Abstract
Objective(s):
One in four persons living with HIV is coinfected with HCV. Biological and behavioral mechanisms may increase HIV viral load among coinfected persons. Therefore, we estimated the longitudinal effect of chronic HCV on HIV suppression after ART initiation among women with HIV (WWH).
Design:
HIV RNA was measured every six months among 441 WWH in the Women’s Interagency HIV Study who initiated ART from 2000–2015.
Methods:
Log-binomial regression models were used to compare the proportion of study visits with detectable HIV RNA between women with and without chronic HCV. Robust sandwich variance estimators accounted for within-person correlation induced by repeated HIV RNA measurements during follow-up. We controlled for confounding and selection bias (due to loss to follow-up and death) using inverse probability-of-exposure-and-censoring weights
Results:
One hundred fourteen women (25%) had chronic HCV before ART initiation. Overall, the proportion of visits with detectable HIV RNA was similar among women with and without chronic HCV (RR: 1.19 (95% CI 0.72, 1.95)). Six months after ART initiation, the proportion of visits with detectable HIV RNA among women with chronic HCV was 1.88 (95% CI 1.41, 2.51) times that among women without HCV, at two years the ratio was 1.60 (95% CI 1.17, 2.19), and by six years there was no difference (1.03; 95% CI 0.60, 1.79).
Conclusions:
Chronic HCV may negatively impact early HIV viral response to ART. These findings reaffirm the need to test persons with HIV for HCV infection, and increase engagement in HIV care and access to HCV treatment among persons with HIV/HCV-coinfection.
Keywords: hepatitis C virus, HIV infection, HCV coinfection, viral load, antiretroviral therapy, women
INTRODUCTION
One in four persons living with HIV in the United States (US) is coinfected with HCV [1]. Quantifying the effect of HCV on HIV suppression is crucial. HIV suppression improves clinical outcomes and reduces HIV transmission [2,3]. Yet, biological and behavioral mechanisms may increase HIV viral load among people with HIV/HCV-coinfection. Common HIV coinfections, such as tuberculosis and herpes simplex virus, type 2, increase HIV viral replication [4–6]. Ongoing alcohol and drug use and decreased ART adherence among coinfected persons may also result in increased HIV viral load [7, 8].
Published studies suggest that persons with HIV/HCV-coinfection do not experience increased time to HIV suppression after ART initiation [9–17] but HCV may be associated with earlier failure of HIV viral control [18]. However, there are several limitations in studies quantifying the effect of HCV on HIV suppression after ART is begun. Many studies that found no association between HCV and HIV suppression did not distinguish between persons with chronic HCV (i.e., seropositive with detectable HCV RNA) and those who cleared infection (i.e., seropositive but RNA negative) [9–14,18]; misclassification of HCV status could result in biased associations. Also, some studies did not account for potential confounding of the relationship between HCV and HIV suppression by alcohol or drug use [11,17].
Evidence regarding the effect of HCV on HIV suppression after ART initiation is especially lacking among women. Women respond differently to ART than men [19–23], often exhibiting more favorable immune responses [21–23] but experiencing greater drug toxicity [20] and more frequent treatment discontinuation [19]. To date, there is only one published study assessing the association between HCV and HIV suppression after ART initiation among women [24]. That study did not find an association between HCV status and HIV suppression, but did not distinguish between women with chronic HCV and women who cleared infection, and follow-up was limited to one-year [24]. The effect of HCV on HIV viral response to ART among women warrants further investigation.
The objective of this study was to estimate the longitudinal effect of chronic HCV on HIV suppression after ART initiation among women for up to 15 years. We hypothesized that women with chronic HCV would be more likely to have detectable plasma HIV RNA than women without HCV during the follow-up period.
METHODS
Study population
We used data collected by the Women’s Interagency HIV Study (WIHS) for this analysis. A full description of the WIHS cohort is provided elsewhere [25,26]. Briefly, the WIHS is a prospective cohort of women living with and at risk for HIV, with follow-up at six-month intervals. At each visit, interviewer-administered questionnaires capture extensive medical, psychosocial, and drug use information. ART use is defined as three or more antiviral medications approved by contemporaneous DHHS guidelines [27]. Between 1994 and 2014, 4,982 women enrolled in the WIHS across ten study sites; 3,677 (74%) were living with HIV at enrollment and 24 (0.5%) acquired HIV during follow-up.
Women with HIV who initiated ART on or after January 1, 2000, and after they were enrolled in the WIHS, were eligible for the present analyses. Women who initiated ART prior to 2000 were excluded to allow assessment of HIV suppression with relatively modern ART regimens. Of the 640 eligible women, we excluded 100 (16%) who did not have HIV RNA measured prior to ART initiation, 29 (4%) without at least one HIV RNA measurement after initiating ART, and 23 (4%) missing HCV infection status. We also excluded 52 HCV-uninfected women less than 30 years of age at ART initiation. There were no women with chronic HCV who were less than 30 years of age at ART initiation and we did not want to bias our findings by extrapolating to women who did not exist in our data [28]. Therefore, the final study sample included 441 women. Demographic and clinical characteristics were similar between women included and excluded from our study, including the proportions with chronic HCV. However, excluded women were less likely to be Black or have liver fibrosis, and more likely to have initiated ART between 2000– 2005.
