Abstract
Introduction
We have previously reported that retinal vessel oxygen saturation is increased in mild-to-moderate dementia of Alzheimer's type when compared with healthy individuals. Mild cognitive impairment (MCI) is the predementia stage of the disease. The main purpose was to investigate if these changes are seen in MCI.
Methods
Retinal vessel oxygen saturation was measured in 42 patients with MCI and 42 healthy individuals with a noninvasive retinal oximeter, Oxymap T1. The groups were paired according to age.
Results
Arteriolar and venular oxygen saturation was increased in MCI patients compared to healthy individuals (arterioles: 93.1 ± 3.7% vs. 91.1 ± 3.4%, P = .01; venules: 59.6 ± 6.1% vs. 54.9 ± 6.4%, P = .001). Arteriovenous difference was decreased in MCI compared to healthy individuals (33.5 ± 4.5% vs. 36.2 ± 5.2%, P = .01).
Discussion
Increased retinal vessel oxygen saturation and decreased arteriovenous difference in MCI could reflect less oxygen extraction by retinal tissue. This indicates that retinal oxygen metabolism may be affected in patients with MCI.
Keywords: Mild cognitive impairment, Alzheimer's disease, Oximetry, Retina, Retinal vessels, Oxygen saturation, Spectrophotometry
Highlights
-
•
The need for reliable, noninvasive techniques for diagnosis of dementia is widely recognized.
-
•
This research indicates that retinal metabolism is decreased in patients in the predementia stage of mild cognitive impairment.
-
•
Retinal oximetry is a novel noninvasive method that could help as a diagnostic tool in dementia.
1. Background
Mild cognitive impairment (MCI) is a broad term that can be defined as an early stage of Alzheimer's disease (AD) as well as of other types of dementias [1]. Some individuals with MCI are however stable and can even recover [2]. Complaints about visual impairment are common in AD patients [3], and retinal vascular abnormalities have been found in patients with AD [4]. There is some evidence of thinning of the retinal nerve fiber layer (RNFL) in MCI [5], [6] and AD [7], [8], [9], [10], [11], [12] as well as with progression from MCI to severe AD [6]. It has been suggested that this may help in diagnosis and also to evaluate progression [13].
Einarsdottir et al. measured difference in retinal oxygen metabolism in mild-to-moderate Alzheimer's dementia and found increased vessel oxygen saturation compared to healthy individuals [14]. In other retinal oximetry studies in retinal atrophic diseases such as in glaucoma, increased venular oxygen saturation and decreased arteriovenous difference (oxygen uptake) were correlated with worse glaucomatous visual fields [15], [16] and thinner RNFLs [17].
Biomarkers for AD have gained increased recognition and include cerebrospinal fluid protein biomarkers (total tau, phospho-tau, and β amyloid-42), amyloid imaging positron emission tomography, and magnetic resonance imaging for evaluation of medial temporal lobe atrophy (MTA). These methods are however invasive and/or expensive, and there is still a need for simpler and reliable biomarkers of the disease. The purpose of the study was to test whether retinal oxygen metabolism is abnormal in AD in the stage of MCI when compared with healthy individuals and to examine whether retinal oximetry can serve as a noninvasive biomarker for early AD.
2. Methods
2.1. Study population
This study is a part of a larger study on progression of cognitive impairment in MCI. All participants signed an informed consent which followed the Tenets of the Declaration of Helsinki (www.wma.net/policy). The study is a case-control study.
Patients were diagnosed at the Memory Clinic of the Geriatric Department, Landspitali University Hospital, Reykjavik, Iceland. The diagnosis of MCI for inclusion in the study was made according to the Petersen criteria [18], [19] based on information on cognitive impairment compared to earlier abilities from the patients and their relatives but without any change in abilities of daily life. The Informant Questionnaire on Cognitive Decline in the Elderly [20] was used in evaluating the changes seen by the relatives during the preceding years. An Mini Mental State Examination score [21] of 24 or above and an Informant Questionnaire on Cognitive Decline in the Elderly score of 4.0 or less were used for inclusion. All patients went through a standardized procedure with neuropsychological testing and brain magnetic resonance imaging for evaluation of MTA. In some cases, analysis of β amyloid and tau proteins from cerebrospinal fluid was also performed.
