Abstract
Background and Purpose:
Stroke is the number one cause of morbidity and mortality in Indonesia. Lacunar infarction is one of cerebral small vessel disease spectrum. This study aimed to present stroke epidemiology in Indonesia and risk factors associated with cerebral small vessel disease.
Methods:
A multicenter prospective cross-sectional study of 18 hospitals in Indonesia was conducted using Stroke Case Report Form from 2012 to 2014. Stroke was diagnosed based on clinical findings confirmed with non-contrast computed tomography of the brain. Subjects were classified into two large groups: ischemic (lacunar and non-lacunar) and hemorrhagic (intracranial and subarachnoid hemorrhage). Other risk factors were assessed on admission.
Results:
We enrolled 5411 patients, of whom 3627 (67.03%) had ischemic stroke and 1784 (32.97%) had hemorrhagic stroke. Male patients were prevalent in both large groups, although found less in subarachnoid hemorrhage group. Among patients with hemorrhagic stroke, 1603 (89.54%) of them had intracerebral hemorrhage and 181 (10.46%) had subarachnoid hemorrhage. From 3627 ischemic stroke patients, 1635 (45.07%) of them had lacunar infarction. We found that age above 55 years old, male gender, hypertension, dyslipidemia, and diabetes were important risk factors associated with lacunar stroke (p < 0.05).
Conclusion:
Ischemic stroke was the leading cause of stroke in Indonesia. In total, 45% of the total ischemic stroke patients had lacunar infarction. Important risk factors associated with lacunar infarction were hypertension, dyslipidemia, diabetes, age over 55, and male population.
Keywords: Cerebral small vessel disease, epidemiology, risk factors
Introduction
Stroke is the leading cause of mortality and morbidity in Indonesia.1,2 One of stroke subtypes is lacunar infarction which can be diagnosed either by clinical examinations in concordance with Bamford criteria or by brain computed tomography (CT) scan which is seen as small hypodensity areas in basal ganglia, brainstem, or subcortical structures. Lacunar infarction is one part of cerebral small vessel disease spectrum. Cerebral small vessel disease’s (CSVD) magnetic resonance imaging (MRI) neuroimaging features include white matter lesions, microbleeds, lacunar infarction, enlargement of perivascular spaces, and cerebral atrophy.3,4 CSVD refers to clinical symptoms and neuroimaging that are thought to arise from damaged arterioles, capillaries, and venules in brain parenchyma.5
The availability of modern and expensive medical equipments in Indonesia is still challenging. The adoption in developing countries relies on government, manufacturers, health care organizations, third-party payers, and medical doctors.6 Therefore, CSVD diagnosis in Indonesia may defy from usual norms which combines clinical examinations and CT scan.
The knowledge of CSVD is important because it can help to reduce the incidence of both vascular dementia and Alzheimer dementia.7 Early diagnosis should be made as soon as possible and manage their risks to reduce the dementia. In this article, we would like to present descriptively the demographic characteristics and stroke sub-types, emphasizing on lacunar infarction (small vessel disease) and the risk factors associated.
Materials and methods
Study population
This was a multicenter hospital-based cross-sectional study using standardized Stroke Case Report Form by the Indonesian Ministry of Health. This study already had ethical clearance from Indonesian Ministry of Health number LB.02.01/5.2/KE.423/2014. All stroke patients included from 1 January 2012 until 31 December 2014 in 18 hospitals gave written informed consent. The hospitals involved in the study are listed in Table 1. Out of the 18 hospitals, 10 hospitals were type A or top referral hospitals, 7 hospitals were type B or referral hospitals, and 1 hospital was type C or community-based hospital with stroke treatment facility.
Table 1.
