Abstract
Purpose of Review:
One of the principal mechanisms by which illness can affect driving safety is by impairing cognition. Nevertheless, despite the substantial evidence demonstrating cognitive impairment in chronic kidney disease (CKD), little is known about the effects of CKD on driving safety.
Objective:
Investigate the current national medical guidelines and research literature with respect to CKD and driving safety.
Sources of Information:
Medline, CINAHL, PEDro, Scopus as of August 2017. The most up to date national driving guidelines and available information provided by the provincial and territorial ministries of transportation across Canada
Findings:
Fives studies of driving fitness in patients with CKD have been published with minimal data available for patients at early stages of the disease. Amongst these studies, only two come from an era when modern end stage renal disease therapies were routinely provided. The first study demonstrated that 40% of 186 surveyed patients on hemodialysis felt uncomfortable driving and that 1/3 of patients were involved in motor vehicle collisions (MVC) since starting dialysis. Of the patients who felt comfortable driving, more than 75% were found to be at increased driving risk. The second study reported that 15% of patients on hemodialysis were involved in MVCs over a three year span and that the “Am I A Safe Driver” assessment tool by the American Medical Association may not capture all patients at high driving risk. Despite these alarming numbers, national guidelines place few driving restrictions on this patient population and only 3 of 11 available provincial or territorial driving forms include kidney disease as a category that physicians should consider when assessing medical fitness to drive.
Limitations:
Our review is limited by the lack of randomized control studies evaluating the effects of CKD on driving safety.
Implications:
Our review demonstrates that driving safety in this patient population remains poorly understood. The limited evidence that does exist, however, suggests that these patients are at substantial risk for unsafe driving. Future research is necessary to determine the impact of CKD-associated cognitive impairment on driving risk, and to parse out the contributions of CKD and its various treatments to driving impairment.
Keywords: chronic kidney disease, driving safety, cognitive impairment, medical guidelines
Abrégé
Motif de la revue:
La réduction de la vigilance engendrée par la maladie est un des principaux mécanismes par lesquels celle-ci peut affecter la sécurité au volant. Cependant, malgré des données probantes faisant état de troubles cognitifs associés à l’insuffisance rénale chronique (IRC), on en sait peu sur l’incidence de l’IRC sur la conduite.
Objectif de la revue:
Examiner les travaux de recherche et les recommandations médicales nationales en matière de sécurité routière en contexte d’IRC.
Sources:
Ont été consultés 1- les articles traitant du sujet publiés en date d’août 2017 sur Medline, CINAHL, PEDro et Scopus; 2- les plus récentes recommandations routières nationales et l’information fournie par les ministères des transports provinciaux et territoriaux du Canada.
Constatations
Cinq études faisant état des aptitudes de conduite de patients atteints d’IRC ont été publiées. Ces études contenaient toutefois peu de données concernant les patients atteints des premiers stades de la maladie. Seules deux études étaient datées d’une époque où on appliquait systématiquement les traitements modernes de l’insuffisance rénale terminale. La première mentionnait que 40 % des 186 patients hémodialysés sondés se disaient mal à l’aise de conduire, et que le tiers avait été impliqué dans un accident de la route depuis le début de leurs traitements de dialyse. Parmi les patients qui se disaient à l’aise de conduire, plus de 75 % se sont avérés des conducteurs à risque. La deuxième étude rapportait que 15 % des patients hémodialysés avaient été impliqués dans une collision automobile sur une période de trois ans. Cette étude ajoutait que l’outil d’évaluation Am I A Safe Driver? (Association médicale américaine) pouvait ne pas dépister tous les patients à risque élevé. Malgré ces chiffres alarmants, les recommandations nationales n’imposent que très peu de restrictions aux patients hémodialysés. De plus, seulement trois des onze formulaires de conduite provinciaux ou territoriaux répertorient la néphropathie comme maladie à considérer lors de l’évaluation médicale des aptitudes de conduite.
Limites de l’étude:
Notre revue est limitée par le manque d’études contrôlées à répartition aléatoire évaluant l’effet de l’IRC sur la conduite.
Conclusion:
Notre revue démontre que la sécurité au volant demeure mal comprise au sein de la population de patients hémodialysés. Les données examinées, quoique parcimonieuses, suggèrent que ces patients posent un risque substantiel à la sécurité routière. Des études additionnelles sont nécessaires pour évaluer l’incidence des troubles cognitifs associés à l’IRC sur les risques d’accidents de la route, et pour établir un lien entre l’IRC (et ses divers modes de traitement) sur la réduction des aptitudes de conduite.
What was known before
Cognitive impairment is associated with unsafe driving. Despite cognitive impairment being remarkably common in chronic kidney disease (CKD), little is known regarding driving safety in this patient population.
What this adds
This review summarizes what is known about driving safety in patients with CKD and highlights areas requiring further investigation.
Introduction
Driving is not only an indispensable daily activity for many individuals, but also carries with it significant risk. Motor vehicle collisions result in approximately 2500 deaths and 180 000 injuries each year in Canada1 with medical illnesses accounting for roughly 18% to 23% of these incidents.2 One of the principal mechanisms by which illness can affect driving safety is by impairing cognition. The impact of cognitive dysfunction on driving safety has been examined for diseases such as Alzheimer’s disease, heart failure, diabetes, and stroke. This research has identified common deficits in specific cognitive domains, which has allowed for the development of evidence-based driving safety guidelines specific to each of these disease states.3-6 Despite substantial evidence demonstrating cognitive impairment in chronic kidney disease (CKD),7 little is known about the effects of CKD on driving safety in a large and growing population of more than 3 million Canadians.8
Cognitive Impairment in CKD
The relationship between CKD and cognitive impairment is a large and well-studied area that has been extensively reviewed in the past7,9-11. For the purposes of this review, we will briefly summarize the known epidemiologic and pathophysiologic links between CKD and cognitive dysfunction.
