Abstract
Background
Haematologic malignancies cause significant morbidity and mortality and are not uncommon in resource-limited-low income countries. However, the types, pattern of presentation and treatment outcomes vary across regions. We assessed the presentation and overall survival over an 11-year period in adult patients presenting with haematologic cancers in Jos, North Central Nigeria
Materials and Methods
This retrospective outcome study evaluated patients who presented with haematologic malignancies between 2005–2015 at the Jos University Teaching Hospital (JUTH), Jos. Variables of interest were abstracted through chart reviews. Descriptive statistics were used to evaluate baseline and follow-up parameters. Overall survival (OS) was assessed using Kaplan-Meier method.
Results
Sixty patients, contributing 25,994 person-days of follow-up were evaluated. The mean age was 43+17 years and 61.7% were males. Thirty-one patients (51.7%) presented with leukemia, 45.0% with lymphoma, and 3.3% with multiple myeloma. Forty-two (70.0%) presented with advanced disease, 5 (5.2%) were HIV positive and 4 (6.7%) had died at the end of follow-up. OS was 84.3% (95% CI: 58.1–94.7). Survival differed by disease group (p=0.01) and having fever at presentation (p=0.02)
Conclusion
We found long-term OS to be impacted by disease type and status of fever at presentation. Disease-specific Strategies to improve early diagnosis and therapies are needed to ensure optimal outcomes in Nigerian patients.
Keywords: Blood cancers, Pattern, Adults, Long-term, Mortality
Background
Haematologic malignancies are malignant neoplasms arising from haemopoietic tissues. They comprise largely of malignancies like leukemia, lymphoma and multiple myeloma, of which leukaemia and lymphoma are the most common globally.1,2 Haematologic malignancies comprise a fifth of the most commonly occurring cancers and the second leading cause of cancer death.3,4 Leukaemia was the fifth most common cause of cancer deaths in men and the sixth most common in women in the U.S in a five-year study between 2007 and 2011.5 Haematologic malignancies were previously thought to be rare in north central Nigeria due to poor understanding of their pathogenesis and clinical presentations.2 No study has been conduct to understand the epidemiology of these diseases in Northern Nigeria. In most cases the aetiology remain largely unknown. However, several factors have been identified to increase the risk of developing these cancers, including immunosuppression or immunodeficiency states,6,7 infections with viruses such as human immunodeficiency virus (HIV), Epstein Barr Virus (EBV), human T- lymphotrophic virus (HTLV) and chronic bacterial infection like Helicobacter pylori [7]. These immune-related risk factors are thought to be involved in a complex interplay of genetic damage in somatic cells arising from mutations or other defects, cytokine dysregulation and chronic antigenic stimulation resulting in development of these malignancies.8 Presentations of these cancers are usually non-specific and may be influenced by associated factors like level of awareness of affected individuals, the type of the disease and other co-morbidities.9 The outcomes in haematologic malignancies can also vary by time of presentation, type of the disease, stage of the tumour at presentation, other co-morbidities, availability and quality of the access to medical care.10
The objective of this study was to determine the pattern of clinical presentation, and overall survival of adult patients seen with haematologic malignancies between 2005 and 2015 in JUTH, Jos, North Central Nigeria.
Materials and Methods
Study setting
JUTH is a tertiary institution located in Jos, the capital city of Plateau State, North-Central Nigeria. It is a 550-bed facility and serves five neighboring states within the region. It offers specialist haematological services on both in and outpatient basis. The patients in this study were referred from clinics within the hospital, and from health facilities within the region between January 2005 and December 2015. The Haematologists at the Department of hematology and blood transfusion managed the patients, where all the cases were recorded in the case registry.
Study Design
This was a retrospective cohort study to determine the clinical presentation, and overall survival of adult patients seen with haematologic malignancies at the JUTH over an eleven-year period.
