Abstract
Fluoropyrimidines such as 5-fluorouracil (5-FU) form the foundation of a wide variety of chemotherapy regimens. 5-FU is in fact the third most commonly used chemotherapeutic agent in the treatment of solid malignancies across the world. As with all chemotherapy, balancing the potential benefits of therapy against the risks of drug-related toxicity is crucial when clinicians and patients make shared decisions about treatment. 5-FU is the second most common chemotherapeutic drug associated with cardiotoxicity after anthracyclines, which can manifest as chest pain, acute coronary syndrome/myocardial infarction or death. Nevertheless a widespread appreciation of 5-FU-related cardiotoxicity and its implications is lacking amongst clinicians. In this review, we outline the incidence, possible risk factors, and likely pathophysiological mechanisms that may account for 5-FU-related cardiotoxicity and also highlight potential management strategies for this poorly understood clinical entity.
Keywords: 5-fluorouracil, cardiotoxicity, chemotherapy, coronary vasospasm, fluoropyrimidine
Introduction
Fluoropyrimidines, which include 5-fluorouracil (5-FU) and capecitabine, form the cornerstone of several different chemotherapy regimens. 5-FU is the third most commonly used chemotherapeutic agent in the treatment of solid malignancies across the world,1,2 including head and neck and gastrointestinal tumors. Fluoropyrimidines also possess radiosensitizing properties and are often used in conjunction with external beam radiotherapy. Nevertheless, as with other chemotherapeutic agents, the potential benefits of fluoropyrimidines have to be weighed against their risks and drug-related toxicities. 5-FU is the second most common drug associated with cardiotoxicity3,4 after anthracyclines. The most common manifestation of cardiotoxicity associated with fluoropyrimidines is chest pain, presenting as atypical chest pain, angina on exertion or rest, and acute coronary syndromes including myocardial infarction.5,6 Other less common manifestations of cardiotoxicity include atrial fibrillation and other arrhythmias,7–9 myocarditis and pericarditis,10 heart failure11 and even death.5,12–14 Fluoropyrimidine-related cardiotoxicity, however, remains a poorly defined entity. We review the incidence, potential risk factors, likely pathophysiological mechanisms and strategies of management of cardiotoxicity related to fluoropyrimidine administration with a particular focus on 5-FU.
Incidence of cardiotoxicity
We searched PubMed for potentially relevant articles published from 1 January 1970 to 31 December 2017, using the following key search terms: fluoropyrimidine, capecitabine, floxuridine, 5-FU, fluorouracil, adverse drug reaction, drug toxicity, cardiotoxicity and cardiovascular events. Searches were enhanced by scanning bibliographies of identified articles, and relevant articles were selected for review. Studies included for selection needed to evaluate, first, the frequency of cardiotoxicity, characterized by cardiac symptoms, electrocardiogram (ECG) changes, troponin elevations, other relevant changes such as echocardiographic changes and cardiac events including myocardial infarction and death; second, patients receiving fluoropyrimidine chemotherapy which could include systemic 5-FU administered as a continuous infusion or as a bolus, oral capecitabine or other fluoropyrimidines given as monotherapy or in combination with other chemotherapeutic agents. Included studies consisted of both prospective studies, including randomized controlled trials, as well as retrospective reviews. When different studies presented data from the same cohorts with, for example, different lengths of follow up, only the most up to date study with the most comprehensive data was included. Articles containing no original data, including reviews and meta-analyses, were also excluded to avoid redundancy. In total, 37 studies were identified with sample sizes ranging from 22 to 1350. Table 1 summarizes the incidence of cardiotoxicity obtained for each study.4–6,13,15–47
Table 1.
Summary of studies evaluating the incidence of cardiotoxicity in patients treated with 5-FU.
| Reference | Sample size | Study design | Drug | Risk estimate |
|---|---|---|---|---|
| Pottage et al.43 | 140 | Prospective study | 5-FU | 2.9% developed cardiotoxicity: 2.1% developed chest pain and ECG changes and 0.8% developed MI |
| Labianca et al.4 | 1083 | Retrospective review | 5-FU | 1.6% of all patients developed angina or MI versus 4.5% in patients with previous cardiac disease |
| Eskilsson et al.42 | 76 | Prospective study | Continuous infusions of 5-FU | 17.1% developed cardiac events: 13.2% experienced angina or ECG changes, 1.3% experienced AF, 1.3% had VF and 1.3% experienced sudden death |
| Rezkalla et al.40 | 25 | Prospective study | Continuous infusions of 5-FU | 4% developed angina, 68% developed asymptomatic ECG changes and 8% experienced sudden death |
| Eskilsson et al.41 | 58 | Prospective study | Continuous infusions of 5-FU | 5.2% developed angina with ECG changes and 6.9% experienced asymptomatic ECG changes |
| Jeremic et al.39 | 80 | Prospective study | 5-FU | 15% developed angina or ECG changes |
| Gradishar et al.36 | 244 | Retrospective review | Continuous infusions of 5-FU | 1.6% experienced angina or ECG changes, 4.1% experienced sudden death and 1.6% developed pulmonary embolism |
| de Forni et al.5 | 367 | Prospective study | Continuous infusions of 5-FU | 7.6% developed cardiotoxicity: 5.4% had chest pain or shortness of breath, 2.2% had unstable angina and 1.1% experienced sudden death |
| Akhtar et al.44 | 100 | Prospective study | Continuous infusions of 5-FU | 8% developed cardiotoxicity: 5% developed angina and 3% developed ECG changes of which one patient had cardiogenic shock |
| Schober et al.38 | 390 | Prospective study | 5-FU | 1.5% developed angina or palpitations, 0.8% had an MI, 0.8% had an arrhythmia, 0.3% had acute heart failure and 0.3% experienced sudden death |
| Keefe et al.37 | 910 | Prospective study | 5-FU | 0.6% developed chest pain and ECG changes |
| Weidmann et al.35 | 231 | Prospective study | 5-FU | 2.6% developed angina and 0.4% developed AF |
| Meyer et al.47 | 483 | Prospective study | Continuous infusions of 5-FU | 1.9% developed cardiotoxicity: 1.3% developed angina, 0.4% developed shock and 0.2% experienced sudden death |
| Orditura et al.34 | 43 | Prospective study | 5-FU | 0% developed any significant ECG changes |
| Blum et al.33 | 162 | Phase II prospective Study | Capecitabine | 0% had any cardiotoxicity |
| Balloni et al.32 | 25 | Prospective study | Fluoro-folate | 0% developed significant changes in diastolic function on echocardiography |
| Blum et al.31 | 74 | Phase II prospective Study | Capecitabine | 0% had any cardiotoxicity |
| Van Cutsem et al.30 | 602 | Phase III randomized controlled trial | Capecitabine versus 5-FU | 0.3% had an MI, 0.3% had heart failure and 0.2% had an arrhythmia |
| Hoff et al.29 | 605 | Phase III randomized controlled trial | Capecitabine versus 5-FU | 0.5% developed angina, 0.2% had myocarditis and 0.2% had an MI |
| Wacker et al.45 | 102 | Prospective study | 5-FU | 19.0% developed angina, 5.1% of which had ‘severe’ symptoms |
| Oztop et al.28 | 22 | Prospective study | 5-FU | 0% had any cardiotoxicity |
| Sudhoff et al.27 | 30 | Prospective study | 5-FU | 0% had any cardiotoxicity |
| Meydan et al.26 | 231 | Prospective study | Continuous infusions of 5-FU | 3.9% developed cardiotoxicity: 2.6% experienced an ACS, 0.9% had heart failure and 0.4% had AF |
| Ceyhan et al.25 | 37 | Prospective study | Continuous infusions of 5-FU | 5.4% developed angina and ECG changes |
| Ng et al.24 | 153 | Two prospective studies | Capecitabine | 6.5% developed cardiotoxicity: 2.6% experienced angina, 2.0% had an MI, 0.7% had heart failure, 0.7% had ventricular tachycardia and 0.7% experienced sudden death |
| Tsibiribi et al.48 | 1350 | Prospective study | 5-FU | 1.1% developed cardiotoxicity: 1% developed angina and 0.1% had an MI |
| Jensen et al.22 | 668 | Retrospective review | Capecitabine or 5-FU | 4.3% developed cardiotoxicity: 0.4% had angina on exertion, 3.6% had angina at rest and 0.3% had an MI |
| Yilmaz et al.21 | 27 | Prospective study | Continuous infusions of 5-FU | 7.4% developed angina |
| Holubec et al.20 | 42 | Prospective study | 5-FU | 14% had significant troponin elevations and 48% had significant BNP elevations |
| Kosmas et al.13 | 644 | Prospective study | Capecitabine or 5-FU | 4.0% of all patients developed angina or ECG changes versus 6.7% of those receiving a continuous infusion |
| Jensen et al.46 | 106 | Prospective study | Continuous infusions of 5-FU | 8.5% developed angina with ECG changes |
| Salepci et al.19 | 31 | Prospective study | Bolus 5-FU | 16.1% developed angina or ECG changes and 3.2% experienced sudden death |
| Koca et al.18 | 52 | Retrospective review | Capecitabine | 34.6% developed cardiac symptoms, 11.5% developed new cardiac signs on exam and 32.6% had new ECG changes |
| Khan et al.17 | 301 | Retrospective review | 5-FU | 19.9% developed cardiac symptoms, 12.0% developed bradycardia and 3.0% died |
| Lestuzzi et al.6 | 358 | Prospective study | Continuous infusions of 5-FU | 5.9% developed angina or ECG changes and amongst 228 patients who underwent a treadmill stress test 6.9% developed exercise-induced ischemia of which 2.6% had angina and 4.3% had silent electrocardiographic ischemia |
| Polk et al.16 | 452 | Retrospective review | Capecitabine | 5.3% developed angina or palpitations, 2.4% developed ECG changes, 0.4% had an MI |
| Kwakman et al.49 | 1973 | Retrospective review of three phase III randomized controlled trials | Capecitabine | 5.9% developed cardiotoxicity: 0.8% developed chest pain, 2.9% developed ischemia or infarction, 2.0% had an arrhythmia and 0.4% had heart failure |
5-FU, 5-fluorouracil; ACS, acute coronary syndrome; AF, atrial fibrillation; BNP, brain natriuretic peptide; ECG, electrocardiograph; MI, myocardial infarction; VF, ventricular fibrillation.