All participants underwent comprehensive eye examination and were excluded if they had retinal or optic nerve disease such as glaucoma and age-related macular degeneration or trauma, diabetes mellitus, or other systemic diseases that can affect the eye. The healthy cohort included individuals with no history of cognitive impairment. All participants were of Caucasian origin. Sixty patients were originally recruited for oximetry. Thirteen patients were excluded because of retinal or optic disc disease or because of nonattendance on measurement day. In all, 47 patients went through oximetry. From the group of 47 patients, we were able to pair 44 with a healthy cohort, according to age (where no more than 7 years were between paired individuals). Of those 44, two were excluded because of bad image quality. Therefore, the final number of included participants was 42 in each group; the MCI group and the group of healthy control subjects.
Each participant answered a questionnaire on medical history, medications, and smoking. Blood pressure and heart rate were measured (Omron M6 Comfort [HEM-7000-E]; Omron Healthcare Europe, Hoofddorp, the Netherlands) as well as finger pulse oximetry (healthy cohort: Ohmeda Biox 3700; Ohmeda, Boulder, CO, USA; patient group: Masimo Rad 57, Masimo Corp., CA, USA) and intraocular pressure (iCare Tonometer TAO1; Tiolat Oy, Helsinki, Finland). Pupils were dilated with 1% tropicamide (Mydriacyl; S.A. Alcon-Couvreur N.V., Puurs, Belgium), which was supplemented with 10% phenylephrine hydrochloride (AK-Dilate; Akorn Inc., Lake Forest, IL, USA).
Magnetic resonance imaging of the brain was obtained from every participant with visual evaluation of atrophy of the medial temporal lobes scoring atrophy from 0 (no atrophy) to 4 (maximal atrophy, [22]). For the purpose of this study, the same experienced radiologist scored all the images consecutively.
Optical coherence tomography (OCT) imaging was performed on most MCI patients. Peripapillary scans were obtained with Topcon 3D OCT 2000 (Topcon Inc. Tokyo, Japan).
All the cases were diagnosed in a consensus meeting of at least three geriatricians. Based on all available information, the participants were grouped into one of two groups:
-
1.
Clinical signs and biomarkers consistent with AD (n = 16)
-
2.
Clinical signs of MCI but without clear biomarkers for AD (n = 25).
One patient had clinical signs and biomarkers that were consistent with early Lewy body dementia.
The consensus diagnosis was made according to ICD-10 (the 10th revision of the International Statistical Classification of Diseases and Related Health Problems by the World health organization). Patients that did not fulfill the diagnosis of MCI by neuropsychological testing but still experienced loss of memory were considered to be in very early stage of MCI and were included in the study. Details on participants can be found in Table 1.
Table 1.
Demographics of the study groups
| Healthy | MCI | Subgroups |
||
|---|---|---|---|---|
| Possible Alzheimer's disease according to biomarkers | MCI without biomarkers of Alzheimer's disease | |||
| Number | 42 | 42 | 16 | 25 |
| Age (years, mean ± SD) | 66 ± 9 | 70 ± 10 | 75 ± 8 | 70 ± 10 |
| Heart rate (beats/min, mean ± SD) | 68 ± 15 | 68 ± 11 | 66 ± 12 | 69 ± 11 |
| Finger oximetry (%, mean ± SD) | 96 ± 2 | 98 ± 8 | 97 ± 2 | 97 ± 1 |
| Systolic pressure (mm Hg, mean ± SD) | 134 ± 19 | 136 ± 23 | 135 ± 23 | 138 ± 24 |
| Diastolic pressure (mm Hg, mean ± SD) | 86 ± 10 | 86 ± 11 | 85 ± 10 | 87 ± 12 |
| MAP (mm Hg, mean ± SD) | 102 ± 12 | 101 ± 17 | 98 ± 22 | 104 ± 14 |
Abbreviations: MAP, mean arterial pressure; MCI, mild cognitive impairment; min, minutes; SD, standard deviation.
NOTE. Heart rate, finger oximetry, and blood pressure measurements were missing for four individuals (three healthy and one MCI).