Chronic HCV was defined as the presence of HCV antibody and HCV RNA prior to ART initiation. HCV-seropositive women with undetectable HCV RNA were classified as HCV-uninfected. In the WIHS, women are screened for HCV with enzyme immunoassays at one of the first three study visits after enrollment. Chronic infection is confirmed with HCV RNA among HCV-seropositive women. The average length of time between HCV testing and ART initiation was six years among participants and we were concerned that chronic HCV may be misclassified due to spontaneous HCV clearance, antiviral treatment, or incident HCV infections during this time. Therefore, we supplemented HCV results with follow-up HCV test results (available for over 80% of women) and HCV treatment initiation. Eleven women who were HCV-uninfected at enrollment had evidence of HCV antibodies and RNA prior to ART initiation and were classified as HCV-infected. Two women classified as HCV-infected at enrollment were reclassified as HCV-uninfected due to subsequent negative RNA tests. Lastly, four women reported HCV treatment prior to ART initiation. Three of the four women had HCV RNA testing performed after ART initiation and were still HCV-infected; we did not change their status. The fourth woman had undetectable HCV RNA before ART initiation and was reclassified as HCV-uninfected.
Our outcome of interest was detectable HIV RNA at each study visit after ART initiation. We defined detectable HIV RNA as >80 copies/mL to accommodate assays used during the study period, which had lower limits of detection ranging from 20–80 copies/mL.
Covariate assessment
Baseline covariates, measured at the last study visit prior to ART initiation, included age, race, year of ART initiation, history of injection drug use, CD4 cell count, HIV RNA and liver fibrosis. Age was included as a continuous variable using restricted quadratic splines with three knots placed at the 5th, 50th, and 95th percentiles [29]. Race was dichotomized as Black or non-Black and history of injection drug use was included as yes/no. Because our sample size was <500, we categorized several continuous variables: year of ART initiation, baseline CD4 cell count, and baseline HIV RNA. Year of ART initiation was considered a proxy measure for ART regimen and categorized as 2000–2005, 2006–2010, and 2011–2015. Baseline CD4 cell counts were grouped as <200, 200–499, ≥500 cells/μL, and HIV RNA was categorized as ≤4000, 4001–10,000, and >10,000 copies/mL. Liver fibrosis was assessed by the FIB-4 index; scores were dichotomized as >1.45 and ≤1.45 to differentiate women with and without significant fibrosis [30–32].
We considered two time-varying covariates as predictors of loss to follow-up or death: alcohol and drug use. We categorized alcohol use as 0, 1–7, and >7 drinks per week since prior study visit. Women were categorized as current drug users if they reported using any illicit or recreational drugs since their last study visit. Time-varying covariates were missing for 4% of follow-up visits; these were replaced with values carried forward from the previous visit.
Statistical methods
We used a log-binomial regression model to estimate the proportion of study visits with detectable HIV RNA during follow-up, and compared women with and without chronic HCV. Robust sandwich variance estimators were used to account for within-person correlation induced by repeated HIV RNA measurements over the follow-up period. We also planned a priori to include a product term between HCV status and time since ART initiation in a second log-binomial model. Therefore, we could assess whether the relationship between chronic HCV and detectable HIV RNA was a function of duration of ART.
Women were followed from the first study visit after ART use until the last visit prior to September 30, 2015, the last possible date that data were available. Women were right-censored at the earliest occurrence of loss to follow-up, death, or last visit prior to September 30, 2015. Loss to follow-up was defined as two consecutively missed study visits. We also censored women with chronic HCV who initiated HCV treatment and HCV-uninfected women who had evidence of HCV antibody and HCV RNA during follow-up.
To control for confounding, we used time-fixed inverse probability-of-exposure weights [33,34]. These weights are fully described in the Appendix. Logistic regression was used to estimate the weights and included age, race, year of ART initiation, FIB-4, baseline HIV RNA, baseline CD4 cell count, and history of injection drug use.
We created time-varying inverse probability-of-censoring weights to account for right-censoring due to death or loss to follow-up [35,36] (see Appendix). Pooled logistic regression models were used to estimate the censoring weights. The models included time, HCV status, race, age, and time-varying alcohol and drug use. Time was measured as the number of semiannual study visits since ART initiation and included in the models using restricted quadratic splines with knots at the 5th, 50th, and 95th percentiles [29].
Robust variance estimates tend to overestimate the variability of inverse probability weighted estimators. We calculated 95% confidence intervals (CIs) for the weighted ratio measures using a nonparametric bootstrap with 200 resamples with replacement.
