Short abstract
Parkinson’s disease (PD) is a neurodegenerative movement disorder involving the selective loss of dopamine-producing neurons in the substantia nigra (SN). Differences in disease presentation, prevalence, and age of onset have been reported between males and females with PD. The content and composition of the major glycosphingolipids, phospholipids, and cholesterol were evaluated in the SN from 12 PD subjects and in 18 age-matched, neurologically normal controls. Total SN ganglioside sialic acid content and water content (%) were significantly lower in the male PD subjects than in the male controls. The content of all major gangliosides were reduced in the male PD subjects to some degree, but the neuronal-enriched gangliosides, GD1a and GT1b, were most significantly reduced. The distribution of phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositol was also significantly lower in the male PD subjects than in the male controls. However, the distribution of myelin-enriched cerebrosides and sulfatides was significantly higher in the male PD subjects than in the male controls suggesting myelin sparing in the male PD subjects. No elevation was detected for astrocytosis-linked GD3. These neurochemical changes provide evidence of selective neuronal loss in SN of the males with PD without robust astrocytosis. In contrast to the SN lipid abnormalities found in the male PD subjects, no significant abnormalities were found in the female PD subjects for SN water content or for any major SN lipids. These data indicate sex-related differences in SN lipid abnormalities in PD.
Keywords: Parkinson’s disease, gangliosides, neurodegeneration, sex differences, myelin
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized primarily by a loss of substantia nigra pars compacta (SNc) dopamine-producing neurons and a significant loss of dopamine in the primary target structures of the SNc, the caudate nucleus, and the putamen (Waters et al., 1988; Jellinger, 1991). The etiology and pathogenesis of PD is complex and not completely known; however, dysfunction of mitochondria, increased oxidative stress resulting in multiple forms of oxidative damage, and defects in the ubiquitin-proteasome system (leading to an increase in misfolded proteins) are often discussed as significant factors contributing to damage of SN neurons in PD patients (Doria et al., 2016). Most likely, a combination of these and other factors contribute to the development of PD.
A significant sex effect has been reported for PD. The incidence and prevalence of PD differ between males and females: Male sex is a significant risk factor for PD (Baldereschi et al., 2000; Van Den Eeden et al., 2003; Shulman and Bhat, 2006), with estimates that up to 1.5 to 2 times as many males than females develop PD (Elbaz et al., 2002; Wooten et al., 2004). Sex differences in the clinical manifestations of PD have also been reported and may influence disease course, response to medications, and quality of life (Baba et al., 2005; Behari et al., 2005; Hristova et al., 2009). In a study of 1,264 patients with PD, men had worse rigidity scores on the Unified Parkinson’s Disease Rating Scale while women had worse postural instability scores (Baba et al., 2005). Other studies have shown that women are more likely than men to present with tremor at PD onset, which may be associated with a more benign disease phenotype (Haaxma et al., 2007; Gillies et al., 2014). However, several studies have reported that female sex is an independent risk factor for the development of levodopa-induced dyskinesias (Warren Olanow et al., 2013; Scott et al., 2016). Thus, although sex differences in PD phenotype exist, they are complex and not entirely consistent across studies.
Substantia nigra cell loss in PD appears to be correlated with disease duration and severity regardless of sex (Fearnley and Lees, 1991; Hughes et al., 1993; Braak et al., 2003). While studies in animals suggest that there may be more dopamine neurons in female SN than in male SN (Cantuti-Castelvetri et al., 2007), this has not been demonstrated in humans. In a study of dopamine neurons microdissected from the SN from male and female PD subjects, sex-related differences in gene expression patterns in residual dopamine neurons were reported (Cantuti-Castelvetri et al., 2007). Although factors underlying the sex differences in dopamine neuron gene expression patterns are unclear, both hormonal and nonhormonal factors have been suggested as possible contributors (Cantuti-Castelvetri et al., 2007).
