total cavopulmonary connection. Heart. 2012;98(12):934–40. 10.1136/heartjnl-2011-301599 [DOI] [PubMed] [Google Scholar]
- 69. de Groot NM, Lukac P, Blom NA, et al. : Long-term outcome of ablative therapy of postoperative supraventricular tachycardias in patients with univentricular heart: a European multicenter study. Circ Arrhythm Electrophysiol. 2009;2(3):242–8. 10.1161/CIRCEP.108.828137 [DOI] [PubMed] [Google Scholar]
- 70. Yap S, Harris L, Silversides CK, et al. : Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol. 2010;56(19):1589–96. 10.1016/j.jacc.2010.04.061 [DOI] [PubMed] [Google Scholar]
- 71. Wilson TG, Shi WY, Iyengar AJ, et al. : Twenty-Five Year Outcomes of the Lateral Tunnel Fontan Procedure. Semin Thorac Cardiovasc Surg. 2017;29(3):347–353. 10.1053/j.semtcvs.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 72. Pihkala JI, Järvelä M, Boldt T, et al. : Fate of fenestration in children treated with fontan operation. Catheter Cardiovasc Interv. 2016;87(6):E233–9. 10.1002/ccd.26324 [DOI] [PubMed] [Google Scholar]
- 73. Dancea A, Justino H, Martucci G: Catheter intervention for congenital heart disease at risk of circulatory failure. Can J Cardiol. 2013;29(7):786–795. 10.1016/j.cjca.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 74. Uzark K, Zak V, Shrader P, et al. : Assessment of Quality of Life in Young Patients with Single Ventricle after the Fontan Operation. J Pediatr. 2016;170:166–72.e1. 10.1016/j.jpeds.2015.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Smaś-Suska M, Dłużniewska N, Weryński P, et al. : What determines the quality of life of adult patients after Fontan procedure? Cardiol J. 2018;25(1):72–80. 10.5603/CJ.a2017.0078 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Idorn L, Jensen AS, Juul K, et al. : Quality of life and cognitive function in Fontan patients, a population-based study. Int J Cardiol. 2013;168(4):3230–5. 10.1016/j.ijcard.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 77. Vahsen N, Bröder A, Hraska V, et al. : Neurodevelopmental Outcome in Children With Single Ventricle After Total Cavopulmonary Connection. Klin Padiatr. 2018;230(1):24–30. 10.1055/s-0043-120526 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Kukreja M, Bryant AS, Cleveland DC, et al. : Health-Related Quality of Life in Adult Survivors After the Fontan Operation. Semin Thorac Cardiovasc Surg. 2015;27(3):299–306. 10.1053/j.semtcvs.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 79. Dulfer K, Bossers SS, Utens EM, et al. : Does functional health status predict health-related quality of life in children after Fontan operation? Cardiol Young. 2016;26(3):459–68. 10.1017/S1047951115000426 [DOI] [PubMed] [Google Scholar]
- 80. Bossers SS, Helbing WA, Duppen N, et al. : Exercise capacity in children after total cavopulmonary connection: lateral tunnel versus extracardiac conduit technique. J Thorac Cardiovasc Surg. 2014;148(4):1490–7. 10.1016/j.jtcvs.2013.12.046 [DOI] [PubMed] [Google Scholar]
- 81. Giardini A, Hager A, Pace Napoleone C, et al. : Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thorac Surg. 2008;85(3):818–21. 10.1016/j.athoracsur.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 82. Egbe AC, Driscoll DJ, Khan AR, et al. : Cardiopulmonary exercise test in adults with prior Fontan operation: The prognostic value of serial testing. Int J Cardiol. 2017;235:6–10. 10.1016/j.ijcard.2017.02.140 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Cordina R, Du Plessis K, Tran D, et al. : Super-Fontan: Is it possible? J Thorac Cardiovasc Surg. 2018;155(3):1192–4. 10.1016/j.jtcvs.2017.10.047 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Dulfer K, Duppen N, Kuipers IM, et al. : Aerobic exercise influences quality of life of children and youngsters with congenital heart disease: a randomized controlled trial. J Adolesc Health. 