Sensitivity Analyses
We conducted several sensitivity analyses to assess the robustness of our findings and reduce possible bias in our estimated effects of chronic HCV on HIV RNA after ART initiation. In the first, we excluded women who reported single or dual antiretroviral use prior to ART initiation. For the second, we only included women who self-reported > 95% adherence to ART during the first six months after initiation. This analysis allowed us to determine if the estimated effect of chronic HCV on HIV RNA could be explained by poorer ART adherence among women with chronic HCV. In the third sensitivity analysis, we reclassified women who were HCV-seropositive with undetectable HCV RNA to HCV-infected, as these women may share some of the same behaviors that lead to poorer control of HIV.
All data analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, US). The analysis was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
RESULTS
Of 441 women, 114 (26%) had chronic HCV before ART initiation (Table 1). The median age was 47 years (interquartile range [IQR] 42, 51) among women with chronic HCV and 41 years (IQR 36, 47) among women without HCV. Two-thirds (67%) of all women were Black and approximately one-third (34%) had less than a high school education. Three-quarters of women had previously used illicit drugs (75%) and the majority of women with chronic HCV (87%) had previously injected drugs. Nine percent of women with chronic HCV were actively injecting drugs at baseline and 1% of women without HCV were.
Table 1.
Baseline characteristics of 441 HIV-infected women initiating ART by HCV status, Women's Interagency HIV Study, January 2000-September 2015.
| HCV+ (n=114) |
HCV- (n=327) | Total (n=441) | ||||
|---|---|---|---|---|---|---|
| Median or n |
IQRa or % | Median or n | IQRa or % | Median or n | IQRa or % | |
| Age at ART initiation (years) |
47 | 42, 51 | 41 | 36, 47 | 43 | 37, 49 |
| Race/ethnicity |
||||||
| White, non-Hispanic |
18 | 16% | 36 | 11% | 54 | 12% |
| Black, non-Hispanic |
77 | 68% | 217 | 66% | 294 | 67% |
| Other b |
19 | 17% | 74 | 23% | 93 | 21% |
| Education c |
||||||
| Less than high school |
48 | 42% | 103 | 32% | 151 | 34% |
| Graduated high school |
29 | 25% | 109 | 33% | 138 | 31% |
| Some college |
37 | 32% | 114 | 35% | 151 | 34% |
| Annual household income <$12,000 |
85 | 77% | 163 | 51% | 248 | 56% |
| Alcohol use d |
||||||
| 0 drinks per week |
60 | 53% | 150 | 46% | 210 | 48% |
| 1 – 7 drinks per week |
32 | 28% | 135 | 41% | 167 | 38% |
| >7 drinks per week |
22 | 19% | 42 | 13% | 64 | 15% |
| Ever used non-injection drugs |
109 | 96% | 221 | 68% | 330 | 75% |
| Ever injected drugs |
99 | 87% | 35 | 11% | 134 | 30% |
| Chronic active HBV e |
1 | <1% | 8 | 2% | 9 | 2% |
| Year of ART initiation f |
||||||
| 2000–2005 |
80 | 70% | 161 | 49% | 241 | 55% |
| 2006–2010 |
28 | 25% | 70 | 21% | 98 | 22% |
| 2011–2015 |
6 | 5% | 96 | 30% | 102 | 23% |
| Single or dual antiretroviral use g |
57 | 50% | 92 | 28% | 149 | 34% |
| CD4 cell count at baseline |
287.5 | 172, 392 | 313 | 194, 454 | 303 | 188, 440 |
| HIV viral load at baseline h |
4.16 | 3.11, 4.84 | 4.23 | 3.34, 4.77 | 4.20 | 3.27 4.79 |
| FIB-4 > 1.45 |
91 | 80% | 70 | 21% | 161 | 37% |
a Interquartile range
b Other race/ethnicity includes Hispanic, Native American/Alaskan Native, Asian/Pacific Islander, and races/ethnicities categorized as other.
c Education categories do not add to the total due to missing information
d Self-reported alcohol use within the last six months
e Chronic, active hepatitis B infection is defined as positive hepatitis B core antibody and positive hepatitis B surface antigen, and measured during one of the first three study visits after enrollment.
f ART is defined as use of three or more antiretroviral medications approved by contemporaneous DHHS guidelines
g Self-reported use of single or dual antiretroviral prior to ART initiation.
h HIV viral load is reported in log10 copies/mL.
Overall, half (55%) of women initiated ART from 2000–2005 and 22% initiated ART from 2006–2010. A greater proportion of women with chronic HCV initiated ART from 2000–2005 (70% vs. 49% among HCV-uninfected). At baseline, the median CD4 cell count was 303 cells/μL (IQR 188, 440) and median HIV RNA was 4.20 log10 copies/mL (IQR 3.27, 4.79). These baseline measures did not differ between women with and without chronic HCV. HCV genotypes were available for 18 women with chronic HCV; 9 of these women (50%) were genotype 1a.
The median follow-up was 11 study visits or 5.6 years. Two hundred twenty-nine (52%) women completed follow-up alive, 86 (20%) died during follow-up, and 110 (25%) were lost to follow-up. Thirteen women with chronic HCV were censored due to HCV treatment initiation and three HCV-uninfected women were censored due to evidence of HCV acquisition during follow-up.