Cognitive impairment refers to a decline in cognitive functioning. This decline may affect 1 or many cognitive domains, which include language, perceptual-motor functioning, executive functioning, learning and memory, social cognition and complex attention.12 Cognitive impairment exists along a spectrum from mild cognitive impairment to dementia. Mild cognitive impairment is defined as chronic impairment in 1 or more domains that does not interfere with activities of daily living. Dementia, on the contrary, is diagnosed only when there is chronic impairment in 2 or more cognitive domains that interferes with work or usual activities.13,14
The insidious onset of symptoms, patients’ denial of impairments, and time limitations of practitioners make cognitive impairment difficult to recognize, particularly at its early stages.15 To overcome barriers to detecting cognitive impairment, simple screening tests are available to help health care professionals initially assess a patient’s cognitive status. Commonly used performance-based screening tests include the Mini-Mental State Examination (MMSE), the Mini-Cog Assessment Instrument (Mini-Cog), and the Montreal Cognitive Assessment (MoCA). More comprehensive standardized cognitive assessments, which involve complex batteries of neuropsychiatric tests that assess individual cognitive domains, are employed by specialists to diagnose cognitive impairment in at-risk patients and to identify which specific cognitive domains are affected.15
Cognitive impairment is remarkably common at all stages of CKD. Both cross-sectional and longitudinal studies suggest that cognitive dysfunction appears to progress as renal function deteriorates.16-19 Importantly, the degree of impairment in any given patient can vary from mild to severe and can affect various cognitive domains. In general, though, the cognitive domains of attention and concentration, processing speed, executive functioning (including mental flexibility and inhibition), and memory are among those most affected by CKD.20
Studies have demonstrated cognitive impairment in both patients on hemodialysis and patients on peritoneal dialysis. Murray et al, for instance, enrolled 338 hemodialysis patients and examined their cognition using a battery of neuropsychological tests, including the Modified Mini-Mental State Examination, the Hopkins Verbal Learning Test–Revised (HVLRT-R), Color Trails 1 and 2, the Stroop Interference test, the Brief Visuospatial Memory Test–Revised (BVMT-R), the Controlled Oral Word Association Test (COWAT), the Clock-Drawing Test, the Wechsler Digit Span, and the Geriatric Depression Scale. The study found that approximately 85% of this cohort was mildly cognitively impaired and 70% demonstrated moderate to severe cognitive dysfunction.21 A more recent study using neuropsychological tests assessing similar cognitive functions had comparable results, with mild cognitive impairment in 89% of patients on hemodialysis and 63% of predialysis patients.22 Interestingly, while studies show that peritoneal dialysis patients also experience cognitive decline, their impairment appears to be both less prevalent and less severe than their hemodialysis counterparts.23-27 Whether this discrepancy is a result of true technique-specific differences, or rather reflects differences in comorbidity burden, needs further investigation.
Despite formal studies demonstrating that many patients with CKD are cognitively impaired, it is likely that cognitive impairment remains significantly underrecognized. In an early study of American hemodialysis patients, for example, only 15% of patients with cognitive impairment had a diagnosis of such in their medical record.28 Similarly, in the Dialysis Outcomes and Practice Patterns Study, only 4% of hemodialysis patients carried a diagnosis of dementia in their medical records.29 This proportion is much lower than the expected prevalence of dementia based on formal assessments of cognitive function in hemodialysis cohorts.18,21,30
Pathophysiology of Cognitive Impairment in CKD
CKD can lead to cognitive impairment due to multiple mechanisms, which can be broadly divided into 2 categories: vascular and nonvascular. Vascular injury and dysfunction are well described phenomena in CKD, due not only to an increased prevalence of traditional risk factors such as hypertension, diabetes, and smoking but also because of direct adverse effects of the uremic environment on endothelial function.31,32 These abnormalities can lead to cerebral macrovascular and microvascular disease that ultimately results in brain hypoperfusion, a phenomenon that is reflected in an increased burden of ischemic cerebral white matter changes and microinfarcts.33 Nonvascular phenomena associated with CKD, such as anemia, hyperparathyroidism, sleep disturbances, and polypharmacy, may also play a role as they have all been linked to cognitive impairment.31
Hemodialysis itself may worsen the cognitive impairment seen in patients with CKD. A common complication of hemodialysis is intradialytic hypotension, which is thought to exacerbate cerebral perfusion defects and ischemic brain injury. Although autoregulation protects against cerebral hypoperfusion when systemic blood pressure falls, the autoregulation process is limited below a mean arterial pressure (MAP) of 60 to 70 mm Hg, a frequent occurrence during hemodialysis.34 These recurrent episodes of dialysis-induced cerebral hypoperfusion can worsen CKD-associated ischemic brain damage, as reflected by the high frequency of white matter changes and cortical atrophy seen in hemodialysis patients.35,36 This small vessel disease is believed to be independently associated with the severity of kidney disease and is a common cause of vascular dementia, typically presenting with impairments in attention, executive function, and processing speed, all of which are required for driving.22 In support of this preferential effect on driving-related cognitive domains, functional magnetic resonance imaging (MRI) has demonstrated reduced perfusion in the frontal lobe of patients on hemodialysis, the domain largely responsible for driving-related functions like memory, movement, and executive function.37 Importantly, this dialysis-induced brain damage may be preventable, as dialysate cooling to 0.5°C below core body temperature stabilized intradialytic MAP and reduced white matter lesions after 1 year, compared with controls receiving normothermic dialysate.38
A final potential deleterious effect of hemodialysis may arise from its episodic nature. Hemodialysis clears uremic toxins only when patients are connected to the dialysis machine. As such, unlike patients on peritoneal dialysis, uremic toxins build up over 48 to 72 hours in a patient on a standard thrice-weekly hemodialysis regimen. During the 48- to 72-hour interdialytic interval, deficits in attention and memory develop, resulting from the accumulation of uremic metabolites.26
Is Cognitive Impairment Associated With Increased Driving Risk?
