Abstract
Rationale:
Atopic dermatitis is a frequent, relapsing, chronic inflammatory skin condition with a deep negative impact on quality of life. Three are the major pathological factors driving its complex pathogenesis: the skin barrier disruption, the altered Th2 cell response and itching. Current management of the disease is often unsatisfactory, unable to induce a complete resolution of signs and symptoms or at least a significant clinical improvement with adequate patient satisfaction.
Patient concerns:
We report the case of a 57-year-old man with severe chronic atopic dermatitis for at least 40 years irresponsive to traditional therapies. The patient was treated in the past with different standard therapeutic regimens without satisfactory and lasting results.
Diagnoses:
The diagnosis of AD was based on the revised criteria of Hanifin and Rjika and the Scoring Atopic Dermatitis was assessed together with laboratory evaluation.
Interventions:
He received off-label omalizumab (300 mg subcutaneous injection repeated at 2-week intervals for six months).
Outcomes:
Scorad and laboratory findings were assessed montly and demonstrated a progressive decrease (SCORAD and ECP levels) togheter with general clinical improvement. Omalizumab, in our patient, determined a significant symptomatic improvement, assessed by SCORAD, simultaneously with a progressive decline in ECP serum levels, whit no side effects, confirming the substantial safety of the drug.
Lessons:
The satisfactory response to omalizumab after the failure of all previous traditional treatments, confirms the efficacy of this biological drug in the therapy of refractory AD with high IgE levels and increased ECP serum levels. The lesson learnt from this case report is that Omalizumab represent an effective and safe alternative to traditional therapies in patients with severe irresponsive atopic dermatitis.
Keywords: atopic dermatitis, eczema, IgE, omalizumab, translational immunology
1. Introduction
Atopic dermatitis (AD) is a chronic skin disease that usually occurs in infancy and persists in adulthood, although it may arise at any age. The disease is characterized by typically pruritic skin lesions, and is usually associated with other allergic manifestations, such as asthma and allergic rhinitis (atopic triad), and often with increased total serum IgE.[1] Several causes contribute to the disease, such as the skin barrier, environmental and temperature mutations, environmental allergens, exogenous irritants, infections, and psychological stress. The disease can occur continuously for long periods, or show some intermissions in its course, with repeated exacerbations. It is very distressing with a significant negative impact on the quality of life. AD is an extremely complex disease, occurring with increasing frequency in any part of the world.[2]
Recently, new research data added one more level of complexity, suggesting a potential role in the modulation of atopic dermatitis by epigenetic regulation, neuro-immunological signals and skin microbiome.
The complexity of the underlying pathogenic mechanisms also explains the remarkable clinical variability of the disease: age of onset, severity, triggering factors, clinical manifestations, natural history, and response to therapy.
Because of the multiple aspects of AD, the best therapeutic approach is still being debated upon. Not all patients respond satisfactorily to the standard therapy with antihistamines, antileukotrienes, topical and/or oral corticosteroids, UV treatments and immunosuppressants such as tacrolimus and cyclosporin. Moreover, the long-term therapy with corticosteroids or immunosuppressants often causes significant side effects that require their suspension. A more tailored therapeutic strategy needs to stratify AD into more homogeneous subpopulations, in order to identify the responding subgroups of patients while developing specific immunotherapeutic regimens in this new era of biologics.
Recently, a number of studies demonstrated the efficacy of omalizumab, a recombinant humanized immunoglobulin (Ig) G1 monoclonal antibody, in the treatment of AD, either in monotherapy[2] or in combination with other treatments.[3–6] Omalizumab specifically binds to the high-affinity FcεRI domain of circulating IgE, thus preventing their binding to the specific receptor on mast cells and other effector cells. Currently approved for the treatment of moderate to severe IgE-mediated asthma[8] and chronic urticaria,[9] the drug is now used off-label also in other pathologies, including atopic dermatitis—regardless of the simultaneous presence of other clinical manifestations of allergy[5]—in order to evaluate its efficacy and safety in the management of this disease.
The patient provided his written informed consent for the publication of this report.
