Abstract
Stroke is a major health burden as it is a leading cause of morbidity and mortality worldwide. Blood flow restoration, through thrombolysis or endovascular thrombectomy, is the only effective treatment but is restricted to a limited proportion of patients due to time window constraint and accessibility to technology. Over the past two decades, research has investigated the basic mechanisms that lead to neuronal death following cerebral ischemia. However, the use of neuroprotective paradigms in stroke has been marked by failure in translation from experimental research to clinical practice. In the past few years, much attention has focused on the immune response to acute cerebral ischemia as a major factor to the development of brain lesions and neurological deficits. Key inflammatory processes after stroke include the activation of resident glial cells as well as the invasion of circulating leukocytes. Recent research on anti-inflammatory strategies for stroke has focused on limiting the transendothelial migration of peripheral immune cells from the compromised vasculature into the brain parenchyma. However, recent trials testing the blockage of cerebral leukocyte infiltration in patients reported inconsistent results. This emphasizes the need to better scrutinize how immune cells are regulated at the blood–brain interface and enter the brain parenchyma, and particularly to also consider alternative cerebral infiltration routes for leukocytes, including the meninges and the choroid plexus. Understanding how immune cells migrate to the brain via these alternative pathways has the potential to develop more effective approaches for anti-inflammatory stroke therapies.
Keywords: choroid plexus, leukocyte infiltration, meninges, neuroinflammation, stroke
Introduction
The inflammatory response to cerebral ischemia is an important element in the onset and progression of stroke. Necrotic cells and cell debris induce neuroinflammation by activating resident microglia and astrocytes.1,2 After this initial glial cell activation, there is an increase in cerebral cytokine and adhesion molecule expression leading to the recruitment of peripheral leukocytes to the lesion site.3–5 Monocytes/macrophages and neutrophils infiltrate the ischemic tissue in large numbers within the first days, whereas lymphocytes appear in the parenchyma later on.1,6 Importantly, previous studies have reported a critical role of T cells in secondary neuroinflammation after brain ischemia.7,8 We and others have shown that blockage of lymphocyte trafficking diminished the infarct volume in different cerebral ischemia models, resulting in improvement of stroke outcome and suggesting a possible therapeutic target.9–11 Several clinical trials have been initiated since then to test pharmacological approaches blocking lymphocyte migration using fingolimod (FTY720), natalizumab (anti-α4-integrin immunoglobulin G4) and enlimomab Intercellular Adhesion Molecule-1 (anti-ICAM-1 antibody) with controversial results. While two trials testing fingolimod in stroke have shown promising results with reduction of infarct volume progression,12,13 both studies testing natalizumab and enlimomab have not been able to demonstrate a significant effect on the respective primary endpoint.14,15 Failure to induce consistent neuroprotection by anti-inflammatory therapies in patients with stroke has been discussed elsewhere.16,17 While several aspects of study design, statistical rigor and translational aspects from mouse to men need to be considered in this regard, the inefficient targeting of lymphocytes, at the predominantly investigated transendothelial migration route, might also account for the lack of efficacy of these anti-inflammatory treatments. A better understanding of how cells of the peripheral immune system access the central nervous system (CNS) and interact with the brain microvasculature would likely lead to the development of more efficient immune interventions for individuals with stroke.
In the stroke field, the transendothelial migration of leukocytes into the ischemic brain, across the compromised blood–brain barrier (BBB), has been investigated most extensively.11,18,19 Interestingly, recent studies from stroke and other brain diseases point to an important role of the alternative cerebral infiltration route, the meningeal compartment and the choroid plexus (ChP).20–22 Specifically, we have demonstrated that a subtype of proinflammatory T cells accumulate early after stroke in the meninges20 and that cerebral ischemia induces the recruitment of lymphocytes from the ChP to the peri-infarct brain tissue.21 However, mechanisms of brain infiltration of leukocytes from these alternative routes and how they may contribute to the infarct development are still unknown.
In this review, we examine the immune mechanisms occurring at the meninges and the ChP in brain diseases, with special attention paid to stroke. In addition to the BBB, the meninges and ChP act as potent regulators for immune cell activation and migration routes into the injured brain. Importantly, their distinct locations, structural differences, different composition of resident immune cells and differential pattern of surface molecule expression in comparison to the BBB may guide leukocytes to utilize preferentially one or the other meningeal or choroidal infiltration routes. Here, we first summarize the anatomical structure of the meningeal and choroidal compartments, which immune cells populate these tissues and how they become activated and migrate into the brain parenchyma. We next discuss how these compartments may regulate the inflammatory response to stroke and influence disease progression, making assumptions for similar mechanisms in humans in the light of current information from postmortem human specimens and patient imaging. Elucidation of the role of the meninges and the ChP in ischemic stroke will advance our understanding of how peripheral immune cells influence stroke pathobiology. Future studies should explore the function of these alternative cerebral routes for leukocytes that could lead to novel pharmacologic interventions involving both meningeal and choroidal compartments in stroke.
