Abstract
We analyzed the changes in absolute lymphocyte count and its changes over time in 139 patients treated with preoperative chemoradiotherapy for locally advanced rectal cancer. The baseline absolute lymphocyte count was defined as the median of absolute lymphocyte count levels measured during 30 days before preoperative chemoradiotherapy. Absolute lymphocyte count at 1 month, 0.5 to 1 year, 1 to 2 years, and 2 to 3 years were determined by the median values of the absolute lymphocyte counts during the respective periods. Absolute lymphocyte count decreased after delivering preoperative chemoradiotherapy, reached minimum level at 1 month, and then gradually increased after the completion of chemoradiotherapy. Baseline absolute lymphocyte count had significant correlations with the absolute lymphocyte count of every period (range of coefficient, 0.41-0.64, P < .001). The overall survival of the group with high baseline absolute lymphocyte count was significantly higher than that of the group with low baseline absolute lymphocyte count (5-year overall survival: 82.4% vs 62.9%, P = .012). In multivariable analyses, the baseline absolute lymphocyte count remained as a significant prognostic factor for overall survival, favoring the group with a high baseline absolute lymphocyte count (hazard ratio = 0.405, P = .017). This study showed that the level of baseline absolute lymphocyte count was an independent prognostic factor, and it correlated with the absolute lymphocyte counts across varying periods of treatments and follow-up in patients treated with preoperative chemoradiotherapy for rectal adenocarcinoma.
Keywords: rectal cancer, blood lymphocyte count, chemoradiotherapy, prognostic factor, neoadjuvant therapy
Introduction
The level of absolute lymphocyte count (ALC) has been demonstrated as one of the significant prognostic factors influencing survival in several malignancies. Pretreatment lymphopenia has been reported as a poor prognostic factor in patients with advanced cancer.1,2 Several studies have shown that treatment-induced lymphopenia is related to the poor survivals in small cell lung cancer, non-small cell lung cancer, pancreatic cancer, nasopharyngeal carcinoma, and high-grade glioma.3 -8 These studies suggest that ALC levels can act as surrogate markers to represent the level of host immunity and predict overall treatment outcomes.
However, ALC levels also have several limitations in serving as prognostic factors and markers for host immunity. This is because ALC levels show physiological variation along the circadian rhythm.9,10 Also, they can be influenced by lymphotoxic agents such as corticosteroids, irradiation, and chemotherapeutics.11 Furthermore, they represent only a small portion of the whole lymphocyte pool and do not provide information regarding their specific subpopulation. However, with a large amount of available laboratory data, collected in a daily routine clinical practice, the immunologic and prognostic values of ALC levels can be easily analyzed in all kinds of malignancies under various clinical settings. These thorough investigations offer valuable insights into enhancing the clinical outcomes of cancer therapies in the era of immunotherapy.
In this context, we aimed to investigate the clinical implications of the ALC in patients with rectal cancer receiving preoperative chemoradiotherapy (CRT). Preoperative CRT has been implemented as one of the standards of care in the treatment of locally advanced rectal cancer.12 Under the setting of preoperative CRT for rectal cancer, some researchers have already reported the potential clinical value of the ALC.13,14 The purpose of this study was to depict a comprehensive picture of ALC levels in preoperative CRT for rectal cancer and to analyze the prognostic value of baseline ALC.
Materials and Methods
Using the tumor registry database of our institution, we identified 139 patients with rectal adenocarcinoma who received preoperative CRT between October 2002 and March 2015. For initial staging workup, colonoscopy with biopsy, abdominopelvic computed tomography (CT) and/or pelvic magnetic resonance imaging (MRI), and chest CT or positron emission tomography (PET)-CT were routinely performed. Preoperative CRT was prescribed for patients who had a perirectal infiltration and/or lymph node metastasis on pelvic CT or MRI and a 0-1 of Eastern Cooperative Oncology Group performance status. Additionally, peripheral blood lymphocyte count of each patient was collected from the complete blood count with a differential count, which was performed as a routine clinical practice.