2. Case presentation
We report the case of a 57-year-old man who has been suffering from severe chronic atopic dermatitis for at least 40 years and who in the past was treated with several standard therapeutic regimens (systemic corticosteroids with associated UVB treatment, sedating and nonsedating H1-blocking antihistamines, leukotriene inhibitors, azathioprine, mycophenolic acid, and methotrexate), without any satisfactory and lasting results. The diagnosis of AD was based on the revised criteria of Hanifin and Rjka.[10]
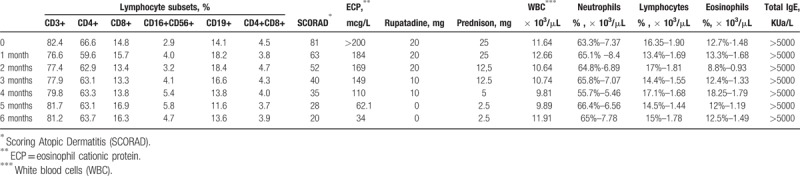
Although the patient was successfully treated for 5 years with 200 mg cyclosporin A per os, this therapy was discontinued, due to the onset of renal failure (progressive increase of blood creatinine levels from 0.9 to 2.3 g/L). Upon physical examination, the patient's skin was very dry everywhere, especially on the face, hands and feet. There was also an intense itching. At the time of the first evaluation, the severity of the disease—as assessed with the eczema area and severity index (EASI) and the Scoring Atopic Dermatitis (SCORAD)—was 83,[11] even if the patient was under chronic treatment with prednisone and rupatadine.
Laboratory analyses revealed a serum IgE level >5000 KUA/L (normal range 0–200 KUA/L) and eosinophil cationic protein (ECP) >200 mcg/L (5.5–15 mcg/L). Complete blood tests, with differential, standard biochemical profile, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum protein electrophoresis, thyroid function screening, serum protein electrophoresis, circulating lymphocyte subset and urine analysis were in the normal ranges, except a moderate increase in creatinine (2.20 g/L), neutrophil leukocytosis [white blood cells (WBC) 11.64 × 103/μL with neutrophils (N) 7.370 × 103/μL – 63.3%, lymphocytes (L) 1.90 × 103/μL – 16.35%] and eosinophils (1.48 × 103/μL – 12.7%). Cancer markers, anti-nuclear antibodies (ANA), anti-extractable nuclear antigens (ENA), anti-neutrophil cytoplasmic antibodies (ANCA), cryoglobulins and stool analysis for parasites were also performed, with negative results. The instrumental exams (urea breath test, electrocardiogram, chest X-ray, abdominal ultrasound scan) didn’t reveal any significant pathological findings.
After receiving the patient's informed consent, in compliance with the rules of our Institution, omalizumab (300 mg subcutaneous injections, repeated at 2-week intervals) was therefore administered off-label for six months.
Prednisone and rupatadine were gradually reduced and discontinued at month 5 of the omalizumab therapy. To evaluate the effectiveness of the omalizumab therapy, the SCORAD and laboratory parameters were assessed monthly (Table 1). After one and three months of therapy, the SCORAD was 63 (reduction of over 23%) and 40 (50% reduction), respectively, until a reduction of 75% (SCORAD 20) at the end of treatment (sixth month). In association with the decrease in the SCORAD, serum ECP levels also decreased significantly. Two months after the discontinuation of therapy, the patient's SCORAD reduction was maintained. In addition, the patient did not have any omalizumab-induced adverse effect. Ethical approval is not necessary in these cases, at our Institution (omalizumab is a safe and effective drug, approved for the treatment of asthma and urticaria).
Table 1.
Monthly laboratory assessment and SCORAD during omalizumab therapy in a case of severe, refractory atopic dermatitis.
3. Discussion
AD is a multifactorial disease, which includes the defective epidermal barrier caused by altered expression of keratinocyte differentiation genes and abnormal content of extracellular lipids, resulting in increased permeation to allergens, irritants and microbes.[12,13,14] In addition to IgE, dendritic cells, basophils and mast cells, also eosinophils are important participators in AD skin inflammation.[10] ECP serum level, which is considered a marker of eosinophil activation, is elevated in AD patients,[15] thus confirming the role of eosinophils in the pathogenesis of the disease.