The alternative CNS barriers for leukocyte trafficking in stroke
Complex endothelial or epithelial barriers separate the brain from blood-derived circulating molecules and effector immune cells. Maintenance of the integrity of these barriers is essential for proper brain function.23 Stroke is associated with a leakage of the BBB which was previously associated with propagating the influx of leukocytes into the brain.24,25 However, to date three distinct routes for leukocyte migration from the blood into the brain parenchyma have been described: through the parenchymal vessels of the BBB, via the meningeal blood circulation and across the epithelial cells of the choroid plexus.26,27
To better interpret the results published in the field, we first aim to clarify certain aspects of the terminology used to describe the different blood–CNS barriers. Specifically, we refer to ‘interface’ as a structure which upon injury allows the passage of immune cells from the intracranial vasculature to the extracellular compartments. Thus, the blood–CNS interface systems refer to the following: the BBB which encloses the parenchymal microvessels and the glial limitans of the brain parenchyma; the blood meningeal interfaces formed between either the pia-arachnoid blood vessels and the cerebrospinal fluid (CSF) of the subarachnoid space (SAS), the pial blood vessels of the pial basement membrane and the glial limitans, and across the dural blood vessels; and the ChP interfaces between the fenestrated capillaries and the stromal side of the ChP epithelial cells, and the apical choroidal region and the CSF of the brain ventricles.28,29 In contrast to this terminology of ‘interface’ which denotes structure on an organ level as entry sites for circulating leukocytes, the term ‘barrier’ refers to specific endothelial or epithelial cell layers which demonstrate a selective permeability for soluble particles, such as the BBB and the blood–CSF barrier (BCSFB), which have been reviewed in detail elsewhere.27,30–32
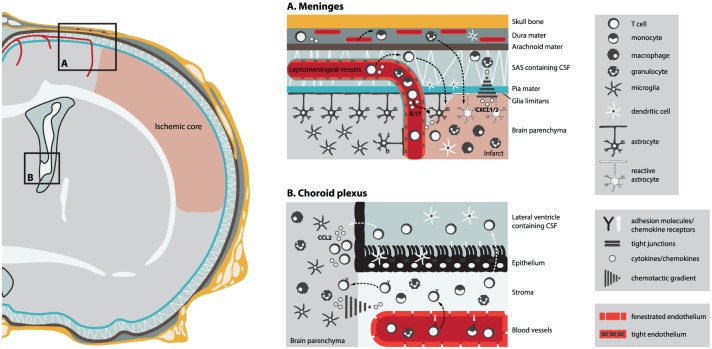
Due to distinct structural characteristics (endothelial versus epithelial cells) and anatomical location (superficial cortical space versus deep ventricular space) as well as different molecule expression (adhesion molecules expression and chemotactic cues) between the blood barrier systems, entry into the CNS might be limited to immune cell subsets that hold the specific molecular keys required to cross these interfaces. Thus, one can hypothesize that targeting a specific route for leukocyte infiltration will have a greater impact on stroke outcome. Here we first describe the structure of the alternative routes for leukocytes to enter the CNS: the meningeal and choroidal paths (Figure 1).
Figure 1.
Meningeal and choroidal cerebral infiltration routes for leukocytes in poststroke neuroinflammation. Cerebral ischemia induces the release of chemotactic cues, such as CXCL1/2 and CCL2, from activated parenchymal glial cells, leading to the recruitment of leukocytes from the meninges and from the choroid plexus into the infarct area. The upregulation of adhesion molecules in the meningeal and choroidal vasculatures after stroke may possibly allow the transmigration of leukocytes from the blood vessel lumen to the brain parenchyma. Arrows indicate routes of possible migration for leukocytes from (A) the meninges: dura mater blood vessels and leptomeningeal vessels (black arrows) and (B) the choroid plexus: ChP stroma (black arrows), possibly the preferred route after stroke, and cerebrospinal fluid (CSF) circulation (white arrows) into the ischemic brain parenchyma.
Structure and function of the blood–CNS interfaces
The meninges
The meninges consist of three layers of connective tissue which enclose the brain and the spinal cord: the dura mater, the arachnoid mater and the pia mater. All components of the meninges show considerable structural and functional heterogeneity. The dura mater is described as two epithelial layers of dense fibrous tissue.33 The outermost periosteal layer is tightly adherent to the calvarium (or skull cap) and plays an important role in the skull bone development.34 The inner dura mater layer is defined as the meningeal dural layer and is firmly attached to the periosteal layer except at distinct regions to form large venous channels known as the dural venous sinuses, which serve as a draining system from the CNS.35 The major bloods vessels that supply the dura run in its outer periosteal surface and vary according to different areas of the skull, among them the internal carotid artery which supplies the more anterior part of the skull.35 Directly attached to the meningeal dural layer is the arachnoid and pia mater which build the SAS between the dura mater and the CNS.29,36 The two structures also known as the leptomeninges are attached by strands of connective tissue, the arachnoid trabeculae. The CSF circulates within the SAS between the arachnoid, composed of tight intercellular junctions, and the pia mater, which is a semipermeable membrane.29 Outflow of CSF from the SAS takes place through arachnoid villi or granulations that evert into the dural sinuses. While this system is efficient for water and small compound clearance, macromolecules and immune cells are preferentially drained through the meningeal lymphatic vessels.37–39 Numerous blood vessels (arteries and veins) are associated with the leptomeninges. They are composed of a monolayer of nonfenestrated endothelial cells attached by intercellular tight junctions and in contrast to vessels in the subpial space they are not covered by astrocyte end feet.40 As leptomeningeal blood vessels enter the brain parenchyma they carry with them arachnoid and pial tissues and together with glial end feet form a cuff known as the perivascular space in which antigen-presenting cells can be found41,42 as well as a concomitant accumulation of lymphocytes.43 Indeed, in disease settings, the meningeal blood vessels appear to be impermeable to defined cell components, such as red blood cells, but permeable to migrating inflammatory cells. The failure of erythrocytes to enter the perivascular spaces in patients with subarachnoid hemorrhage suggests that the pia mater does form an effective barrier to large molecules and nonmigratory cells. However, in inflammatory conditions affecting the meninges, such as infective meningitis, polymorphonuclear leukocytes and macrophages are seen throughout the subarachnoid space as well as in the subpial and perivascular spaces.44
As mentioned above, meningeal blood vessels run along the dura mater, in the SAS and at the pia basement membrane, and eventually penetrate the brain parenchyma. Previous studies investigating lymphocyte egress from the vasculature in these various meningeal compartments have studied the contribution of the dura mater as well as the leptomeninges (arachnoid and pia mater), which results in some discrepancy on information about the blood–meningeal interface at the different meningeal levels across various brain diseases. This particular focus on substructures of the meninges could also be attributed to the use of specific techniques investigating meningeal lymphocyte transmigration and need to be kept in mind when drawing conclusions about the role of the ‘meninges’ in a specific disease setting as the described findings most often reflect only events at a particular meningeal compartment. In particular, multiphoton in vivo imaging studies focus mainly on the leptomeningeal compartment,45 flow cytometric analysis of cell composition is widely performed on the dura mater and arachnoid mater,46 while histological studies might investigate the various meningeal structures.47