Study Population
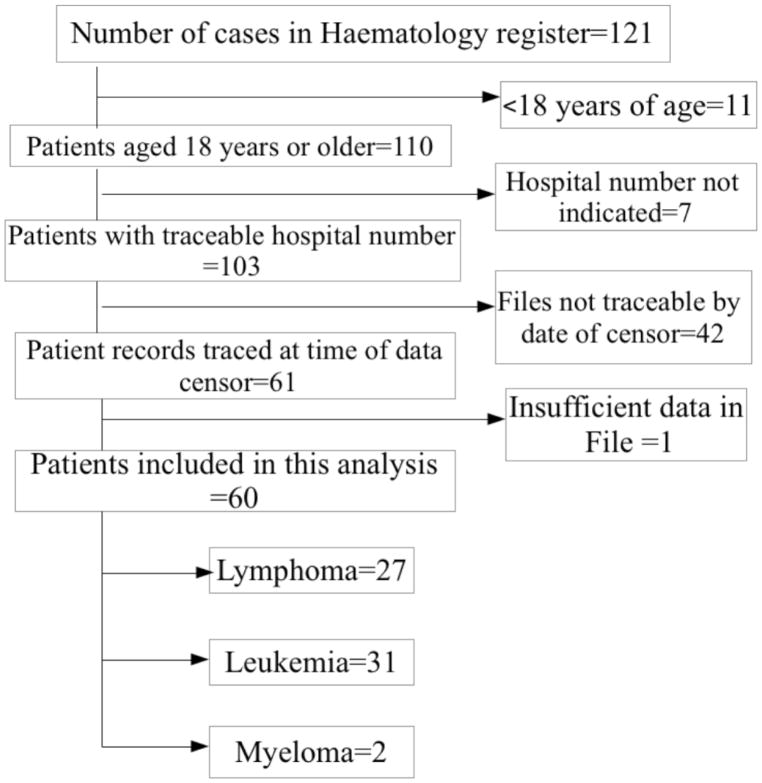
There were 121 cases recorded in the case register of the department of haematology and blood transfusion over the study period. The inclusion criteria were those on the register with documentation of diagnosis by bone marrow aspiration cytology or histology of tissue biopsy. Fifty-nine cases were excluded from this analysis for failure to meet the inclusion criteria as indicated in the flow chart (Figure 1).
FIGURE 1.
FLOW CHART OF PATIENTS INCLUDED IN THE STUDY
Data collection
Chart reviews were conducted and data were captured on a designed template. Information extracted included socio-demographic parameters, clinical features, haematologic parameters, histologic/cytological diagnosis, and disease staging. Additionally, information on date of diagnosis, treatment status, and regimen received were captured. HIV status and baseline haematological parameters were also abstracted. For patients who received chemotherapy, treatment was classified into single-agent alkylator therapy (+/− steroid), non-purine analogue-alkylating agent based combination, antibody-only or other therapy. Time spent in follow-up (days) was calculated from the date of histologic/cytological confirmation of disease until the end of follow-up (31st December 2015) or the occurrence of death (all-cause mortality), which was the outcome of interest as documented in the case files. We used the following definitions to assess baseline haematological parameters: 1) anaemia-defined as haematocrit <30%, 2) leucocytosis-defined as total white cell count >10 x109 /L, 3) thrombocytopaenia as absolute platelet count <90 x109 /L. The haematologic malignancies were classified into three groups namely lymphoma, leukaemia and myeloma. We further grouped these conditions into early or late stage as follows; for chronic myeloid leukaemia (CML), early stage disease was chronic phase while late disease was either accelerated or acute phase or blastic transformation. For chronic lymphocytic leukaemia (CLL), early disease was Binet A while late disease was Binet B or C. For lymphoma, early disease was stage IA or IIA while late disease was stage IIB, III or IV (Ann-Abor classification).
Statistical analysis
Descriptive statistics was used to present baseline data and they were expressed as proportions, mean (±SD), and median (IQR). The burden of haematologic malignancies was estimated using the number of all cancers that presented at JUTH during the same period as reference population. Bivariate analysis was used to describe the association with socio-demographic features and burden of haematologic malignancies in JUTH. Outcome was assessed based on status of patients, whether alive or dead at the last visit or follow up. Overall survival was assessed using Kaplan-Meier method. STATA version 13.1 software package (STATA Corp, College Station, Texas) was used for the statistical analysis. A p-value < 0.05 was considered significant.