Omalizumab is a recombinant humanized murine monoclonal antibody that binds to IgE and prevents the binding of IgE to FcεRI (high-affinity IgE receptor), thereby reducing the amount of free IgE available to trigger the allergic cascade. The treatment of atopic subjects with omalizumab resulted in a marked down-regulation of FcεRI receptors on basophils.
Although it is not yet completely clear how omalizumab specifically acts in the various diseases in which it has proved itself effective, it has been suggested that this biological drug acts by binding to the circulating IgE, thus resulting in a reduction in free IgE levels and in the down-regulation of IgE receptors. The final biological result is the prevention of mast cell or basophil activation and mediator release, leading to the interruption of the allergic cascade.[15]
Omalizumab is also thought to induce the abduction of incoming allergens and IgE immune complexes, to down-regulate IgE receptors on dendritic cells, and to decrease IgE production and binding, thus preventing the IgE-mediated improvement of the mast cell activity and exerting a non-specific desensitization of mast cells, basophils and dendritic cells in the skin of AD patients.[16] In addition, omalizumab seems to have a role in removing the eosinophil-mediated skin inflammation in AD.[15] All these possible mechanisms of action help to explain the effectiveness of omalizumab in inhibiting the more chronic aspects of allergic inflammation in AD patients.
Considering the complex and multifactorial pathogenesis of the disease, and even more the manifold pharmacological mechanisms of omalizumab, its effective dose is difficult to establish. Therefore, the dose of omalizumab that we administered to our patient was 300 mg every two weeks; this dosage was decided arbitrarily, on the basis of the recommended dosage of the drug in the treatment of Chronic Spontaneous Urticaria (300 mg), but at closer intervals (every two weeks), considering the severity of the disease, with an in-between choice, with regard to the extremely variable data reported in the literature by other authors (150–600 mg/2 weeks).[5]
In our patient, in concomitance with the symptomatic improvement, assessed by the SCORAD, omalizumab also causes a progressive decline in ECP serum levels. None of the side effects commonly seen in patients treated with omalizumab [headache (15%), dizziness (9%) and acute urticaria (9%)][7] occurred in our patient, thus confirming the substantial safety of the drug.
In clinical studies, serum free IgE levels were reduced in a dose-dependent manner within one hour following the first dose, and were maintained between doses. One year after the discontinuation of omalizumab, IgE levels had returned to pre-treatment levels, with no observed rebound in IgE levels after the washout of the medicinal product.
The laboratory methods commonly used (as in our case) for the measurement of total IgE do not distinguish between free IgE and those linked to omalizumab or the effector cells. Therefore the monitoring of the total IgE dosage before, during and after the therapy with omalizumab (also reported in Table 1) is of little significance. The high initial level of total IgE may instead be significant.
AD is a T cell-mediated inflammatory disease, mainly with an altered Th2 cell response;[2] although, in the course of the omalizumab therapy, some authors[17] described a reduction in the CD4+/CD8+ ratio, we did not found any significant changes in the CD4+ and CD8+ lymphocyte subsets, as reported in Table 1.
The satisfactory response to omalizumab—after the failure of all previous traditional treatments—confirms the effectiveness of this biological drug in the therapy of refractory AD with high IgE levels and increased ECP serum levels.
Although many studies reported the effectiveness of omalizumab in the treatment of AD, its use for this pathology has not yet been officially authorized. Based on our experience and literature data,[5–8,12,15,16] omalizumab may therefore be an effective and safe alternative to traditional therapies in patients with a severe form of the disease, unresponsive to other therapeutic measures, or suffering from the undesirable side effects of such therapies. AD patients with severe refractory disease in whom systemic treatment was not successful could be the best candidates for the omalizumab therapy.
Acknowledgments
The authors would like to thank Attubato S.r.l. for English language assistance. Unconditional support for language assistance was funded by Novartis Farma, Italy
Author contributions
Conceptualization: Maria Maddalena Sirufo, Massimo De Martinis, Lia Ginaldi.
Data curation: Maria Maddalena Sirufo, Massimo De Martinis, Lia Ginaldi.
Formal analysis: Maria Maddalena Sirufo, Massimo De Martinis, Lia Ginaldi.