The choroid plexus
The ChP is not only involved in CSF production but also in homeostasis of the brain parenchyma by supplying the brain with nutrients, by clearing toxic compounds and by surveying the immunological status of the brain.48 The ChP is found in all four cerebral ventricles and potentially allows cell trafficking from the choroid blood vessels and the CSF to the brain parenchyma.31 Structurally, the ChP is composed of a single layer of epithelial cells continuous with the ependymal cells that line the ventricles. At the apical side, the ChP epithelium has numerous villi increasing the surface area for a substantial flux of solutes and water from the blood through the ChP epithelium to the ventricles. Reduction in size of microvilli due to aging has been related to reduced ability to produce CSF, which may affect brain function.49,50 The basal side of the ChP epithelium faces the ChP stroma, which is a highly vascularized tissue, in which capillaries have a fenestrated endothelium facilitating nonrestrictive substance secretion and absorption in contrast to the tightly sealed ChP epithelium. Tight junction and adhesion molecules are found between adjacent choroidal cells restricting the passage of solutes from the blood circulation into the CSF.51,52 This highly polarized ChP structure, defined by the fenestrated vasculature and tight epithelium, forms the ChP interfaces which are possible gates allowing cell migration in disease setting.48,53 Both blood flow and CSF production by the ChP epithelium are regulated by sympathetic and parasympathetic innervation, probably via the glossopharyngeal and vagus nerves.54 Vagal nerve stimulation inhibits the release of proinflammatory cytokines55,56 and improves stroke outcome.57 One can assume that activation of the vagal nerve after stroke may have a direct impact on the ability of ChP to produce cytokines/chemokines58 and further influence cell migration, although further investigations are needed.
The ChP receives its blood supply from the anterior and posterior circulation. The anterior choroidal artery, which branches from the internal carotid or middle cerebral artery, supplies the ChP from the lateral ventricle. The posterior choroidal arteries, which branch from the posterior cerebral artery, supply the lateral ventricle as well as the third ventricle, whereas the ChP of the fourth ventricle is supplied by the anterior and posterior inferior cerebellar arteries.59 Ischemic stroke has been reported in patients in the territory of the anterior choroidal artery and more rarely in the lateral and medial posterior choroidal arteries.60,61 Whether changes in the function of the ChP supplied by these choroidal arteries contribute to the neurological deficits is unclear. Several studies reported that cerebral ischemia induced in rodents led to necrosis of the ChP. Likewise, proximal middle cerebral artery occlusion induced ischemic lesions of the ipsilateral ChP21 and permanent global ischemia induces choroidal cell death resulting in ChP atrophy and preceding neuronal cell death in the hippocampus.62–64 Apart from direct injury to the ChP, both transient global ischemia and common carotid artery occlusion models caused ChP tissue disruption with intraventricular leakage of high molecular weight blood molecules.65 Additionally, pathogenic processes in Alzheimer’s disease contribute to the low efficacy of the ChP to clear amyloid β (Aß) peptides, resulting in an accumulation of Aß in the brain. This, in turn, enhances the progression of the disease.66 These studies indicate that damage to choroidal cells may result in disruption of the ChP selective barrier, leading to the entry of neurotoxic substances, inflammatory mediators or migration of immune cells to the CSF.
Immune cell populations of the meninges and ChP
Under physiological conditions, brain resident microglia are present in large numbers, whereas fewer immune cells from the innate and adaptive immune system can be found in the brain parenchyma.67 Immune surveillance of several regions of the CNS occurs at steady state by patrolling T cells and dendritic cells (DCs).27 The CSF of healthy individuals contains between 1000 and 3000 leukocytes per ml, and is enriched in memory T cells compared with blood and secondary lymphatic organs.68 Interestingly, several reports have shown that at steady state immune cells of the innate and adaptive system are present in the meninges and choroid plexus.69 They are located in the CSF and lymphatics and may act either as patrolling cells detecting subtle changes in brain homeostasis or as a drainage system to signal a peripheral immune response during injury.70,71 Also, similarly to parenchymal blood vessels, cells circulating in the meningeal and choroidal vessels may bridge the impaired or inflamed vasculature and infiltrate the brain parenchyma upon injury (Figure 1). Moreover, resident immune cells of the dural compartment or the subpial structure of the meninges as well as in the ChP stroma may sense cytokines/chemokines released from the injured brain tissue. Such resident immune cells at the borders of the brain might either invade the parenchyma themselves after activation by brain-released stimuli or influence neighboring cells towards either a pro- or anti-inflammatory phenotype.27,53 These different aspects are discussed in more detail in the following sections.
T lymphocytes
Recently, we have demonstrated the importance of the meninges as a site of T-cell accumulation and the ChP as an alternative route for lymphocyte brain infiltration after stroke.20,21 γδT cells are nonconventional lymphocytes which display several innate cell-like features allowing them to become rapidly activated following ischemic injury.72 Using the transgenic mouse model expressing a Kikume Green-Red photoconvertible fluorescent protein in all cell types,73 we showed that intestinal T cells are mobilized from the gut and were exclusively located in the meningeal compartment after stroke 20 Specifically, immunohistochemistry and flow cytometry analysis revealed that interleukin (IL)-17-producing γδT cells increased in the meninges early after stroke onset20 and preceded their accumulation in the ischemic area.8,74 This was associated with an upregulation of chemotactic genes CXCL1/CXCL2 contributing to brain injury, possibly through the promotion of neutrophil infiltration.20 Together, the meninges could function as a checkpoint in postischemic inflammation and orchestrate leukocyte infiltration into the brain parenchyma via chemotactic cues.