Different reports describe a wide range of estimates for the incidence of cardiotoxicity associated with the administration of a fluoropyrimidine. This variability is the likely result of differences in the drug being studied, as well as the dose and method of administration, the use of other potentially cardiotoxic chemotherapeutic agents as well as concurrent radiotherapy, patients’ clinical characteristics including the presence of pre-existing coronary artery disease or traditional cardiovascular risk factors, and variability in the definitions of cardiotoxicity. Fluoropyrimidine cardiotoxicity tends to occur most commonly during the first cycle of administration.14,24,47 The median time to initiation of symptoms is 12 h following infusion initiation, though cardiotoxicity could occur anytime during infusion or even up to 1–2 days after infusion.50 As an example, in a report of 106 patients receiving short-term infusional 5-FU as a component of the FOLFOX (folinic acid, 5-FU and oxaliplatin) regimen, nine developed chest pain during treatment, and the onset was during courses 1, 2, 6, and 8 in three, four, one, and one patient, respectively.46 Symptoms and ECG changes may disappear quickly after drug discontinuation or last several days.
Potential mechanisms leading to 5-FU-related cardiotoxicity
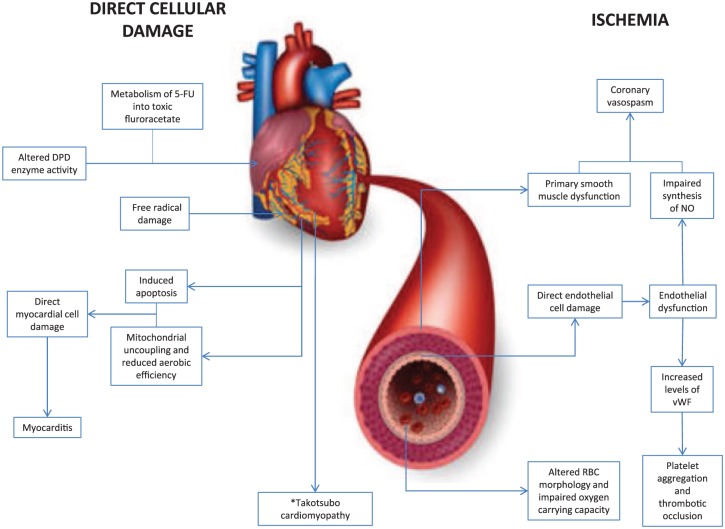
A number of mechanisms are thought to be responsible for 5-FU-related cardiotoxicity, some of which are inter-related. The two most likely contributors are ischemia and drug-related myocardial toxicity (Figure 1).
Figure 1.
Diagram outlining the two potential mechanisms by which 5-fluorouracil could lead to cardiotoxicity: direct cellular damage and ischemia.
5-FU, 5-fluorouracil; DPD, dihydropyrimidine dehydrogenase; NO, nitric oxide; RBC, red blood cell; vWF, von Willebrand factor. *Takatsubo cardiomyopathy is typically seen as a structural cardiomyopathic process, though there is some evidence which suggests that ischemia may contribute to the pathophysiology of this process. This remains a controversial area that requires further investigation.
Coronary vasospasm is a leading theory for 5-FU-related myocardial ischemia. Patients with coronary vasospasm may have ECG findings suggestive of coronary occlusion, including ST-segment elevation as well as biochemical evidence of myocardial injury with troponin elevation even in the absence of occlusive macrovascular disease on angiography or computed tomography (CT) imaging of the coronary vessels. Indeed, patients with 5-FU-related cardiotoxicity have consistently been shown to be lacking significant coronary stenosis on angiography.51–55 In some cohorts, coronary artery vasospasm has directly been visualized during coronary angiography,56–58 as has brachial artery vasoconstriction immediately following the administration of 5-FU injections.19,27 Vasospasm can be related to an endothelial-dependent mechanism (endothelial dysfunction) or an endothelial-independent mechanism (primary smooth muscle dysfunction). Endothelial dysfunction is the unique yet almost universal ‘reaction to injury’ of the vasculature to a variety of insults and clinical circumstances,59,60 and represents the first stage of atherosclerosis. In clinical practice, endothelial dysfunction is most often recognized by an abnormal vasodilatory response to increased flow (shear stress) or endothelial-dependent vasodilating agents such as acetylcholine.61–64 Ordinarily, acetylcholine induces vasodilation through a biochemical process that releases nitric oxide from endothelial cells, which diffuses to the smooth muscle lining the vessel and causes muscle relaxation and in turn vessel dilatation through the cyclic-guanosine monophosphate (GMP) pathway.65 Thus, any damage to the endothelial cells interferes with this process and when acetylcholine is infused, it instead leads to paradoxical vasoconstriction. In the coronary arteries, endothelial function is assessed by way of invasive pharmacologic provocation during coronary angiography with excessive vasoconstriction representing endothelial dysfunction.63,66–68 Endothelial-independent primary smooth muscle dysfunction leads to vasoconstriction in a similar way, but in the presence of a functionally intact endothelium, and can also be assessed with invasive pharmacologic provocation using nitroglycerin.66 Like atherosclerosis, endothelial dysfunction is a systemic disorder affecting peripheral arteries as well as the coronary vessels, allowing clinicians the opportunity to measure endothelial function noninvasively using flow-mediated dilatation of the brachial arteries, which correlates well with invasively measured coronary endothelial function.69 Thus, peripherally observed vasoconstriction related to 5-FU is anticipated to correlate with coronary vasoconstriction, although direct observation with coronary angiography would be required to confirm this. Furthermore, in an in-vitro study, the investigators exposed rabbit aorta rings to different chemical substances and were able to demonstrate concentration-dependent vasoconstriction in response to 5-FU and preserved relaxation of the vascular wall in response to acetylcholine,70 suggesting impaired primary smooth muscle function and preserved endothelial function. In addition, studies have demonstrated the presence of brachial artery vasoconstriction27 or angina with ECG changes with57,58 or without71 directly observed coronary vasospasm on initial challenge with 5-FU, further suggesting a role for smooth muscle function in 5-FU-related coronary vasospasm.
Nevertheless, there have been some inconsistencies with the theory of vasospasm and 5-FU administration. Coronary vasospasm has not been consistently demonstrated at angiography during symptomatic episodes, even after reintroducing 5-FU in subjects known to have had previous cardiac symptoms following 5-FU administration.72,73 Amongst patients suspected of having 5-FU-related cardiotoxicity, vasospasm was not demonstrated on pharmacologic provocation with the alkaloid ergonovine,74 an agent that has previously been used to assess coronary vasomotor function through its action on smooth muscle serotonergic receptors, which in turn leads to muscle contraction and vasoconstriction under physiologic conditions.75 Furthermore, in one study of patients who reported angina in response to a 5-FU challenge who had ECG changes suggestive of ischemia, those who underwent concurrent echocardiography were shown to have global akinesia, incompatible with a characteristic territorial distribution of a major coronary artery.5 Despite the systemic distribution of 5-FU, multivessel coronary vasospasm is uncommon in patients receiving 5-FU.76,77 Indeed, when assessing patients with stable angina de novo at coronary angiography, epicardial vasospasm is also typically observed in a single vessel,63,66 which is often the vessel supplying the largest territory of myocardium. This may be related to an oxygen supply–demand mismatch, but this explanation has not been fully elucidated yet. The discordance between echocardiographic and angiographic findings could undermine the epicardial vessel vasospasm theory in patients receiving 5-FU, though does not preclude microvascular vasospasm. Endothelial-dependent and -independent dysfunction also affects the coronary microvasculature, often in the absence of affecting the epicardial vessels78 where it leads to diffuse as opposed to segmental ischemia. Since the coronary microvasculature cannot be directly visualized, its function is assessed through measurements of coronary blood velocity and flow using intravascular Doppler guidewires also with pharmacologic provocation at angiography.66 Thus a failure to observe epicardial coronary vasospasm may be insufficient to exclude a vasospastic pathology and investigators studying patients with angina in response to 5-FU should also consider microvascular function in their assessment. Other inconsistencies with the vasospasm hypothesis include the fact that while vasoconstriction may be observed in patients during or immediately after 5-FU injection, clinical features of toxicity do not typically manifest until after the end of an infusion or even hours to days later.1,46,50 Further, the role of antianginal therapy such as calcium channel blockers to prevent symptoms remains unclear. While some studies demonstrated that pretreatment of patients with previous 5-FU-induced angina with calcium channels prevented subsequent angina or coronary spasm on rechallenge with 5-FU, this has not been consistently shown in other studies.14,41,73 There have been no randomized clinical trials to date that have evaluated the role of calcium channel blockers in patients with 5-FU-related coronary vasospasm and angina, and so further clarification is required here. Lastly, endothelin, a potent vasoconstrictor also produced by endothelial cells and cardiomyocytes, was found to be elevated in one study of patients treated with 5-FU, particularly those experiencing cardiotoxicity including angina79 further suggesting 5-FU may be responsible for a coronary vasospasm process. However, though a further study was also able to demonstrate elevated levels of a precursor molecule of endothelin in patients treated with 5-FU this was not confined to patients who developed vasospasm.19 It may be that these studies are confounded by differences in levels of endothelin produced from the coronary endothelium and that from other tissues such as the lungs where endothelin is also produced. Further, endothelin produced by the endothelium is secreted towards the vessel smooth muscle and it may be that only small amounts of endothelial endothelin reach the vessel lumen and contribute to measured serum levels. While a relationship between 5-FU and coronary vasospasm seems plausible, further clinical studies are required in subjects experiencing cardiotoxicity to further clarify this relationship.