2.2. Retinal oximetry
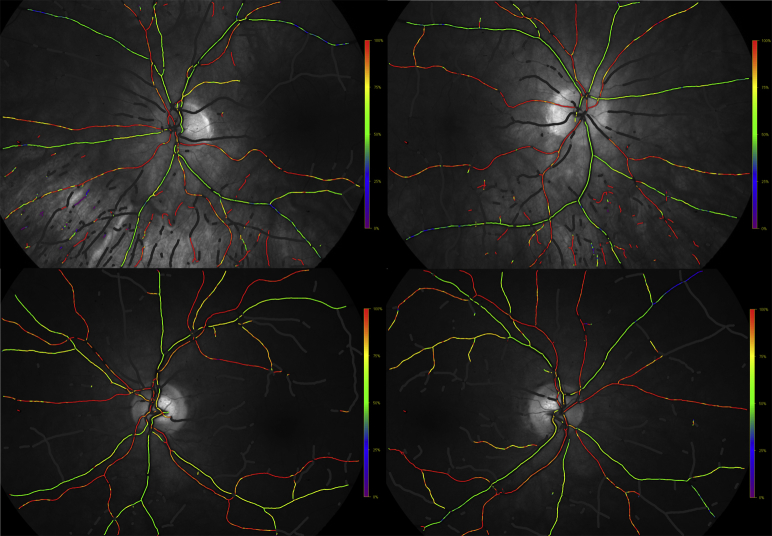
Oximetry was performed with a dual wavelength, noninvasive spectrophotometric oximeter, Oxymap T1 (Oxymap ehf., Reykjavik, Iceland). The oximeter has been described in details elsewhere [23]. In short, the oximeter consists of a conventional fundus camera (Topcon TRC-50DX, Topcon Corporation, Tokyo, Japan) with two attached digital cameras. Two images of the retina at two different wavelengths, 570 nm (insensitive to oxygen saturation) and 600 nm (sensitive to oxygen saturation), are simultaneously acquired, and retinal vessel oxygen saturation is calculated from those two images (Fig. 1).
Fig. 1.
Fundus oximetry images from (above) a healthy individual and (below) an MCI patient. These two individuals were paired according to age. Average retinal oxygen saturation was calculated from left and right eye for each individual. Abbreviation: MCI, mild cognitive impairment.
2.2.1. Analysis of oximetry images
For every individual, both eyes were analyzed with a specialized analysis program for Oxymap T1, Oxymap Analyzer (version 2.2.1, revision 10927, Oxymap ehf., Reykjavik, Iceland). Excluded were images with image quality graded below five (according to the Oxymap Analyzer program). The optic disc was located in the center of analyzed images. Oxygen saturation was measured in all major retinal blood vessels that were between two, concentric circles, 1.5–3 disc diameter, circled around the optic disc. The minimum length of a measured vessel segment was 50 pixels. The diameter of the same vessels was measured. For each individual, mean values from both eyes were averaged and the average was used for further analysis (one value for venules and one value for arterioles per individual).
2.3. Analysis of MTA scores
The patient group was divided into two subgroups according to the severity of MTA scores, MTA score ≥2, and MTA score <2.
2.4. Statistical analysis
All statistical analysis was performed with GraphPad Prism, version 7.0 (GraphPad Software Inc., La Jolla, California, USA). Two-tailed, unpaired t-test was used for comparison of the two groups. P < .05 was considered statistically significant.