In a second study, we investigated the ChP as a possible route for T-cell entry into the brain parenchyma by distal occlusion of the middle cerebral artery in mice. This model of cortical stroke has the advantage of not affecting the blood flow in the anterior choroidal artery, which provides blood supply to the ChP in the lateral ventricle. We have reported that T cells were the main population in the ipsilateral ChP of the lateral ventricle after an ischemic stroke compared with other myeloid cells. In the same study, we showed a cluster of T cells in the peri-infarcted cortex between the lateral ventricle and the lesion core. To assess the migration of T cells from the ChP to the brain parenchyma after stroke, we specifically photo labelled T cells in the ipsilateral ChP using a T-cell photoactivated mouse model. We detected that approximately two thirds of all peri-lesional T cells in the peri-infarcted cortex 24 h after stroke have been photoactivated, indicating their choroidal invasion pathways in contrast to only one third of T cells migrating via other invasion routes (e.g. transendothelial or meningeal).21 Interestingly, blocking the CSF circulation by intraventricular Matrigel injection did not affect T-cell invasion after stroke, suggesting that choroidal T cells translocate preferentially from the ChP stroma to the brain parenchyma but not transmigrating to the CSF compartment. These data support the hypothesis that the ChP acts as a predominant route of lymphocyte infiltration after an ischemic stroke. In contrast to previous findings, the reduced number of invading CD3+ T cells did not affect the volume of the infarct.21 It is plausible that compensatory mechanisms, such as the involvement of other peripheral immune cells or resident microglia, tampered with the reduced infiltration of T cells and thus failed to significantly contribute to inflammatory lesion expansion.
Previous studies in animal models of primary autoimmune CNS disease have guided current studies on the role of the ChP in stroke. Experimental autoimmune encephalomyelitis (EAE), the murine model of multiple sclerosis, was induced by intravenous transfer of CD4+ effector T cells reactive against the myelin component, myelin basic protein (MBP). The fate of these T cells was followed across the leptomeninges of the spinal cord by two-photon imaging.75,76 T cells appear crawling intraluminally in the meningeal vessels of the subarachnoid space, followed by their transendothelial migration to the perivascular space of the pia mater. There, MBP T-cell receptor-restricted CD4+ cells encounter perivascular macrophages or DCs with antigen presentation capabilities. Upon contact with these antigen-presenting cells, effector T cells increased the expression of proinflammatory mediators and this expression profile remained stable after T-cell infiltration into the CNS parenchyma. In light of these findings in EAE, it would be of great interest to investigate the meningeal route for T-cell transmigration after stroke. It is conceivable that the preferred route of infiltration for T-cell subpopulations would be disease dependent. In a recent study, Schläger and colleagues found that the meningeal route for brain infiltration of CD4+ T cells in EAE was preferentially used as very few cells were observed in the ChP.77 In contrast, the ChP route was mainly involved in T-cell brain accumulation after stroke. Indeed, we have demonstrated that the experimental infarction of the ChP significantly decreased T-cell migration to the ischemic area.21
The recruitment of circulating T cells in the meningeal compartments and the ChP is mediated by the sequential interaction of different adhesion and chemokine/cytokine cues, leading to the interaction between circulating immune cells and endothelial or epithelial cells.78 In particular, the cellular and molecular events leading to lymphocyte cerebral infiltration across the meningeal75,77 and the ChP79,80 interfaces have been described in EAE, and involved the disruption of the endothelial tight junctions and the upregulation of different adhesion molecules on the outer cell membrane of vascular endothelial cells: selectins (P, E and L selectins), immunoglobulins and integrins Vascular Cell Adhesion Molecule-1 (VCAM-1), Very Late Antigen-4 (VLA-4), (ICAM-1), which lead to leukocyte rolling on the surface of endothelial cells, adhesion to the endothelial wall and paracellular or transcellular migration.32,81 This sequence of events shares similarities with some features of lymphocyte recruitment across the BBB.32 The differential regulation of specific adhesion molecules or chemotactic cues involved in T-cell migration at the distinct interfaces of the parenchymal vessels, the meninges and the ChP has been discussed in detail before32,82,83 and is beyond the scope of this review. Nevertheless, it would be of interest to investigate the molecular basis of leukocyte infiltration through the meningeal vessels and ChP tissue in a stroke context. For instance, it has been reported that the C-C chemokine ligand-20 (CCL20), the ligand for the C-C chemokine receptor-6 (CCR6), is constitutively expressed in the ChP but not on the endothelium of CNS parenchymal capillaries.84 Interestingly, infiltration of CCR6+ T helper (Th)-17 via the choroid plexus was shown to be essential for the initial phase of EAE.84 Although little is known about how leukocytes are recruited into the brain after stroke, we have shown that intracerebral T-cell migration from the ChP to the peri-infarcted cortex is driven by a CCL2 chemokine gradient between these two compartments, mainly produced by parenchymal macrophages and microglia.21
Dendritic cells
DCs have been located in the meninges and ChP of the CNS.85,86 Specifically, DCs were identified on the internal region of the dura mater and on the surface of the pia mater facing the SAS. DCs thereby have ready access to CSF-circulating antigens.86 Similarly, the ChP stroma contains high numbers of major histocompatibility complex class II expressing macrophages and DCs.42,87,88 Immature DCs reside in between the ChP epithelial cells and extend their dendrites into the CSF88 where they have a sentinel function by sampling the CSF micromilieu for antigens. DCs can further present antigens to T cells entering the ChP stroma.87 This continuous immunosurveillance by choroidal DCs allows specific responses to disease and injury. In EAE an increase of CD11c+ DCs was observed in the ChP as well as in the leptomeninges of the spinal cord prior to the onset of EAE symptoms.76,89 Specifically, meningeal DCs were shown to activate CD4+ T cells before EAE disease manifestation was observed.76 These findings suggest that the meninges represent an important site for primary interaction of DCs with antigen-specific T cells. However, it remains to be defined whether meningeal and choroidal DCs upon activation are actively recruited to the brain parenchyma after EAE and sustain the autoreactive immune response during the progression of the disease. Stroke involves DCs either as antigen-presenting cells90 or independently to their antigen-presenting function,91 thus one can speculate a similar role for meningeal and choroidal DCs in stroke, as previously demonstrated in autoimmune CNS models. These findings on functional DC–T-cell interaction in the meninges indicate that the meninges are a potent polarizing compartment for T cells via antigen presentation and costimulation by DCs activity before their entry into the injured brain. Alternatively, activation of DCs in these compartments due to an injury may participate in the peripheral immune system response after antigen presentation to T cells of the cervical lymph nodes,92 which still remains to be conclusively investigated.