Results
Sixty (60) patients with haematologic malignancies were included in this study. The mean age of the study population was 43±17 years. Majority (61.7%) were males and most (86%) of the patients had received formal education. About two-thirds (65.5%) were married and 50.8% were employed at the time of presentation (Table 1). Majority (71.2%) of the patients presented late and the main clinical features they presented with were lymph node enlargement (63.2%), fever/pyrexia (48.3%), splenomegaly (47.4%) and hepatomegaly (42.1%).
Table 1.
Socio-demographic Characteristics of adults with Haematologic malignancies seen at the Jos University Teaching Hospital, Nigeria between 2005 and 2015 (n=60)
| Socio-demographic
characteristics. | |
|---|---|
| Variable | n (%) |
| Mean Age, years | 43+17 |
| Sex | |
| Female | 23 (38.3) |
| Male | 37 (61.7) |
| Education attained | |
| No formal education | 7 (14.0) |
| Primary | 7 (14.0) |
| Secondary | 17 (34.0) |
| Tertiary | 19 (38.0) |
| Marital status | |
| Single | 15 (25.9) |
| Married | 38 (65.5) |
| Widowed | 4 (6.9) |
| Divorced | 1 (1.7) |
| Occupation | |
| Unemployed | 20 (33.9) |
| Self-employed | 30 (50.8) |
| Civil servant | 9 (15.3) |
| Clinical characteristics | |
| Disease group | |
| Leukemia | 31 (51.7) |
| Lymphoma | 27 (45.0) |
| Myeloma | 2 (3.3) |
| Disease stage | |
| Early | 17 (28.8) |
| Advanced | 42 (71.2) |
| Presenting features | |
| Lymph node enlargement | 36 (63.2) |
| Fever/Pyrexia | 28 (48.3) |
| Splenomegaly | 27 (47.4) |
| Hepatomegaly | 24 (42.1) |
| Abdominal mass | 12 (21.1) |
| Epistaxis | 3 (5.4) |
| Gum bleeds | 2 (3.6) |
| Laboratory indices | |
| Median Haematocrit (IQR) | 28 (21–35) |
| Median TWCC (IQR) | 30,000 (6000–110,000) |
| Median platelet (IQR) | 204,500 (110,000–350,000) |
| Median neutrophils (%)* | 40 (18–59) |
| Median lymphocytes (%)* | 31.5 (12–69) |
| Proportion with abnormal laboratory values | |
| Anaemia | 30 (50.0) |
| Leucocytosis | 33 (55.0) |
| Thrombocytopaenia | 9 (15.0) |
| HIV status | |
| Positive | 3 (5.2) |
| Negative | 33 (56.9) |
| Unknown | 22 (37.9) |
| Follow up data | |
| Median follow-up time,(days), IQR | 182 (50–737) |
| Person-time contributed (days) | 25, 994 |
| Received cytotoxic therapy (%) | 50 (86.2) |
| Regimen received (%): | |
| Non-purineanalogue-alkylating agent based combination | 19 (38.0) |