Driving requires the integration of multiple cognitive domains to be performed safely.39,40 The cognitive domains include those commonly affected by CKD, such as attention and concentration, reaction time, executive function, and memory. These cognitive domains are essential for basic driving-related tasks such as starting, turning, reversing, and stopping, as well as for more complex functions such as obeying traffic laws and responding to driving hazards.40 A more comprehensive list of the cognitive functions relevant to driving and the driving-related tasks for which those cognitive functions are required are summarized in Table 1.
Table 1.
Cognitive Domains Associated With Driving-Related Tasks.
| Cognitive function | Associated driving-related tasks |
|---|---|
| Visuospatial skills | Ability to determine spatial relationship between one’s car and objects in its environment (road lines, objects on the road, curb, other cars). Ability to position automobile correctly and maneuver it. |
| Orientation | Ability to recognize infrastructure and navigate routes. |
| Judgment | Ability to assess danger associated with specific driving conditions, such as driving when roads are slippery or overtaking another vehicle |
| Complex reaction time | Ability to respond to traffic situations on the road (eg, another driver crossing lanes) |
| Working memory | Ability to handle and update information based on external stimuli |
| Long-term memory | Ability to remember specific locations, road rules, and routes |
| Executive function | Ability to plan routes, problem solve, make driving-related decisions |
| Attention and concentration | Ability to identify pertinent environmental stimuli, while ignoring others. |
Whether cognitive dysfunction might affect driving safety has been best explored in the elderly, as increased age is a known risk factor for cognitive impairment.39-43 Studies on the effect of cognitive dysfunction on driving safety among the elderly stem from the well-documented curvilinear relationship that exists between age and motor vehicle accidents (MVAs): young drivers and drivers older than the age of 65 years are at higher risk for collisions when compared with middle-aged drivers.41,42 In the elderly, multiple epidemiologic studies have demonstrated an association between cognitive function and driving risk.43,44 Importantly, cognitive test scores remain an independent predictor of driving performance in the elderly, even when controlling for age, race, and driving frequency. These tests scores suggest that cognitive function itself is an important determinant of driving safety.43 In an effort to understand which cognitive domains are most important for driving safety in the elderly population, researchers have performed more detailed neuropsychological testing and have found that decreased cognitive processing speed, psychomotor functioning, visuospatial performance, and executive functioning correlated with driving impairment.41 Other studies have demonstrated that tests of complex attention, including selective and divided attention, as well as spatial ability and reaction time, are important predictors of driving performance.42 Furthermore, the capacity to divide and shift attention, processing speed, and the ability to detect road hazards, including pedestrians, have all been shown to decline with age and impact driving safety.45
In addition to the elderly, studies on driving safety have examined several chronic disease states in which cognitive impairment has been implicated. A recent meta-analysis of almost 1300 patients with mild or very mild Alzheimer’s disease demonstrated poor performance in driving simulation and on-road testing when compared with healthy age-matched controls.46 The most significant predictors of driving performance in these patients were executive function (as measured by The Maze Test, TMT-B, verbal fluency), attention (as measured by Useful Field of View), visuospatial (as measured by Rey Osterrieth Complex Figure copy), and global cognition (as measured by MMSE). The relationship between cognition and driving performance has also been demonstrated in patients with nonneurologic diseases such as heart failure. In a single-center, cross-sectional study, specific cognitive deficits associated with heart failure were shown to be independently associated with poor driving performance.47 Specifically, attention and executive function were associated with lane deviations and centerline crossings, while psychomotor dysfunction was correlated with reduced ability to avoid collisions. Interestingly, these cognitive deficits were believed to be at least in part the result of cerebral hypoperfusion,48 a pathophysiologic feature shared between heart failure and CKD.
In this review, we discuss the available literature with respect to CKD and driving safety, and the existing guidelines for assessing and reporting driving impairment in patients with CKD.
Methods
We performed a comprehensive scoping review of the available literature with respect to CKD-induced cognitive impairment and driving safety guided by a health sciences librarian.49,50 Our review approach was divided into 5 stages, including (1) identifying the research question, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collating, summarizing, and reporting the results.
Identifying Relevant Peer-Reviewed Academic and Grey Literature
We first performed a search of the peer-reviewed academic literature. To identify relevant studies, we used 3 search phrases: “chronic kidney disease,” “cognitive dysfunction,” and “automobile driving.” We first focused on defining search terms for CKD. The Medical Subject Head (MeSH) term for CKD is “Renal Insufficiency, Chronic.” We expanded the search to include all references tagged with this subject heading, as well as those references tagged with any narrower subject headings. We then expanded our search using truncation symbols and Boolean operators to combine search terms when appropriate. Ultimately, we used the following terms: chronic renal insufficiency, chronic renal disease, chronic kidney disease, and CKD. We added field searching abbreviations to these terms to search for these key phrases in the title, abstract, and author-supplied keyword fields. In addition, we included the following terms for the MeSH term “Renal Dialysis”: hemodialysis, dialysis, hemodiafiltration, hemofiltration, kidney transplant, and renal transplant. For our second search area (automobile driving), our search terms included the following: automobile driving, traffic accidents, motor vehicles, drive, driving, traffic accident, traffic safety, and vehicle. To limit irrelevant results generated from widely used terms, such as “drive” and “vehicle,” we solely searched for these terms in the title and author-supplied keyword fields. For our third search area (cognitive dysfunction), we included the following search terms: cognitive dysfunction, cognitive impairment, cognitive decline, and mental deterioration. We used the same criteria for expanding our search and narrowing for specific terms as noted above.