Macrophages
Monocytes/Macrophages have been observed under physiological conditions in all layers of the meninges85,93 and in the ChP.42,86 In contrast to DCs, macrophages, by their location within the connective tissue of the meningeal compartment and ChP, are less exposed to the CSF, suggesting a tissue macrophage function which is distinct from that of DCs. The phenotypes of macrophages are dictated by tissue-specific signals and the health or disease state.93 Particularly, when mice were tested for spatial memory, they showed an accumulation of IL-4-producing T cells in the meninges over time.22 Importantly, acute depletion of T cells from the meningeal space or the deletion of IL-4 from T cells resulted in impairment of spatial memory and led to a proinflammatory skew in meningeal macrophages, pointing to an important role for meningeal macrophages in learning and memory. The spatial memory performance could be rescued by using IL-4-primed anti-inflammatory macrophages.22 Likewise, depletion of meningeal and perivascular macrophages using clodronate liposomes increased clinical symptoms and bacterial load in a mouse model of pneumococcal meningitis.94 Interestingly, the meninges were associated with neuroprotection following an ischemic stroke via preconditioned monocytes.95 Low doses of the proinflammatory mediator lipopolysaccharide (LPS) before an ischemic injury induced protection after transient occlusion of the middle cerebral artery, a phenomenon known as preconditioning.95,96 This effect was recapitulated by adoptive transfer of monocytes isolated from LPS-preconditioned mice. Specifically, the neuroprotective monocytes were recruited to meningeal vessels, where they dampened the expression of proinflammatory genes involved in the activation of neutrophils and chemotactic factors such as Csf3,97 indicating an essential role of the meningeal compartment as a key immunoregulator in ischemic brain injury. Moreover, a recent study reported an increase in monocytes/macrophages in the CSF and ChP after stroke.98 The study found that GFP+CD11b+ macrophages expressing preferentially an M2-like phenotype were located between the lateral ventricle and the ischemic core. To address the role of M2-like macrophages after cerebral ischemia, in vitro M2-polarized cells were directly injected into the lateral ventricle. Both motor and cognitive functions were improved post stroke in animals treated with M2-polarized macrophages, although the infarct volume remained unaffected.98 Importantly, Ge and colleagues demonstrated an upregulation of several adhesion molecule and chemokine genes in the ChP after stroke, which are associated with the homing and migration of monocytes.98 Similarly, traumatic brain injury led to a release of the chemokine CCL2 from the ChP in a polarized manner, leading to the accumulation of neutrophils and monocytes in the ChP stroma and their transmigration into the injured cortex.58,99
Neutrophil granulocytes
Experimental stroke induced an increase in neutrophil granulocyte counts in the infarct region between 1 and 3 days after stroke onset, depending on the investigated stroke model.1,100 Interestingly, neutrophils were detected in the leptomeninges as well as in the cortical basal lamina and perivascular space a few hours after ischemia induction prior to brain infiltration.20,101 In addition, ischemic tissue from fatal cases of human stroke revealed positive immunostaining for neutrophils in the leptomeninges and perivascular spaces.101 Traumatic brain injury (TBI) induces leakage of blood vessels of the subarachnoid space as well as in the perivascular space, leading to cell death of meningeal cells and disruption of the glia limitans.102 Within an hour after injury, myeloid cells, mainly neutrophil granulocytes, were highly motile and interacted with dead cells in the meningeal compartment.102 Interestingly, in this study, application of the reactive oxygen species scavenger glutathione on the surface of the skull, allowing its penetration through the porous structure of the skull bone, resulted in survival of meningeal myeloid cells as well as glia limitans preservation, a process associated with a reduction in meningeal cell death.102 Another study reported that neutrophils were found both in the leptomeninges and ChP of the nondamaged area as well as the injured side. However, neutrophils remained within the leptomeninges and ChP of the nontraumatized hemisphere, whereas neutrophils accumulated in the ipsilateral hemisphere. These results suggest that the leptomeninges and ChP are possible gates for neutrophil infiltration into the injured area after brain injury, similar to the parenchymal vasculature.103 Interestingly, invasion of neutrophils after TBI was regulated by an upregulation of tumor necrosis factor α and IL-1β in the ipsilateral ChP, causing the production of neutrophil chemoattractants in the ChP, such as cytokine-induced neutrophil chemoattractant (CINC). CINC upregulation leads to a temporary neutrophil recruitment to the ipsilateral ChP 24 h after TBI, since no neutrophils could be noted at 48 h post lesion.58
Mast cells
Mast cells are resident immune cells of the meninges, located in the perivascular space and in the dura mater, where they act as early regulators of barrier integrity. Mast cells are activated early after cerebral ischemia and contribute to the BBB breakdown by their gelatinase activity.104 Also depletion of the mast cell population has been shown to dampen granulocyte and macrophage infiltration after stroke, whereas T cells and microglia were not affected.105 Taken together, meningeal mast cells can exacerbate stroke outcome in mice, highlighting a critical function for the meningeal compartment after stroke while the specific interaction of mast cells with other immunocompetent cells, neurons and glial cells after stroke still needs to be elucidated.