For 3-dimensional radiation therapy (RT), all patients underwent a simulation using a CT scan with 5-mm slice intervals. The clinical target volume (CTV) included the gross tumor, mesorectum, presacral space, and regional lymphatics. The planning target volume was expanded in all directions from the CTV by a margin of 0.5 to 1.0 cm. Radiation therapy was delivered with a dose of 45 to 50.4 Gy with a 3-field technique using posterior and bilateral beams.
Three cycles of chemotherapy were routinely administered. Two cycles of chemotherapy were administered concurrently with RT. After the completion of RT, 1 cycle of chemotherapy was repeated. At 4 to 6 weeks after the completion of the last cycle of chemotherapy, surgical resection was performed with low anterior resection or abdominoperineal resection (APR). By the discretion of a surgeon, 2 to 3 cycles of chemotherapy were added after surgery.
For the first year after surgery, the patients were followed up every 3 months. For the next 2 years, they were followed up every 6 months, after which they were checked up annually. We performed an abdominopelvic CT scan at each follow-up visit, and an optional PET-CT scan was performed annually.
We analyzed the patterns of ALC levels across the whole period of diagnosis, treatments, and follow-up. For each patient, we found a representative ALC value during each period by measuring the median ALC value during each respective period. Specifically, we set the start day of preoperative CRT as a reference time point, and we selected the following representative values from 5 different periods in each patient: (1) baseline value (−30 to 0 day), (2) value at 1 month (20-40 day), (3) value at 0.5 to 1 year, (4) value at 1 to 2 years, and (5) value at 2 to 3 years. With other clinical parameters affecting overall survival (OS) and disease-free survival (DFS), we analyzed the prognostic values of ALC levels. We divided the 2 groups according the median value of ALC. The comparison between 2 groups was analyzed with χ2 or Fisher exact test for categorical variables and Student t test or Mann-Whitney U test for continuous variables. The survival time was defined as the interval between the start date of preoperative CRT and date of last follow-up visit or events of death or recurrence. Survivals were calculated by the Kaplan-Meier method. The log-rank test and proportional hazard regression model (enter method) were used for univariable and multivariable analyses, respectively. Variables with a P value of less than .1 by a univariable analysis were entered for a multivariable analysis. We