Using these terms, we first employed the following search strategy: “chronic kidney disease” AND “cognitive dysfunction” AND “automobile driving,” retrieving zero results. To broaden our strategy, we next searched using the following strategy: “chronic kidney disease” AND “cognitive dysfunction” OR “automobile driving”. We did not limit articles by language; where possible, we obtained English translations of foreign articles. We focused our review to the Medline, CINAHL, PEDro, and Scopus databases from their inception to August 9, 2017. In addition to the articles we retrieved from our literature search, we hand-searched the references of all the relevant articles to ensure that we did not exclude any important literature.51
We also searched the grey literature, which includes material produced by all levels of government, academics, business, and industry, but not controlled by commercial publishers.52 We reviewed the websites or archives of relevant governmental agencies, such as the Canadian Council of Motor Transport Administrators and the Canadian Medical Association. Furthermore, we consulted online resources provided by the provincial and territorial Ministries of Transportation across Canada. For all provinces and territories with resources unavailable online, we contacted Ministry of Transportation offices directly by phone and/or email to obtain relevant documents to our review. We also consulted with relevant CKD interest groups, such as the Kidney Foundation of Canada, the National Kidney Foundation, the National Kidney Disease Education Program, and Caring for Australians with Renal Impairment. For inclusion in our review, the grey literature needed to (1) focus on fitness to drive, (2) be directed toward health care professionals, and (3) address the relationship between CKD and unsafe driving. The Authority, Accuracy, Coverage, Objectivity, Date and Significance (AACODS) checklist was used to critically appraise and report findings of the grey literature.53
Literature Selection and Data Abstraction
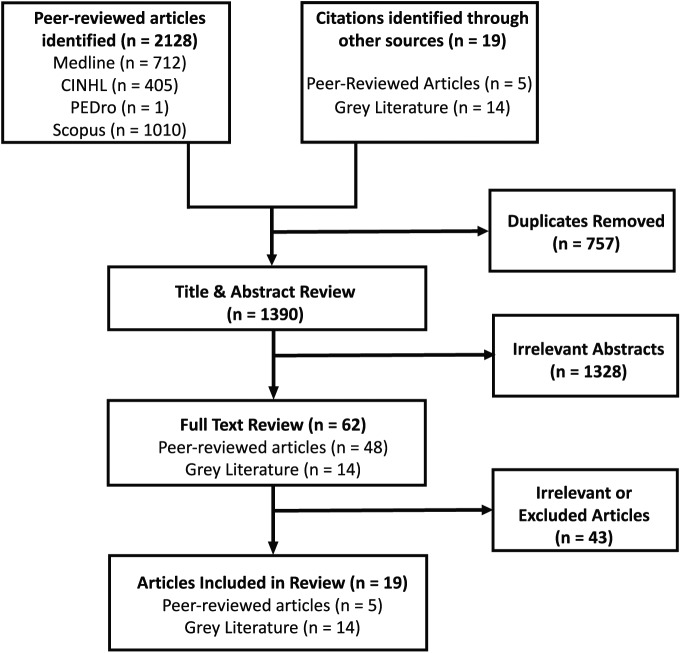
We divided our literature selection process between review of (1) peer-reviewed academic articles and (2) the grey literature, consisting of unpublished or published reports or forms from health organizations and governmental agencies. Two of the authors (D.K. and L.G.) carried out the review of the literature and screened the titles and abstracts for mention of CKD and/or renal replacement therapy, cognitive dysfunction, and their effects on driving. If we considered an abstract relevant, we reviewed the full article. The results of our literature search and study selection are outlined in Figure 1. Following removal of duplicates, the total number of citations returned by our search was 1390. Following our review of these titles and abstracts, there were 62 articles suspected to be relevant to our research question that we subsequently reviewed in full. We excluded 36 articles for irrelevance as they did not specifically address our research question, and we were unable to obtain 7 articles and therefore did not analyze them. We ultimately identified 5 published articles which we included in our review (Table 2). In addition, from our search of unpublished or published reports of health organizations and governmental agencies, we identified 14 reports or forms that were included in our review.
Figure 1.
Literature review strategy.
Table 2.
Published Articles Included in Review.
| Author(s) (year) | Title | Findings | Limitations |
|---|---|---|---|
| Ysander (1966)54 | “The safety of drivers with chronic disease” | 2.5% of patients with “renal disease” were involved in a road accident, compared with 7.7% of healthy age-matched controls. | Patients were considered to have renal disease solely by the presence of proteinuria and/or hypertension suggesting milder forms of chronic kidney disease. |
| Gyalog (1972)55 | “Investigations on the ability to transport in diabetics and chronically hemodialysed patients” | Driving impairment detected following twice-weekly 10-hour hemodialysis populations. Recommend patients avoid driving for 24 hours after dialysis | Full article unavailable No accessible methods or results |
| Schewe et al (1982)56 | “Examinations concerning the psychophysiological capacity of dialysis patients with regard to driving ability” | Patients often experienced resting tremor, but intentional movements were uncompromised. Recommend patients are fit to drive. | Small sample size (n = 15) No descriptions of tests performed Cognition not formally assessed |
| Vats and Duffy (2010)57 | “Assessment of self-perceived risk and driving safety in chronic dialysis patients” | 40% of patients on dialysis were uncomfortable driving, but almost half continued to operate a motor vehicle. 79% of the patients who felt comfortable driving had absolute or relative risk factors for unsafe driving | Information gathered by patient survey and, thus, was subject to recall bias. Absence of control groups. |
| Varela et al (2015)58 | “A diagnostic screening tool for identifying safe drivers among dialysis patients” | American Medical Association’s survey was sensitive, but not specific, for identifying patients with absolute or relative risk factors for unsafe driving | Information gathered by survey with no objective assessment of driving ability |
In our scoping review, we applied a common analytical framework and collected standardized information from each study. We recorded the (1) authors’ names, (2) year of publication, (3) study location, (4) study population, (5) aims of the study, (6) methodology, and (7) outcomes of the study. In an effort to allow for comparison of studies, we applied a consistent approach to reporting study findings. Furthermore, we aimed to identify gaps in the methodology and findings of these reports.