Conclusion and perspectives
Although numerous preclinical trials showed an improvement of the stroke outcome when circulating lymphocytes were reduced, the first clinical trials testing anti-inflammatory therapies in stroke have generated conflicting results. While fingolimod (FTY720) could reduce the infarct volume, natalizumab and enlimomab failed to show an effect on their primary endpoints.12–15 One of the reasons for these discrepancies might be due to the incomplete concept that lymphocytes infiltrate into the brain mainly via the transendothelial route of parenchymal capillaries, without considering the meninges and ChP. Natalizumab (anti-CD49d) and enlimomab (anti-ICAM-1) aimed to block the lymphocyte entrance to the CNS by blocking specific adhesion molecules required for trasnendothelial migration across parenchymal vessels. However, CD49d as well as ICAM-1 are both expressed at the apical side of the ChP epithelium but not on ChP endothelial cells.79 Hence, stromal lymphocytes in the ChP vasculature have no access to these adhesion molecules and blocking of these will not affect the ChP infiltration route. In contrast, fingolimod reduces the number of circulating lymphocytes independent of adhesion molecule expression at the various migration routes, which might explain why currently the only positive results on treatment efficacy are obtained with this drug in patients with stroke compared with natalizumab and enlimomab.
Our increasing understanding of the highly complex and anatomically as well as molecularly diverse structures of the three main invasion routes to the postischemic brain, the parenchymal vasculature, the meninges and the choroid plexus, reveals the previously unrecognized diversity of selective invasion routes for leukocyte subpopulations and poses a problem on efficiently targeting proinflammatory, neurotoxic leukocyte entry. Therefore, the results of current clinical trials aiming at blocking poststroke leukocyte migration need to be interpreted in light of the uncertainty regarding the involved invasion routes and their particular selectivity for specific subpopulations. Designing more efficient drugs targeting cerebral leukocyte invasion after stroke will require a much more detailed understanding of the contribution of the different invasion routes, their specifications in expression of chemotactic cues and adhesion molecules, and identification of site-specific pharmacological targets.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Excellence cluster of the German research foundation ‘‘Munich Cluster for Systems Neurology (SyNergy)’’ to AL.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Corinne Benakis  https://orcid.org/0000-0001-6463-7949
https://orcid.org/0000-0001-6463-7949
Contributor Information
Corinne Benakis, Institute for Stroke and Dementia Research, University Medical Center Munich, Feodor-Lynen-Str. 17, Munich 81377, Germany.
Gemma Llovera, Institute for Stroke and Dementia Research, University Medical Center Munich, Munich, Germany.
Arthur Liesz, Institute for Stroke and Dementia Research, University Medical Center Munich, Munich, Germany Munich Cluster for Systems Neurology (SyNergy), Munich, Germany.
References
- 1. Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009; 40: 1849–1857. [DOI] [PubMed] [Google Scholar]
- 2. Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003; 4: 399–414. [DOI] [PubMed] [Google Scholar]
- 3. Chamorro A, Meisel A, Planas AM, et al. The immunology of acute stroke. Nat Rev Neurol 2012; 8: 401–410. [DOI] [PubMed] [Google Scholar]
- 4. Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci 2012; 1268: 21–25. [DOI] [PubMed] [Google Scholar]
- 5. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benakis C, García-Bonilla L, Iadecola C, et al. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front Cell Neurosci 2014; 8: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liesz A, Zhou W, Na S-Y, et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci 2013; 33: 17350–17362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med 1999; 15: 946–950. [DOI] [PubMed] [Google Scholar]
- 9. Llovera G, Hofmann K, Roth S, et al. Results of a preclinical randomized controlled multicenter trial (pRCT): Anti-CD49d treatment for acute brain ischemia. Sci Transl Med 2015; 7: 299ra121–299ra121. [DOI] [PubMed] [Google Scholar]
- 10. Neumann J, Riek-Burchardt M, Herz J, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol 2014; 129: 259–277. [DOI] [PubMed] [Google Scholar]
- 11. Liesz A, Zhou W, Mracskó É, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain Pathology 2011; 134: 704–720. [DOI] [PubMed] [Google Scholar]
- 12. Zhu Z, Fu Y, Tian D, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation 2015; 132: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu Y, Zhang N, Ren L, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci USA 2014; 111: 18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elkins J, Veltkamp R, Montaner J, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol 2017; 16: 217–226. [DOI] [PubMed] [Google Scholar]
- 15. Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke Results of the Enlimomab Acute Stroke Trial. Neurology 2001; 57: 1428–1434. Epub ahead of print 2001. DOI: 10.1212/WNL.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 16. Veltkamp R, Gill D. Clinical trials of immunomodulation in ischemic stroke. Neurotherapeutics 2016; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 18. Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest 2010; 120: 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37: 13–25. [DOI] [PubMed] [Google Scholar]
- 20. Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med 2016; 22: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Llovera G, Benakis C, Enzmann G, et al. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol 2017; 1–18. [DOI] [PubMed] [Google Scholar]
- 22. Derecki NC, Cardani AN, Yang CH, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med 2010; 207: 1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sweeney MD, Sagare AP, Zlokovic BV. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018; 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pinheiro MAL, Kooij G, Mizee MR, et al. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. BBA Mol Basis Dis 2015; 1–11. [DOI] [PubMed] [Google Scholar]