Abnormalities in the content and composition of various lipids have been reported in subjects with PD. Gangliosides are a family of sialic-acid-containing glycosphingolipids that are enriched in neuronal membranes of the central nervous system (CNS; Derry and Wolfe, 1967; Ledeen, 1985; Svennerholm et al., 1989). Gangliosides are excellent biochemical markers for assessing changes in neural composition. Reductions in ganglioside GM1, using a cholera toxin immunostaining procedure, have been reported in the SN in PD subjects, potentially involving dopamine and non-dopamine cells (Wu et al., 2012; Hadaczek et al., 2015). Ganglioside GD3 is enriched in reactive astrocytes and is indicative of reactive astrocytosis in association with neuronal degeneration in the mature CNS (Seyfried et al., 1984; Seyfried and Yu, 1985). Alterations in the content and composition of gangliosides can provide insight on the structural integrity of synaptic, axonal, and glial cell membranes (Seyfried et al., 1982; Seyfried and Yu, 1985; Benjamins et al., 2012). Reductions in gangliosides GM1 and GD1a were found in occipital lobe of PD subjects using a cholera toxin immunostaining procedure (Hadaczek et al., 2015). In addition to changes in gangliosides, elevations of sphingomyelin, linked to its presence in Lewy bodies, and reductions in phosphatidylethanolamine (PE) and phosphatidylcholine (PC), linked to abnormalities in dopaminergic neurons, were reported previously in SN of PD subjects (den Jager, 1969; Riekkinen et al., 1975). Our study presents the first neurochemical analysis on the content and composition of glycolipids, phospholipids, and cholesterol in SN from a cohort of male and female PD subjects. The lipid abnormalities found in the male PD subjects were not found in the female PD subjects.
Materials and Methods
Brain Tissues
Coded or anonymous biological specimens were obtained through the NIH NeuroBioBank and sourced from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, and from the Human Brain and Spinal Fluid Resource Center, VA, West Los Angeles Healthcare Center, 11301 Wilshire Blvd., Los Angeles, CA, which is sponsored by NINDS/NIMH, the National Multiple Sclerosis Society, and the Department of Veterans Affairs, and the Harvard Brain Tissue Resource Center, which is supported in part by HHSN-271-2013-00030C. The clinical diagnosis of Parkinson’s disease was confirmed at autopsy by presence of gross depigmentation of the SN and microscopic confirmation of SN cell loss and presence of Lewy bodies in the SN and normal findings in other brain regions sampled. Frozen tissue blocks containing the SN were stored at −80°C and warmed to −20°C, and samples containing the SNc were dissected from the blocks, placed in sterile Eppendorf tubes, and then rapidly refrozen in powdered dry ice. Standard BL2 procedures for handling human tissues were observed.
Lipid Isolation and Purification
Total lipids were isolated and purified from lyophilized SN samples by using modifications of previously described procedures (Seyfried et al., 1978; Hauser et al., 2004; Denny et al., 2006). Lyophilized tissues were transferred into 50 mL glass screw capped test tubes and rehydrated with 0.5 mL of dH2O. Lipids were extracted from the tissue by adding 5 mL CHCl3:CH3OH (1:1, v/v) and samples were placed on a magnetic stirrer at room temperature overnight (Hauser et al., 2004). Samples were centrifuged at 2500 r/min for 20 min, and the supernatant was collected. The pellet and stirring bar were washed in 2.0 mL CHCl3:CH3OH (1:1, v/v), centrifuged again, and the second supernatant was added to the first. The supernatant was brought up to 19.6 mL at a ratio of 30:60:8 CHCl3:CH3OH:dH2O (v/v/v).
Neutral lipids were separated from acidic lipids and gangliosides using DEAE Sephadex (A-25, GE Healthcare, Uppsala, Sweden) column chromatography as described previously (Denny et al., 2006). The total lipid mixture was applied to a DEAE-Sephadex column with a bed volume of 1.2 mL. Neutral lipids were eluted from the column with 20 mL of Solvent A, as previously described (Seyfried et al., 1978). This fraction contained cholesterol, phosphatidylcholine (PC), phosphatidylethanolamine (PE), ceramide, sphingomyelin, and cerebrosides. Acidic lipids and gangliosides were then eluted from the column with 30 mL of Solvent B. Neutral lipids were dried by rotary evaporation and resuspended in 10 mL CHCl3:CH3OH 2:1 (v/v). The acidic lipid fraction containing gangliosides was dried and separated into acidic lipids and gangliosides by Folch partitioning (Folch et al., 1957; Kasperzyk et al., 2005; Akgoc et al., 2015). Once separated, the upper phase containing the gangliosides (GM3, GM2, GM1, GD3, GD1a, GT1a, GD1b, GT1b, and GQ1b) and the lower phase containing the acidic phospholipids lipids and sulfatides were dried under nitrogen and resuspended in 10 mL CHCl3:CH3OH 2:1 (v/v).