The physiological role of the endothelium is not limited to modifying vascular tone and lumen caliber to meet blood-oxygen demand but extends to regulating coagulation and thrombus formation. Damaged endothelium exposes tissue factor, initiating platelet aggregation that is further propagated by the release of von Willebrand factor and fibrin aggregation, resulting in occlusive thrombi. Thus, it follows along the endothelial dysfunction hypothesis that 5-FU may lead to thrombotic occlusive disease and indeed studies of rabbit endothelium exposed to 5-FU have demonstrated sites of platelet aggregation and fibrin formation.80,81 Despite this, coronary angiography amongst patients with chest pain after receiving 5-FU has consistently failed to demonstrate occlusive disease.51–55 Nevertheless, there is evidence that 5-FU may play a role in influencing intravascular coagulation. One study showed that 44% of patients with solid tumor had plasma von Willebrand levels above the reference range before treatment with 5-FU and 92% had elevated levels after treatment.82 These findings suggest that 5-FU could play a role in influencing the coagulation-fibrinolytic system, though cancer in itself is considered a hypercoagulable state and these patients have been shown to have elevated serum levels of von Willebrand factor at baseline,55,56 which could act as a confounding variable. Nevertheless regulating the initiation of thrombus formation represents an additional facet of endothelial function and studies have characterized abnormal endothelial function by identifying altered levels of endothelium-derived markers such as von Willebrand factor and fibronectin,83,84 suggesting further that endothelial dysfunction in its broader sense could be responsible for 5-FU-related cardiotoxicity. Additionally, ischemia in its wider sense may still play a role in 5-FU-related cardiotoxicity, even in the absence of occlusive vascular vasospasm or thrombus. Animal models have demonstrated reversible changes in erythrocyte morphology, notably to echinocytes, associated with increased membrane fluidity and altered metabolism resulting in more rapid depletion of stored oxygen and decreased ATP levels impairing the ability of erythrocytes to carry oxygen to the myocardium.85,86 However, as with other observations in animal models, whether these changes occur in vivo as well, and can be attributed to cardiotoxicity in patients receiving 5-FU treatment remains to be proven. Experimental models are necessary in uncovering pathological mechanisms and testing a broad range of hypotheses that could otherwise be challenging or even unethical to attempt in human subjects but remain limited in their small sample size as well as in their direct clinical application to cardiotoxicity in humans. Isolated cells in vitro and isolated organs may not behave the same as in a live human subject and more trials in clinical settings will be required.
Other pathological mechanisms that could contribute to 5-FU-induced cardiotoxicity include factors that cause direct cellular damage. Animal studies have demonstrated gross pathological changes to cardiomyocytes in a dose-dependent fashion48 as well as directly to endothelial cells,87 which could represent the initial insult and subsequent ‘reaction to injury’ that leads to endothelial dysfunction. However, not all of these pathological changes have been corroborated in human subjects experiencing symptoms of cardiotoxicity. Further, biopsies of this nature are difficult to obtain and in patients with cancer who have received a multitude of different treatments it would be difficult to discriminate the role of 5-FU in any changes identified. Experimental models therefore represent a necessary alternative. The nature of these changes is thought to be caused by induction of apoptosis with a notable absence of necrosis as opposed to that seen with direct cytotoxicity,88 as is the mechanism in neoplastic cells, which could suggest an alternative mechanism of action of 5-FU or a different response of different cell types to the same agent. Other animal models have demonstrated specific biochemical changes in cardiomyocytes, including increased oxygen consumption, depletion of high-energy phosphate compounds and citrate accumulation,89–91 effects which occur independent of changes in blood and oxygen supply. This is thought to be caused by reduced aerobic efficiency secondary to 5-FU-related mitochondrial uncoupling,90 which in turn leads to hypoxic cell injury. Other studies focusing on damage caused at the cellular level have postulated the oxidative stress theory, demonstrating increased levels of reactive oxygen species such as superoxide anions in rat cardiomyocytes after treatment with 5-FU88 and diminished activity of antioxidant agents such as sodium oxide dismutase and glutathione peroxidase in guinea pigs treated with 5-FU.92 Toxic free radical species then lead to oxidation of proteins, lipids and other macromolecules, leading to disturbed cellular function; increased levels of some of these end products have been identified in experimental models.92 This has also been proposed as a potential mechanism for endothelial cell damage and in one study the chemical probucol, which increases superoxide dismutase and glutathione peroxidase activity in animals, protected rabbit endothelial cells from putative free radical species induced lipid peroxidation after treatment with 5-FU.87 All experimental data investigating the role of oxidative stress in 5-FU cardiotoxicity are not consistent however. Iron is an important element that catalyzes the Haber–Weiss reaction that allows reactive oxygen species to generate toxic free radicals and studies have not consistently demonstrated altered iron levels in animal models treated with 5-FU.92 No studies however have looked at the role of iron chelation therapies in mitigating 5-FU-related cardiotoxicity or even 5-FU-related free radical production and this may be a useful next step in clarifying the role of this process. Further evidence suggesting a direct toxic effect of 5-FU relates to its metabolism whereby 5-FU is initially converted to α-fluoro-β-alanine (FBAL) and subsequently to fluoroacetate, the presence of which has been correlated with cardiotoxicity.93,94 This is further supported when coadministration of dihydropyrimidine dehydrogenase (DPD) enzyme inhibitors, which inhibit the metabolism of 5-FU to FBAL, lead not only to significantly reduced levels of FBAL and its metabolites95 but also prevent recurrent cardiotoxicity in patients who previously experienced 5-FU-related cardiotoxicity.93 Following on from this, inherited enzyme polymorphisms and variations in enzyme pathways could influence 5-FU metabolism, leading to individualized susceptibility to toxicity. This has been demonstrated previously whine reduced DPD enzyme activity has led to accumulation of 5-FU and subsequent myelosuppression, diarrhea, stomatitis and neurotoxicity.96,97 It might follow therefore that individuals with DPD mutations resulting in aberrant enzyme activity would be susceptible to cardiotoxicity, and there have been reports of DPD mutations in patients with 5-FU-related cardiotoxicity.98 However, whether the cardiotoxicity could be attributed to the mutation is difficult to prove and as yet this association remains unclear.
Gross evidence of myocarditis has been demonstrated in rabbits exposed to 5-FU48 where left ventricular hypertrophy, foci of myocardial necrosis, thickening of intra-myocardial arterioles, and disseminated apoptosis in myocardial and endothelial cells have all been demonstrated. In this study, the use of a high single dose of 5-FU was intended to differentiate the acute toxic effects of 5-FU, which resulted in thrombogenesis and spasm due to endothelial lesions, from delayed cardiotoxicity after four injections at 7-day intervals, which lead to apoptosis of myocardial and endothelial cells without evidence of spasm. These results support an alternative mechanism for 5-FU cardiotoxicity beyond vasospasm and ischemia. In human subjects as well,99,100 biventricular dilatation and diffusely scatter necrosis with an inflammatory infiltrate and proliferation of the sarcoplasmic reticulum with marked vacuolization, similar to that found with doxorubicin cardiotoxicity, was also demonstrated at autopsy amongst subjects who had been treated with 5-FU. It may be that this condition represents the consequences of some or a combination of all of the pathological processes described above. Further clinical evidence of a cardiomyopathic process has been shown in studies demonstrating echocardiographic evidence of left ventricular dysfunction101 and neuroendocrine changes characterized by elevated plasma brain natriuretic peptide and lactic acid levels in patients treated with 5-FU46 even in the absence of a significant change to left ventricular ejection fraction, suggestive of a subclinical process. In other studies, 5-FU has been associated with transient myocardial dysfunction associated with apical ballooning akin to a Takotsubo cardiomyopathy,52,100,102 which typically arises in the context of excess sympathetic stimulation and occurs with chest pain, electrocardiographic ST-segment elevation and cardiac enzyme elevation mimicking acute myocardial infarction. Indeed a proportion of patients with chest pain suggestive of an acute coronary syndrome after receiving 5-FU and who are shown to have normal coronaries may have this syndrome.