- 25. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics 2016; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol 2012; 33: 579–589. [DOI] [PubMed] [Google Scholar]
- 27. Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 2003; 3: 569–581. [DOI] [PubMed] [Google Scholar]
- 28. Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol 2017; 1–13. [DOI] [PubMed] [Google Scholar]
- 29. Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol 2012; 18: 123–131. [DOI] [PubMed] [Google Scholar]
- 30. Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J 2014; 33: 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Engelhardt B, Sorokin L. The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 2009; 31: 497–511. [DOI] [PubMed] [Google Scholar]
- 32. Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol 2005; 26: 485–495. [DOI] [PubMed] [Google Scholar]
- 33. Ross MD, Burkel W. Electron microscopic observations of the nucleus, glial dome, and meninges of the rat acoustic nerve. Am J Anat 1997; 130: 73–91. [DOI] [PubMed] [Google Scholar]
- 34. Jiang X, Iseki S, Maxson RE, et al. Tissue origins and interactions in the mammalian skull vault. Dev Biol 2002; 241: 106–116. [DOI] [PubMed] [Google Scholar]
- 35. Adeeb N, Mortazavi MM, Tubbs RS, et al. The cranial dura mater: a review of its history, embryology, and anatomy. Childs Nerv Syst 2012; 28: 827–837. [DOI] [PubMed] [Google Scholar]
- 36. Nabeshima S, Reese TS, Landis DM, et al. Junctions in the meninges and marginal glia. J Comp Neurol 1975; 164: 127–169. [DOI] [PubMed] [Google Scholar]
- 37. Ma Q, Ineichen BV, Detmar M, et al. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 2017; 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 2011; 128: 309–316. [DOI] [PubMed] [Google Scholar]
- 39. Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 2016; 353: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neuwelt EA, Bauer B, Fahlke C, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 2011; 12: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 2008; 67: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 42. McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol 1999; 405: 553–562. [PubMed] [Google Scholar]
- 43. Korn T, Kallies A. T cell responses in the central nervous system. Nat Rev Immunol 2017; 1–16. [DOI] [PubMed] [Google Scholar]
- 44. Hutchings M, Weller RO. Anatomical relationships of the pia mater to cerebral blood vessels in man. J Neurosurg 1986; 65: 316–325. [DOI] [PubMed] [Google Scholar]
- 45. Lodygin D, Flügel A. Intravital real-time analysis of T cell activation in health and disease. Cell Calcium 2016; 1–41. [DOI] [PubMed] [Google Scholar]
- 46. Derecki N, Kipnis J. Mouse meninges isolation for FACS. Protocol Exchange. Epub ahead of print 8 September 2014. DOI: 10.1038/protex.2014.030. [DOI] [Google Scholar]
- 47. Derecki N, Louveau A, Kipnis J. Dissection and immunostaining of mouse whole-mount meninges. Protocol Exchange. Epub ahead of print 2 June 2015. DOI: 10.1038/protex.2015.047. [DOI] [Google Scholar]
- 48. Ghersi-Egea J-F, Strazielle N, Catala M, et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol 2018; 135: 337–361. [DOI] [PubMed] [Google Scholar]
- 49. Serot JM, Foliguet B, Béné MC, et al. Choroid plexus and ageing in rats: a morphometric and ultrastructural study. Eur J Neurosci 2001; 14: 794–798. [DOI] [PubMed] [Google Scholar]
- 50. Preston JE. Ageing choroid plexus-cerebrospinal fluid system. Microsc Res Tech 2001; 52: 31–37. [DOI] [PubMed] [Google Scholar]
- 51. Wolburg H, Wolburg-Buchholz K, Liebner S, et al. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett 2001; 307: 77–80. [DOI] [PubMed] [Google Scholar]
- 52. Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat Rev Immunol 2015; 16: 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol 2013; 13: 206–218. [DOI] [PubMed] [Google Scholar]
- 54. Lindvall M, Owman C. Autonomic nerves in the mammalian choroid plexus and their influence on the formation of cerebrospinal fluid. J Cereb Blood Flow Metab 1981; 1: 245–266. [DOI] [PubMed] [Google Scholar]
- 55. Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–462. [DOI] [PubMed] [Google Scholar]
- 56. Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci 2017; 20: 156–166. [DOI] [PubMed] [Google Scholar]
- 57. Yang Y, Yang LY, Orban L, et al. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain Stimul 2018; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Szmydynger-Chodobska J, Strazielle N, Zink BJ, et al. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J Cereb Blood Flow Metab 2009; 29: 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Uz A, Erbil KM, Esmer AF. The origin and relations of the anterior choroidal artery: an anatomical study. Folia Morphol (Warsz) 2005; 64: 269–272. [PubMed] [Google Scholar]
- 60. Ois A, Cuadrado-Godia E, Solano A, et al. Acute ischemic stroke in anterior choroidal artery territory. Journal of the Neurol Sci 2009; 281: 80–84. [DOI] [PubMed] [Google Scholar]
- 61. Neau JP, Bogousslavsky J. The syndrome of posterior choroidal artery territory infarction. Ann Neurol 1996; 39: 779–788. [DOI] [PubMed] [Google Scholar]
- 62. Ferrand Drake M. Cell death in the choroid plexus following transient forebrain global ischemia in the rat. Microsc Res Tech 2001; 52: 130–136. [DOI] [PubMed] [Google Scholar]
- 63. Dienel GA. Regional accumulation of calcium in postischemic rat brain. J Neurochem 1984; 43: 913–925. [DOI] [PubMed] [Google Scholar]
- 64. Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 1982; 11: 491–498. [DOI] [PubMed] [Google Scholar]
- 65. Ennis SR, Keep RF. The effects of cerebral ischemia on the rat choroid plexus. J Cereb Blood Flow Metab 2005; 26: 675–683. [DOI] [PubMed] [Google Scholar]
- 66. Krzyzanowska A, Carro E. Pathological alteration in the choroid plexus of Alzheimer’s disease: implication for new therapy approaches. Front Pharmacol 2012; 3: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Prinz M, Priller J, Sisodia SS, et al. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 2011; 13: 1227–1235. [DOI] [PubMed] [Google Scholar]
- 68. Kivisäkk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA 2003; 100: 8389–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nature Neuroscience 2017; 20: 136–144. [DOI] [PubMed] [Google Scholar]