tested interactions among potential confounders by adding an interaction term to the Cox proportional hazards regression model. Two-sided P values less than .05 were considered statistically significant. All statistical analyses were performed using R statistical packages.15 This study was performed in accordance with the Helsinki II Declaration and were reviewed and approved by the institutional review board of Ajou University Hospital (AJIRB-MED-MDB-16-460). Acquisition of written informed consent was exempted.
Results
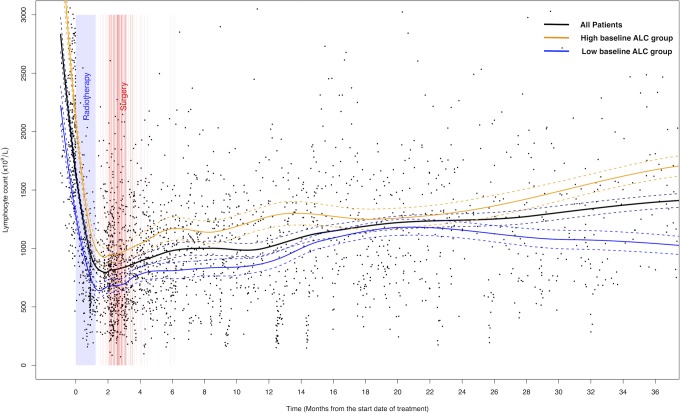
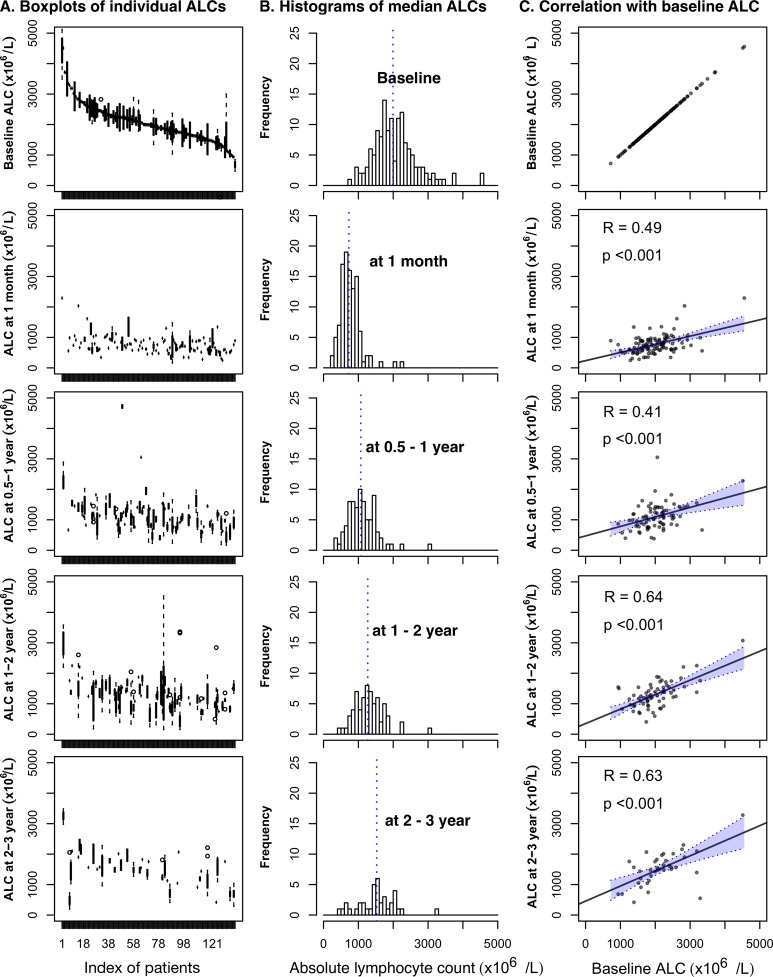
Peripheral blood ALC of 139 patients across the whole period of diagnosis, treatments, and follow-up are illustrated in Figure 1. The ALC trend line showed an abrupt decrease just after the start of preoperative CRT, reached its minimum level around 1 month, and then gradually increased over the period of surgery, adjuvant chemotherapy, and follow-up time. The median values of ALC at baseline, 1 month, 0.5 to 1 year, 1 to 2 years, and 2 to 3 years were 1993/µL (range, 720-4557), 728/µL (range, 247-2291), 1071/µL (range, 381-3050), 1269/µL (range, 403-3074), and 1527/µL (range, 419-3280), respectively (Figure 2A and B). The level of ALC showed inter- and intraindividual variabilities at every period. The interindividual variability decreased at a period of 1 month, and afterward it increased over time. Baseline ALC showed significant correlations with the lymphocyte counts at every time point (range of correlation coefficient, 0.41-0.64, P < .001; Figure 2C).
Figure 1.
Scatter plot of peripheral blood lymphocyte count over time. Solid lines are local regression trend lines and dotted lines indicate 95% confidence intervals of each trend line.
Figure 2.
Inter- and intrapatient variability of peripheral blood lymphocyte counts of 5 periods. (A) Boxplots of each patient’s ALC; indices of patients are ordered by the median ALC of each patient (interpatient variability). The vertical bar of each patient’s boxplot denotes the interquartile range of ALC (intrapatient variability). (B) Histograms of median ALC (interpatient variability). (C) Correlations of ALCs between baseline and other periods (intrapatient variability).
Using the median value of baseline ALC (1993/µL), we divided patients into 2 groups as high versus low baseline ALC. Between the groups with high and low baseline ALC, there was no significant difference in patient characteristics (Table 1). The mean ALC for the groups with high and low baseline count were 2530/µL and 1588/µL, respectively (P < .001).
Table 1.