| Single-agent alkylator therapy (+/− steroid) | 16 (32.0) |
| Antibody or other therapy | 15 (30.0) |
| Outcome (%) | |
| Alive | 56 (93.3) |
| Died | 4 (6.7) |
| Overall survivial (95% CI) | 84.3 (58.1–94.7) |
TWCC-Total white cell count
Subset of TWCC
NB: Compared to patients alive at the time of censoring data, patients who died were similar in age (p=0.84), sex (p=0.53) disease stage (p=0.20) and Haematocrit status (p=0.85), platelet counts (p=0.85) and follow-up time (p=0.62)
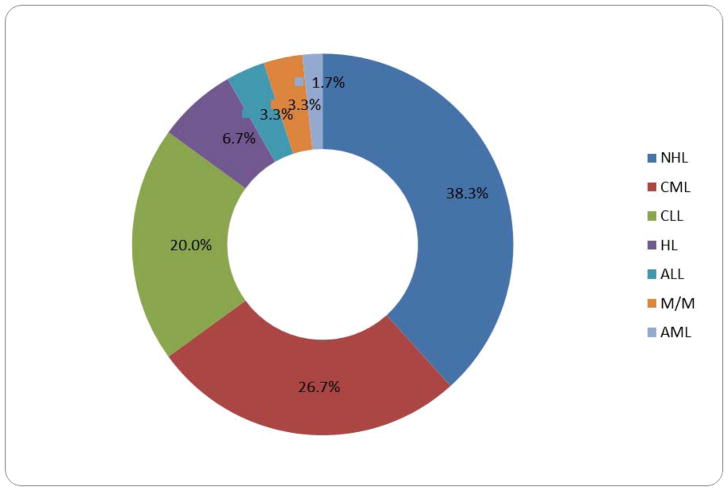
Haematologic malignancies constituted 4.4% of all malignancies at JUTH within the study period. The annual burden of haematologic malignancies was 0.4%. Among the haematologic malignancies, the highest burden was for leukeamia (51.7%), and then lymphoma (45.0%), while multiple myeloma made up only 3.3%. The distribution of histologic diagnosis of the various malignancies is shown in Figure 2. Non-Hodgkins lymphoma accounted for 38.3% of histologic diagnoses, followed by CML (26.7%) and CLL (20.0%). Other histologic types occurred in lower frequencies as shown in the doughnut plot.
FIGURE 2.
DOUGHNUT PLOT OF DISTRIBUTION OF CASES OF HAEMATOLOGIC MALIGNANCIES BY HISTOLOGIC TYPE IN ADULTS SEEN AT THE JOS UNIVERSITY TEACHING HOSPITAL, NIGERIA BETWEEN 2005 AND 2015 (n=60)
With regards to cytotoxic therapy, 86.2% received chemotherapy; comprising of non-purine analogue-alkylating agent-based combination in 38%, single-agent alkylator therapy (+/− steroid) in 32% and antibody-only or other therapy in 30% of those that accessed specific treatment.
The median follow-up time for the whole cohort was 182 (IQR: 50–737) days. Patients with leukaemia had shorter median follow-up periods (75, IQR: 21–382) compared to patients with lymphoma (398, IQR: 103–1221) and multiple myeloma (161, IQR: 73–249), p=0.03
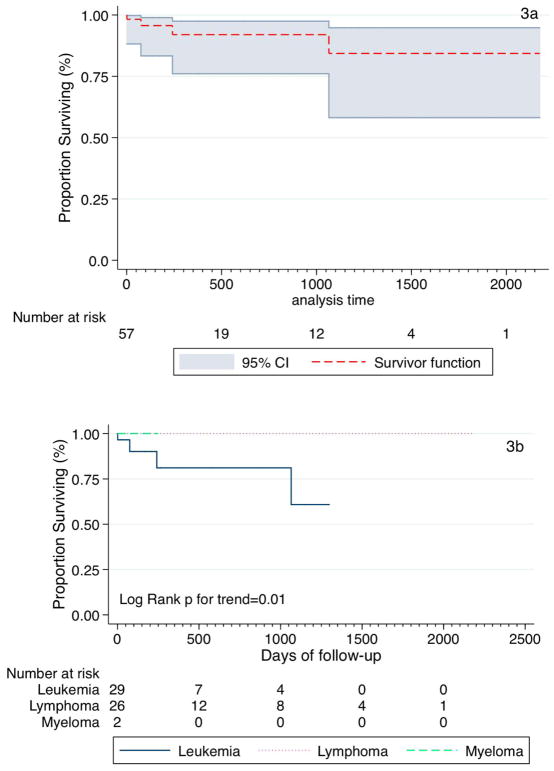
Fifty-seven of the 60 patients had complete time-updated data and contributed 25, 994 days of follow-up at the end of the observation period. Overall survival was 84.3%; 95% CI: 58.1–94.7% (Figure 3a). When survival was stratified by socio-demographic and clinical variables survival function differed only with disease group (p=0.01, Figure 3b) and fever (p=0.02), with age achieving borderline significance (Table 2).