Risk factors for cardiotoxicity
While risk factors for cardiotoxicity in patients taking fluoropyrimidines are incompletely understood, a number of patient- and drug-administration-related factors have emerged from observational studies, though there have been some conflicting results. In one retrospective study the authors demonstrated that 72% of all patients affected by 5-FU-related cardiotoxicity, which was defined as chest pain on rest or exertion or myocardial infarction with cardiac enzyme elevation, were aged greater than 55 years. However, the authors also showed that pre-existing renal disease defined as a creatinine clearance of less than 30 ml min−1 and pre-existing cardiovascular disease were also risk factors for cardiotoxicity, which may very well have confounded the association with age.22 Other studies have failed to demonstrate age as an independent risk factor for cardiotoxicity,4,14 but have suggested that underlying cardiac disease including history of ischemic heart disease or myocardial infarction24,47 or structural disease4 could be linked with toxicity. These studies, however, had low event rates, an observational study design, and defined pre-existing cardiovascular disease through patient-reported history rather than using objective reproducible criteria. It is also important to note that most cases of cardiotoxicity occur in patients who do not have pre-existing cardiovascular disease. In one series in which patients with colorectal cancer were treated with infusions of 5-FU, 9 out of 106 patients had cardiotoxicity with only one having significant pre-existing cardiovascular disease; meanwhile amongst 7 patients with significant pre-existing cardiovascular disease none developed cardiotoxicity.46 This was demonstrated again in another study in which none of a cohort of 102 unselected consecutive patients receiving 5-FU chemotherapy who developed angina had a known history of coronary artery disease. It may be that risk factors for cardiovascular disease such as hypertension, hyperlipidemia and history of smoking are more predictive of cardiotoxicity than a history of pre-existing cardiac disease itself. One review demonstrated that of 377 cases of 5-FU-associated cardiotoxicity, only 14% had a previous history of cardiac disease while 37% had at least one traditional risk factor for cardiovascular disease, of which smoking was the most common.14 The precise roles that pre-existing cardiac disease and risk factors for cardiovascular disease play in determining risk for cardiotoxicity in response to 5-FU treatment remain uncertain, and to date there are insufficient data to discriminate risk well enough to justify withholding therapy in this population. Ideally clinically relevant risk factors could be identified and compiled into a risk stratification model that clinicians could use to inform treatment decisions in patients being considered for treatment with 5-FU. Further clinical trials identifying such risk factors and demonstrating their clinical utility in risk prediction are required.
Studies have consistently demonstrated that the schedule of administration of 5-FU influences risk for cardiotoxicity, with infusional regimens being associated with greater risk compared with bolus regimens. For patients receiving infusional regimens of 5 days or longer, the incidence of toxicity varies between 2% and 18%,5,14,47 with some differences in risk between studies accounted for by differences in treatment duration, dose, additional chemotherapy agents and patient characteristics. Meanwhile, amongst patients receiving short-term infusional chemotherapy as part of the FOLFOX chemotherapy regimen for gastrointestinal cancer, the incidence of cardiotoxicity was 8.5%,46 while that in patients receiving bolus regimens was no greater than 3%,3,4 suggesting that net duration of therapy may play a role in toxicity. Indeed, 5-FU is cleared rapidly from the bloodstream with a half life of 15–20 min, suggesting that continuous infusions would allow for drug accumulation or at least a continual exposure and therefore a greater propensity for cardiotoxicity in a way that might not occur with bolus administration. Alternatively, capecitabine, which is an orally available 5-FU prodrug that is metabolized in tissues expressing the enzyme thymidine phosphorylase and is pharmacokinetically similar to administering a continuous infusion of 5-FU, is associated with a comparable potential to induce coronary vasospasm with an incidence of cardiotoxicity of 3–9%,13,24,49 which is similar to that for short-term infusion therapy. This lends more weight to the concept that cardiotoxicity related to 5-FU is influenced by drug pharmacokinetics and metabolism. However, this theory requires greater clarification in clinical studies, particularly because a compelling relationship between the administered dose of 5-FU and cardiotoxicity has not been demonstrated,47 nor have plasma circulating levels of 5-FU in patients presenting with cardiotoxicity following 5-FU administration been significantly different to those in patients without toxicity.103
Management of cardiotoxicity
Figure 2 outlines a suggested approach to the management of cardiotoxicity related to 5-FU administration. 5-FU-related cardiotoxicity is potentially fatal14 and so the first step should be to discontinue chemotherapy immediately, and then to treat symptoms empirically with antianginal therapy such as calcium channel blockers or nitrates. This approach has been shown to abort symptoms in up to 69% of effected subjects.14,22 In one case series, 11 patients with suspected fluoropyrimidine-induced coronary vasospasm were all successfully rechallenged with the culprit drug and completed their planned first-line chemotherapy following cardioprotective pretreatment with two calcium channel blockers and a long-acting nitrate in conjunction with careful cardiac monitoring.104 The pivotal next step is determining whether the cardiac symptoms can be reasonably attributed to 5-FU, which is a challenging situation as further administration of the drug could lead to potentially avoidable morbidity and mortality, while withholding effective chemotherapy unnecessarily could compromise the patient’s chance of cure. No definitive test can establish a causal link between 5-FU and cardiotoxicity and thus clinical judgment is required. Auxiliary testing may be of use but has limitations; ECG can detect new ischemic changes but lacks sensitivity,105 echocardiography can demonstrate segmental or global hypokinesia5,7 but may be normal, and laboratory testing for cardiac enzymes or brain natriuretic peptide may be elevated7,46 but could also be normal, suggesting that cardiotoxicity related to 5-FU is not always severe enough to lead to myocardial necrosis. In the outpatient setting ambulatory rhythm monitoring amongst patients receiving 5-FU might be a useful strategy to identify patients having cardiotoxicity by capturing transient arrhythmias or electrocardiographic signs of ischemia. In one study, 27 patients receiving 5-FU as part of the de Gramont regimen for gastrointestinal cancers underwent ECG Holter monitoring before and during the first 24 h of chemotherapy. The investigators found that during treatment patients had a significant decrease in mean heart rate and a significant increase in number of atrial and ventricular premature complexes per hour compared with before treatment,21 which could increase the risk of arrhythmia. In another study, 25 patients receiving infusions of 5-FU were monitored with continuous ambulatory ECG monitoring both before infusion and during infusion. The authors showed that while 24% of patients had asymptomatic ST-segment changes before infusion, 68% had these changes during infusion (p < 0.002) and the incidence of ischemic episodes per patient per hour as well as the duration of ECG changes was significantly higher during the infusion.40 Ambulatory ECG monitoring may therefore be a useful tool to confirm cases of cardiotoxicity and may even be able to identify cases of subclinical cardiotoxicity, but the exact role this test should play in clinical practice remains unclear and needs to be further studied. Often therefore the most relevant clue may be the temporal association between the administration of 5-FU and symptoms of cardiotoxicity. Clinicians should however be mindful that even though the median time to initiation of symptoms is 12 h after infusion initiation, cardiotoxicity could occur anytime during infusion or even up to 1–2 days after infusion,48 and in fact may not necessarily manifest during the first cycle of administration.46 Reproducibility of symptoms on empiric rechallenge may be useful but could be life threatening and thus is not routinely recommended. When 5-FU-attributable toxicity is likely, the clinician must decide whether an alternative chemotherapy regimen excluding 5-FU is acceptable, which is often the safest option. If a 5-FU-based regimen is preferable, a reasonable approach would be to determine if an alternative underlying pathological process that could explain the patient’s presentation could be identified and potentially reversed. In the case of angina, patients with risk factors for cardiovascular disease should undergo coronary angiography. If angiography reveals clinically significant occlusive disease that could explain the symptoms, an attempt at revascularization followed by a drug rechallenge would be reasonable. Even if these findings cannot directly explain the patient’s presentation, treating the occlusive disease could play a role in helping the patient better tolerate a rechallenge. Patients without conventional cardiovascular risk factors could be screened for coronary disease using a noninvasive test such as CT coronary angiography and managed accordingly. For patients with clinically insignificant coronary disease/normal coronaries, a presumptive diagnosis of 5-FU-related cardiotoxicity is made and further 5-FU should be avoided if possible (see below). Of note, the presence of significant coronary stenosis does not exclude the possibility of superimposed 5-FU-related cardiotoxicity and any readministration of 5-FU must be undertaken cautiously with close monitoring. Invasive pharmacologic provocation at coronary angiography could yield diagnostically useful information by identifying coronary endothelial dysfunction or primary smooth muscle dysfunction evidenced by excessive vasospasm in response to acetylcholine or nitroglycerin respectively. This testing has been shown to be safe106 and is the reference standard for diagnosing functional coronary abnormalities.107 Nevertheless, invasive testing of this nature requires specialist resources and expertise, is currently not widely available, and its role in this setting has not been validated. At present the American College of Cardiology/American Heart Association assigns this testing a class IIB recommendation in appropriately selected patient groups.108 Further, the role of invasive pharmacologic provocation in risk-stratifying patients with suspected 5-FU cardiotoxicity is unclear, as there is currently no evidence that a positive provocation test predicts cardiotoxicity. Indeed successfully identifying endothelial dysfunction or primary smooth muscle dysfunction does not necessarily yield the mechanism behind each patient’s symptoms as this testing can only identify coronary vasospasm, which is one potential mechanism of 5-FU-induced cardiotoxicity and ignores the potential role of other mechanisms such as direct cellular toxicity. Further studies clarifying the potential role of invasive pharmacologic testing are required.