Patient Characteristics Between the Groups With High and Low Level of Baseline Lymphocyte Count.
| High Baseline Count (≥1993 × 109/L), n = 70 | Low Baseline Count (<1993 × 109/L), n = 69 | P Value | |
|---|---|---|---|
| Age (year) | .158 | ||
| Mean (SD) | 54.4 (11.9) | 57.6 (12.2) | |
| Gender | .804 | ||
| Male | 55 | 52 | |
| Female | 15 | 17 | |
| Baseline lymphocyte count, ×109/L | <.001 | ||
| Mean (SD) | 2530 (537) | 1588 (288) | |
| Initial CEA | .605 | ||
| Mean (SD) | 13.6 (29.2) | 16.1 (29.0) | |
| Clinical T stage | .563 | ||
| T2 | 5 | 6 | |
| T3 | 56 | 50 | |
| T4 | 9 | 13 | |
| Clinical N stage | .995 | ||
| N0 | 8 | 8 | |
| N1 | 31 | 30 | |
| N2 | 31 | 31 | |
| Histologic gradea | .137 | ||
| WD | 9 | 3 | |
| MD | 51 | 51 | |
| PD | 6 | 10 | |
| Type of surgery | .672 | ||
| LAR | 37 | 33 | |
| APR | 33 | 36 | |
| Pathologic T stage | .563 | ||
| T0 | 13 | 12 | |
| T1 | 4 | 1 | |
| T2 | 15 | 17 | |
| T3 | 37 | 36 | |
| T4 | 1 | 3 | |
| Pathologic N stage | .133 | ||
| N0 | 54 | 49 | |
| N1 | 10 | 17 | |
| N2 | 6 | 2 | |
| Radiation dose, Gy | .686 | ||
| Mean (SD) | 48.4 (3.6) | 48.7 (3.9) | |
| Cycles of chemotherapy | 1.165 | ||
| Median (range) | 3 (1-3) | 3 (1-3) | |
| Use of GM-CSF | .426 | ||
| Yes | 2 | 5 | |
| No | 68 | 64 |
Abbreviations: APR, abdominoperineal resection; CEA, carcinoembryonic antigen; GM-CSF, granulocyte-macrophage colony-stimulating factor; LAR, low anterior resection; MD, moderately differentiated; PD, poorly differentiated; SD, standard deviation; WD, well differentiated.
a Histologic grade was available in 130 patients.
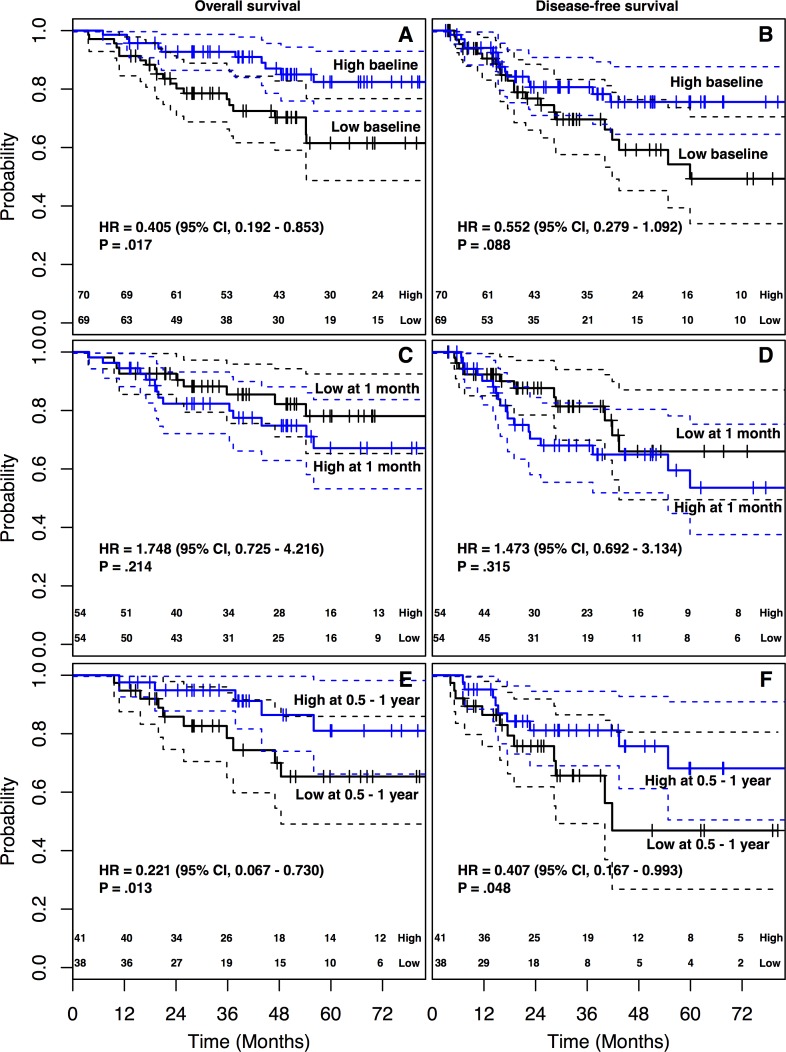