3. Results
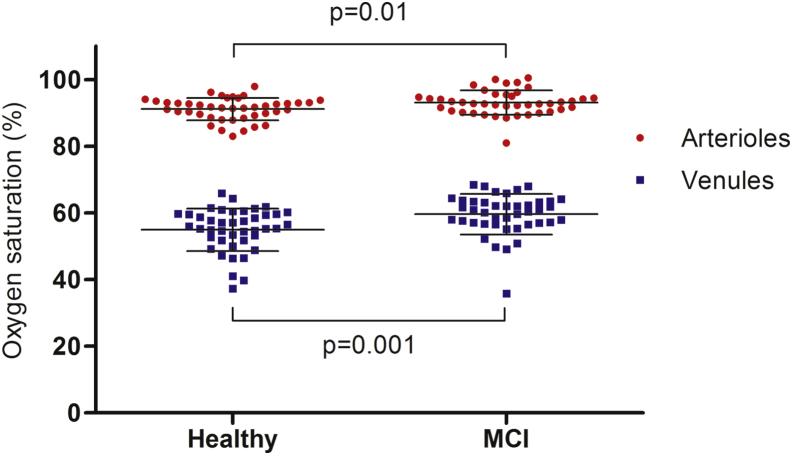
When the whole MCI group (n = 42) was compared to healthy individuals (n = 42), retinal oxygen saturation in arterioles was statistically significantly higher in MCI patients (93.1 ± 3.7% vs. 91.1 ± 3.4%, mean ± SD, n = 42, P = .01). The saturation in venules were also statistically significantly increased in MCI compared to healthy individuals (59.6 ± 6.1% vs. 54.9 ± 6.4%, P = .001, Fig. 2). The arteriovenous difference in oxygen saturation was lower in MCI patients compared to healthy individuals (33.5 ± 4.5% vs. 36.2 ± 5.2%, P = .01).
Fig. 2.
Retinal vessel oxygen saturation in arterioles (red) and venules (blue). There was an increase in both arteriolar and venular oxygen saturation in MCI patients when compared with healthy individuals. Abbreviation: MCI, mild cognitive impairment.
No difference could be found between groups in vessel width for either arterioles (P = .6) or venules (P = .5, Table 2). There was also no difference in image quality, as measured by the oximetry software, between MCI patients (quality grade 7.9 ± 0.8) and healthy individuals (7.7 ± 1.0, P = .3).
Table 2.
Mean retinal oxygen saturation values and vessel width for healthy individuals and patients with MCI
| Healthy (n = 42) | MCI (n = 42) | P value (unpaired t-test) | |
|---|---|---|---|
| Oxygen saturation (%) | |||
| Arterioles | 91.1 ± 3.4 | 93.1 ± 3.7 | .01 |
| Venules | 54.9 ± 6.4 | 59.6 ± 6.1 | .001 |
| AV difference | 36.2 ± 5.2 | 33.5 ± 4.5 | .01 |
| Vessel width (pixels) | |||
| Arterioles | 11.9 ± 0.8 | 12.0 ± 1.0 | .6 |
| Venules | 15.0 ± 1.4 | 15.1 ± 1.2 | .9 |
Abbreviation: AV, arteriovenous; MCI, mild cognitive impairment.
NOTE. Also represented are P values for comparison between groups with an unpaired t-test.
No difference in retinal vessel oxygen saturation and arteriovenous difference was found between those that had MTA score of 2 or more and those that had less atrophy (P = .1–.7). There was a correlation between decreased arteriolar oxygen saturation and increased MTA scores (P = .01) but not between MTA scores and venular oxygen saturation (P = .07) and arteriovenous difference (P = .6). There was no correlation between retinal vessel oxygen saturation and the thickness of peripapillary retina (measured with OCT, P = .1–.4).
When the two subgroups (possible AD according to biomarkers and MCI without biomarkers of AD) were compared to paired, healthy individuals, an increase in venular oxygen saturation was found in patients with MCI without biomarkers of AD (P = .005), and the arteriovenous difference was decreased in the same subgroup (P = .01), Table 3. Arteriolar vessel oxygen saturation was not significantly different from healthy in either subgroup although there was a trend toward increased saturation in patients with neuropsychological state of MCI subgroup when compared with healthy (P = .07). Vessel width (Table 3) and image quality in the subgroups were not significantly different from healthy individuals.
Table 3.
Retinal oxygen saturation and vessel width subgroups of patients
| Subgroups |
||
|---|---|---|
| Possible Alzheimer's disease according to biomarkers (n = 16) | MCI without biomarkers of Alzheimer's disease (n = 25) | |
| Oxygen saturation (%) | ||
| Arterioles | 93.2 ± 5.0 | 93.0 ± 2.7 |
| Venules | 58.6 ± 7.8 | 60.2 ± 5.0∗ |
| AV difference | 34.6 ± 5.0 | 32.8 ± 4.1† |
| Vessel width (pixels) | ||
| Arterioles | 12.0 ± 1.0 | 12.0 ± 1.0 |
| Venules | 15.1 ± 1.1 | 15.0 ± 1.2 |
Abbreviation: AV, arteriovenous; MCI, mild cognitive impairment.