Results
As noted above, CKD leads to deficits in many cognitive domains that are of importance in determining driving safety in other, more well-studied chronic disease states. Furthermore, cerebral hypoperfusion, a common feature of CKD due both to uremia-associated cerebrovascular disease and hemodialysis-associated reductions in cerebral blood flow, has been implicated as a major pathophysiologic contributor to cognitive dysfunction–induced driving impairment in disease states such as heart failure. Together, these data suggest that CKD-induced cognitive dysfunction may affect driving safety in patients with CKD. To date, however, the effects of CKD-associated cognitive impairment on driving safety remain largely unexplored.
Is Driving Safety Impaired in Patients With CKD-Induced Cognitive Impairment?
To better understand the links between CKD-induced cognitive dysfunction and driving fitness, we performed a review of the existing literature. Our search identified only 5 published studies of driving fitness in patients with CKD, with half of these studies from an era predating the use of current therapies such as high flux dialysis, aggressive blood pressure control, and erythropoiesis stimulation.
One early study focused on the effects of a variety of chronic medical conditions on driving safety. This study, published in 1966, investigated the frequency of patients with chronic disease that were involved in MVAs.54 Over a span of 4.5 years, 2.5% of patients with “renal disease” were shown to be involved in a road accident, as compared with 7.7% of the healthy age-matched control population. Unfortunately, in this retrospective cohort, the definition of renal disease was the presence of proteinuria and/or hypertension, 2 characteristics that provide little information on the severity of renal dysfunction.
The first report of the effects of end-stage renal disease (ESRD) on driving ability was a German thesis dissertation published in 1972, in which the author documented driving impairment following twice-weekly 10-hour hemodialysis sessions and recommended that patients avoid driving for 24 hours postdialysis.55 A decade later, driving fitness was assessed in patients dialyzed using a more modern regimen (3 to 4 hours thrice weekly).56 The investigators examined motor, but not cognitive, function before and after dialysis, and found that while patients often experienced resting tremor, their intentional movements were largely uncompromised.
Since these early studies, the typical patient with CKD has changed markedly. Today, patients are older and have more medical comorbidities, many of which can independently impair cognitive function.59 Renal replacement therapies have also changed, with the development of a diversified repertoire of dialytic modalities that can be tailored to patients’ individual needs and preferences.60 Despite these advances in dialytic therapies, limited research has been performed in the intervening years to examine whether driving safety in patients with CKD has similarly evolved.
In the last 3 decades, only 2 studies have examined driving fitness in ESRD patients, with no studies in patients with predialysis CKD. The first of these reports, published in 2010, surveyed 186 hemodialysis patients (average age 67.8 years) across 6 American dialysis units.57 Although 40% of patients were uncomfortable driving, almost half continued to do so. Alarmingly, 79% of the patients who reported feeling comfortable driving had absolute or relative risk factors for unsafe driving, including a history of fainting during driving, falling asleep at the wheel, sleep apnea or loud snoring, weakness prior to dialysis, and a history of hypoglycemic episodes. Furthermore, approximately one-third of patients reported being involved in a MVA since beginning dialysis.57
The second study enrolled 106 dialysis patients (average age 53.4 years) from a single American center and examined the utility of the American Medical Association’s (AMA) “Am I A Safe Driver” survey.58 The authors found that the AMA survey was sensitive but not specific for identifying patients with absolute or relative risk factors for unsafe driving, as defined in the 2010 study. However, as no gold standard for assessing driving risk in ESRD patients exists, it is unclear whether the AMA survey captured all patients at high driving risk. Of note, 15% of the dialysis patients in the AMA survey reported being involved in a MVA in the last 3 years,58 a marginally higher rate than the 3% to 4% per year collision rate for age-matched drivers in the general American population.61 Whether the self-reported MVA rate in this dialysis cohort is accurate remains unclear. It is conceivable that patients may have underreported their collision rates due to concern that their answers could jeopardize their driving privileges.
Clearly, the nature and causes of driving impairment in CKD and ESRD remain understudied. Given the importance of driving for independent living and the potential for serious harm to patients and the general public, further investigation of these issues is urgently needed.
What Are the Current Driving Guidelines in Patients With CKD?
Our review identified “grey” literature that consisted mostly of guidelines and provincial report forms related to the management of driving capacity in patients with medical illnesses, specifically focusing on sections pertaining to CKD. Reflecting the concern that CKD may be associated with impaired driving safety, the Canadian Council of Motor Transport Administrators (CCMTA) and Canadian Medical Association (CMA) have published guidelines to help clinicians assess driving safety (Table 3). The CCMTA’s “Determining Driver Fitness in Canada” manual states that patients with mild renal dysfunction (stage 1-2 CKD) likely have the functions necessary to drive. These patients are thus at low risk for driving impairment, whereas patients with moderate to severe dysfunction (stage 3-5 CKD) are at significant risk for cognitive dysfunction and driving impairment.62 Despite this categorization, the CCMTA recommends that all patients with stages 1 to 4 CKD should be assessed for “functional abilities” required for safe driving to qualify for a driver’s license. The CCMTA also recommends that patients with ESRD undergo this same initial exam, as well as annual reassessments, which would require the patients to demonstrate that they abstain from driving when dialysis is delayed, are under regular medical supervision, and are following their prescribed dialysis regimen. Reflecting the lack of evidence in this area and validated assessment tools, no guidance is provided as to what the components of these examinations should be, nor are the definitions of “functional abilities” and “regular medical supervision” specified.
Table 3.