The follow-up time of 139 patients ranged from 3.6 to 125.9 months (median, 49.6), and the 5-year DFS and OS were 63.4% and 72.5%, respectively. The OS of the group with high baseline ALC was significantly higher than that of the group with low baseline ALC (5-year OS: 82.4% vs 62.9%, log-rank P = .012; Figure 3A). The group with high baseline ALC showed favorable DFS over the low baseline ALC group (5-year DFS: 75.6% vs 49.3%, log-rank P = .072; Figure 3B). Between the 2 groups, there were no significant differences in locoregional recurrence (7.1% vs 7.2%, P = 1.000), distant metastasis (11.4% vs 21.7%, P = .159), and both locoregional and distant failure rate (1.4% vs 1.4%, P = 1.000). There was no significant difference in the rate of cancer-related death between the groups with high and low baseline ALC (10.1% vs 18.6%, P = .241).
Figure 3.
Kaplan-Meier survival curves according to the level of ALC at baseline, 1 month, and 0.5 to 1 year. (A) Overall survival. (B) Disease-free survival.
Furthermore, we analyzed the prognostic values of the baseline ALC with other clinical parameters. Univariable analyses for clinical parameters affecting survivals are presented in Table 2. Pathologic responses (pathologic T/N stage) after preoperative CRT were significant factors affecting both DFS and OS. In multivariate analyses, pathologic N stage was a significant prognostic factor for both DFS (hazard ratio [HR] = 3.258, P = .001) and OS (HR = 4.309, P < .001; Table 3). Pathologic T stage showed significant prognostic value for OS (HR = 3.110, P = .049) but not for DFS (HR = 2.118, P = .100). The baseline ALC remained a significant prognostic factor for OS, favoring the group with the ALC high group (HR = 0.405, P = .017). Concerning DFS, the baseline ALC showed marginally significant prognostic value (HR = 0.552, P = .088). The level of ALC at 1 month showed no significant differences in both OS and DFS between the high and low ALC groups (Figure 3C and D). At 0.5 to 1 year, there were significant differences in ALC for survival, which also favored the high ALC group (Figure 3E and F).
Table 2.
Univariable Analyses for Clinical Variables Affecting Survivals.