FIGURE 3.
KAPLAN-MEIER ESTIMATES OF OVERALL SURVIVAL (A) AND SURVIVAL BY DISEASE GROUP (B)
Table 2.
Stratified Survival Analysis by Socio-demographic Characteristics (n=60)
| Variable | SF | 95% CI | p-value |
|---|---|---|---|
| Overall survival | 84.3 | 58.1–94.7 | |
| Sex | 0.10 | ||
| Female | 88.8 | 43.3–92.3 | |
| Male | 79.5 | 39.5–94.5 | |
| Age, years | 0.05 | ||
| 18–64 | 90.0 | 70.8–96.8 | |
| ≥65 | 50.0 | 60.0–91.0 | |
| Disease group* | 0.01 | ||
| Leukemia | 60.8 | 17.6–86.7 | |
| Lymphoma | 1.00 | - | |
| Myeloma | 1.00 | - | |
| Disease stage | 0.17 | ||
| Early | 1.00 | - | |
| Late | 77.2 | 43.3–92.3 | |
| Fever | 0.02 | ||
| Yes | 62.6 | 18.1–87.9 | |
| No | 1.00 | - | |
| Splenomegaly | 0.71 | ||
| Yes | 96.0 | 74.8–99.4 | |
| No | 78.8 | 43.0–93.5 | |
| Hepatomegaly | 0.54 | ||
| Yes | 88.2 | 59.0–97.1 | |
| No | 81.6 | 41.9–95.3 | |
| Lymphadenopathy | 0.71 | ||
| Yes | 87.6 | 65.1–96.0 | |
| No | 75.0 | 12.7–96.0 | |
| Abdominal mass | 0.65 | ||
| Yes | 91.6 | 53.9–98.7 | |
| No | 82.5 | 51.1–94.6 | |
| HIV status | 0.87 | ||
| Positive | 1.00 | - | |
| Negative | 87.4 | 64.9–95.9 | |
| Unknown | 66.6 | 54.1–94.5 | |
| Anaemia | 0.86 | ||
| Yes | 86.2 | 54.6–96.4 | |
| No | 82.6 | 37.7–96.3 | |
| Thrombocytopaenia | 0.43 | ||
| Yes | 66.6 | 5.4–94.5 | |
| No | 86.0 | 56.2–96.3 | |
| Leucocytosis | 0.35 | ||
| Yes | 74.2 | 36.2–91.5 | |
| No | 96.0 | 74.8–99.4 | |
| Cytotoxic treatment | 0.28 | ||
| Yes | 84.6 | 55.1–95.4 | |
| No | 87.5 | 38.7–98.1 |
SF-Survivor function
CI-Confidence interval
p-value: for Log-Rank test
Log Rank for trend of SF (3 or more groups)
Discussion
Haematologic malignancies are not as rare in our environment as previously thought. These conditions are responsible for significant morbidity and mortality in individuals affected. In this study, they represented 4.4% of all cancer cases presenting in JUTH within the period under review and an annual burden of 0.4%. This burden of disease observed was lower than that reported by Kagu et al in Maiduguri, North Eastern Nigeria where an overall burden of 6.05% was reported.11 The burden in our study was also lower than that reported from a study in Benin, South Southern Nigeria where 17.4% burden was observed.12
Whereas non-Hodgkin’s lymphoma was the most frequent type of haematologic malignancy seen in the studies from Maiduguri and Benin, Nigeria,11,12 the most frequent haematologic malignancy observed in our study was leukaemia. This has confirmed previous findings of geographical variation in the pattern of haematologic malignancies even within the same country.8,13 The finding of leukaemia as the most frequently occurring haematologic malignancy in this study was however similar to the report by Idris et al in Abbottabad, Pakistan14 and in another study from Bangladesh.15 The mean age at presentation in this study was 47±17 years. Our patients presented at an older age compared with the patients in a Pakistani study that presented at a younger age of 22.5 years.14 The age at presentation in our patients is however similar to that described in patients from an Ethiopian study.16 A male preponderance was observed in this study wherein the male patients constituted 61.7% of the cases while the females constituted only 38.3% of patients giving a male to female ratio of 1.6:1. This is in agreement with previous studies that reported the tendency of haematologic malignancies to occur more frequently in males than females.11, 17–18
The spectrum of clinical presentation in patients with haematologic malignancies varies depending on the type and stage of disease at which diagnosis was made. While some patients are asymptomatic, others present with symptoms attributable to effects of cytopaenia and/or organ enlargement from infiltration with malignant cells. In this study, majority of the patients presented with lymph node enlargement. This is at variance with some studies in which the commonest features at presentation were non-specific features of cytopaenias (fatigue, fever and bleeding tendency).14,16 Other less frequent features like splenomegaly and hepatomegaly seen in our patients have also been described in a study among aviators in India.19 In addition, non-specific features