2014;55(1):65–72. 10.1016/j.jadohealth.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 85. McCrindle BW, Williams RV, Mital S, et al. : Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child. 2007;92(6):509–14. 10.1136/adc.2006.105239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Duppen N, Etnel JR, Spaans L, et al. : Does exercise training improve cardiopulmonary fitness and daily physical activity in children and young adults with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. Am Heart J. 2015;170(3):606–14. 10.1016/j.ahj.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 87. Takken T, Hulzebos HJ, Blank AC, et al. : Exercise prescription for patients with a Fontan circulation: current evidence and future directions. Neth Heart J. 2007;15(4):142–7. 10.1007/BF03085970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brassard P, Bédard E, Jobin J, et al. : Exercise capacity and impact of exercise training in patients after a Fontan procedure: a review. Can J Cardiol. 2006;22(6):489–95. 10.1016/S0828-282X(06)70266-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hedlund ER, Lundell B, Söderström L, et al. : Can endurance training improve physical capacity and quality of life in young Fontan patients? Cardiol Young. 2018;28(3):438–46. 10.1017/S1047951117002360 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. Cordina RL, O'Meagher S, Karmali A, et al. : Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013;168(2):780–8. 10.1016/j.ijcard.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 91. Laohachai K, Winlaw D, Selvadurai H, et al. : Inspiratory Muscle Training Is Associated With Improved Inspiratory Muscle Strength, Resting Cardiac Output, and the Ventilatory Efficiency of Exercise in Patients With a Fontan Circulation. J Am Heart Assoc. 2017;6(8): pii: e005750. 10.1161/JAHA.117.005750 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Khairy P, Ouyang DW, Fernandes SM, et al. : Pregnancy outcomes in women with congenital heart disease. Circulation. 2006;113(4):517–24. 10.1161/CIRCULATIONAHA.105.589655 [DOI] [PubMed] [Google Scholar]
- 93. Mair DD, Puga FJ, Danielson GK: Late functional status of survivors of the Fontan procedure performed during the 1970s. Circulation. 1992;86(5 Suppl):II106–9. [PubMed] [Google Scholar]
- 94. Pike NA, Evangelista LS, Doering LV, et al. : Clinical profile of the adolescent/adult Fontan survivor. Congenit Heart Dis. 2011;6(1):9–17. 10.1111/j.1747-0803.2010.00475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mohammad Nijres B, Murphy JJ, Diab K, et al. : Routine Cardiac Catheterization Prior to Fontan Operation: Is It a Necessity? Pediatr Cardiol. 2018;39(4):818–23. 10.1007/s00246-018-1825-8 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Ro PS, Rychik J, Cohen MS, et al. : Diagnostic assessment before Fontan operation in patients with bidirectional cavopulmonary anastomosis: are noninvasive methods sufficient? J Am Coll Cardiol. 2004;44(1):184–7. 10.1016/j.jacc.2004.02.058 [DOI] [PubMed] [Google Scholar]
- 97. Fogel MA, Pawlowski TW, Whitehead KK, et al. : Cardiac magnetic resonance and the need for routine cardiac catheterization in single ventricle patients prior to Fontan: a comparison of 3 groups: pre-Fontan CMR versus cath evaluation. J Am Coll Cardiol. 2012;60(12):1094–102. 10.1016/j.jacc.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 98. Valsangiacomo Buechel ER, Grosse-Wortmann L, Fratz S, et al. : Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: an expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur Heart J Cardiovasc Imaging. 2015;16(3):281–97. 10.1093/ehjci/jeu129 [DOI] [PubMed] [Google Scholar]