- 70. Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 2012; 12: 623–635. [DOI] [PubMed] [Google Scholar]
- 71. Marin IA, Kipnis J. Central nervous system: (immunological) ivory tower or not? Neuropsychopharmacology 2017; 42: 28–35. Epub ahead of print 10 August 2016. DOI: 10.1038/npp.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gelderblom M, Weymar A, Bernreuther C, et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 2012; 120: 3793–3802. [DOI] [PubMed] [Google Scholar]
- 73. Kotani M, Kikuta J, Klauschen F, et al. Systemic circulation and bone recruitment of osteoclast precursors tracked by using fluorescent imaging techniques. J Immunol 2013; 190: 605–612. [DOI] [PubMed] [Google Scholar]
- 74. Gelderblom M, Arunachalam P, Magnus T. γδ T cells as early sensors of tissue damage and mediators of secondary neurodegeneration. Front Cell Neurosci 2014; 8: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bartholomäus I, Kawakami N, Odoardi F, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 2009; 461: 94–98. [DOI] [PubMed] [Google Scholar]
- 76. Kivisäkk P, Imitola J, Rasmussen S, et al. Localizing central nervous system immune surveillance: Meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol 2009; 65: 457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schläger C, Körner H, Krueger M, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 2016; 530: 349–353. [DOI] [PubMed] [Google Scholar]
- 78. Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol Mech Dis 2011; 6: 323–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Steffen BJ, Breier G, Butcher EC, et al. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol 1996; 148: 1819–1838. [PMC free article] [PubMed] [Google Scholar]
- 80. Carrithers MD, Visintin I, Viret C, et al. Role of genetic background in P selectin-dependent immune surveillance of the central nervous system. J Neuroimmunol 2002; 129: 51–57. [DOI] [PubMed] [Google Scholar]
- 81. Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 2008; 32: 200–219. [DOI] [PubMed] [Google Scholar]
- 82. Engelhardt B. Immune cell entry into the central nervous system: Involvement of adhesion molecules and chemokines. J Neurol Sci 2008; 274: 23–26. [DOI] [PubMed] [Google Scholar]
- 83. Sallusto F, Impellizzieri D, Basso C, et al. T-cell trafficking in the central nervous system. Immunol Rev 2012; 248: 216–227. [DOI] [PubMed] [Google Scholar]
- 84. Reboldi A, Coisne C, Baumjohann D, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 2009; 10: 514–523. [DOI] [PubMed] [Google Scholar]
- 85. Mrdjen D, Pavlovic A, Hartmann FJ, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 2018; 1–23. [DOI] [PubMed] [Google Scholar]
- 86. McMenamin PG, Wealthall RJ, Deverall M, et al. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res 2003; 313: 259–269. [DOI] [PubMed] [Google Scholar]
- 87. Nathanson JA, Chun LL. Immunological function of the blood-cerebrospinal fluid barrier. Proc Natl Acad Sci USA 1989; 86: 1684–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Serot JM, Foliguet B, Béné MC, et al. Ultrastructural and immunohistological evidence for dendritic-like cells within human choroid plexus epithelium. Neuroreport 1997; 8: 1995–1998. [DOI] [PubMed] [Google Scholar]
- 89. Serafini B, Columba-Cabezas S, Di Rosa F, et al. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol 2010; 157: 1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Felger JC, Abe T, Kaunzner UW, et al. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun 2010; 24: 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gelderblom M, Gallizioli M, Ludewig P, et al. IL-23 (Interleukin-23)–Producing conventional dendritic cells control the detrimental IL-17 (Interleukin-17) response in stroke. Stroke 2018; 49: 155–164. [DOI] [PubMed] [Google Scholar]
- 92. Mohammad MG, Tsai VWW, Ruitenberg MJ, et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest 2014; 124: 1228–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol 2016; 17: 9–17. [DOI] [PubMed] [Google Scholar]
- 94. Polfliet MMJ, Zwijnenburg PJG, van Furth AM, et al. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J Immunol 2001; 167: 4644–4650. [DOI] [PubMed] [Google Scholar]
- 95. García-Bonilla L, Brea D, Benakis C, et al. Endogenous protection from ischemic brain injury by preconditioned monocytes. bioRxiv 2018; 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Iadecola C, Kahles T, Gallo E, et al. Neurovascular protection by ischemic tolerance: role of nitric oxide. J Physiol 2011; 4137–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. García-Bonilla L, Racchumi G, Murphy M, et al. Endothelial CD36 contributes to postischemic brain injury by promoting neutrophil activation via CSF3. J Neurosci 2015; 35: 14783–14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ge R, Tornero D, Hirota M, et al. Choroid plexus-cerebrospinal fluid route for monocyte-derived macrophages after stroke. J Neuroinflammation 2017; 14: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Szmydynger-Chodobska J, Strazielle N, Gandy JR, et al. Posttraumatic invasion of monocytes across the blood-cerebrospinal fluid barrier. J Cereb Blood Flow Metab 2012; 32: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang RL, Chopp M, Chen H, et al. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J Neurol Sci 1994; 125: 3–10. [DOI] [PubMed] [Google Scholar]
- 101. Pérez-de Puig I, Miró-Mur F, Ferrer-Ferrer M, et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 2014; 129: 239–257. [DOI] [PubMed] [Google Scholar]
- 102. Roth TL, Nayak D, Atanasijevic T, et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature 2014; 505: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Carlos TM, Clark RSB, Franicola-Higgins D, et al. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol 1997; 61: 279–285. [DOI] [PubMed] [Google Scholar]
- 104. Mattila OS, Strbian D, Saksi J, et al. Cerebral mast cells mediate blood-brain barrier disruption in acute experimental ischemic stroke through perivascular gelatinase activation. Stroke 2011; 42: 3600–3605. [DOI] [PubMed] [Google Scholar]
- 105. Arac A, Grimbaldeston MA, Nepomuceno ARB, et al. Evidence that meningeal mast cells can worsen stroke pathology in mice. Am J Pathol 2014; 184: 2493–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]