The list of hospitals involved in the study.
| No. | Hospital | Province |
|---|---|---|
| 1 | Dr Zainoel Abidin Hospital | Aceh |
| 2 | Dr M Djamil Hospital | West Sumatera |
| 3 | Bukit Tinggi National Stroke Hospital | West Sumatera |
| 4 | Reksodiwiryo Hospital | West Sumatera |
| 5 | Dr Cipto Mangunkusumo Hospital | DKI Jakarta |
| 6 | Dr Hasan Sadikin Hospital | West Java |
| 7 | Bekasi City Hospital | West Java |
| 8 | Dr Kariadi Hospital | Central Java |
| 9 | Dr Moewardi Hospital | Central Java |
| 10 | Mardi Rahayu Hospital | Central Java |
| 11 | Prof. Dr. Margono Soekarjo Hospital | Central Java |
| 12 | Dr Sardjito Hospital | DI Yogyakarta |
| 13 | Bethesda Hospital | DI Yogyakarta |
| 14 | Dr Soetomo Hospital | East Java |
| 15 | Dr Saiful Anwar Hospital | East Java |
| 16 | Premier Surabaya Hospital | East Java |
| 17 | Sanglah Hospital | Bali |
| 18 | St. Antonius Hospital | West Kalimantan |
Diagnosis categories
The diagnosis of stroke was taken from the medical record made by registered neurologists. The definition of stroke was focal or global neurological signs due to vascular origin that lasted at least 24 h. Stroke severity was assessed by the National Institutes of Health Stroke Scale (NIHSS) and standard imaging ordered was brain CT scan without contrast. Hemorrhagic stroke was defined if there was any sign of hyperdensities on brain CT scan either in brain parenchyma or subarachnoid spaces. All patients underwent standardized laboratory examination, including hematology indices, coagulation assay, chest X-ray, and electrocardiography (ECG). Ischemic stroke was classified in concordance with Bamford (Oxfordshire Community Stroke Project) criteria. For this study purpose, the classification was simplified as stroke with lacunae involvement (or lacunar group) and non-lacunar stroke. Lacunar stroke was defined if there were pure motor deficits including face, arm, and leg without speech, sensory disturbances, or ataxic hemiplegia. On CT scan, there might be found at least one small area of hypodensities with diameter 3–20 mm and not directly correlated with current clinical manifestations.4 Clinical findings alone without CT abnormalities were included because the probability of lacunar infarction seen on CT scan between 24 and 72 h was 28%.8 New-onset lacunar infarction patients without any CT scan finding were also included as lacunar group.
Risk factors including hypertension, smoking, diabetes, and dyslipidemia were assessed in all subjects. They were defined as follows. Hypertension was present if systemic blood pressure was elevated above 140 mmHg and/or diastolic blood pressure above 90 mmHg for at least 1 week before stroke onset or was currently consuming anti-hypertensive medication. Diabetes mellitus was diagnosed in accordance with American Diabetes Association (ADA) definition where at least two random glucose tests showed above 200 mg/dL or fasting blood glucose was above 126 mg/dL. Dyslipidemia was present if total serum cholesterol was above 200 mg/dL or triglycerides (TGs) serum was above 150 mg/dL, or low-density lipoprotein (LDL) was above 130 mg/dL or high-density lipoprotein (HDL) below 40 mg/dL or currently taking statin medication. Smoking was assessed during history taking.
Statistical analysis
Data were analyzed using SPSS for Windows version 20. Data were presented in mean and standard deviation if normally distributed. Otherwise, they were presented in median and minimum-maximum value. The mean difference between lacunar and non-lacunar subgroup was analyzed using Student’s t test if data followed Gaussian distribution. Otherwise, they were compared using Mann–Whitney test. Risk factors of lacunar infarction were determined using binary logistic regression analysis only if they showed significance <0.20 in bivariate analysis.
Results
Between 1 January 2012 and 28 December 2014, there were 5411 stroke patients enrolled in this study. The proportion of male gender across all groups was 3059 (56.53%). Male predominance was prevalent in ischemic stroke and ICH, except those with subarachnoid hemorrhage (SAH; Table 2). The mean age of patients with ischemic and hemorrhagic stroke was 59.74 ± 11.79 and 57.29 ± 12.48 years, respectively. The highest age group population who suffered from stroke was above 60 years of age, which encompassed 1610 (44.39%) ischemic stroke patients and 657 (36.83%) hemorrhagic stroke patients.
Table 2.