NOTE. *P = .005; †P = .01 when compared to paired, healthy cohort.
4. Conclusion
The results from the study suggest that retinal arteriolar and venular oxygen saturation is higher in MCI than in healthy subjects. The arteriovenous difference was decreased in MCI compared with healthy individuals. Patients with MCI without biomarkers of AD (subgroup) had higher venular oxygen saturation and decreased arteriovenous difference when compared with paired, healthy individuals. This confirms and extends earlier reports by our research group [14].
Increased venular oxygen saturation and decreased arteriovenous difference could indicate decreased oxygen extraction. Other groups have measured thinning of the RNFL in AD and in MCI [5], [6], [7], [8], [9], [11], [12], [24], [25], [26]. Increased venular oxygen saturation and decreased arteriovenous difference could indicate less metabolic activity in the tissue, which could be due to thinner RNFL. In our study, OCT was only performed on the patient group but not the healthy group; so unfortunately, we do not have the comparison of RNFL thickness between these two groups. The arterioles also have higher oxygen saturation in MCI compared to healthy individuals.
There are some possible explanations for the increase in retinal vessel oxygen saturation. As previously stated, thinner RNFL with resultant decreased metabolic activity could be one of the reasons. Fluorodeoxyglucose positron emission tomography has demonstrated that metabolism could be decreased in the brain of MCI patients [27], [28]. If metabolism and oxygen consumption is decreased in MCI, this could also explain the decreased arteriovenous difference that was measured in the study. Other reasons could also explain increased venular oxygen saturation. According to Thal et al., 82%–98% AD patients have cerebral amyloid angiopathy, which leads to thickening of vessel walls [29]. This leads to decreased diffusion of glucose and oxygen from the vessels, which could increase venular oxygen saturation. This could also explain the increase in arteriolar oxygen saturation, that is, arterioles loose oxygen by diffusion and this loss would decrease with thicker walls. Similar results have been reported in diabetic retinopathy (increased arteriolar and venular oxygen saturation), and it has been suggested that thickening of the vessel wall could partly explain those results [30]. Another potential explanation for increased arteriolar oxygen saturation could be an effect from a thinner RNFL (as discussed previously) and a resultant decreased oxygen uptake by tissue adjacent to an arteriole. Decreased oxygen concentration gradient between the central retinal artery and the adjacent central retinal vein could also decrease the expected oxygen counter current diffusion of oxygen between them.
Retinal oxygen saturation has been previously measured in mild and moderate AD by our group [14]. We measured increased vessel oxygen saturation in moderate AD when compared with healthy individuals, which is in agreement with the results from this study. Berisha et al. measured smaller retinal venular diameter in mild-to-moderate AD compared to healthy individuals. They also measured decreased blood flow in retinal venules in AD [9]. Feke et al. measured decrease in blood flow in AD and to a smaller degree but still statistically significant, in MCI when compared to healthy individuals [31]. They did not measure difference in the blood column diameter in MCI when compared to healthy individuals, which is in agreement with our results. In agreement with Berisha et al., they did, however, measure decreased diameter in AD. If blood flow is decreased (such as Berisha et al. and Feke et al. measured) and arteriovenous difference is also decreased, such as was measured in this study, this could indicate less oxygen extraction by the retinal tissue.
Most of the patients could be divided into two subgroups (possible AD according to biomarkers and MCI without biomarkers of AD). In both subgroups, the mean in arteriolar and venular oxygen saturation was higher than for healthy individuals. This increase in saturation reached statistical significance only in the group with MCI without biomarkers of AD. The low number of patients in each subgroup means that strong conclusions cannot be drawn about possible differences.
There are a few limitations to the study. The mean age of the control group was lower by 4 years compared to the mean age of MCI patients. It has been shown that age can affect image quality, which consequently affects measured oxygen saturation values. For example, Geirsdottir et al. measured decreased venular oxygen saturation with increased age [23]. In this study, arteriolar and venular oxygen saturation was higher in the MCI group compared to the younger healthy group, which means that the age difference between the two groups is an unlikely explanation for the difference seen in saturation. Image quality was also evaluated, and there was no difference found between the groups.