including fever and bleeding tendencies which were described in patients from other studies were also seen in our patients. The most frequent abnormal laboratory finding in our patients in this study was leucocytosis. This was contrary to the finding reported in a study from Nepal in which 90% of the patients with haematologic malignancies presented with cytopaenias such as bicytopaenia and pancytopaenia.20 Cytopaenias are reflection of the extent of bone marrow inadequacy resulting from infiltration of the bone marrow with the malignant cells. Leucocytosis was commonly seen among patients in this study and was closely followed by anaemia, a manifestation of cytopaenia. Thrombocytopaenia was not a frequent presentation in our patients as reported elsewhere.20
In this study, 5.2% of the patients were positive for human immunodeficiency virus (HIV). All those with a positive HIV status were found in the lymphoma group. This prevalence of HIV was higher than that reported in a study done in Ile-Ife, Southwest Nigeria among lymphoma patients in which 2.3% of the patients were HIV positive,21 but lower than the reported 26.5% positive HIV status in another study from Cameroon.22 The difference in HIV positive status may be explained by regional variation of HIV prevalence in the general population. The finding of HIV positive status among the lymphoma cohort confirmed previous reports of infections with viruses such as HIV as risk factor for the development of lymphoma.7,23 Interestingly, no patient with leukaemia, which had the highest burden for haematologic malignancies in our study was HIV positive.
Late presentation was also observed among this cohort of patients, with 70.0% presenting with advanced stages of their disease. Patients with malignancies in resource-limited settings have been documented to present late. In a recent study of women with invasive cervical cancer from this centre, 87.5% of the cases presented with advanced disease.24 A study on AIDS-related lymphoma conducted in southwest Nigeria also documented majority of the patients presenting in advanced stages of the disease.21 The reasons for late presentation of haematologic malignancies in our setting are multi-factorial, and ranges from patient factors, to delays in referral as well as paucity of adequate screening, diagnostic and therapeutic facilities.10, 25–27
We found overall 11-year survival to be high in our cohort, with differences in survival rate by disease group. Patients with leukaemia had the lowest probability of survival compared to those with lymphoma and multiple myeloma. The factors that impact on cancer survival are usually clinical and disease related but a recent publication on survival in haematologic cancers among young persons reported social factors having a negative impact on cancer survival.28–29
A key strength of our study is the fact that we reported on 11-year survival among patients with haematologic malignancies in a resource-limited setting and found overall survival to be over 80%. However, our study had some limitations. Firstly, the use of routine clinical data resulted in our inability to assess additional outcome measures (like rates of remission and disease free survival) due to missing/incomplete data. This limited our survival analysis to overall survival alone. We were however able to compare patients who were excluded due to missing data and those included in our final analyses and found them to have similar socio-demographic and disease characteristics. A prospective cohort will enable us fill in the gaps experienced in this study. Secondly, we were limited by the use of institutional data as there are no local cancer registries that would have enabled us compare trends in incidence and outcome. We do believe however that because the patients presented in this study were drawn from multiple centres/sites, our results are a fair representation of overall survival and can be generalized to our setting. Thirdly, our classification of the various cancers into three broad groups (leukaemia, lymphoma and myeloma) may have resulted in under and or over estimation of survival rates for some of the patients. Using grouping by histologic type may have been better.