Subject characteristics.
| Ischemic stroke (n = 3627) |
Hemorrhagic stroke (n = 1784) |
|||
|---|---|---|---|---|
| Lacunar (n = 1635) | Non-lacunar (n = 1992) | SAH (n = 181) | ICH (n = 1603) | |
| Male, n (%) | 970 (59.33) | 1099 (55.17) | 84 (46.41) | 906 (56.52) |
| Age, years | 59.74 ± 11.79 | 57.29 ± 12.48 | ||
| Age, n (%) | ||||
| <40 years | 75 (4.57) | 96 (4.80) | 16 (8.53) | 121 (7.52) |
| 41–50 years | 262 (16.04) | 345 (17.33) | 35 (19.51) | 352 (21.98) |
| 51–60 years | 543 (33.18) | 696 (34.96) | 62 (34.15) | 541 (33.73) |
| >60 years | 755 (46.20) | 855 (42.91) | 68 (37.81) | 589 (36.77) |
| Systolic blood pressure, mmHg | 154.01 ± 26.98 | 152.49 ± 27.45 | 163.36 ± 31.35 | 166.64 ± 29.29 |
| Diastolic blood pressure, mmHg | 91.85 ± 14.30 | 91.45 ± 13.80 | 95.53 ± 15.16 | 96.88 ± 15.27 |
| Risk factors, n (%) | ||||
| Hypertension | 1330 (81.35) | 1542 (77.41) | 150 (82.87) | 1429 (89.15) |
| Diabetes | 376 (23.00) | 498 (25.00) | 23 (12.71) | 186 (11.60) |
| Dyslipidemia | 261 (15.96) | 318 (15.96) | 17 (9.39) | 171 (10.67) |
| Smoking | 468 (28.62) | 545 (27.36) | 40 (22.10) | 432 (26.95) |
| ECG LVH, n (%) | 230 (14.07) | 262 (13.15) | 26 (14.36) | 278 (17.34) |
SAH: subarachnoid hemorrhage; ICH: intracerebral hemorrhage; ECG LVH: electrocardiographic left ventricular hypertrophy.
Data presented in mean ± SD.
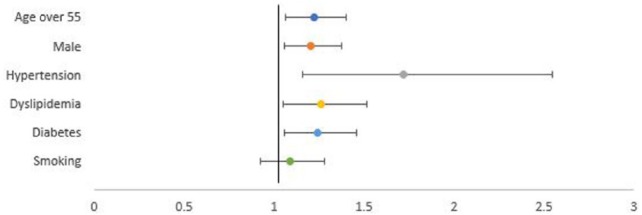
Eventually, 3627 (67.03%) patients had ischemic stroke and further analysis showed 1635 (45.07%) had evidence of lacunar infarction on non-contrast CT brain. We tried to find the best fit model to predict risks in developing lacunar infarction. Age above 55 years old (p = 0.001), male gender (p = 0.007), hypertension diagnosed at admission (p = 0.234), dyslipidemia (p = 0.004), diabetes (p < 0.001), and smoking (p = 0.209) were eligible for logistic regression analysis. Results are shown in Table 3. All were associated with lacunar infarction, except smoking (odds ratio (OR), 1.086; 95% confidence interval (CI), 0.922−1.279). The most important risk factors for lacunar infarction were hypertension, followed by dyslipidemia, diabetes, age over 55, and male gender (Table 3 and Figure 1).
Table 3.
Risk factors associated with lacunar infarction (n = 1563).
| Risk factors | OR (95% CI) | SE | p |
|---|---|---|---|
| Age ⩾55 | 1.223 (1.065–1.403) | 0.070 | 0.004 |
| Male gender | 1.204 (1.055–1.374) | 0.067 | 0.006 |
| Hypertension | 1,719 (1,161–2.545) | 0.200 | 0.007 |
| Diabetes | 1.241 (1.056–1.461) | 0.083 | 0.010 |
| Dyslipidemia | 1.261 (1.049–1.517) | 0.094 | 0.014 |
| Smoking | 1.086 (0.922–1.279) | 0.084 | 0.325 |
OR: odds ratio; CI: confidence interval; SE: standard error.
Figure 1.
Risk factors associated with lacunar infarction.