Unfortunately, data on RNFL thickness for the healthy cohort did not exist, so any RNFL thickness comparison between healthy and MCI group was impossible. We performed a correlation between the thickness of the RNFL and retinal oxygen saturation for the MCI cohort but found no correlation. Arteriolar saturation decreased with increased size of MTA score.
In conclusion, patients with MCI have higher retinal vessel oxygen saturation and lower arteriovenous difference when compared to healthy individuals. This could indicate less oxygen extraction in MCI. Further studies with larger number of MCI patients are needed to fully evaluate whether retinal metabolism is altered in early AD and if retinal oxygen values could serve as an early biomarker for the disease.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using PubMed. There has only been one publication in a peer reviewed journal on retinal oximetry and Alzheimer's disease, which is a study conducted by our group. Most of the studies on mild cognitive impairment and the eye are based on retinal nerve fiber layer thickness and blood flow. The relevant publications have been appropriately cited.
-
2.
Interpretation: Our findings demonstrate an increase in retinal venous oxygen saturation and decrease in arteriovenous difference using a simple, noninvasive technique. This could indicate a decrease in retinal metabolism in patients with mild cognitive impairment.
-
3.
Future directions: It is important to measure a larger cohort to determine whether these changes in retinal metabolism can be used as a biomarker for early Alzheimer's disease.
Acknowledgments
Olof Birna Olafsdottir received a grant from the Icelandic Centre for Research. The sponsor did not have any role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report, and in the decision to submit the article for publication.
References
- 1.Apter J.T., Shastri K., Pizano K. Update on disease-modifying/preventive therapies in Alzheimer's disease. Curr Geriatr Rep. 2015;4:312–317. doi: 10.1007/s13670-015-0141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 3.Cunha L.P., Almeida A.L., Costa-Cunha L.V., Costa C.F., Monteiro M.L. The role of optical coherence tomography in Alzheimer's disease. Int J retina vitreous. 2016;2:24. doi: 10.1186/s40942-016-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung C.Y., Ong Y.T., Ikram M.K., Ong S.Y., Li X., Hilal S. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimers Dement. 2014;10:135–142. doi: 10.1016/j.jalz.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Paquet C., Boissonnot M., Roger F., Dighiero P., Gil R., Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2007;420:97–99. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 6.Liu D., Zhang L., Li Z., Zhang X., Wu Y., Yang H. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer's disease. BMC Neurol. 2015;15:14. doi: 10.1186/s12883-015-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parisi V., Restuccia R., Fattapposta F., Mina C., Bucci M.G., Pierelli F. Morphological and functional retinal impairment in Alzheimer's disease patients. Clin Neurophysiol. 2001;112:1860–1867. doi: 10.1016/s1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- 8.Danesh-Meyer H.V., Birch H., Ku J.Y., Carroll S., Gamble G. Reduction of optic nerve fibers in patients with Alzheimer disease identified by laser imaging. Neurology. 2006;67:1852–1854. doi: 10.1212/01.wnl.0000244490.07925.8b. [DOI] [PubMed] [Google Scholar]
- 9.Berisha F., Feke G.T., Trempe C.L., McMeel J.W., Schepens C.L. Retinal abnormalities in early Alzheimer's disease. Invest Ophthalmol Vis Sci. 2007;48:2285–2289. doi: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- 10.Berisha F., Feke G.T. Postural changes in intraocular pressure. Ophthalmology. 2007;114:1413. doi: 10.1016/j.ophtha.2007.03.049. author reply-4. [DOI] [PubMed] [Google Scholar]
- 11.Marziani E., Pomati S., Ramolfo P., Cigada M., Giani A., Mariani C. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer's disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:5953–5958. doi: 10.1167/iovs.13-12046. [DOI] [PubMed] [Google Scholar]