| Variable | 5-Year DFS (%) | P Value (log-rank) | 5-Year OS (%) | P Value (log-rank) |
|---|---|---|---|---|
| Age (<56 vs ≥56) | 75.9 vs 57.4 | .825 | 75.8 vs 68.9 | .174 |
| Gender (male vs female) | 64.9 vs 59.2 | .448 | 73.8 vs 67.4 | .611 |
| Baseline lymphocyte count (low vs high) | 49.3 vs 75.6 | .072 | 62.9 vs 82.4 | .012 |
| Initial CEA (<4.5 vs ≥4.5) | 70.3 vs 55.1 | .052 | 79.5 vs 64.3 | .072 |
| Clinical T stage (1-2 vs 3-4) | 80.8 vs 61.8 | .523 | 68.2 vs 72.8 | .776 |
| Clinical N stage (0 vs 1-2) | 62.9 vs 63.6 | .961 | 66.7 vs 73.1 | .625 |
| Histologic grade (WD/MD vs PD) | 64.7 vs 56.5 | .210 | 73.6 vs 58.9 | .088 |
| Type of surgery (APR vs LAR) | 65.8 vs 62.7 | .761 | 68.6 vs 77.0 | .246 |
| Pathologic T stage (0-2 vs 3-4) | 80.8 vs 49.6 | .001 | 91.3 vs 58.6 | <.001 |
| Pathologic N stage (0 vs 1-2) | 73.7 vs 30.4 | <.001 | 84.8 vs 30.7 | <.001 |
Abbreviations: APR, abdominoperineal resection; CEA, carcinoembryonic antigen; DFS, disease-free survival; LAR, low anterior resection; MD, moderately differentiated; OS, overall survival; PD, poorly differentiated; WD, well differentiated.
Table 3.
Multivariable Analyses for Clinical Variables Affecting Survivals.
| Variable | DFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Histologic grade (WD/MD vs PD) | 1.089 | 0.412-2.883 | .863 | |||
| Initial CEA (<4.5 vs ≥4.5) | 1.315 | 0.643-2.690 | .452 | 1.497 | 0.698-3.211 | .300 |
| Baseline lymphocyte count (low vs high) | 0.552 | 0.279-1.092 | .088 | 0.405 | 0.192-0.853 | .017 |
| Pathologic T stage (0-2 vs 3-4) | 2.118 | 0.865-5.182 | .100 | 3.110 | 1.007-9.610 | .049 |
| Pathologic N stage (0 vs 1-2) | 3.258 | 1.575-6.741 | .001 | 4.309 | 1.984-9.357 | <.001 |
Abbreviations: CEA, carcinoembryonic antigen; CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; MD, moderately differentiated; OS, overall survival; PD, poorly differentiated; WD, well differentiated.
Acute toxicities related to CRT are summarized in Table 4. Lymphopenia and anemia with grade ≥3 developed more in the group with low baseline ALC than in the high baseline ALC group.
Table 4.
Acute Treatment-Related Toxicities Between the Groups With High and Low Level of Baseline Lymphocyte Count.
| Toxicity of CRT (grade)a | High Baseline Count (≥1993 × 109/L), n = 70, No. of Patients (%) | Low Baseline Count (<1993 × 109/L), n = 69, No. of Patients (%) | P Value |
|---|---|---|---|
| Neutopenia | .439 | ||
| 0 | 43 (63.2) | 40 (59.7) | |
| 1 | 12 (17.6) | 11 (16.4) | |
| 2 | 10 (14.7) | 7 (10.4) | |
| 3 | 2 (2.9) | 5 (7.5) | |
| 4 | 1 (1.5) | 4 (6.0) | |
| Lymphopenia | <.001 | ||
| 0 | 7 (10.3) | 0 (0.0) | |
| 1 | 10 (14.7) | 5 (7.5) | |
| 2 | 29 (42.6) | 14 (20.9) | |
| 3 | 20 (30.9) | 40 (59.7) | |
| 4 | 1 (1.5) | 8 (11.9) | |
| Thromocytopenia | .295 | ||
| 0 | 60 (88.2) | 52 (76.5) | |
| 1 | 7 (10.3) | 13 (19.1) | |
| 2 | 1 (1.5) | 2 (2.9) | |
| 3 | 0 (0.0) | 1 (1.5) | |
| Anemia | .030 | ||
| 0 | 11 (16.2) | 9 (13.2) | |
| 1 | 33 (48.5) | 19 (27.9) | |
| 2 | 19 (27.9) | 27 (39.7) | |
| 3 | 5 (7.4) | 13 (19.1) | |
| Nausea/vomiting | .733 | ||
| 0-2 | 51 (72.9) | 53 (76.8) | |
| 3 | 19 (27.1) | 16 (23.2) | |
| Diarrhea | .627 | ||
| 0-2 | 60 (85.7) | 62 (89.9) | |
| 3 | 10 (14.3) | 7 (10.1) | |
| Gastroparesis | .483 | ||
| 0-2 | 68 (97.1) | 69 (100.0) | |
| 3 | 2 (2.9) | 0 (0.0) |
Abbreviation: CRT, chemoradiotherapy.
a Toxicity grade of the Common Terminology Criteria for Adverse Events version 4.03.