Canadian Driving Guidelines Recommendations for Driving With CKD.
| Canadian Council of Motor Transport Administrators | Canadian Medical Association | |
|---|---|---|
| Eligibility for a license | Stage 1-4 CKD Medical assessments show no residual effects The functional abilities necessary to drive are intact Stage 5 CKD Medical assessments show no residual effects The functional abilities necessary to drive are intact The conditions for maintaining a license are met |
Stage 1-4 CKD Not discussed Stage 5 CKD Individual assessment for relevant comorbidities, medications, and adverse symptoms associated with their treatments |
| Medical reassessment | Stage 1-4 CKD Require routine medical review or more frequently at the discretion of the authority Stage 5 CKD Must have an annual medical review |
Stage 1-4 CKD Not discussed Stage 5 CKD Commercial drivers must have an annual medial review |
| Conditions for maintaining a license | Stage 1-4 CKD None Stage 5 CKD Routinely follow dialysis regimen Do not drive if dialysis is delayed Remain under regular medical supervision |
Stage 1-4 CKD Not discussed Stage 5 CKD Not discussed |
Note. CKD = chronic kidney disease.
The other major resource for Canadian physicians is the “Driver’s Guide: Determining Medical Fitness to Drive,” published by the CMA.63 These guidelines state that patients with ESRD are safe to operate a motor vehicle, once adjusted to a “stable dialysis regimen,” but should avoid driving if dialysis is delayed or a new medical issue develops that has not yet been assessed by a physician. Furthermore, the guidelines suggest that physicians should consider comorbidities, medications, and adverse symptoms associated with dialysis treatments. The CMA advises physicians to counsel all CKD patients on medical issues that may occur while adjusting to a new dialysis regimen and that can impair driving ability, such as electrolyte imbalances, infection, hypotension, weakness, or ischemic coronary events. The guidelines do not set out in detail how these assessments should be performed nor does it specifically mention the effects of CKD-induced cognitive impairment on driving risk.
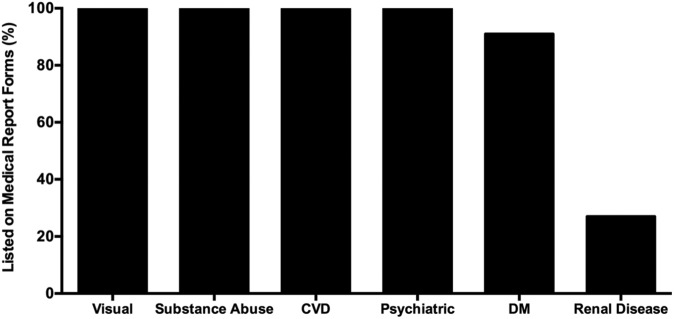
Finally, as driving safety is provincially regulated, physicians must complete “medical assessment of driving” forms to report potential driving impairment to their respective Ministry of Transportation. Although cardiovascular, neurological, psychiatric, and substance abuse disorders are included on all fitness to drive assessment forms (Figure 2), only 3 of the 11 available provincial or territorial forms include kidney disease as a category that physicians should consider when assessing medical fitness to drive. Clearly, current Canadian driving guidelines with regard to CKD vary across the country and are limited in scope. Moreover, because they are based on a paucity of evidence, they are vaguely worded and, in most cases, do not specifically address CKD-induced cognitive impairment. Finally, these guidelines focus primarily on monitoring dialysis compliance and fail to address the possibility that treatments for ESRD and the comorbidities of ESRD might also affect cognitive function and driving safety.
Figure 2.
Relative representation of medical disorders on provincial and territorial “Medical Assessment of Driving” Forms.
Note. CVD = cardiovascular disease; DM = diabetes mellitus.
Conclusion and Future Directions
Driving is an important component of independent life and yet is associated with significant risk. Despite the increasing prevalence of CKD and the well-documented presence of CKD-induced cognitive impairment, driving safety in this frail population remains poorly understood. The little evidence that does exist, however, suggests that (1) these patients are at substantial risk for unsafe driving57,58 and (2) CKD tends to commonly affect cognitive domains that are important for driving safety, such as attention, concentration, memory, and executive function.20
Although clinicians may be aware of the potential for driving impairment in patients with CKD, many do not raise these concerns with their patients or with their provincial Ministry of Transportation. Clinicians’ failure to raise concerns may be explained by our poor understanding of how CKD and its treatments might affect driving fitness, a paucity of education on how to assess driving impairment, and a desire to maintain a positive clinician-patient relationship.64,65 Compounding this situation, existing guidelines on how to assess driving fitness in CKD patients are vague, and the majority of provincial or territorial driving safety assessment forms do not highlight CKD and its associated treatments as indications for driving safety evaluation. With all these factors considered, it is not surprising that physicians struggle to detect potential unsafe driving in patients with CKD.
Our review highlights a number of areas needing further investigation. First, studies to determine whether CKD-associated cognitive dysfunction adversely affects driving capacity are clearly needed, and if so, the stage(s) of CKD at which risk significantly increases must be identified. Second, given the highly reported prevalence of cognitive impairment in CKD, the importance of screening for cognitive dysfunction as a means to identify patients at risk for driving impairment needs to be addressed. Third, as CKD is a pathologic state that is the result of many different etiologies, it will be important to analyze whether different causes of chronic kidney injury affect driving ability in different ways. Similarly, studies are needed to determine whether the various pharmacologic and nonpharmacologic treatments used in patients with CKD affect cognitive function and driving ability differently.
As driving requires the use of multiple motor, sensory, and cognitive domains, we suggest, as a first step, the use of validated driving simulator tests that integrate all of these functions. We suggest that these tests should be used in conjunction with bedside tests of cognition, motor, and sensory functions to clarify the effects of different stages and types of CKD, as well as its therapies, on driving ability. Mechanistic studies will also be needed to parse out the individual contributions of CKD and its various treatments to driving impairment. Furthermore, as there is no literature regarding how clinicians taking care of CKD patients assess driving safety in their patients, we believe that surveying these clinicians with respect to their current assessment practices is an important area for further investigation. Importantly, although our review focused on cognitive impairment, the design of these future studies will need to take into account not only CKD-associated cognitive dysfunction but also the many other noncognitive risk factors present in CKD patients, including physical disability and frailty that can also affect driving safety. These studies will ultimately provide a much-needed foundation of knowledge to refine driving guidelines and raise clinician awareness regarding screening practices in this fragile, at-risk population.