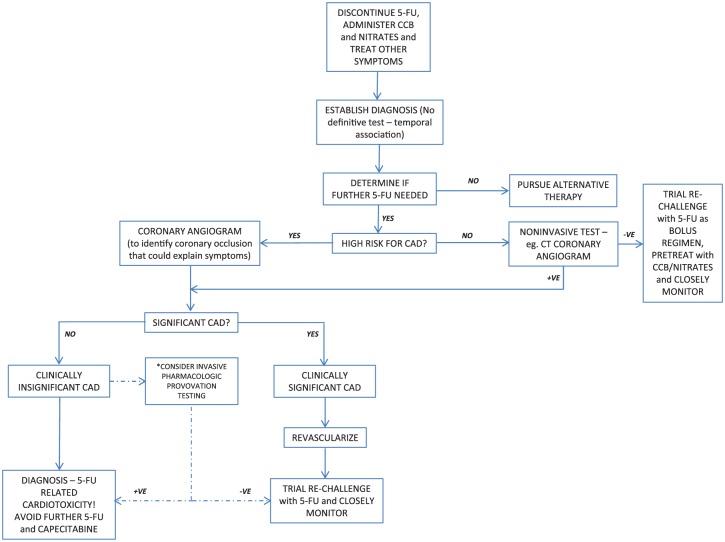
Figure 2.
Flow diagram demonstrating a suggested treatment approach for patients who have experienced suspected cardiotoxicity related to 5-fluorouracil chemotherapy.
5-FU, 5-fluorouracil; CAD, coronary artery disease; CCB, calcium channel blocker. *When significant CAD has not been demonstrated clinicians could consider invasive pharmacologic provocation testing to identify reproducible coronary vasospasm, though this should only be undertaken by experienced operators in appropriately resourced centers.
Table 2 outlines potential management strategies that have been shown to be effective and those which are ineffective or for which evidence is lacking when approaching chemotherapy for patients with suspected 5-FU-related cardiotoxicity. Important principles that should be considered include treatment intent and the availability of treatment regimens containing non-fluoropyrimidine or alternative fluoropyrimidine with more acceptable toxicity profiles. When rechallenging with a fluoropyrimidine is being considered, clinicians and patients should undergo a detailed and informed discussion about the potential risks and benefits of such a strategy. Further, appropriate precautions including pretreating patients with cardioprotective medication such as calcium channel blockers and nitrates and careful cardiac monitoring could help to mitigate risk. Additionally, if the risk to benefit ratio of rechallenging with 5-FU is deemed acceptable, using a bolus regimen has consistently been proven to be safer than a continuous infusion and should be the method of choice for drug administration.6,7,109,110
Table 2.
Summary of medical treatments for 5-FU-related cardiotoxicity.
| Effective treatment methods | Rechallenge | • Controversial - Recurrence up to 90%; death up to 13%5,14 • Avoid if possible • Individualized decision weighing risks and benefits • Patients with significant CAD who undergo revascularization: can retreat if close observation and if benefits > risks • Patients with nonsignificant CAD: avoid 5-FU if possible - if not possible and risk/benefit ratio acceptable attempt cautious challenge with BOLUS regimen6,7,109,110 - pretreat with 48 h of aspirin, CCB and long-acting nitrate - careful observation and continuous ECG monitoring - discontinue 5-FU if any symptoms/signs of cardiac event |
| Use of alternative non-FU drugs | • Difficult in patients with gastrointestinal cancers when FU is an integral component of treatment • Good options in metastatic disease, i.e. for colorectal cancer: - Irinotecan alone - Irinotecan plus oxaliplatin, cetuximab or panitumumab (for patients with RAS/BRAF wild-type tumors) - Trifluridine-tipiracil, regorafenib and ramucirumab or raltitrexed alone or in combination regimens111,112 • In patients with adjuvant therapy for resected, node-positive colorectal cancer: - Global standard is oxaliplatin + FU containing regimen (also recommended for patients with node-negative disease but presumed to be at high enough risk) - In those with FU-related cardiotoxicity, trial of FU BOLUS containing regimen (Roswell Park weekly regimen)113,114 - or if patients need oxaliplatin use FLOX (FU, leucovorin and oxaliplatin) with pretreatment using antianginal therapy, empiric aspirin and close monitoring |
|
| Use of alternative FU drugs | • UFT - Contains tefagur which is a FU prodrug, and uracil which competitively inhibits the degradation of FU - < 1% incidence of cardiotoxicity115,116 - Not available in the USA (available in Japan and other Asian and South American countries) • S-1 - Contains tefagur, gimeracil which inhibits the enzyme DPD that breaks down FU and oteracil which inhibits phosphorylation of FU - No reported cardiotoxicity to date117–119 - Not available in the USA (available in several Asian and European countries) |
|
| Alternative treatment modalities | • Patients who are not candidates for alternative chemotherapy drugs or have limited disease could undergo locally directed therapy, e.g. surgery, radiofrequency ablation radioembolization or transarterial chemoembolization • Requires careful patient selection and evaluation of risks and benefits |
|
| Ineffective treatment methods | Dose reduction | • The dose dependence of 5-FU-related cardiotoxicity is unclear14,22,110 |
| Prophylactic treatment with CCB or nitrates | • Much data have shown no benefit with either CCB or nitrates3,14,100,111,120 or CCB in isolation41
• Some data have shown a benefit with prophylactic CCB69,121,122 and prophylactic nitrates110 • Overall conflicting data derived from retrospective studies and case series or reports • No randomized trial evaluating the role of CCBs or nitrates in this setting • Could be used in selected situations when they are unlikely to lead to harm |
|
| Antidote therapy? | • Toxicity is thought to be related to metabolite FUTP - Uridine is a naturally occurring nucleoside: competes with FUTP for incorporation into RNA - Can reduce FU toxicity to normal tissues123 - Has not been studied in FU-related cardiotoxicity - Can cause phlebitis and requires central access for administration • Uridine triacetate is an oral active prodrug of uridine - Higher bioavailability - Approved by FDA in 2015 (for FU or capecitabine overdose in patients with severe/life-threatening toxicity of cardiovascular or central nervous system) - Could be used in severe toxicity124 |
CAD, coronary artery disease; CCB, calcium channel blocker; DPD, Dihydropyrimidine Dehydrogenase; ECG, electrocardiograph; FDA, US Food and Drug Administration; FU, fluorouracil; FUTP, 5-fluorouridine triphosphate; UFT, Uracil-tegafur.
Preventing 5-FU-induced coronary vasospasm in the first place is a further approach that clinicians could consider by determining in advance which patients have endothelial dysfunction using noninvasive testing such as ultrasound-guided flow-mediated dilation. Those with suspected endothelial dysfunction demonstrated noninvasively could then be pretreated in a prophylactic fashion with calcium channel blockers or other vasodilatory cardiovascular medication prior to administering 5-FU. This approach may prevent symptoms and adverse health consequences amongst patients receiving 5-FU and could additionally have benefits in healthcare resource allocation and cost-effectiveness. However, to date this technique has not been investigated as part of any controlled trial and should be studied and validated before being recommended as part of routine clinical practice.
Conclusion
Cardiotoxicity related to 5-FU administration is a poorly understood but relatively common clinical entity that deserves special consideration given the frequent use of this chemotherapeutic agent and the potential morbidity and mortality associated with its use. Patients with pre-existing cardiovascular disease receiving continuous infusions of FU, as opposed to a bolus-based regimen, may be at increased risk. The mechanism of 5-FU-related cardiotoxicity is incompletely understood and may arise from a combination of ischemia related to coronary vasospasm and direct myocardial cell toxicity, though further clinical studies in human subjects are required to clarify this. Management of affected patients focuses on determining whether 5-FU can reasonably be attributed to the cardiotoxicity, identifying and treating other coexisting coronary disease and determining if further 5-FU is required or whether acceptable alternative treatments can be safely used. When further doses of 5-FU are required, clinicians should proceed cautiously, consider using prophylactic antianginal therapy and monitor patients closely with a low threshold to discontinue therapy. Randomized clinical trials comparing different approaches to managing these patients will be essential to clarify the optimal strategy.