Discussion
In our study, stroke in general was prevalent in males, except those with SAH. We also found that age above 60 years were dominant in each stroke subtype although when it was combined with age 51–60 years resulted in more than half study population. Zhang et al.9 concluded that the incidence, prevalence, and mortality due to stroke both increase with age and are greater in males compared to females in Europe and the United States. Most epidemiological studies of stroke in Asia also showed similar results.10–12 SAH is unique where female gender is more prevalent with ratio from 1.2:1 to 3.1:1.13
Ischemic stroke is the most common type of stroke accounting for 67.03% of the total study population. Studies from Asian countries indicate that the proportion of ICH is higher than in Caucasians with approximately 20%–30% being hemorrhagic.14 Similar results were also reported in South Asian countries like India (68%–80%), Pakistan (69%–78%), Sri Lanka (75%), and Bangladesh (54%–80%).11 On the other hand, in the United States, the prevalence of ischemic stroke was 87% and hemorrhagic stroke was only 13%.15 A nationwide Danish stroke registry concluded that 89.9% had ischemic stroke and 10.1% had hemorrhagic stroke.16 In Caucasian populations, approximately 80% of all strokes are ischemic, 10%–15% intracerebral hemorrhage (ICH), 5% SAH, and the rest is due to other causes of stroke.
In our study, 45.07% of the total ischemic stroke had lacunar infarction corresponding to previous studies which stated that lacunar stroke is the highest cause of stroke in Asia.1 Other Asian countries gave similar results where the prevalence of ischemic stroke is higher and lacunar infarction had high prevalence among the ischemic stroke sub-types.11,13,17–19 Mehndiratta et al.20 did a review on the geographical variations and temporal trends of stroke in Asia. In that review, among ischemic strokes, China, Taiwan, and Pakistan had high proportion of small vessel disease.20 In comparison, the prevalence of lacunar infarction as the overall cause of stroke in China was 33.1% while the prevalence in Japan was as high as 54.1%.21,22
Our study also analyzed the risk factors associated with lacunar infarction and concluded that hypertension diagnosed at admission is also the highest risk factor associated with lacunar infarction. Hypertension is a traditional risk factor for small vessel disease.3,23,24 Shear stress results in arteriolosclerosis development in penetrating vessels. Antihypertensive drugs are beneficial in reducing dementia risk.25 Unfortunately, the lesions still persist although patients have consumed antihypertensive drugs.23 Otherwise, in older patients, hypertension was not associated with white matter lesion progression.23
We used age over 55 to bridge two major age groups which were 51–60 and above 60 years old. With regard to Asian countries, developing age was correlated with CSVD development and progression.26 Age above 75 years was reported as the most severe CSVD condition.27
Our study reported the association between male gender and CSVD. The reports on sex are variable which depend on study design and CSVD evaluation techniques used.24,28–31 Staals et al.24 also found the combination of developing age, male gender, and hypertension as risk factors for CSVD development.
Diabetes was also considered as an important risk factor of isolated lacunar infarction.32 Diabetic patients with retinopathy were strongly associated with CSVD development and severity.33 It is also associated with poorer prognosis in lacunar stroke.34 We consecutively included all stroke patients in this study while other studies might selectively include subjects.
Apparently, we found no association between smoking and lacunar infarction development. We only explored whether patients had ever smoked in their life, but did not explore how long and how many cigarettes burned every day. Other studies found that current smoking was positively associated with CSVD.23,24 Smoking is associated with microstructure disruption in white matter and development of white matter hyperintensity.23,35
Serum total cholesterol was also a risk factor for lacunar stroke.36 TGs level both with and without lowering drugs was important in white matter lesion development.37 It is hypothesized that TGs produce inflammation and endothelial dysfunction during lipolysis, reduce small artery compliance, and disrupt blood–brain barrier.38–40
Our result is interesting to be more explored in the future. As mentioned in Stroke Prevention by Aggresive Reduction of Cholesterol Levels (SPARCL) study, the risk of secondary hemorrhagic stroke was increased if patients with lacunar stroke or hemorrhagic stroke consumed high-dose statins.41 Our data also showed that the proportion of dyslipidemia in hemorrhagic stroke was lower than ischemic stroke. We preferred the non-pharmacological approach to manage dyslipidemia in lacunar infarction or CSVD. Although it is still non-conclusive, low plasma omega-3 level was associated with CSVD radiological findings and cognitive function.42,43 Furthermore, we prescribed cilostazol as the treatment of choice in patients with CSVD, instead of aspirin.44
Diverse risk factors associated with CSVD may be explained by Boiten et al.45 It was proposed that there are two types of CSVD. The first one is multiple small lacunar infarct associated with leukoaraiosis and hypertension where the underlying pathogenesis is diffuse arteriopathy.46 The second one is single large lacunar infarct without leukoaraiosis where atherosclerosis is the underlying mechanism. These two different mechanisms are proposed by Kim and Kim47 as the probable cause of high lacunar infarction prevalence in Asian population is possibly due to these two different mechanisms. The latter group is associated with proximal atherosclerosis stenosis in other major arteries; therefore, it is more correctly classified into large-artery disease. This still needs further validation.