Acknowledgments
The authors would like to thank Teruko Kishibe for her assistance in our review of the literature.
Footnotes
Ethics Approval and Consent to Participate: Not applicable
Consent for Publication: Not applicable
Availability of Data and Materials: Not applicable
Authors’ Note: David M. Kepecs and Lauren Glick contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: No competing financial interests exist. S.A.S. is supported by a Kidney Research Scientist Core Education and National Training (KRESCENT) Program Post-Doctoral Fellowship (co-funded by the Kidney Foundation of Canada, Canadian Society of Nephrology, and Canadian Institutes of Health Research). D.A.Y. was supported by a KRESCENT New Investigator Award. He is currently supported by a Canadian Diabetes Association Clinician Scientist Award and a Canadian Institutes of Health Research New Investigator Award.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Canadian Motor Vehicle Traffic Collision Statistics: 2010. Statistics Ottawa, ON, Canada; 2010. [Google Scholar]
- 2. Lindsay VBM. Medical Conditions as a Contributing Factor in Crash Causation; Adelaide, South Australia, Australia, 2008. [Google Scholar]
- 3. Simpson C, Dorian P, Gupta A, et al. Assessment of the cardiac patient for fitness to drive: drive subgroup executive summary. Can J Cardiol. 2004;20:1314-1320. [PubMed] [Google Scholar]
- 4. Begg I, Yale J, Houlden R, Rowe R, McSherry J. Canadian diabetes association’s clinical practice guidelines for diabetes and private and commercial driving. Can J Diabetes. 2003;27:128-140. [Google Scholar]
- 5. Hogan DB, Bailey P, Carswell A, et al. Management of mild to moderate Alzheimer’s disease and dementia. Alzheimers Dement. 2007;3:355-384. [DOI] [PubMed] [Google Scholar]
- 6. Molnar F. Driving & Dementia eLearning Module. Markham, ON: Canadian Geriatrics Society. [Google Scholar]
- 7. Shen Z, Ruan Q, Yu Z, Sun Z. Chronic kidney disease-related physical frailty and cognitive impairment: a systemic review. Geriatr Gerontol Int. 2017;17:529-544. [DOI] [PubMed] [Google Scholar]
- 8. Arora P, Vasa P, Brenner D, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013;185:E417-E123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berger I, Wu S, Masson P, et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med. 2016;14:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iyasere O, Okai D, Brown E. Cognitive function and advanced kidney disease: longitudinal trends and impact on decision-making. Clin Kidney J. 2017;10:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bronas UG, Puzantian H, Hannan M. Cognitive impairment in chronic kidney disease: vascular milieu and the potential therapeutic role of exercise. Biomed Res Int. 2017;2017:2726369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. 2014;10:634-642. [DOI] [PubMed] [Google Scholar]
- 13. Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142. [DOI] [PubMed] [Google Scholar]
- 14. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 15. Galvin JE, Sadowsky CH, Nincds A. Practical guidelines for the recognition and diagnosis of dementia. J Am Board Fam Med. 2012;25:367-382. [DOI] [PubMed] [Google Scholar]
- 16. Davey A, Elias MF, Robbins MA, Seliger SL, Dore GA. Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrol Dial Transplant. 2013;28:1810-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant. 2009;24:2446-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863-1869. [DOI] [PubMed] [Google Scholar]
- 19. Kurella M, Yaffe K, Shlipak MG, Wenger NK, Chertow GM. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45:66-76. [DOI] [PubMed] [Google Scholar]
- 20. Koushik NS, McArthur SF, Baird AD. Adult chronic kidney disease: neurocognition in chronic renal failure. Neuropsychol Rev. 2010;20:33-51. [DOI] [PubMed] [Google Scholar]
- 21. Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216-223. [DOI] [PubMed] [Google Scholar]
- 22. Post JB, Jegede AB, Morin K, Spungen AM, Langhoff E, Sano M. Cognitive profile of chronic kidney disease and hemodialysis patients without dementia. Nephron Clin Pract. 2010;116:c247-c155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolfgram DF, Szabo A, Murray AM, Whittle J. Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int. 2015;35:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ozcan H, Yucel A, Avsar UZ, et al. Kidney transplantation is superior to hemodialysis and peritoneal dialysis in terms of cognitive function, anxiety, and depression symptoms in chronic kidney disease. Transplant Proc. 2015;47:1348-1351. [DOI] [PubMed] [Google Scholar]
- 25. Tilki HE, Akpolat T, Tunali G, Kara A, Onar MK. Effects of haemodialysis and continuous ambulatory peritoneal dialysis on P300 cognitive potentials in uraemic patients. Ups J Med Sci. 2004;109:43-48. [DOI] [PubMed] [Google Scholar]
- 26. Williams MA, Sklar AH, Burright RG, Donovick PJ. Temporal effects of dialysis on cognitive functioning in patients with ESRD. Am J Kidney Dis. 2004;43:705-711. [DOI] [PubMed] [Google Scholar]
- 27. Wolcott DL, Wellisch DK, Marsh JT, Schaeffer J, Landsverk J, Nissenson AR. Relationship of dialysis modality and other factors to cognitive function in chronic dialysis patients. Am J Kidney Dis. 1988;12:275-284. [DOI] [PubMed] [Google Scholar]
- 28. Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30:41-49. [DOI] [PubMed] [Google Scholar]
- 29. Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2006;21:2543-2548. [DOI] [PubMed] [Google Scholar]
- 30. Foster R, Walker S, Brar R, et al. Cognitive impairment in advanced chronic kidney disease: the Canadian frailty observation and interventions trial. Am J Nephrol. 2016;44:473-480. [DOI] [PubMed] [Google Scholar]