- 99. Glatz AC, Rome JJ, Small AJ, et al. : Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes. Circ Cardiovasc Imaging. 2012;5(2):218–25. 10.1161/CIRCIMAGING.111.966986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Grosse-Wortmann L, Drolet C, Dragulescu A, et al. : Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: a multimodality study. J Thorac Cardiovasc Surg. 2012;144(6):1329–36. 10.1016/j.jtcvs.2012.03.032 [DOI] [PubMed] [Google Scholar]
- 101. Lawley CM, Broadhouse KM, Callaghan FM, et al. : 4D flow magnetic resonance imaging: role in pediatric congenital heart disease. Asian Cardiovasc Thorac Ann. 2018;26(1):28–37. 10.1177/0218492317694248 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 102. Bächler P, Valverde I, Pinochet N, et al. : Caval blood flow distribution in patients with Fontan circulation: quantification by using particle traces from 4D flow MR imaging. Radiology. 2013;267(1):67–75. 10.1148/radiol.12120778 [DOI] [PubMed] [Google Scholar]
- 103. Rathod RH, Prakash A, Kim YY, et al. : Cardiac magnetic resonance parameters predict transplantation-free survival in patients with fontan circulation. Circ Cardiovasc Imaging. 2014;7(3):502–9. 10.1161/CIRCIMAGING.113.001473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ghelani SJ, Harrild DM, Gauvreau K, et al. : Comparison Between Echocardiography and Cardiac Magnetic Resonance Imaging in Predicting Transplant-Free Survival After the Fontan Operation. Am J Cardiol. 2015;116(7):1132–8. 10.1016/j.amjcard.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 105. Whitehead KK, Pekkan K, Kitajima HD, et al. : Nonlinear power loss during exercise in single-ventricle patients after the Fontan: insights from computational fluid dynamics. Circulation. 2007;116(11 Suppl):I165–71. 10.1161/CIRCULATIONAHA.106.680827 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 106. Park PW, Atz AM, Taylor CL, et al. : Speckle-Tracking Echocardiography Improves Pre-operative Risk Stratification Before the Total Cavopulmonary Connection. J Am Soc Echocardiogr. 2017;30(5):478–84. 10.1016/j.echo.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 107. Saiki H, Eidem BW, Ohtani T, et al. : Ventricular-Arterial Function and Coupling in the Adult Fontan Circulation. J Am Heart Assoc. 2016;5(9): pii: e003887. 10.1161/JAHA.116.003887 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 108. Dori Y, Keller MS, Fogel MA, et al. : MRI of lymphatic abnormalities after functional single-ventricle palliation surgery. AJR Am J Roentgenol. 2014;203(2):426–31. 10.2214/AJR.13.11797 [DOI] [PubMed] [Google Scholar]
- 109. Dori Y, Keller MS, Rome JJ, et al. : Percutaneous Lymphatic Embolization of Abnormal Pulmonary Lymphatic Flow as Treatment of Plastic Bronchitis in Patients With Congenital Heart Disease. Circulation. 2016;133(12):1160–70. 10.1161/CIRCULATIONAHA.115.019710 [DOI] [PubMed] [Google Scholar]
- 110. Itkin M, Piccoli DA, Nadolski G, et al. : Protein-Losing Enteropathy in Patients With Congenital Heart Disease. J Am Coll Cardiol. 2017;69(24):2929–37. 10.1016/j.jacc.2017.04.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 111. Cromme-Dijkhuis AH, Hess J, Hählen K, et al. : Specific sequelae after Fontan operation at mid- and long-term follow-up. Arrhythmia, liver dysfunction, and coagulation disorders. J Thorac Cardiovasc Surg. 1993;106:1126–32. [PubMed] [Google Scholar]
- 112. Alsaied T, Alsidawi S, Allen CC, et al. : Strategies for thromboprophylaxis in Fontan circulation: a meta-analysis. Heart. 2015;101(21):1731–7. 10.1136/heartjnl-2015-307930 [DOI] [PubMed] [Google Scholar]
- 113. McCrindle BW, Manlhiot C, Cochrane A, et al. : Factors associated with thrombotic complications after the Fontan procedure: a secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J Am Coll Cardiol. 2013;61(3):346–53. 10.1016/j.jacc.2012.08.1023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 114. Yang H, Bouma BJ, Mulder BJM: Is Initiating NOACs for Atrial Arrhythmias Safe in Adults with Congenital Heart Disease? Cardiovasc Drugs Ther. 2017;31(4):413–7. 10.1007/s10557-017-6745-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 115. Wilson TG, Iyengar AJ, Winlaw DS, et al. : Use of ACE inhibitors in Fontan: Rational or irrational? Int J Cardiol. 2016;210:95–9. 10.1016/j.ijcard.2016.02.089 [DOI] [PubMed] [Google Scholar]