Acknowledgments
All authors contributed significantly to this manuscript and have read and approved of the final version.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Jaskanwal D. Sara, Department of Cardiovascular Diseases, Mayo College of Medicine, 200 First Street SW, Rochester, MN 55905-0001, USA.
Jasvinder Kaur, Kent Oncology Centre, Maidstone and Tunbridge Wells NHS Trust, Maidstone, Kent, UK.
Ryan Khodadadi, Department of Internal Medicine, Mayo College of Medicine, Rochester, MN, USA.
Muneeb Rehman, Department of Internal Medicine, Mayo College of Medicine, Rochester, MN, USA.
Ronstan Lobo, Department of Internal Medicine, Mayo College of Medicine, Rochester, MN, USA.
Sakti Chakrabarti, Department of Medical Oncology, Mayo College of Medicine, Rochester, MN, USA.
Joerg Herrmann, Department of Cardiovascular Diseases, Mayo College of Medicine, Rochester, MN, USA.
Amir Lerman, Department of Cardiovascular Diseases, Mayo College of Medicine, Rochester, MN, USA.
Axel Grothey, Department of Medical Oncology, Mayo College of Medicine, Rochester, MN, USA.
References
- 1. Grem JL. 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs 2000; 18: 299–313. [DOI] [PubMed] [Google Scholar]
- 2. Myers CE. The pharmacology of the fluoropyrimidines. Pharmacol Rev 1981; 33: 1–15. [PubMed] [Google Scholar]
- 3. Anand AJ. Fluorouracil cardiotoxicity. Ann Pharmacother 1994; 28: 374–378. [DOI] [PubMed] [Google Scholar]
- 4. Labianca R, Beretta G, Clerici M, et al. Cardiac toxicity of 5-fluorouracil: a study on 1083 patients. Tumori 1982; 68: 505–510. [DOI] [PubMed] [Google Scholar]
- 5. de Forni M, Malet-Martino MC, Jaillais P, et al. Cardiotoxicity of high-dose continuous infusion fluorouracil: a prospective clinical study. J Clin Oncol 1992; 10: 1795–1801. [DOI] [PubMed] [Google Scholar]
- 6. Lestuzzi C, Vaccher E, Talamini R, et al. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann Oncol 2014; 25: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 7. Stewart T, Pavlakis N, Ward M. Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Intern Med J 2010; 40: 303–307. [DOI] [PubMed] [Google Scholar]
- 8. Hrovatin E, Viel E, Lestuzzi C, et al. Severe ventricular dysrhythmias and silent ischemia during infusion of the antimetabolite 5-fluorouracil and cis-platin. J Cardiovasc Med (Hagerstown) 2006; 7: 637–640. [DOI] [PubMed] [Google Scholar]
- 9. Talapatra K, Rajesh I, Rajesh B, et al. Transient asymptomatic bradycardia in patients on infusional 5-fluorouracil. J Cancer Res Ther 2007; 3: 169–171. [DOI] [PubMed] [Google Scholar]
- 10. Calik AN, Celiker E, Velibey Y, et al. Initial dose effect of 5-fluorouracil: rapidly improving severe, acute toxic myopericarditis. Am J Emerg Med 2012; 30: 257.e1–e3. [DOI] [PubMed] [Google Scholar]
- 11. Robben NC, Pippas AW, Moore JO. The syndrome of 5-fluorouracil cardiotoxicity. An elusive cardiopathy. Cancer 1993; 71: 493–509. [DOI] [PubMed] [Google Scholar]
- 12. Manojlovic N, Babic D, Stojanovic S, et al. Capecitabine cardiotoxicity–case reports and literature review. Hepatogastroenterology 2008; 55: 1249–1256. [PubMed] [Google Scholar]
- 13. Kosmas C, Kallistratos MS, Kopterides P, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol 2008; 134: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf 2009; 8: 191–202. [DOI] [PubMed] [Google Scholar]
- 15. Kwakman JJ, Simkens LH, Mol L, et al. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: a retrospective analysis of the CAIRO studies of the Dutch Colorectal Cancer Group. Eur J Cancer 2017; 76: 93–99. [DOI] [PubMed] [Google Scholar]
- 16. Polk A, Shahmarvand N, Vistisen K, et al. Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open 2016; 6: e012798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan MA, Masood N, Husain N, et al. A retrospective study of cardiotoxicities induced by 5-fluouracil (5-FU) and 5-FU based chemotherapy regimens in Pakistani adult cancer patients at Shaukat Khanum Memorial Cancer Hospital & Research Center. J Pak Med Assoc 2012; 62: 430–434. [PubMed] [Google Scholar]
- 18. Koca D, Salman T, Unek IT, et al. Clinical and electrocardiography changes in patients treated with capecitabine. Chemotherapy 2011; 57: 381–387. [DOI] [PubMed] [Google Scholar]
- 19. Salepci T, Seker M, Uyarel H, et al. 5-Fluorouracil induces arterial vasoconstrictions but does not increase angiotensin II levels. Med Oncol 2010; 27: 416–420. [DOI] [PubMed] [Google Scholar]
- 20. Holubec L, Jr, Topolcan O, Finek J, et al. Dynamic monitoring of cardio-specific markers and markers of thyroid gland function in cancer patients–a pilot study. Anticancer Res 2007; 27: 1883–1886. [PubMed] [Google Scholar]
- 21. Yilmaz U, Oztop I, Ciloglu A, et al. 5-fluorouracil increases the number and complexity of premature complexes in the heart: a prospective study using ambulatory ECG monitoring. Int J Clin Pract 2007; 61: 795–801. [DOI] [PubMed] [Google Scholar]
- 22. Jensen SA, Sorensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol 2006; 58: 487–493. [DOI] [PubMed] [Google Scholar]
- 23. Tsibiribi P, Descotes J, Lombard-Bohas C, et al. Cardiotoxicity of 5-fluorouracil in 1350 patients with no prior history of heart disease. Bull Cancer 2006; 93: E27–E30. [PubMed] [Google Scholar]
- 24. Ng M, Cunningham D, Norman AR. The frequency and pattern of cardiotoxicity observed with capecitabine used in conjunction with oxaliplatin in patients treated for advanced colorectal cancer (CRC). Eur J Cancer 2005; 41: 1542–1546. [DOI] [PubMed] [Google Scholar]
- 25. Ceyhan C, Meydan N, Barutca S, et al. Ultrasound tissue characterization by integrated backscatter for analyzing Fluorouracil induced myocardial damage. Echocardiography 2005; 22: 233–238. [DOI] [PubMed] [Google Scholar]
- 26. Meydan N, Kundak I, Yavuzsen T, et al. Cardiotoxicity of de Gramont’s regimen: incidence, clinical characteristics and long-term follow-up. Jpn J Clin Oncol 2005; 35: 265–270. [DOI] [PubMed] [Google Scholar]
- 27. Sudhoff T, Enderle MD, Pahlke M, et al. 5-Fluorouracil induces arterial vasocontractions. Ann Oncol 2004; 15: 661–664. [DOI] [PubMed] [Google Scholar]
- 28. Oztop I, Gencer M, Okan T, et al. Evaluation of cardiotoxicity of a combined bolus plus infusional 5-fluorouracil/folinic acid treatment by echocardiography, plasma troponin I level, QT interval and dispersion in patients with gastrointestinal system cancers. Jpn J Clin Oncol 2004; 34: 262–268. [DOI] [PubMed] [Google Scholar]
- 29. Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 2001; 19: 2282–2292. [DOI] [PubMed] [Google Scholar]
- 30. Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 2001; 19: 4097–4106. [DOI] [PubMed] [Google Scholar]
- 31. Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 2001; 92: 1759–1768. [DOI] [PubMed] [Google Scholar]
- 32. Balloni L, Porta C, Rossi S, et al. Left ventricular function in colon cancer patients receiving adjuvant fluoro-folate chemotherapy: an echocardiographic study. Oncol Rep 2000; 7: 887–890. [DOI] [PubMed] [Google Scholar]
- 33. Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 1999; 17: 485–493. [DOI] [PubMed] [Google Scholar]
- 34. Orditura M, De Vita F, Sarubbi B, et al. Analysis of recovery time indexes in 5-fluorouracil-treated cancer patients. Oncol Rep 1998; 5: 645–647. [DOI] [PubMed] [Google Scholar]
- 35. Weidmann B, Jansen W, Heider A, et al. 5-Fluorouracil cardiotoxicity with left ventricular dysfunction under different dosing regimens. Am J Cardiol 1995; 75: 194–195. [DOI] [PubMed] [Google Scholar]
- 36. Gradishar W, Vokes E, Schilsky R, et al. Vascular events in patients receiving high-dose infusional 5-fluorouracil-based chemotherapy: the University of Chicago experience. Med Pediatr Oncol 1991; 19: 8–15. [DOI] [PubMed] [Google Scholar]
- 37. Keefe DL, Roistacher N, Pierri MK. Clinical cardiotoxicity of 5-fluorouracil. J Clin Pharmacol 1993; 33: 1060–1070. [DOI] [PubMed] [Google Scholar]
- 38. Schober C, Papageorgiou E, Harstrick A, et al. Cardiotoxicity of 5-fluorouracil in combination with folinic acid in patients with gastrointestinal cancer. Cancer 1993; 72: 2242–2247. [DOI] [PubMed] [Google Scholar]