Table 2 depicts the crude and weighted risk ratios for detectable HIV RNA comparing women with and without chronic HCV. There were 5,523 total study visits and HIV RNA was detected at 2,064 visits (37%). The percentage of study visits with detectable HIV RNA was higher among women with chronic HCV (47%, vs. 34%). After weighting, the proportion of study visits with detectable HIV RNA among women with chronic HCV was 1.19 (95% CI 0.72, 1.95) times that of HCV-uninfected women.
Table 2.
Estimated associations between HCV infection and detectable HIV viral load among 441 women enrolled in the Women's Interagency HIV Study, January 2000-September 2015.
| HCV+ (n=114) |
HCV- (n=327) |
Overall (n=441) |
|
|---|---|---|---|
| Total study visits |
1,509 | 4,014 | 5,523 |
| Visits with detectable HIV viral load |
708 | 1,356 | 2,064 |
| Proportion of visits with detectable HIV viral load (95% CI) |
0.47 (0.44, 0.49) | 0.34 (0.32, 0.35) | 0.37 (0.36, 0.38) |
| Crude risk ratio (95% CI) |
1.39 (1.15, 1.68) | 1 | -- |
| Weighted risk ratio (95% CI) a,b |
1.19 (0.72, 1.95) | 1 | -- |
a Weights account for the following set of time-fixed and time-varying covariates: age at ART initiation, race, history of injection drug use, year of ART initiation, CD4 cell count at ART initiation, HIV viral load at ART initiation, FIB-4 at ART initiation, current alcohol use, current drug use, and time.
b 95% CI was estimated with a nonparametric bootstrap using 200 samples with replacement.
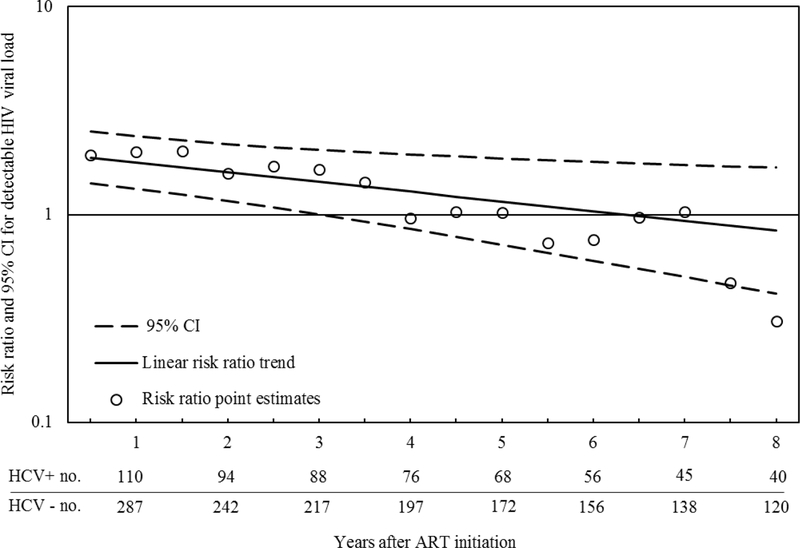
The results of the log-binomial model with a product term between HCV status and time since ART initiation are depicted in Figure 1. Approximately six months after ART initiation, the proportion of visits with detectable HIV RNA among women with chronic HCV was 1.88 (95% CI 1.41, 2.51) times that among women without HCV, at two years the ratio was 1.60 (95% CI 1.17, 2.18), and by six years there was essentially no difference (1.03; 95% CI 0.60, 1.79). The p-value for a test of linear trend was 0.02, which indicates the observed trend of risk ratios by time was unlikely due to chance.
FIGURE 1.
Estimated association between chronic HCV infection and detectable HIV RNA at each 0.5-year interval following ART initiation among 441 HIV-infected women enrolled in the Women’s Interagency HIV Study, January 2000-September 2015. The open circles represent the risk ratio point estimates. The solid black line represents the linear trend for the risk ratio point estimates and the dotted lines represent the 95% CI for the trend line. This CI was estimated with a nonparametric bootstrap using 200 samples with replacement. The line at 1 represents the null effect for a ratio measure.
There were 149 women (34%) who reported single or dual antiretroviral use prior to initiation of an effective ART regimen. After excluding these women, the association between chronic HCV and HIV RNA after ART initiation was similar to the main analysis. The proportion of visits with detectable HIV RNA among women with chronic HCV was 1.46 times that among HCV-uninfected women (95% CI 0.74, 2.93). A similar trend in ratio measures over time was also observed (p=0.04).
We also observed similar results when we limited our sample to only those women who reported taking their ART medications as prescribed during the first six months of ART (n=359). The proportion of visits with detectable HIV RNA among women with chronic HCV was 1.05 (95% CI 0.62, 1.78) times that among HCV-uninfected women. There was a similar trend in risk ratios over time as well (p=0.05); at six months the ratio was 1.68 (95% CI 1.14, 2.44), at three years the ratio was 1.43 (95% CI 0.98, 2.07), and by six years there was no difference (0.95; 95% CI 0.53, 1.69).