This study is the first to report stroke profile in Indonesia and most developing countries might face, particularly lacunar stroke, but is still far from perfect. First, it was only based on brain CT scan without contrast where MRI is the more preferred choice. Second, the study sample size was considered low for Indonesia because there was still economic burden for patients even to undertake CT scan. Therefore, we only provided basic characteristic data in this study. This registry did not enroll patients where brain CT scan was not performed. Third, this was only cross-sectional study and we did not explore the causal relationship between risk factors and CSVD development and progression. Fourth, vessel studies (carotid ultrasound, transcranial Doppler (TCD), computed tomography angiography (CTA), or magnetic resonance angiography (MRA)), echocardiography, and continuous ECG monitoring were not performed, so that non-lacunar stroke might be under-estimated.
Conclusion
In this study, we concluded that the prevalence of ischemic stroke in Indonesia is 67% which was higher than hemorrhagic stroke, with 45% of ischemic stroke patients with lacunar infarction. The most important risk factors for lacunar infarction were hypertension, followed by diabetes, dyslipidemia, age over 55, and male population.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Indonesian Ministry of Health (Approval number LB.02.01/5.2/KE.423/2014).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Indonesian Ministry of Health and the Indonesian Neurological Association.
Informed consent: Written informed consent was obtained from all subjects before the study.
ORCID iD: Salim Harris  https://orcid.org/0000-0002-4271-2933
https://orcid.org/0000-0002-4271-2933
References
- 1. Hoy DG, Rao C, Hoa NP, et al. Stroke mortality variations in South-East Asia: empirical evidence from the field. Int J Stroke 2013; 8: 21–27. [DOI] [PubMed] [Google Scholar]
- 2. Kusuma Y, Venketasubramanian N, Kiemas LS, et al. Burden of stroke in Indonesia. Int J Stroke 2009; 4: 379–380. [DOI] [PubMed] [Google Scholar]
- 3. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 4. Wardlaw JM, Smith C, Dichgans M. Mechanisms underlying sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12: 70060–70067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rincon F, Wright CB. Current pathophysiological concepts in cerebral small vessel disease. Front Aging Neurosci 2014; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silva HP, Viana ALD. Health technology diffusion in developing countries: a case study of CT scanners in Brazil. Health Policy Plan 2011; 26: 385–394. [DOI] [PubMed] [Google Scholar]
- 7. Makin SDJ, Turpin S, Dennis MS, et al. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry 2013; 84: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown JJ, Hesselink JR, Rothrock JF. MR and CT of lacunar infarcts. Am J Neuroradiol 1988; 9: 477–482. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Chapman AM, Plested M, et al. The incidence, prevalence, and mortality of stroke in France, Germany, Italy, Spain, the UK, and the US: a literature review. Stroke Res Treat 2012; 2012: 436125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suwanwela NC. Stroke epidemiology in Thailand. J Stroke 2014; 16: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wasay M, Khatri IA, Kaul S. Stroke in South Asian countries. Nat Rev Neurol 2014; 10: 135–143. [DOI] [PubMed] [Google Scholar]
- 12. Kooi CW, Peng HC, Aziz ZA, et al. A review of stroke research in Malaysia from 2000–2014. Med J Malaysia 2016; 71: 58–69. [PubMed] [Google Scholar]
- 13. Hamdan A, Barnes J, Mitchell P. Subarachnoid hemorrhage and the female sex: analysis of risk factors, aneurysm characteristics, and outcomes. J Neurosurg 2014; 121: 1367–1373. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization (WHO). The global burden of disease: 2004 update. Geneva: WHO. [Google Scholar]
- 15. Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics 2011; 8: 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andersen KK, Olsen TS, Dehlendorff C, et al. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke 2009; 40: 2068–2072. [DOI] [PubMed] [Google Scholar]