The resorcinol assay was used to analyze sialic acid content in the ganglioside fraction as previously described (Svennerholm, 1957; Miettinen and Takki-Luukkainen, 1959; Hauser et al., 2004). Dried gangliosides were base treated with 1.0 mL of 0.15 N NaOH at 37°C for 90 min. Salts were removed from the samples using C18 reverse-phase Bond Elute column as previously described (Williams and McCluer, 1980; Hauser et al., 2004). Gangliosides were resuspended in 5.0 mL CHCl3:CH3OH 1:1 (v/v) and an aliquot was analyzed for sialic acid content.
High-Performance Thin-Layer Chromatography
All lipids were evaluated qualitatively with high-performance thin-layer chromatography (HPTLC) as previously described (Ando et al., 1978; Macala et al., 1983; Kasperzyk et al., 2005; Denny et al., 2006). These are highly sensitive procedures for the quantitative and quantitative analysis of all major brain lipids. Each lane was spotted with 1.5 µg sialic acid for gangliosides, 35 µg of dry weight for neutral lipids, and 100 µg of dry weight for acidic lipids. Purified lipid standards were purchased from Matreya Inc. (Pleasant Gap, PA, USA) or Sigma (St Louis, MO, USA) or were a gift from Dr. Robert Yu (Medical College of Georgia, Augusta, GA, USA). To enhance precision, oleyl alcohol was added as an internal standard to the neutral and acidic lipid standards and samples. Lipids were spotted on 10 × 20 cm Silica gel HPTLC plates (E. Merck, Darmstadt, Germany) using a Camag Linomat V semiautomatic TLC spotter (Camag Scientific Inc., Wilmington, NC). Plates were developed as previously described (Hauser et al., 2004). The quantification of individual lipids was performed as previously described (Arthur et al., 2013). Total brain ganglioside distribution was normalized to 100%, and the percentage distribution was used to calculate sialic acid concentration of each ganglioside. Neutral and acidic lipid density values were normalized to the internal standard to calculate individual lipid concentration.
Statistical Analysis
Two-tailed Student's t-test was used to calculate statistical significance between controls and PD subjects within each sex and to compare controls and PD subjects across sex. Pearson’s product–moment correlations were also used to evaluate relationships between ganglioside content, brain water content, disease duration, patient age, and postmortem interval (PMI).
Results
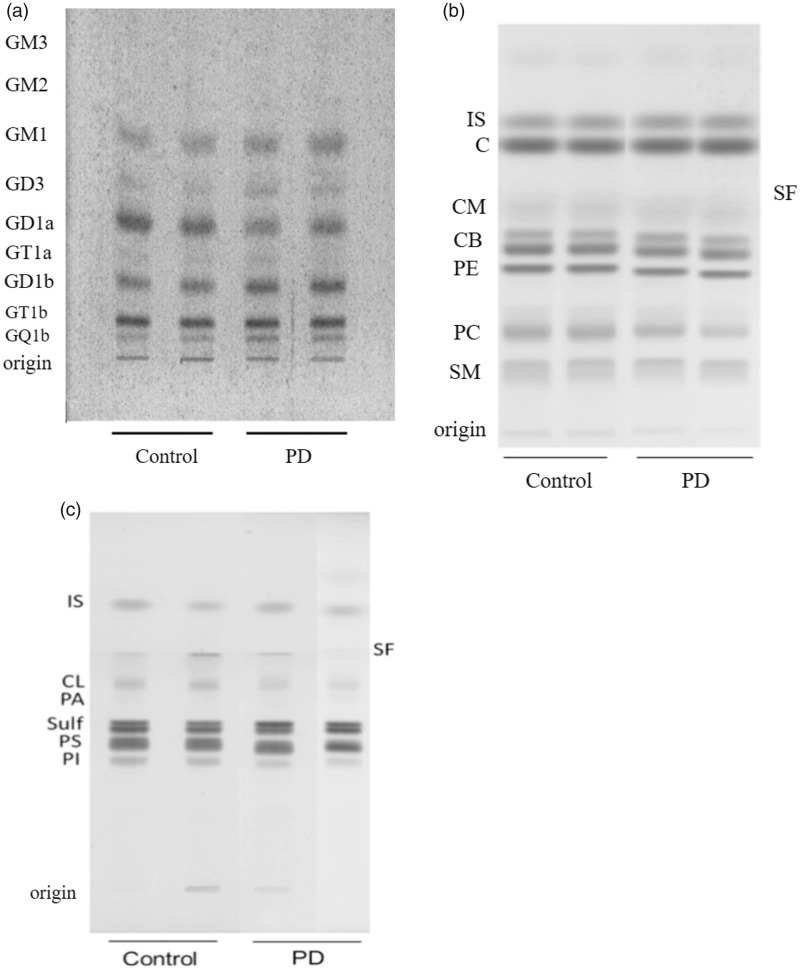