Lastly, we observed similar findings when we reclassified 33 women who were HCV antibody positive with undetectable HCV RNA as HCV-infected. The proportion of visits with detectable HIV RNA among HIV/HCV-coinfected women was 1.29 times that among HIV-monoinfected women (95% CI 0.84, 1.96). A similar trend in ratio measures over time was also observed (p=0.07).
DISCUSSION
In this longitudinal study of women with HIV initiating ART, the proportion of study visits with detectable HIV RNA was similar among women with and without chronic HCV. However, women with chronic HCV were more likely to have detectable HIV RNA up to two years after ART initiation than women without HCV.
Behavioral and biological mechanisms may explain the increased risk for detectable HIV RNA among women with chronic HCV during the first few years after ART initiation. Women with HCV may have poorer ART adherence and use alcohol and drugs more frequently [7,8]. When we repeated our analysis among women reporting >95% adherence during the first six months after ART initiation, results were similar to the main analysis. We controlled for potential confounding of the relationship between chronic HCV and HIV RNA by history of injection drug use, and included time-varying drug and alcohol use in the models used to estimate inverse probability-of-censoring weights. Thus, the observed association between chronic HCV and detectable HIV RNA is not fully explained by behavioral differences between women with and without chronic HCV. Persons living with HIV/HCV-coinfection experience greater immune system dysregulation [37, 38] and HCV has also been shown to replicate in extrahepatic lymphoid cells [39–41]. Therefore, biological interaction between HIV and HCV is possible, and could decrease ART effectiveness during the first few years after uptake.
Most of the previous studies assessing the effect of HCV on HIV treatment outcomes did not find that HCV negatively impacts HIV suppression [9–17]. Yet, a recent study by Hua et al. found that HCV was associated with earlier failure of HIV control, which was defined as two consecutive detectable HIV RNA measurements 16 or more weeks after ART initiation [18]. In that study significantly more HIV/HCV-coinfected patients experienced viral failure by 48 weeks after ART initiation [18]. These results also provide evidence that HCV may negatively impact early HIV viral response to ART.
There are several key differences between the present study and previous research assessing the effect of HCV on HIV suppression after ART. Previous studies compared time to HIV suppression after ART initiation among persons with and without HCV [9–17]. WIHS data are interval censored at six-month time periods, and the median time to first suppressed viral load in our analytic sample was approximately six months. Therefore, we could not replicate these analyses with our data. However, other studies have not assessed the association between HCV and HIV RNA using repeated measures of HIV RNA during follow-up, and our study provides a unique view of the longitudinal effect of chronic HCV on HIV RNA.
We defined HCV infection with confirmation of detectable HCV RNA. Approximately 15–25% of persons spontaneously clear their HCV infection (i.e. HCV RNA becomes undetectable), but remain HCV antibody-positive [42]. Previous studies often defined HCV by HCV antibody status only [9–14,18] and were not estimating the effect of chronic HCV infection on HIV RNA.
An additional distinction between previous research and the present study is that our analysis only included women. Women experience more frequent ART regimen discontinuation [19] and drug toxicity [20] than men. Despite this, only one previous study assessed the effect of HCV on HIV viral response after ART initiation among women. In unadjusted analyses, Marcus et al. found that the cumulative incidence of HIV suppression one year after ART initiation was lower among HIV/HCV-coinfected women than among HIV-monoinfected women [24]. After adjustment for confounding, there was no difference in HIV viral response by HCV status [24] but follow-up was limited to one year after ART initiation [24].
Our study has several limitations. We used observational data and the possibility of uncontrolled confounding remains. HCV may also potentiate hepatoxicity after ART initiation [43,44], which could lead to more frequent regimen changes and interruptions in therapy among HIV/HCV-coinfected persons [45,46]. Although we controlled for clinical characteristics at ART initiation and year of ART initiation (as a proxy measure for ART regimen), we did not assess the frequency of ART regimen changes due to toxicity in our cohort. Drug toxicities and regimen changes could contribute to the increased risk for detectable HIV RNA among women with chronic HCV in our cohort.
Approximately 25% of our sample were lost to follow-up and 20% died. Our estimated measures of effect would be biased if there is differential loss to follow-up or death among women with chronic HCV or among women with detectable HIV RNA [47,48]. To reduce selection bias, we created inverse probability-of-censoring weights. If the models used to construct the censoring weights are correctly specified and the observed variables fully explain selection that is associated with the exposure and outcome, those who were censored would be exchangeable with those who remained under study in our weighted sample, and bias would be averted [49]. Nevertheless, the extent to which we achieved exchangeability is not testable in observational data.
There was also an average of six years between baseline HCV testing and ART initiation. This could result in poorer clinical outcomes among women with chronic HCV due to worsening liver function [37,38,50–53] and misclassification of HCV status. We included the FIB-4 index in our exposure weights to control for confounding by declining liver function among women with chronic HCV. We also minimized HCV misclassification by supplementing HCV tests at enrollment with follow-up results and self-reported HCV treatment initiation. Over 80% of women in our analytic sample had at least one follow-up result available. Only 1% of women who were HCV-infected at enrollment cleared their infection prior to ART initiation and were reclassified to HCV-uninfected, and 2% of women who were HCV-uninfected at enrollment were reclassified to HCV-coinfected. We were not able to account for women classified as HCV-infected at ART initiation who cleared HCV infection during follow-up.