- 116. Wilson TG, Iyengar AJ, d'Udekem Y: The Use and Misuse of ACE Inhibitors in Patients with Single Ventricle Physiology. Heart Lung Circ. 2016;25(3):229–36. 10.1016/j.hlc.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 117. Morchi GS, Ivy DD, Duster MC, et al. : Sildenafil Increases Systemic Saturation and Reduces Pulmonary Artery Pressure in Patients with Failing Fontan Physiology. Congenit Heart Dis. 2009;4(2):107–11. 10.1111/j.1747-0803.2008.00237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Giordano R, Palma G, Poli V, et al. : First experience with sildenafil after Fontan operation: short-term outcomes. J Cardiovasc Med (Hagerstown). 2015;16(8):552–5. 10.2459/JCM.0b013e328361390a [DOI] [PubMed] [Google Scholar]
- 119. Oldenburger NJ, Mank A, Etnel J, et al. : Drug therapy in the prevention of failure of the Fontan circulation: a systematic review. Cardiol Young. 2016;26(5):842–50. 10.1017/S1047951115002747 [DOI] [PubMed] [Google Scholar]
- 120. Mori H, Park IS, Yamagishi H, et al. : Sildenafil reduces pulmonary vascular resistance in single ventricular physiology. Int J Cardiol. 2016;221:122–7. 10.1016/j.ijcard.2016.06.322 [DOI] [PubMed] [Google Scholar]
- 121. Hebert A, Mikkelsen UR, Thilen U, et al. : Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) study. Circulation. 2014;130(23):2021–30. 10.1161/CIRCULATIONAHA.113.008441 [DOI] [PubMed] [Google Scholar]
- 122. Shang X, Lu R, Zhang X, et al. : Efficacy of Bosentan in patients after Fontan procedures: a double-blind, randomized controlled trial. J Huazhong Univ Sci Technol Med Sci. 2016;36(4):534–40. 10.1007/s11596-016-1621-8 [DOI] [PubMed] [Google Scholar]
- 123. Ovaert C, Thijs D, Dewolf D, et al. : The effect of bosentan in patients with a failing Fontan circulation. Cardiol Young. 2009;19(4):331–9. 10.1017/S1047951109990023 [DOI] [PubMed] [Google Scholar]
- 124. Schuuring MJ, Vis JC, van Dijk AP, et al. : Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. Eur J Heart Fail. 2013;15(6):690–8. 10.1093/eurjhf/hft017 [DOI] [PubMed] [Google Scholar]
- 125. Park I: Efficacy of pulmonary vasodilator therapy in patients with functionally single ventricle. Int Heart J. 2015;56Suppl:S26–30. 10.1536/ihj.14-392 [DOI] [PubMed] [Google Scholar]
- 126. Agnoletti G, Gala S, Ferroni F, et al. : Endothelin inhibitors lower pulmonary vascular resistance and improve functional capacity in patients with Fontan circulation. J Thorac Cardiovasc Surg. 2017;153(6):1468–75. 10.1016/j.jtcvs.2017.01.051 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 127. Chopski SG, Moskowitz WB, Stevens RM, et al. : Mechanical Circulatory Support Devices for Pediatric Patients With Congenital Heart Disease. Artif Organs. 2017;41(1):E1–E14. 10.1111/aor.12760 [DOI] [PubMed] [Google Scholar]
- 128. Carlo WF, Villa CR, Lal AK, et al. : Ventricular assist device use in single ventricle congenital heart disease. Pediatr Transplant. 2017;21(7):e13031. 10.1111/petr.13031 [DOI] [PubMed] [Google Scholar]
- 129. Blume ED, VanderPluym C, Lorts A, et al. : Second annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) report: Pre-implant characteristics and outcomes. J Heart Lung Transplant. 2018;37(1):38–45. 10.1016/j.healun.2017.06.017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 130. Lorts A, Eghtesady P, Mehegan M, et al. : Outcomes of children supported with devices labeled as "temporary" or short term: A report from the Pediatric Interagency Registry for Mechanical Circulatory Support. J Heart Lung Transplant. 2018;37(1):54–60. 10.1016/j.healun.2017.10.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 131. Rossano JW, Goldberg DJ, Fuller S, et al. : Successful use of the total artificial heart in the failing Fontan circulation. Ann Thorac Surg. 2014;97(4):1438–40. 10.1016/j.athoracsur.2013.06.120 [DOI] [PubMed] [Google Scholar]