We compared survivals of the group showing grade ≥3 CRT-related lymphopenia (< 500/µL) with those of grade 0 to 2 (≥500/µL), and there were no significant differences in OS (5-year OS: 67.9% vs 79.7%, log-rank P = .089) and DFS (5-year DFS: 56.5% vs 71.5%, log-rank P = .607) between the 2 groups.
Discussion
In this study, we investigated the patterns of ALC levels from the time of diagnosis to the time of long-term follow-up and observed that the peripheral blood ALC had a specific change pattern depending on the phase of treatments. Specifically, ALC dropped immediately after the start of preoperative CRT, reached its minimum level at 1 month after the start of CRT, and gradually increased although not normalizing even after 3 years. That ALC abruptly fell to its minimum levels during preoperative CRT implies that CRT is a major cause of decrease in ALC. In fact, it is well known that CRT induces radiation-related lymphopenia in various malignancies,3–8 and the decrease in count during the acute phase is contributed mainly by irradiation of circulating lymphocytes when passing through the radiation fields.11,16 After the completion of preoperative CRT, the level of ALC increased gradually even during the time of surgery and adjuvant chemotherapy. Although the end of radiation leads to a gradual increase in lymphocyte count, lymphocyte homeostasis via interleukin-7 may also contribute to this recovery.17 However, full recovery to the baseline level was not shown until 3 years after the start of preoperative CRT, possibly due to the long-lasting bone marrow suppression of pelvic irradiation.18
It has been reported that the level of ALC sampled at various points in time would affect clinical outcomes. Moreover, there are several ALC-related parameters showing prognostic values such as the neutrophil-to-lymphocyte ratio,19 platelet-to-lymphocyte ratio,20 and the prognostic nutritional index.21 These reports regarding the prognostic value of the ALC and its derived metrics suggest that the level of ALC itself could be a useful marker to represent host antitumor immunity. However, despite its potential as an immunologic marker, the level of ALC has been considered an unreliable parameter because of its dynamic changes even within a span of few hours. Therefore, we implemented the median level of ALC during periods of specific clinical circumstances to overcome and adjust for its variability. We observed the variability of ALC levels at each time-period (Figure 2A), which implies that there may be a sampling bias. Because our data was not collected by standardized protocols, but from routine clinical practices, our results for adopting median value may be also biased. However, the consistent correlation between baseline ALC and ALC values of other periods implies that our method can be a reliable strategy in analyzing the ALC data collected in other various clinical settings. To ensure further reliability and clinical validity, further investigations using standardized sampling protocols are warranted.
The consistent correlations among the ALC levels in differing periods of time suggest that although absolute value might change depending on clinical circumstances, the relative level in the whole population remains stable. The results of this study showed that the baseline ALC had a significant prognostic value in patients treated with preoperative CRT for rectal cancer. Considering the correlation of ALC levels is relatively stable, the prognostic value of ALC should also be stable regardless of its timings. Our results showed that the ALC at 0.5 to 1 year after the start of preoperative CRT had a significant prognostic value (Figure 2E and F). However, there were no significant differences in DFS and OS with the count at 1 month (Figure 2C and D). This loss in prognostic value of the ALC at 1 month may be explained by the loss of discriminating power due to the relatively narrow range of ALC values. These findings suggest that the optimal time to measure lymphocyte counts for prognostic value would be during periods of high standard deviation, such as the periods of diagnostic workups.