- 12.Bambo M.P., Garcia-Martin E., Pinilla J., Herrero R., Satue M., Otin S. Detection of retinal nerve fiber layer degeneration in patients with Alzheimer's disease using optical coherence tomography: searching new biomarkers. Acta Ophthalmol. 2014;92:e581–e582. doi: 10.1111/aos.12374. [DOI] [PubMed] [Google Scholar]
- 13.Larrosa J.M., Garcia-Martin E., Bambo M.P., Pinilla J., Polo V., Otin S. Potential new diagnostic tool for Alzheimer's disease using a linear discriminant function for Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:3043–3051. doi: 10.1167/iovs.13-13629. [DOI] [PubMed] [Google Scholar]
- 14.Einarsdottir A.B., Hardarson S.H., Kristjansdottir J.V., Bragason D.T., Snaedal J., Stefansson E. Retinal oximetry imaging in Alzheimer's disease. J Alzheimers Dis. 2015;49:79–83. doi: 10.3233/JAD-150457. [DOI] [PubMed] [Google Scholar]
- 15.Olafsdottir O.B., Hardarson S.H., Gottfredsdottir M.S., Harris A., Stefansson E. Retinal oximetry in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2011;52:6409–6413. doi: 10.1167/iovs.10-6985. [DOI] [PubMed] [Google Scholar]
- 16.Olafsdottir O.B., Vandewalle E., Abegao Pinto L., Geirsdottir A., De Clerck E., Stalmans P. Retinal oxygen metabolism in healthy subjects and glaucoma patients. Br J Ophthalmol. 2014;98:329–333. doi: 10.1136/bjophthalmol-2013-303162. [DOI] [PubMed] [Google Scholar]
- 17.Vandewalle E., Pinto L.A., Olafsdottir O.B., De Clerck E., Stalmans P., Van Calster J. Oximetry in glaucoma: correlation of metabolic change with structural and functional damage. Acta Ophthalmol. 2014;92:105–110. doi: 10.1111/aos.12011. [DOI] [PubMed] [Google Scholar]
- 18.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 19.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 20.Jorm A.F., Korten A.E. Assessment of cognitive decline in the elderly by informant interview. Br J Psychiatry. 1988;152:209–213. doi: 10.1192/bjp.152.2.209. [DOI] [PubMed] [Google Scholar]
- 21.Folstein M.F., Folstein S.E., McHugh P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Scheltens P., Launer L.J., Barkhof F., Weinstein H.C., van Gool W.A. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol. 1995;242:557–560. doi: 10.1007/BF00868807. [DOI] [PubMed] [Google Scholar]
- 23.Geirsdottir A., Palsson O., Hardarson S.H., Olafsdottir O.B., Kristjansdottir J.V., Stefansson E. Retinal vessel oxygen saturation in healthy individuals. Invest Ophthalmol Vis Sci. 2012;53:5433–5442. doi: 10.1167/iovs.12-9912. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari L., Huang S.C., Magnani G., Ambrosi A., Comi G., Leocani L. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer's disease. J Alzheimers Dis. 2017;56:1101–1107. doi: 10.3233/JAD-160886. [DOI] [PubMed] [Google Scholar]
- 25.Choi S.H., Park S.J., Kim N.R. Macular ganglion cell -inner plexiform layer thickness is associated with clinical progression in mild cognitive impairment and Alzheimers disease. PLoS One. 2016;11:e0162202. doi: 10.1371/journal.pone.0162202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesler A., Vakhapova V., Korczyn A.D., Naftaliev E., Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Clin Neurol Neurosurg. 2011;113:523–526. doi: 10.1016/j.clineuro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Mosconi L., Tsui W.H., Herholz K., Pupi A., Drzezga A., Lucignani G. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer's disease, and other dementias. J Nuclear Med. 2008;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perani D., Della Rosa P.A., Cerami C., Gallivanone F., Fallanca F., Vanoli E.G. Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. NeuroImage Clin. 2014;6:445–454. doi: 10.1016/j.nicl.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thal D.R., Griffin W.S., de Vos R.A., Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathologica. 2008;115:599–609. doi: 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- 30.Hardarson S.H., Olafsdottir O.B., Karlsson R.A., Beach J.M., Eysteinsson T., Benediktsson J.A. Retinal vessel oxygen saturation in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1328. [Google Scholar]
- 31.Feke G.T., Hyman B.T., Stern R.A., Pasquale L.R. Retinal blood flow in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement (Amst) 2015;1:144–151. doi: 10.1016/j.dadm.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]