- 39. Jeremic B, Jevremovic S, Djuric L, et al. Cardiotoxicity during chemotherapy treatment with 5-fluorouracil and cisplatin. J Chemother 1990; 2: 264–267. [DOI] [PubMed] [Google Scholar]
- 40. Rezkalla S, Kloner RA, Ensley J, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol 1989; 7: 509–514. [DOI] [PubMed] [Google Scholar]
- 41. Eskilsson J, Albertsson M. Failure of preventing 5-fluorouracil cardiotoxicity by prophylactic treatment with verapamil. Acta Oncol 1990; 29: 1001–1003. [DOI] [PubMed] [Google Scholar]
- 42. Eskilsson J, Albertsson M, Mercke C. Adverse cardiac effects during induction chemotherapy treatment with cis-platin and 5-fluorouracil. Radiother Oncol 1988; 13: 41–46. [DOI] [PubMed] [Google Scholar]
- 43. Pottage A, Holt S, Ludgate S, et al. Fluorouracil cardiotoxicity. Br Med J 1978; 1: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akhtar SS, Salim KP, Bano ZA. Symptomatic cardiotoxicity with high-dose 5-fluorouracil infusion: a prospective study. Oncology 1993; 50: 441–444. [DOI] [PubMed] [Google Scholar]
- 45. Wacker A, Lersch C, Scherpinski U, et al. High incidence of angina pectoris in patients treated with 5-fluorouracil. A planned surveillance study with 102 patients. Oncology 2003; 65: 108–112. [DOI] [PubMed] [Google Scholar]
- 46. Jensen SA, Hasbak P, Mortensen J, et al. Fluorouracil induces myocardial ischemia with increases of plasma brain natriuretic peptide and lactic acid but without dysfunction of left ventricle. J Clin Oncol 2010; 28: 5280–5286. [DOI] [PubMed] [Google Scholar]
- 47. Meyer CC, Calis KA, Burke LB, et al. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy 1997; 17: 729–736. [PubMed] [Google Scholar]
- 48. Becker K, Erckenbrecht JF, Haussinger D, et al. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs 1999; 57: 475–484. [DOI] [PubMed] [Google Scholar]
- 49. Yung LT, McCrea WA. Capecitabine induced acute coronary syndrome. BMJ Case Rep 2009. DOI: 10.1136/bcr.09.2008.0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Basselin C, Fontanges T, Descotes J, et al. 5-Fluorouracil-induced Tako-Tsubo-like syndrome. Pharmacotherapy 2011; 31: 226. [DOI] [PubMed] [Google Scholar]
- 51. Tajik R, Saadat H, Taherkhani M, et al. Angina induced by 5-fluorouracil infusion in a patient with normal coronaries. Am Heart Hosp J 2010; 8: E111–E112. [DOI] [PubMed] [Google Scholar]
- 52. Camaro C, Danse PW, Bosker HA. Acute chest pain in a patient treated with capecitabine. Neth Heart J 2009; 17: 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Atar A, Korkmaz ME, Ozin B. Two cases of coronary vasospasm induced by 5-fluorouracil. Anadolu Kardiyol Derg 2010; 10: 461–462. [DOI] [PubMed] [Google Scholar]
- 54. Alter P, Herzum M, Soufi M, et al. Cardiotoxicity of 5-fluorouracil. Cardiovasc Hematol Agents Med Chem 2006; 4: 1–5. [DOI] [PubMed] [Google Scholar]
- 55. Shoemaker LK, Arora U, Rocha Lima CM. 5-fluorouracil-induced coronary vasospasm. Cancer Control 2004; 11: 46–49. [DOI] [PubMed] [Google Scholar]
- 56. Luwaert RJ, Descamps O, Majois F, et al. Coronary artery spasm induced by 5-fluorouracil. Eur Heart J 1991; 12: 468–470. [DOI] [PubMed] [Google Scholar]
- 57. Heistad DD, Armstrong ML, Marcus ML, et al. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res 1984; 54: 711–718. [DOI] [PubMed] [Google Scholar]
- 58. Lopez JA, Armstrong ML, Piegors DJ, et al. Effect of early and advanced atherosclerosis on vascular responses to serotonin, thromboxane A2, and ADP. Circulation 1989; 79: 698–705. [DOI] [PubMed] [Google Scholar]
- 59. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 2003; 23: 168–175. [DOI] [PubMed] [Google Scholar]
- 60. Reddy KG, Nair RN, Sheehan HM, et al. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol 1994; 23: 833–843. [DOI] [PubMed] [Google Scholar]
- 61. Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000; 101: 948–954. [DOI] [PubMed] [Google Scholar]
- 62. Vita JA, Treasure CB, Nabel EG, et al. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation 1990; 81: 491–497. [DOI] [PubMed] [Google Scholar]
- 63. Schwartz BG, Economides C, Mayeda GS, et al. The endothelial cell in health and disease: its function, dysfunction, measurement and therapy. Int J Impot Res 2010; 22: 77–90. [DOI] [PubMed] [Google Scholar]
- 64. Hasdai D, Cannan CR, Mathew V, et al. Evaluation of patients with minimally obstructive coronary artery disease and angina. Int J Cardiol 1996; 53: 203–208. [DOI] [PubMed] [Google Scholar]
- 65. Hasdai D, Gibbons RJ, Holmes DR, Jr, et al. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation 1997; 96: 3390–3395. [DOI] [PubMed] [Google Scholar]
- 66. Hasdai D, Holmes DR, Jr, Higano ST, et al. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc 1998; 73: 1133–1140. [DOI] [PubMed] [Google Scholar]
- 67. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 2005; 568: 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mosseri M, Fingert HJ, Varticovski L, et al. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res 1993; 53: 3028–3033. [PubMed] [Google Scholar]
- 69. Kleiman NS, Lehane DE, Geyer CE, Jr, et al. Prinzmetal’s angina during 5-fluorouracil chemotherapy. Am J Med 1987; 82: 566–568. [DOI] [PubMed] [Google Scholar]
- 70. Burger AJ, Mannino S. 5-Fluorouracil-induced coronary vasospasm. Am Heart J 1987; 114: 433–436. [DOI] [PubMed] [Google Scholar]
- 71. Mizuno Y, Hokamura Y, Kimura T, et al. A case of 5-fluorouracil cardiotoxicity simulating acute myocardial infarction. Jpn Circ J 1995; 59: 303–307. [DOI] [PubMed] [Google Scholar]
- 72. Freeman NJ, Costanza ME. 5-Fluorouracil-associated cardiotoxicity. Cancer 1988; 61: 36–45. [DOI] [PubMed] [Google Scholar]
- 73. Henry PD, Yokoyama M. Supersensitivity of atherosclerotic rabbit aorta to ergonovine. Mediation by a serotonergic mechanism. J Clin Invest 1980; 66: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim SM, Kwak CH, Lee B, et al. A case of severe coronary spasm associated with 5-fluorouracil chemotherapy. Korean J Intern Med 2012; 27: 342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Spencker S, Schmittel A, Westermann D, et al. [Angina pectoris and ST-elevation after chemotherapy with 5-fluorouracil]. Internist (Berl) 2007; 48: 69–72, 74. [DOI] [PubMed] [Google Scholar]
- 76. Sara JD, Widmer RJ, Matsuzawa Y, et al. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015; 8: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 77. Thyss A, Gaspard MH, Marsault R, et al. Very high endothelin plasma levels in patients with 5-FU cardiotoxicity. Ann Oncol 1992; 3: 88. [DOI] [PubMed] [Google Scholar]
- 78. Kinhult S, Albertsson M, Eskilsson J, et al. Antithrombotic treatment in protection against thrombogenic effects of 5-fluorouracil on vascular endothelium: a scanning microscopy evaluation. Scanning 2001; 23: 1–8. [DOI] [PubMed] [Google Scholar]
- 79. Cwikiel M, Zhang B, Eskilsson J, et al. The influence of 5-fluorouracil on the endothelium in small arteries. An electron microscopic study in rabbits. Scanning Microsc 1995; 9: 561–576. [PubMed] [Google Scholar]
- 80. Jensen SA, Sorensen JB. 5-fluorouracil-based therapy induces endovascular injury having potential significance to development of clinically overt cardiotoxicity. Cancer Chemother Pharmacol 2012; 69: 57–64. [DOI] [PubMed] [Google Scholar]
- 81. Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999; 19: 972–978. [DOI] [PubMed] [Google Scholar]
- 82. Schalkwijk CG, Poland DC, van Dijk W, et al. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia 1999; 42: 351–357. [DOI] [PubMed] [Google Scholar]
- 83. Spasojevic I, Maksimovic V, Zakrzewska J, et al. Effects of 5-fluorouracil on erythrocytes in relation to its cardiotoxicity: membrane structure and functioning. J Chem Inf Model 2005; 45: 1680–1685. [DOI] [PubMed] [Google Scholar]