This study is the first to present neurochemical data on the content and composition of glycolipids, phospholipids, and cholesterol in SN tissue from male and female PD subjects and age-matched controls. There were no significant differences between the ages or PMI for the controls and PD subjects, but disease duration before death was noticeably less for the female PD patients than for the male PD patients (Table 1). A highly significant reduction in SN water content was observed between the control males and the PD males (Table 1). No difference in SN water content was observed between the control females and the PD females. Total ganglioside content was significantly lower by 23% in the PD males than in the control males (Table 1). No significant difference for total ganglioside content was found between the control females and the PD females (Table 1). Gangliosides GD1a, GD1b, and GT1b were significantly lower by 33%, 20%, and 32%, respectively, in the PD males than in the control males (Table 2 and Figure 1). None of the other differences in ganglioside distribution reached statistical significance. A slight, but nonsignificant elevation was seen for GD3 distribution in the PD males. In the control subjects, only GD1a levels were significantly different between males and females, with levels in control males approximately 52% higher than in control females (p = .0496). It is important to mention that a similar amount of total gangliosides was spotted (1.5 µg) for each sample on the HPTLC shown in Figure 1(a). This is done to reveal qualitative differences in distribution and not quantitative differences, which are determined from the density of each ganglioside band relative to the total amount of gangliosides in the tissue. No significant qualitative or quantitative differences were found between the control females and PD females for the distribution of any ganglioside species (Table 2). A highly significant correlation was found between total SN ganglioside content (μg/100 mg dry weight) and SN water content both within sexes and across sexes (r = 0.78, p < .00001). No significant correlations were found either within sexes or across sexes for ganglioside content, PMI, disease duration, or age. Also, either no correlation or only marginal correlations were found between age and disease duration or between PMI and disease duration.
Table 1.
Total SN Ganglioside Concentration, Water Content, and Disease Duration in Male and Female Control and PD Subjects.
| Subjects | Age (years) | Disease duration (years) | PMI (hr) | Wet Wt (mg) | Water content (%) | Total ganglioside sialic acid (μg/100 mg dry wt) |
|---|---|---|---|---|---|---|
| Male control | ||||||
| 1 | 57 | NA | 24 | 43.9 | 76.0 | 245 |
| 2 | 76 | NA | 16 | 68.8 | 77.0 | 265 |
| 3 | 80 | NA | 14 | 38.2 | 77.9 | 275 |
| 4 | 76 | NA | 11 | 35.7 | 77.6 | 220 |
| 5 | 79 | NA | 10 | 44.0 | 82.1 | 345 |
| 6 | 76 | NA | 16 | 30.6 | 77.5 | 268 |
| 7 | 80 | NA | 14 | 31.4 | 77.7 | 272 |
| 8 | 84 | NA | 12 | 18.1 | 78.5 | 246 |
| 9 | 70 | NA | 12 | 42.6 | 76.1 | 204 |
| 10 | 78 | NA | 5 | 61.8 | 78.6 | 188 |