The observed associations between chronic HCV and HIV RNA may not generalize to all women with HIV on ART in the US. Our target population was women who initiated ART after they enrolled in the WIHS and after year 2000. We used these criteria in order to observe HIV RNA results at regular intervals, provide minimal missing information on confounders, and to assess the effect of chronic HCV on viral response to relatively modern ART regimens. These criteria resulted in fewer than 500 women who were eligible for our study. Although women in the WIHS are representative of the HIV epidemic among women in the US with respect to demographic characteristics [26] we cannot guarantee that the distribution of effect measure modifiers, like ART adherence, are similar. Therefore, the ability to generalize our findings to all women with HIV in the US is limited [54].
CONCLUSION
The results of this study provide a unique view of the longitudinal effects of chronic HCV on HIV viral response to ART among women. Overall, the proportion of visits with detectable HIV RNA was similar between women with and without chronic HCV. However, women with chronic HCV were more likely to have detectable HIV RNA up to two years after ART initiation than women without HCV. This finding suggests that chronic HCV may negatively impact early HIV viral response to ART. Despite new treatment options for persons with HCV, 45–85% of those with HCV are unaware of their infection [55–58] and considerable barriers to HCV treatment remain [59,60]. Thus, our findings reaffirm the need to test persons with HIV for HCV infection, and increase engagement in HIV care and access to HCV treatment among persons with HIV/HCV-coinfection.
ACKNOWLEDGEMENTS:
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), and P30-AI-050410 (UNC CFAR).
Appendix: Inverse probability of treatment and censoring weights
To control for confounding of the relationship between chronic HCV and detectable HIV RNA, we used stabilized time-fixed inverse probability-of-exposure weights denoted as:
These weights create a pseudo-population in which chronic HCV is no longer associated with measured covariates, assuming no statistical model misspecification [33,34]. The numerator of the exposure weight represents the probability of having the exposure that participant i factually had; the denominator is the probability of having the exposure that participant i factually had conditional on is a vector of covariate values at baseline for participant i, assumed to be sufficient to control for confounding. Logistic regression was used to estimate the denominator of the exposure weight.
Time-varying inverse probability-of-censoring weights are denoted as:
These weights account for selection bias due to right-censoring from loss to follow-up and death [35]. We fit separate weight models for right-censoring due to death and loss to follow-up to allow the parameter estimates to differ for each censoring mechanism [36]. The numerator of the censoring weights represent the probability of remaining in the study at visit k, conditional on exposure and . The denominators of the censoring weight are the conditional probability of remaining free from censoring, where is a vector of time-fixed and time-varying covariate histories measured up to visit k-1. Pooled logistic regression models were used to estimate the censoring weights.
The log binomial regression models were weighted by the product of the treatment and censoring weights (. The average of the estimated weights was 1.05 (standard deviation: 1.78) and they ranged from 0.20 to 25.57. We obtained 95% confidence intervals (CIs) for the weighted ratio measures using a nonparametric bootstrap with 200 resamples with replacement. The estimated weights in the 200 samples ranged from 0.06 to 535.95. We trimmed the weights at the 0.5th and 99.5th percentile to reduce the variability of the estimated effect of chronic HCV on detectable HIV RNA.
Footnotes
Author contributions: S.J.W. and S.R.C. designed the study. A.E. compiled the data. S.J.W. performed the data analysis. S.J.W., S.R.C., D.W., A.E., C.B.H. and A.A.A. had significant contributions to the writing of this article. The article was extensively reviewed, edited, and approved for submission by all other co-authors, who are part of the Women’s Interagency HIV Study and had patients involved in the study.