In conclusion, this study has shown that haematologic malignancies are not uncommon in our environment and that overall survival is over 80%, even in the face of limited cancer management resources. We also observed similar disease patterns as have been previously documented in prior studies. The findings of this study can be used as a guide to plan future prospective studies that will enable the collection of more comprehensive data which will speak to some of the limitations encountered in this study and enable better planning and implementation of cancer prevention and treatment programs.
Acknowledgments
We are grateful to the patients who contributed data to this study and the staff of the health records department for case file retrieval.
Funding
This study was supported by Northwestern University and Jos University Research Training Program in HIV and Malignancies (award number D43TW009575) from the Fogarty International Center, National Institutes of Health (NIH), USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes for Health.
References
- 1.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from Haematological Malignancy Research Network. British J Cancer. 2011;105:1684–1692. doi: 10.1038/bjc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandong BM, Madaki AKJ, Mannaseh AN. Malignant diseases in Jos: a follow up. Ann Afr Med. 2003;2(2):49–53. [Google Scholar]
- 3.Fitzmaurice C, Allen C, Barber RM, et al. for Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns R, Leal J, Sullivan R, Luengo-Fernandez R. Economic burden of malignant blood disorders across Europe: a population-based cost analysis. Lancet Haematol. 2016;3(8):e362–370. doi: 10.1016/S2352-3026(16)30062-X. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J, Pivodic L, Miccinesi G, et al. International study of the place of death of people with cancer; a population-level comparison of 14 countries across 4 continents using death certificate data. British J Cancer. 2015;113:1397–1404. doi: 10.1038/bjc.2015.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann L, El-Haddad A, Barr RD. Global Approach to Hematologic Malignancies. Hematol Oncol Clin North Am. 2016;30(2):417–432. doi: 10.1016/j.hoc.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Park LS, Tate JP, Rodriguez-Barradas MC, et al. Cancer incidence in HIV-infected versus uninfected veterans: comparison of cancer registry and ICD-9 diagnoses. J AIDS Clin Res. 2014;5(7):1000318. doi: 10.4172/2155-6113.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamid GA. The pattern of haematological malignancies at the Al-Gamhouria Teaching Hospital, Aden, Yemen, from 2008 to 2010. Turk J Hematol. 2012;29:342–347. [Google Scholar]
- 9.Savage DG, Szydlo RM, Goldman JM. Clinical features at presentation in 340 patients with chronic myeloid leukaemia seen at a referral Centre over a 16-year period. British J Haematol. 1997;96:111–116. doi: 10.1046/j.1365-2141.1997.d01-1982.x. [DOI] [PubMed] [Google Scholar]
- 10.Abel GA, Friese RC, Magazu LS, et al. Delays in referral and diagnosis for chronic hematologic malignancies: A literature review. Leukemia and Lymphoma. 2008;49(7):1352–1359. doi: 10.1080/10428190802124281. [DOI] [PubMed] [Google Scholar]
- 11.Kagu MB, Ahmed SG, Bukar AA, Mohammed AA, Mayun AA, Musa AB. Spectrum of haematologic malignancies and survival outcomes of adult lymphomas in Maiduguri, northeastern Nigeria-a fourteen-year review. Afr J Med Med Sci. 2013;42(1):5–14. [PubMed] [Google Scholar]