- 17. Toyoda K. Epidemiology and registry studies of stroke in Japan. J Stroke 2013; 15: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lv P, Jin H, Liu Y, et al. Comparison of risk factor between lacunar stroke and large artery atherosclerosis stroke: a cross-sectional study in China. PLoS ONE 2016; 11: e0149605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Syed NA, Khealani BA, Ali S, et al. Ischemic stroke subtypes in Pakistan: the Aga Khan University stroke data bank. J Pak Med Assoc 2003; 53: 584–588. [PubMed] [Google Scholar]
- 20. Mehndiratta MM, Khan M, Mehndiratta P, et al. Stroke in Asia: geographical variations and temporal trends. J Neurol Neurosurg Psychiatry 2014; 85: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 21. Turin TC, Kita Y, Rumana N, et al. Ischemic stroke subtypes in a Japanese population: Takashima stroke registry, 1988-2004. Stroke 2010; 41: 1871–1876. [DOI] [PubMed] [Google Scholar]
- 22. Tsai CF, Thomas B, Sudlow CLM. Epidemiology of stroke and its subtypes in Chinese vs white populations. Neurology 2013; 81: 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Dijk EJ, Prins ND, Vrooman HA, et al. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke 2008; 39: 2712–2719. [DOI] [PubMed] [Google Scholar]
- 24. Staals J, Makin SDJ, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014; 83: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 26. Hilal S, Mok V, Youn YC, et al. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry 2017; 88: 669–674. [DOI] [PubMed] [Google Scholar]
- 27. Simoni M, Li L, Paul NLM, et al. Age- and sex-specific rates of leukoaraiosis in TIA and stroke patients population-based study. Neurology 2012; 79: 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009; 8: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vermeer SE, Longstreth WT, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007; 6: 611–619. [DOI] [PubMed] [Google Scholar]
- 30. Poels MMF, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 2010; 41: S103–S116. [DOI] [PubMed] [Google Scholar]
- 31. Launer LJ. Epidemiology of white matter lesions. Top Magn Reson Imaging 2004; 15: 365–367. [DOI] [PubMed] [Google Scholar]
- 32. Khan U, Porteous L, Hassan A, et al. Risk factor profile of cerebral small vessel disease and its subtypes. J Neurol Neurosurg Psychiatry 2007; 78: 702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanahuja J, Alonso N, Diez J, et al. Increased burden of cerebral small vessel disease in patients with type 2 diabetes and retinopathy. Diabetes Care 2016; 39: 1614–1620. [DOI] [PubMed] [Google Scholar]
- 34. Palacio S, McClure LA, Benavente OR, et al. Lacunar strokes in patients with diabetes mellitus: risk factors, infarct location, and prognosis. Stroke 2014; 45: 2689–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gons RAR, Van Norden AGW, De Laat KF, et al. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain 2011; 134: 2116–2124. [DOI] [PubMed] [Google Scholar]
- 36. Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation 2008; 118: 2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schilling S, Tzourio C, Dufouil C, et al. Plasma lipids and cerebral small vessel disease. Neurology 2014; 83: 1844–1852. [DOI] [PubMed] [Google Scholar]
- 38. Wang L, Gill R, Pedersen TL, et al. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res 2009; 50: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12: 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006; 37: 1391–1398. [DOI] [PubMed] [Google Scholar]
- 41. Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355: 549–559. [DOI] [PubMed] [Google Scholar]
- 42. Song T-J, Chang Y, Shin M-J, et al. Low levels of plasma omega 3-polyunsaturated fatty acids are associated with cerebral small vessel diseases in acute ischemic stroke patients. Nutr Res 2015; 35: 368–374. [DOI] [PubMed] [Google Scholar]
- 43. Suwa M, Yamaguchi S, Komori T, et al. The association between cerebral white matter lesions and plasma omega-3 to omega-6 polyunsaturated fatty acids ratio to cognitive impairment development. Biomed Res Int 2015; 2015: 153437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shinohara Y, Katayama Y, Uchiyama S, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010; 9: 959–968. [DOI] [PubMed] [Google Scholar]
- 45. Boiten J, Lodder J, Kessels F. Two clinically distinct lacunar infarct entities? A hypothesis. Stroke 1993; 24: 652–656. [DOI] [PubMed] [Google Scholar]
- 46. Smith EE. Leukoaraiosis and stroke. Stroke 2010; 41: S139–S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim BJ, Kim JS. Ischemic stroke subtype classification: an Asian viewpoint. J Stroke 2014; 16: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]