REFERENCES
- 1.The HIV-CAUSAL Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010; 24:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J Infect Dis 2013; 207(Suppl 1):S1–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toossi Z, Mayanja-Kizza H, Hirsh CS, Edmonds KL, Spahlinger T, Hom DL, et al. Impact of Tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol 2001; 123:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray RH, Li X, Wawer MJ, Serwadda D, Sewankambo NK, Wabwire-Mangen F, et al. Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis 2004; 189:1209–1215. [DOI] [PubMed] [Google Scholar]
- 6.Barnabas RV, Webb EL, Weiss HA, Wasserheit JN. The Role of co-infections in HIV epidemic trajectory and positive prevention: a systematic review and meta-analysis. AIDS 2011; 25:1559–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braitstein P, Justice A, Bangsberg DR, Yip B, Alfonso V, Schechter MT, et al. Hepatitis C coinfection is independently associated with decreased adherence to antiretroviral therapy in a population-based HIV cohort. AIDS 2006; 20:323–321. [DOI] [PubMed] [Google Scholar]
- 8.Shuper PA, Joharchi N, Irving H, Fletcher D, Kovacs C, Loutfy M, et al. Differential predictors of ART adherence among HIV-monoinfected versus HIV/HCV-coinfected individuals. AIDS Care 2016; 28:954–962. [DOI] [PubMed] [Google Scholar]
- 9.Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer P, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet 2000; 356:1800–1805. [DOI] [PubMed] [Google Scholar]
- 10.De Luca A, Bugarini R, Lepri AC, Puotic M, Girardi E, Antinori A, et al. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med 2002; 162:2125–2132. [DOI] [PubMed] [Google Scholar]
- 11.Lincoln D, Petoumenos K, Dore GJ. HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med 2003; 4:241–249. [DOI] [PubMed] [Google Scholar]
- 12.Carmo RA, Guimaraes MDC, Moura AS, Neiva AM, Versiani JB, Lima LV, et al. The influence of HCV coinfection on clinical, immunological and virological responses to HAART in HIV-patients. Braz J Infect Dis 2008; 12:173–179. [DOI] [PubMed] [Google Scholar]
- 13.Weis N, Lindhardt BO, Kronborg G, Hansen AB, Laursen AL, Christensen PB, et al. Impact of hepatitis C virus coinfection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: a nationwide cohort study. Clin Infect Dis 2006; 42:1481–1487. [DOI] [PubMed] [Google Scholar]
- 14.Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, Horban A, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis 2005; 192:992–1002. [DOI] [PubMed] [Google Scholar]
- 15.Klein MB, Lalonde RG, Suissa S. The impact of hepatitis C virus coinfection on HIV progression before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2003; 33:365–372. [DOI] [PubMed] [Google Scholar]
- 16.Turner J, Bansi L, Gilson R, Gazzard B, Walsh J, Pillay D, Orkin C, et al. The prevalence of hepatitis C virus (HCV) infection in HIV-positive individuals in the UK – trends in HCV testing and the impact of HCV on HIV treatment outcomes. J Viral Hepat 2010; 17:569–577. [DOI] [PubMed] [Google Scholar]
- 17.Stapleton JT, Bennet K, Bosch RJ, Polgreen PM,Swindells. Effect of antiretroviral therapy and hepatitis C co-infection on changes in lipid levels in HIV-infected patients 48 weeks after initiation of therapy. HIV Clin Trials 2007; 8:429–436. [DOI] [PubMed] [Google Scholar]
- 18.Hua L, Andersen JW, Daar ES, Glesby MJ, Hollabaugh K, Tierney C. HCV/HIV co-infection and responses to initial antiretroviral treatment. AIDS 2013; 27:2725–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber TJ, Geretti AM, Anderson J, Schwenk A, Phillips AN, Bansi L, et al. Outcomes in the first year after initiation of first-line HAART among heterosexual men and women in the UK CHIC Study. Antivir Ther 2011; 16:805–814. [DOI] [PubMed] [Google Scholar]
- 20.Collazos J, Asensi V, Carton JA. Sex differences in the clinical, immunological and virological parameters of HIV infected patients treated with HAART. AIDS 2007; 21:835–843. [DOI] [PubMed] [Google Scholar]
- 21.Zaragoza-Macias E, Cosco D, Nguyen ML, Del Rio C, Lennox J. Predictors of success with highly active antiretroviral therapy in an antiretroviral-naive urban population. AIDS Res Hum Retroviruses 2010; 26:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giordano TP, Wright JA, Hasan MQ, White AC, Jr, Graviss EA, Visnegarwala F. Do sex and race/ethnicity influence CD4 cell response in patients who achieve virologic suppression during antiretroviral therapy? Clin Infect Dis 2003; 37:433–437. [DOI] [PubMed] [Google Scholar]
- 23.Maskew M, Brennan AT, Westreich D, McNamara L, MacPhail AP, Fox MP. Gender differences in mortality and CD4 count response among virally suppressed HIV-positive patients. J Womens Health (Larchmt) 2013; 22:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus JL, Leyden WA, Chao CR, Xu L, Quesenberry CP, Tien PC, et al. Differences in response to antiretroviral therapy by sex and hepatitis C infection status. AIDS Patient Care STDS 2015; 29:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. Epidemiology 1998; 9:117–125. [PubMed] [Google Scholar]