- 31. Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial. 2008;21:29-37. [DOI] [PubMed] [Google Scholar]
- 32. Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. 2008;294:F1-F9. [DOI] [PubMed] [Google Scholar]
- 33. Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol. 2013;24:353-363. [DOI] [PubMed] [Google Scholar]
- 34. Madero M, Sarnak MJ. Does hemodialysis hurt the brain? Semin Dial. 2011;24:266-268. [DOI] [PubMed] [Google Scholar]
- 35. Drew DA, Bhadelia R, Tighiouart H, et al. Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2013;61:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seliger SL, Sarnak MJ. Subclinical vascular disease of the brain in dialysis patients. Am J Kidney Dis. 2007;50:8-10. [DOI] [PubMed] [Google Scholar]
- 37. Chen HJ, Zhang LJ, Lu GM. Multimodality MRI findings in patients with end-stage renal disease. Biomed Res Int. 2015;2015:697402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 2015;26:957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lundqvist A, Alinder J, Alm H, Gerdle B, Levander S, Ronnberg J. Neuropsychological aspects of driving after brain lesion: simulator study and on-road driving. Appl Neuropsychol. 1997;4:220-230. [DOI] [PubMed] [Google Scholar]
- 40. Spiers HJ, Maguire EA. Neural substrates of driving behaviour. NeuroImage. 2007;36:245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shanmugaratnam S, Kass SJ, Arruda JE. Age differences in cognitive and psychomotor abilities and simulated driving. Accid Anal Prev. 2010;42:802-808. [DOI] [PubMed] [Google Scholar]
- 42. Andrews EC, Westerman SJ. Age differences in simulated driving performance: compensatory processes. Accid Anal Prev. 2012;45:660-668. [DOI] [PubMed] [Google Scholar]
- 43. Stutts JC, Stewart JR, Martell C. Cognitive test performance and crash risk in an older driver population. Accid Anal Prev. 1998;30:337-346. [DOI] [PubMed] [Google Scholar]
- 44. Odenheimer GL, Beaudet M, Jette AM, Albert MS, Grande L, Minaker KL. Performance-based driving evaluation of the elderly driver: safety, reliability, and validity. J Gerontol. 1994;49:M153-M119. [DOI] [PubMed] [Google Scholar]
- 45. Boot WR, Stothart C, Charness N. Improving the safety of aging road users: a mini-review. Gerontology. 2014;60:90-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hird MA, Egeto P, Fischer CE, Naglie G, Schweizer TA. A systematic review and meta-analysis of on-road simulator and cognitive driving assessment in Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 2016;53:713-729. [DOI] [PubMed] [Google Scholar]
- 47. Alosco ML, Spitznagel MB, Cleveland MJ, Gunstad J. Cognitive deficits are associated with poorer simulated driving in older adults with heart failure. BMC Geriatr. 2013;13:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gruhn N, Larsen FS, Boesgaard S, et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530-2533. [DOI] [PubMed] [Google Scholar]
- 49. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Meth. 2005;8:19-32. [Google Scholar]
- 50. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database Syst Rev. 2011;MR000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Program GC. Fourth International Conference on Grey Literature: New Frontiers in Grey Literature Washington, DC: Grey Literature Network Service; 1999. [Google Scholar]
- 53. Tyndall J. AACODS Checklist. Adelaide, South Australia, Australia: Flinders University; 2010. [Google Scholar]
- 54. Ysander L. The safety of drivers with chronic disease. Br J Ind Med. 1966;23:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gyalog G. Investigations on the Ability to Transport in Diabetics and Chronically Hemodialysed Patients. Mainz, Germany: University of Mainz; 1972. [Google Scholar]
- 56. Schewe G, Eisenhauer T, Leber HW, Lindner U, Ludwig O, Schuster R. [Studies on psychophysical ability of dialysis patients with regard to the question of driving aptitude]. Beitr Gerichtl Med. 1982;40:249-264. [PubMed] [Google Scholar]
- 57. Vats H, Duffy DP. Assessment of self-perceived risk and driving safety in chronic dialysis patients. Dial Transplant. 2010;39:63-68. [Google Scholar]
- 58. Varela D, Mallawaarachchi I, Blandon P. A diagnostic screening tool for identifying safe drivers among dialysis patients. Clin Nephrol. 2015;83:22-28. [DOI] [PubMed] [Google Scholar]
- 59. Davis JW, Chung R, Juarez DT. Prevalence of comorbid conditions with aging among patients with diabetes and cardiovascular disease. Hawaii Med J. 2011;70:209-213. [PMC free article] [PubMed] [Google Scholar]
- 60. Boateng EA, East L. The impact of dialysis modality on quality of life: a systematic review. J Ren Care. 2011;37:190-200. [DOI] [PubMed] [Google Scholar]
- 61. Tefft B. Motor vehicle crashes, injuries, and deaths in relation to driver age: United States, 1995-2010. Project Summary report. Washington, DC: AAA Foundation for Traffic Safety; 2012. [Google Scholar]
- 62. Determining Driver Fitness in Canada. Ottawa, ON: Canadian Council of Motor Transport Administrators (CCMTA); 2015. [Google Scholar]
- 63. CMA. Driver’s Guide: Determining Medical Fitness to Operate Motor Vehicles. 8th ed. Ottawa, ON: Canadian Medical Association; 2012. [Google Scholar]
- 64. Jang RW, Man-Son-Hing M, Molnar FJ, et al. Family physicians’ attitudes and practices regarding assessments of medical fitness to drive in older persons. J Gen Intern Med. 2007;22:531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Redelmeier DA, Yarnell CJ, Thiruchelvam D, Tibshirani RJ. Physicians’ warnings for unfit drivers and the risk of trauma from road crashes. N Engl J Med. 2012;367:1228-1236. [DOI] [PubMed] [Google Scholar]