- 132. Rossano JW, Woods RK, Berger S, et al. : Mechanical support as failure intervention in patients with cavopulmonary shunts (MFICS): rationale and aims of a new registry of mechanical circulatory support in single ventricle patients. Congenit Heart Dis. 2013;8(3):182–6. 10.1111/chd.12053 [DOI] [PubMed] [Google Scholar]
- 133. Poh CL, Chiletti R, Zannino D, et al. : Ventricular assist device support in patients with single ventricles: the Melbourne experience. Interact Cardiovasc Thorac Surg. 2017;25(2):310–6. 10.1093/icvts/ivx066 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 134. Horne D, Conway J, Rebeyka IM, et al. : Mechanical circulatory support in univentricular hearts: current management. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015;18(1):17–24. 10.1053/j.pcsu.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 135. Weinstein S, Bello R, Pizarro C, et al. : The use of the Berlin Heart EXCOR in patients with functional single ventricle. J Thorac Cardiovasc Surg. 2014;147(2):697–704; discussion 70–45. 10.1016/j.jtcvs.2013.10.030 [DOI] [PubMed] [Google Scholar]
- 136. Mauchley DC, Mitchell MB: Transplantation in the Fontan patient. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015;18(1):7–16. 10.1053/j.pcsu.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 137. Rossano JW, Shaddy RE: Heart transplant after the Fontan operation. Cardiol Young. 2013;23(6):841–6. 10.1017/S1047951113001662 [DOI] [PubMed] [Google Scholar]
- 138. Kanter KR: Heart Transplantation in Children after a Fontan Procedure: Better than People Think. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016;19(1):44–9. 10.1053/j.pcsu.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 139. Martin MH, Feinstein JA, Chan FP, et al. : Technical feasibility and intermediate outcomes of using a handcrafted, area-preserving, bifurcated Y-graft modification of the Fontan procedure. J Thorac Cardiovasc Surg. 2015;149(1):239–45.e1. 10.1016/j.jtcvs.2014.08.058 [DOI] [PubMed] [Google Scholar]
- 140. Trusty PM, Wei Z, Tree M, et al. : Local Hemodynamic Differences Between Commercially Available Y-Grafts and Traditional Fontan Baffles Under Simulated Exercise Conditions: Implications for Exercise Tolerance. Cardiovasc Eng Technol. 2017;8(3):390–9. 10.1007/s13239-017-0310-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 141. Trusty PM, Restrepo M, Kanter KR, et al. : A pulsatile hemodynamic evaluation of the commercially available bifurcated Y-graft Fontan modification and comparison with the lateral tunnel and extracardiac conduits. J Thorac Cardiovasc Surg. 2016;151(6):1529–36. 10.1016/j.jtcvs.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 142. Mainwaring RD, Reddy VM, Hanley FL: Completion of the Three-Stage Fontan Pathway Without Cardiopulmonary Bypass. World J Pediatr Congenit Heart Surg. 2014;5(3):427–33. 10.1177/2150135114536908 [DOI] [PubMed] [Google Scholar]
- 143. LaPar DJ, Mery CM, Peeler BB, et al. : Short and long-term outcomes for bidirectional glenn procedure performed with and without cardiopulmonary bypass. Ann Thorac Surg. 2012;94(1):164–70; discussion 170–1. 10.1016/j.athoracsur.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 144. Ovroutski S, Sohn C, Miera O, et al. : Improved early postoperative outcome for extracardiac Fontan operation without cardiopulmonary bypass: a single-centre experience. Eur J Cardiothorac Surg. 2013;43(5):952–7. 10.1093/ejcts/ezs535 [DOI] [PubMed] [Google Scholar]