Despite no differences of patient characteristics between groups with high and low baseline ALC, the results of this study show that the baseline ALC was an independent prognostic factor affecting OS and a marginally significant value for DFS. These findings suggest that the level of ALC might be related to the level of host immunity, which could suppress the cancer progression.22 However, superior OS favoring the high ALC group was not fully explained by the high trend of its DFS, suggesting the presence of other lymphocyte-associated factors affecting OS. Low ALC may have limited the use of additional salvage chemotherapy or lowered its treatment responses. Also, lymphopenia may have contributed to the higher risk of infection. These unexplained factors should be investigated in terms of immunologic perspective because ALC can be a prognostic or predictive factor in the use of immunotherapy.23
The results of this study are limited due to its retrospective design with a lack of information regarding detailed lymphocyte subpopulations. Moreover, ALC was checked at large periods of time and it made impossible to determine the specific time of checking of ALC for prognostic value. However, this study described the trend of ALC across the whole clinical course and showed the prognostic value in rectal cancer treated with preoperative CRT. Given the huge available amount of laboratory data during clinical practices and trials, further studies may be conducted to confirm the immunologic and prognostic value of ALC in various clinical settings including the use of immunotherapy.
In conclusion, baseline ALC was significantly correlated with other counts across the periods of treatments and even follow-up. The baseline ALC was an independent significant prognostic factor in patients treated with preoperative CRT for rectal adenocarcinoma. Further studies are warranted to investigate the correct timing of ALC count and the ALC value, which can be used to stratify patients.
Acknowledgments
The authors thank Stephen Kim for assistance with manuscript editing.
Abbreviations
- ALC
absolute lymphocyte count
- APR
abdominoperineal resection
- CRT
chemoradiotherapy
- CT
computed tomography
- CTV
clinical target volume
- DFS
disease-free survival
- HR
hazard ratio
- MRI
magnetic resonance imaging
- OS
overall survival
- PET
positron emission tomography
- RT
radiation therapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the new faculty research fund of Ajou University School of Medicine.
References
- 1. Lissoni P, Brivio F, Fumagalli L, et al. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers. 2004;19(2):135–140. [DOI] [PubMed] [Google Scholar]
- 2. Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho O, Oh YT, Chun M, Noh OK, Lee HW. Radiation-related lymphopenia as a new prognostic factor in limited-stage small cell lung cancer. Tumour Biol. 2016;37(1):971–978. [DOI] [PubMed] [Google Scholar]
- 4. Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31(3):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30(8):571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wild AT, Ye X, Ellsworth SG, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38(3):259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho O, Oh YT, Chun M, Noh OK, Hoe JS, Kim H. Minimum absolute lymphocyte count during radiotherapy as a new prognostic factor for nasopharyngeal cancer. Head Neck. 2016;38(suppl 1):E1061–E1067. [DOI] [PubMed] [Google Scholar]
- 8. Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13(3):190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki S, Toyabe S, Moroda T, et al. Circadian rhythm of leucocytes and lymphocytes subsets and its possible correlation with the function of the autonomic nervous system. Clin Exp Immunol. 1997;110(3):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94(3):571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. [DOI] [PubMed] [Google Scholar]
- 13. Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heo J, Chun M, Noh OK, et al. Sustaining blood lymphocyte count during preoperative chemoradiotherapy as a predictive marker for pathologic complete response in locally advanced rectal cancer. Cancer Res Treat. 2016;48(1):232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. R Development Core Team. R: A language and environment for the statistical computing, R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/. 2016.
- 16. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31(2):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11(5):330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cozzarini C, Chiorda BN, Sini C, et al. Hematologic toxicity in patients treated with postprostatectomy whole-pelvis irradiation with different intensity modulated radiation therapy techniques is not negligible and is prolonged: preliminary results of a longitudinal, observational study. Int J Radiat Oncol Biol Phys. 2016;95(2):690–695. [DOI] [PubMed] [Google Scholar]
- 19. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6): dju124. [DOI] [PubMed] [Google Scholar]
- 20. Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204–1212. [DOI] [PubMed] [Google Scholar]
- 21. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688–2692. [DOI] [PubMed] [Google Scholar]
- 22. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. [DOI] [PubMed] [Google Scholar]
- 23. Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]