- 12.Nwannadi IA, Alao OO, Bazuaye GN, Halim NKO. The Epidemiology of Haematological Malignancies at the University of Benin Teaching Hospital: A Ten-Year Retrospective Study. The Internet Journal of Epidemiology. 2010;9(2) doi: 10.5580/1fbb. [DOI] [Google Scholar]
- 13.Babatunde AS, Amiwero CE, Olatunji PO, Durotoye IA. Pattern of Haematological malignancies in Ilorin, Nigeria: Ten year review. Internet J Haematol. 2009;5(2) doi: 10.5580/28cc. [DOI] [Google Scholar]
- 14.Idris M, Shah SH, Fareed J, Gul N. An experience with sixty cases of haematological malignancies; a clinic haematological correlation. J Ayub Med Coll Abbottabad. 2004;16(4):51–54. [PubMed] [Google Scholar]
- 15.Hossain MS, Iqbal MS, Khan MA, Rabbani MG, Khatun H, Munira S, et al. Diagnosed haematological malignancies in Bangladesh – a retrospective analysis of over 5000 cases from 10 specialized hospital. BMC Cancer. 2014;14:438. doi: 10.1186/1471-2407-14-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weldetsadik AT. Clinical characteristics of patients with haematological malignancies at Gondar University hospital, North West Ethiopia. Ethiop Med J. 2013;51(1):25–31. [PubMed] [Google Scholar]
- 17.Obafunwa JO, Akinsete I. Malignant lymphoma in Jos, Nigeria: A ten-year study. Central Afr J Med. 1992;38(1):17– 25. [PubMed] [Google Scholar]
- 18.Williams CKO. Neoplastic diseases of the haemopoietic system in Ibadan: preliminary report of a prospective study. Afr J Med Sci. 1985;14:89–94. [PubMed] [Google Scholar]
- 19.Ganjoo RK, Chadha DS, Sharma SK, Kasthuri AS. Spectrum of hematological malignancies in aviators-A clinical series. Ind J Aerospace Med. 2009;53(1):45–51. [Google Scholar]
- 20.Jha A. Spectrum of hematological malignancies and peripheral cytopenias. J Nepal Health Res Counc l. 2013;1(25):273–278. [PubMed] [Google Scholar]
- 21.Bolarinwa RA, Ndakotu MA, Oyekunle AA, Salawu L, Akinola NO, Durosinmi MA. AIDS-Related Lymphomas in Nigeria. Brazilian Journal of Infectious Diseases. 2009;13(5):359–361. doi: 10.1590/S1413-86702009000500009. [DOI] [PubMed] [Google Scholar]
- 22.Mbanya DN, Minkoulou EM, Fezeu L, Kaptue L. Impact of HIV-1 infection on survival in patients with haematological malignancies in Yaoundé, Cameroon. Trop Doct. 2007;37(3):151–152. doi: 10.1258/004947507781524836. [DOI] [PubMed] [Google Scholar]
- 23.American Cancer Society. Global Cancer Facts & Figures. 3. Atlanta: American Cancer Society; 2015. pp. 24–26. [Google Scholar]
- 24.Musa J, Nankat J, Achenbach CJ, et al. Cervical cancer survival in a resource- limited setting-North Central Nigeria. Infectious Agents and Cancer. 2016;11:15. doi: 10.1186/s13027-016-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel-Wahab M, Bourque JM, Pyanda Y, et al. Status of radiotherapy resources in Africa: an international atomic energy agency analysis. Lancet Oncol. 2013;14:e168–175. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- 26.Kingham TP, Alatise OI, Vanderpuye V, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013;14:e158–167. doi: 10.1016/S1470-2045(12)70472-2. [DOI] [PubMed] [Google Scholar]
- 27.Gopal S, Wood WA, Lee SJ, et al. Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood. 2012;119:5078–5087. doi: 10.1182/blood-2012-02-387092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efficace F, Cartoni C, Niscola P, et al. Predicting survival in advanced hematologic malignancies: do patient-reported symptoms matter? Eur J Haematol. 2012;89(5):410–416. doi: 10.1111/ejh.12004. [DOI] [PubMed] [Google Scholar]
- 29.Borate U, Mineishi S, Costa LJ, et al. Non-biological factors affecting survival in younger patients with acute myeloid leukemia. Cancer. 2015 doi: 10.1002/cncr.29436. [DOI] [PubMed] [Google Scholar]