- 84. Spasojevic I, Jelic S, Zakrzewska J, et al. Decreased oxygen transfer capacity of erythrocytes as a cause of 5-fluorouracil related ischemia. Molecules 2008; 14: 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tsibiribi P, Bui-Xuan C, Bui-Xuan B, et al. Cardiac lesions induced by 5-fluorouracil in the rabbit. Hum Exp Toxicol 2006; 25: 305–309. [DOI] [PubMed] [Google Scholar]
- 86. Kinhult S, Albertsson M, Eskilsson J, et al. Effects of probucol on endothelial damage by 5-fluorouracil. Acta Oncol 2003; 42: 304–308. [DOI] [PubMed] [Google Scholar]
- 87. Lamberti M, Porto S, Marra M, et al. 5-Fluorouracil induces apoptosis in rat cardiocytes through intracellular oxidative stress. J Exp Clin Cancer Res 2012; 31: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Matsubara I, Kamiya J, Imai S. Cardiotoxic effects of 5-fluorouracil in the guinea pig. Jpn J Pharmacol 1980; 30: 871–879. [DOI] [PubMed] [Google Scholar]
- 89. Millart H, Brabant L, Lorenzato M, et al. The effects of 5-fluorouracil on contractility and oxygen uptake of the isolated perfused rat heart. Anticancer Res 1992; 12: 571–576. [PubMed] [Google Scholar]
- 90. Tamatsu H, Nakazawa M, Imai S, et al. 31P-topical nuclear magnetic resonance (31P-TMR) studies of cardiotoxic effects of 5-fluorouracil (5-FU) and 5’-deoxy-5-fluorouridine (5’-DFUR). Jpn J Pharmacol 1984; 34: 375–379. [DOI] [PubMed] [Google Scholar]
- 91. Durak I, Karaayvaz M, Kavutcu M, et al. Reduced antioxidant defense capacity in myocardial tissue from guinea pigs treated with 5-fluorouracil. J Toxicol Environ Health A 2000; 59: 585–589. [DOI] [PubMed] [Google Scholar]
- 92. Muneoka K, Shirai Y, Yokoyama N, et al. 5-Fluorouracil cardiotoxicity induced by alpha-fluoro-beta-alanine. Int J Clin Oncol 2005; 10: 441–443. [DOI] [PubMed] [Google Scholar]
- 93. Arellano M, Malet-Martino M, Martino R, et al. The anti-cancer drug 5-fluorouracil is metabolized by the isolated perfused rat liver and in rats into highly toxic fluoroacetate. Br J Cancer 1998; 77: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Guo XD, Harold N, Saif MW, et al. Pharmacokinetic and pharmacodynamic effects of oral eniluracil, fluorouracil and leucovorin given on a weekly schedule. Cancer Chemother Pharmacol 2003; 52: 79–85. [DOI] [PubMed] [Google Scholar]
- 95. Diasio RB, Beavers TL, Carpenter JT. Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J Clin Invest 1988; 81: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Maring JG, van Kuilenburg AB, Haasjes J, et al. Reduced 5-FU clearance in a patient with low DPD activity due to heterozygosity for a mutant allele of the DPYD gene. Br J Cancer 2002; 86: 1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shahrokni A, Rajebi MR, Saif MW. Toxicity and efficacy of 5-fluorouracil and capecitabine in a patient with TYMS gene polymorphism: a challenge or a dilemma? Clin Colorectal Cancer 2009; 8: 231–234. [DOI] [PubMed] [Google Scholar]
- 98. Sasson Z, Morgan CD, Wang B, et al. 5-Fluorouracil related toxic myocarditis: case reports and pathological confirmation. Can J Cardiol 1994; 10: 861–864. [PubMed] [Google Scholar]
- 99. Kuropkat C, Griem K, Clark J, et al. Severe cardiotoxicity during 5-fluorouracil chemotherapy: a case and literature report. Am J Clin Oncol 1999; 22: 466–470. [DOI] [PubMed] [Google Scholar]
- 100. Patel B, Kloner RA, Ensley J, et al. 5-Fluorouracil cardiotoxicity: left ventricular dysfunction and effect of coronary vasodilators. Am J Med Sci 1987; 294: 238–243. [DOI] [PubMed] [Google Scholar]
- 101. Grunwald MR, Howie L, Diaz LA., Jr. Takotsubo cardiomyopathy and Fluorouracil: case report and review of the literature. J Clin Oncol 2012; 30: e11–e14. [DOI] [PubMed] [Google Scholar]
- 102. Kwakman JJ, Simkens LH, Mol L, et al. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: a retrospective analysis of the CAIRO studies of the Dutch Colorectal Cancer Group. Eur J Cancer 2017; 76: 93–99. [DOI] [PubMed] [Google Scholar]
- 103. Thyss A, Milano G, Schneider M, et al. Circulating drug levels in patients presenting cardiotoxicity to 5-FU. Eur J Cancer Clin Oncol 1988; 24: 1675–1676. [DOI] [PubMed] [Google Scholar]
- 104. Clasen SC, Ky B, O’Quinn R, et al. Fluoropyrimidine-induced cardiac toxicity: challenging the current paradigm. J Gastrointest Oncol 2017; 8: 970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Polk A, Vaage-Nilsen M, Vistisen K, et al. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 2013; 39: 974–984. [DOI] [PubMed] [Google Scholar]
- 106. Reriani M, Sara JD, Flammer AJ, et al. Coronary endothelial function testing provides superior discrimination compared with standard clinical risk scoring in prediction of cardiovascular events. Coron Artery Dis 2016; 27: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Osto E, Coppolino G, Volpe M, et al. Restoring the dysfunctional endothelium. Curr Pharm Des 2007; 13: 1053–1068. [DOI] [PubMed] [Google Scholar]
- 108. Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e663–e828. [DOI] [PubMed] [Google Scholar]
- 109. Saif MW, Garcon MC, Rodriguez G, et al. Bolus 5-fluorouracil as an alternative in patients with cardiotoxicity associated with infusion 5-fluorouracil and capecitabine: a case series. In Vivo 2013; 27: 531–534. [PubMed] [Google Scholar]
- 110. Cianci G, Morelli MF, Cannita K, et al. Prophylactic options in patients with 5-fluorouracil-associated cardiotoxicity. Br J Cancer 2003; 88: 1507–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ransom D, Wilson K, Fournier M, et al. Final results of Australasian Gastrointestinal Trials Group ARCTIC study: an audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann Oncol 2014; 25: 117–121. [DOI] [PubMed] [Google Scholar]
- 112. Bozkurt O, Karaca H, Ciltas A, et al. Efficacy and safety of raltitrexed combinations with uracil-tegafur or mitomycin C as salvage treatment in advanced colorectal cancer patients: a multicenter study of Anatolian Society of Medical Oncology (ASMO). Asian Pac J Cancer Prev 2014; 15: 1845–1849. [DOI] [PubMed] [Google Scholar]
- 113. Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005; 23: 8671–8678. [DOI] [PubMed] [Google Scholar]
- 114. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011; 29: 3768–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kikuchi K, Majima S, Murakami M. [Clinical survey on cardiotoxicity of tegafur (FT-207)–compilation of a nationwide survey]. Gan To Kagaku Ryoho 1982; 9: 1482–1488. [PubMed] [Google Scholar]
- 116. Marsh JC, Catalano P, Huang J, et al. Eastern Cooperative Oncology Group phase II trial (E4296) of oral 5-fluorouracil and eniluracil as a 28-day regimen in metastatic colorectal cancer. Clin Colorectal Cancer 2002; 2: 43–50. [DOI] [PubMed] [Google Scholar]
- 117. Nagashima F, Ohtsu A, Yoshida S, et al. Japanese nationwide post-marketing survey of S-1 in patients with advanced gastric cancer. Gastric Cancer 2005; 8: 6–11. [DOI] [PubMed] [Google Scholar]
- 118. Koizumi W, Kurihara M, Nakano S, et al. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 2000; 58: 191–197. [DOI] [PubMed] [Google Scholar]
- 119. Lee JL, Kang YK, Kang HJ, et al. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer 2008; 99: 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Akpek G, Hartshorn KL. Failure of oral nitrate and calcium channel blocker therapy to prevent 5-fluorouracil-related myocardial ischemia: a case report. Cancer Chemother Pharmacol 1999; 43: 157–161. [DOI] [PubMed] [Google Scholar]
- 121. Ambrosy AP, Kunz PL, Fisher GA, et al. Capecitabine-induced chest pain relieved by diltiazem. Am J Cardiol 2012; 110: 1623–1626. [DOI] [PubMed] [Google Scholar]
- 122. Oleksowicz L, Bruckner HW. Prophylaxis of 5-fluorouracil-induced coronary vasospasm with calcium channel blockers. Am J Med 1988; 85: 750–751. [DOI] [PubMed] [Google Scholar]
- 123. Klubes P, Cerna I, Meldon MA. Uridine rescue from the lethal toxicity of 5-fluorouracil in mice. Cancer Chemother Pharmacol 1982; 8: 17–21. [DOI] [PubMed] [Google Scholar]
- 124. Ma WW, Saif MW, El-Rayes BF, et al. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer 2017; 123: 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]