- 27.DHHS/Henry J. Kaiser Family Foundation Panel on Clinical Practices for the Treatment of HIV infection. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. July 2016. revision. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 28.Westreich D, Cole SR. Invited commentary: positivity in practice. Am J Epidemiol 2010; 171:674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ. Splines for trend analysis and continuous confounder control. Epidemiology 2011; 22:874–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterling RK, Lissen E, Clumeck N, Correa MC, Montaner J, Sulkowski M, et al. Development of a simple noninvasive indext to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 31.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhallui-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–36. [DOI] [PubMed] [Google Scholar]
- 32.Matta B, Lee TH, Patel K. Use of Non-invasive Testing to Stage Liver Fibrosis in Patients with HIV . Curr HIV/AIDS Rep 2016; 13:279–288. [DOI] [PubMed] [Google Scholar]
- 33.Hernan MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health 2006; 60:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–560. [DOI] [PubMed] [Google Scholar]
- 35.Buchanan AL, Hudgens MG, Cole SR, Lau B, Adimora AA. Worth the weight: using inverse probability weighted Cox models in AIDS research. AIDS Res Hum Retroviruses 2014; 30:1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cain LE, Cole SR. Inverse probability-of-censoring weights for the correction of time-varying noncompliance in the effect of randomized highly active antiretroviral therapy on incident AIDS or death. Stat Med 2009; 28:1725–1738. [DOI] [PubMed] [Google Scholar]
- 37.Liberto MC, Zicca E, Pavia G, Quirino A, Marascio N, Torti C, et al. Virological mechanisms in the coinfection between HIV and HCV. Mediators Inflamm 2015; 2015:320532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotman Y, Liang TJ. Coinfection with hepatitis C virus and human immunodeficiency virus: virological, immunological, and clinical outcomes. J Virol 2009; 83:7366–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackard JT, Smeaton L, Hiasa Y, Horiike N, Onji M, Jamieson DJ, et al. Detection of hepatitis C virus (HCV) in serum and peripheral-blood mononuclear cells from HCV-monoinfected and HIV/HCV-coinfected persons. J Infect Dis 2005; 192:258–265. [DOI] [PubMed] [Google Scholar]
- 40.Laskus T, Radkowski M, Piasek A, Nowicki M, Horban A, Cianciara J, et al. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: evidence of active replication in monocytes/macrophages and lymphocytes. J Infect Dis 2000; 181:442–448. [DOI] [PubMed] [Google Scholar]
- 41.Revie D, Salahuddin SZ. Human cell types important for hepatitis C virus replication in vivo and in vitro: old assertions and current evidence. Virol J 2011; 8:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. Hepatitis C FAQs for Health Professionals. Last updated on March 11, 2016. Accessed from Centers for Disease Control and Prevention on April 7, 2016 http://www.cdc.gov/hepatitis/hcv/hcvfaq.htm
- 43.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA 2000; 283:74–80. [DOI] [PubMed] [Google Scholar]
- 44.Martinez E, Blanco JL, Arnaiz JA, Perez-Cuevas JB, Mocroft A, Cruceta A, et al. Hepatotoxicity in HIV-1-infected patients receiving nevirapine-containing antiretroviral therapy. AIDS 2001; 15:1261–1268. [DOI] [PubMed] [Google Scholar]
- 45.Grint D, Peters L, Rockstroh JK, de Wit S, Mitsura VM, Knysz B, et al. Increased incidence of antiretroviral drug discontinuation among patients with viremic hepatitis C virus coinfection and high hyaluronic acid, a marker of liver fibrosis. AIDS 2014; 28:577–587. [DOI] [PubMed] [Google Scholar]
- 46.Ripamonti D,Arici C, Pezzotti P, Maggiolo F, Ravasio L, Suter F. Hepatitis C infection increases the risk of the modification of first highly active antiretroviral therapy in HIV-infected patients. AIDS 2004; 18:334–337. [DOI] [PubMed] [Google Scholar]
- 47.Collett D Modelling Survival Data in Medical Research, 2E Boca Raton, FL: Chapman and Hall/CRC; 2003. [Google Scholar]
- 48.Hernán MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004; 15(5):615–625. [DOI] [PubMed] [Google Scholar]
- 49.Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ. Selection bias due to loss to follow-up in cohort studies. Epidemiology. 2016; 27:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements 2002; 19:1–46. [PubMed] [Google Scholar]
- 51.Lesens O, Deschenes M, Steben M, Belanger G, Tsoukas CM. Hepatitis C virus is related to progressive liver disease in HIV-positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis 1999; 179:1254–1258. [DOI] [PubMed] [Google Scholar]
- 52.Soto B, Sanchez-Quijano A, Rodrigo L, del Olma JA, Garcia-Bengoechea M, Hernandex-Quero J, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol 1997; 26:1–5. [DOI] [PubMed] [Google Scholar]
- 53.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001; 33:562–569. [DOI] [PubMed] [Google Scholar]
- 54.Hernán MA, VanderWeele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011; 22:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roblin DW, Smith BD, Weinbaum CM, Sabin M. Hepatitis C virus screening practices and prevalence in a managed care organization. Am J Managed Care 2011; 17:548–555. [PubMed] [Google Scholar]
- 57.Southern WN, Drainoni ML, Smith BD, Christiansen CL, McKee D, Gifford AL, et al. Hepatitis C testing practices and prevalence in a high-risk urban ambulatory care setting. J Viral Hepat 2011; 18:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 2009; 50:1750–1755. [DOI] [PubMed] [Google Scholar]
- 59.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–223. [DOI] [PubMed] [Google Scholar]
- 60.Canary LA, Klevens RM, Holmberg SD. Limited access to new hepatitis C virus treatment under state Medicaid programs. Ann Intern Med 2015; 163:226–228. [DOI] [PubMed] [Google Scholar]