Investigation: Maria Maddalena Sirufo.
Methodology: Maria Maddalena Sirufo, Massimo De Martinis, Lia Ginaldi.
Resources: Maria Maddalena Sirufo, Massimo De Martinis, Lia Ginaldi.
Validation: Lia Ginaldi, Massimo De Martinis, Maria Maddalena Sirufo.
Visualization: Massimo De Martinis, Maria Maddalena Sirufo, Lia Ginaldi.
Writing – original draft: Maria Maddalena Sirufo, Massimo De Martinis, Lia Ginaldi.
Writing – review & editing: Maria Maddalena Sirufo, Massimo De Martinis, Lia Ginaldi.
Footnotes
Abbreviations: AD = atopic dermatitis, ANA = antinuclear antibodies, ANCA = antineutrophil cytoplasmic antibodies, CRP = C-reactive protein, EASI = eczema area and severity index, ECP = eosinophil cationic protein, ENA = antiextractable nuclear antigens, ESR = erithrocyte sedimentation rate, L = lymphocytes, N = neutrophils, SCORAD = Scoring Atopic Dermatitis, WBC = white blood cells.
The authors have no conflicts of interest to disclose.
References
- [1].Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol 2017;139:S49–57. [DOI] [PubMed] [Google Scholar]
- [2].Nomura T, Honda T, Kabashima K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int Immunol 2018;doi: 10.1093/intimm/dxy015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [3].Forman SB, Garrett AB. Success of omalizumab as monotherapy in adult atopic dermatitis: case report and discussion of the high-affinity immunoglobulin E receptor, FcepsilonRI. Cutis 2007;80:38–40. [PubMed] [Google Scholar]
- [4].Fernández-Anton Martinez MC, Leis-Dosil V, Alfageme-Roldán F, et al. Omalizumab for the treatment of atopic dermatitis. Actas Dermosifiliogr 2012;103:624–8. [DOI] [PubMed] [Google Scholar]
- [5].Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol 2017;56:18–26. [DOI] [PubMed] [Google Scholar]
- [6].Velling P, Skowasch D, Pabst S, et al. Improvement of quality of life in patients with concomitant allergic asthma and atopic dermatitis: one year follow-up of omalizumab therapy. Eur J Med Res 2011;15:407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lane JE, Cheyney JM, Lane TN, et al. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol 2006;54:68–72. [DOI] [PubMed] [Google Scholar]
- [8].Babu KS, Polosa R, Morjaria JB. Anti-IgE—emerging opportunities for omalizumab. JExpert Opin Biol Ther 2013;13:765–77. [DOI] [PubMed] [Google Scholar]
- [9].Maurer M, Rosén K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 2013;368:924–35. [DOI] [PubMed] [Google Scholar]
- [10].Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014;70:338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stalder JF, Barbarot S, Wollenberg A, et al. PO-SCORAD Investigators Group. Patient-oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy 2011;66:1114–21. [DOI] [PubMed] [Google Scholar]
- [12].Bierber T. Atopic dermatitis. Ann Dermatol 2010;22:125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].D’Auria E, Banderali G, Barberi S, et al. Atopic dermatitis: recent insight on pathogenesis and novel therapeutic target. Asian Pac J Allergy Immunol 2016;34:98–108. [DOI] [PubMed] [Google Scholar]
- [14].De Martinis M, Sirufo MM, Ginaldi L. Allergy and aging: an old/new emerging health issue. Aging Dis 2017;8:162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Romano C, Sellitto A, De Fanis U, et al. Omalizumab for difficult-to-treat dermatological conditions: clinical and immunological features from a retrospective real-life experience. Clin Drug Investing 2015;35:159–68. [DOI] [PubMed] [Google Scholar]
- [16].Chang TW, Chen JB, Chu CY. The pharmacological mechanism of omalizumab in patients with very high IgE levels—clues from studies on atopic dermatitis. Dermatologica Sinica 2012;30:147–53. [Google Scholar]
- [17].Caruso C, Gaeta R, Valluzzi L, et al. Omalizumab efficacy in a girl with atopic eczema. Allergy 2009;65:278–9. [DOI] [PubMed] [Google Scholar]