- 145. Talwar S, Muthukkumaran S, Choudhary SK, et al. : A complete extracorporeal circulation-free approach to patients with functionally univentricular hearts provides superior early outcomes. World J Pediatr Congenit Heart Surg. 2014;5(1):54–9. 10.1177/2150135113507091 [DOI] [PubMed] [Google Scholar]
- 146. Sett SS, Lafaro RJ: Extracardiac Fontan Operation Through a Right Thoracotomy. Ann Thorac Surg. 2017;104(2):e147–e149. 10.1016/j.athoracsur.2017.03.017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 147. Akintuerk H, Michel-Behnke I, Valeske K, et al. : Stenting of the arterial duct and banding of the pulmonary arteries: basis for combined Norwood stage I and II repair in hypoplastic left heart. Circulation. 2002;105(9):1099–103. 10.1161/hc0902.104709 [DOI] [PubMed] [Google Scholar]
- 148. Schranz D, Bauer A, Reich B, et al. : Fifteen-year single center experience with the "Giessen Hybrid" approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol. 2015;36(2):365–73. 10.1007/s00246-014-1015-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zheng J, Li Z, Li Q, et al. : Meta-analysis of Fontan procedure: Extracardiac conduit vs. intracardiac lateral tunnel. Herz. 2018;43(3):238–45. 10.1007/s00059-017-4553-6 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 150. Kogon B: Is the extracardiac conduit the preferred Fontan approach for patients with univentricular hearts? The extracardiac conduit is the preferred Fontan approach for patients with univentricular hearts. Circulation. 2012;126(21):2511–5; discussion 2515. 10.1161/CIRCULATIONAHA.111.076398 [DOI] [PubMed] [Google Scholar]
- 151. Khairy P, Poirier N: Is the extracardiac conduit the preferred Fontan approach for patients with univentricular hearts? The extracardiac conduit is not the preferred Fontan approach for patients with univentricular hearts. Circulation. 2012;126(21):2516–25; discussion 2525. 10.1161/CIRCULATIONAHA.111.075036 [DOI] [PubMed] [Google Scholar]
- 152. Pace Napoleone C, Oppido G, Angeli E, et al. : Results of the modified Fontan procedure are not related to age at operation. Eur J Cardiothorac Surg. 2010;37(3):645–50. 10.1016/j.ejcts.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 153. d'Udekem Y, Xu MY, Konstantinov IE: The optimal age at Fontan procedure and the 'ticking clock' theory: do we have an answer? Eur J Cardiothorac Surg. 2011;39(1):144; author reply 144–5. 10.1016/j.ejcts.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 154. Dabal RJ, Kirklin JK, Kukreja M, et al. : The modern Fontan operation shows no increase in mortality out to 20 years: a new paradigm. J Thorac Cardiovasc Surg. 2014;148(6): 2517–23.e1. 10.1016/j.jtcvs.2014.07.075 [DOI] [PubMed] [Google Scholar]
- 155. Devanagondi R, Suntharos P, Boyle GJ, et al. : Protein Losing Enteropathy After Cardiac Transplantation Successfully Treated by Stent Implantation. World J Pediatr Congenit Heart Surg. 2017;8(6): 754–757. 10.1177/2150135116658452 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 156. Brizard CP, Lane GK, Alex G, et al. : Original Surgical Procedure for the Treatment of Protein-Losing Enteropathy in Fontan Patients: Report of Two Midterm Successes. Circulation. 2016;134(8):625–7. 10.1161/CIRCULATIONAHA.116.023424 [DOI] [PubMed] [Google Scholar]
- 157. Kreutzer C, Kreutzer G: The Lymphatic System: The Achilles Heel of the Fontan-Kreutzer Circulation. World J Pediatr Congenit Heart Surg. 2017;8(5):613–623. 10.1177/2150135117720685 [DOI] [PubMed] [Google Scholar]
- 158. Menon S, Chennapragada M, Ugaki S, et al. : The Lymphatic Circulation in Adaptations to the Fontan Circulation. Pediatr Cardiol. 2017;38(5):886–892. 10.1007/s00246-017-1576-y [DOI] [PubMed] [Google Scholar]
- 159. Hraška V: Decompression of thoracic duct: new approach for the treatment of failing Fontan. Ann Thorac Surg. 2013;96(2):709–11. 10.1016/j.athoracsur.2013.02.046 [DOI] [PubMed] [Google Scholar]