| Mean | 75.6 ± 2.4 | 13 ± 2 | 41.5 ± 4.7 | 77.9 ± 0.5 | 252.8 ± 14.0 | |
| Male PD | ||||||
| 1 | 75 | UK | 6 | 77.4 | 72.2 | 180 |
| 2 | 71 | 16 | 22 | 115.9 | 73.0 | 173 |
| 3 | 67 | 10 | 10 | 63.6 | 75.0 | 222 |
| 4 | 82 | 13 | 13 | 47.7 | 74.8 | 190 |
| 5 | 95 | 13 | 10 | 106.1 | 76.1 | 203 |
| 6 | 83 | 29 | 7 | 60.8 | 75.5 | 170 |
| 7 | 76 | UK | 16 | 33.9 | 76.4 | 227 |
| Mean | 78.4 ± 3.5 | 16.2 ± 3.3 | 12 ± 2 | 72.2 ± 11.3 | 74.7 ± 0.6** | 195.0 ± 8.7** |
| Female control | ||||||
| 1 | 74 | NA | 12 | 58.6 | 75.3 | 207 |
| 2 | 75 | NA | 15 | 86.8 | 76.8 | 161 |
| 3 | 80 | NA | 10 | 65.8 | 78.6 | 273 |
| 4 | 64 | NA | 20 | 51.9 | 79.8 | 286 |
| 5 | 74 | NA | 12 | 30.5 | 74.1 | 149 |
| 6 | 71 | NA | 24 | 79.5 | 74.8 | 161 |
| 7 | 69 | NA | 25 | 56.4 | 77.8 | 243 |
| 8 | 74 | NA | 12 | 98.2 | 76.3 | 190 |
| Mean | 72.6 ± 1.7 | 16 ± 2 | 66.0 ± 7.6 | 76.7 ± 0.7 | 208.8 ± 18.8 | |
| Female PD | ||||||
| 1 | 72 | 6 | 15 | 44.0 | 75.2 | 200 |
| 2 | 65 | 3 | 15 | 51.5 | 79.2 | 237 |
| 3 | 76 | 13 | 14 | 65.5 | 78.6 | 246 |
| 4 | 85 | UK | 11 | 82.5 | 74.1 | 133 |
| 5 | 81 | 5 | 27 | 63.0 | 79.2 | 228 |
| Mean | 75.8 ± 3.5 | 6.8 ± 2.2 | 16 ± 3 | 61.3 ± 6.6 | 77.3 ± 1.1 | 208.8 ± 20.5 |
Note. Values represent the mean ± SEM. Asterisks indicate significant difference from same sex controls at **p < .01 as determined by the two-tailed t test. SN = substantia nigra; PMI = postmortem interval; NA= not applicable, UK= unknown.
Table 2.
Distribution of SN Gangliosides in Male and Female Control and PD Subjects.
| Gangliosides |
Males |
Females |
||
|---|---|---|---|---|
| Ctl, n=10 | PD, n=7 | Ctl, n=8 | PD, n=5 | |
| GM3 | 3.2 ± 0.3 | 3.2 ± 0.3 | 1.8 ± 0.6 | 1.7 ± 1.1 |
| GM2 | 5.7 ± 0.5 | 4.9 ± 0.3 | 3.7 ± 1.5 | 2.0 ± 1.3 |
| GM1 | 41.7 ± 2.2 | 37.4 ± 1.8 | 37.1 ± 3.6 | 36.9 ± 4.2 |
| GD3 | 19.4 ± 1.7 | 18.4 ± 1.5 | 18.8 ± 2.4 | 19.2 ± 2.1 |
| GD1a | 78.1 ± 6.7 | 52.2 ± 3.6** | 51.3 ± 11.4 | 50.5 ± 11.3 |
| GT1a | 7.9 ± 0.6 | 7.2 ± 0.6 | 7.1 ± 0.4 | 7.7 ± 0.5 |
| GD1b | 42.3 ± 2.6 | 33.7 ± 2.1* | 39.9 ± 1.5 | 42.3 ± 3.0 |
| GT1b | 44.3 ± 3.3 | 30.3 ± 1.9** | 38.5 ± 1.54 | 37.9 ± 3.0 |
| GQ1b | 10.2 ± 1.1 | 7.8 ± 0.6 | 10.5 ± 0.6 | 10.6 ± 0.5 |
Note. All values are expressed as µg/100 mg dry weight sialic acid/mg dry weight and represent the mean ± SEM. The asterisks indicate significant difference from controls of the same sex at *p < .05 and at **p < .01 as determined by the two-tailed t test. SN = substantia nigra.
Figure 1.
(a) HPTLC of gangliosides in substantia nigra (SN) samples from male control and PD subjects. The amount of ganglioside sialic acid spotted per lane was equivalent to approximately 1.5 µg. The plate was developed by a single ascending run with CHCl3:CH3OH:dH2O (55:45:10 by vol) containing 0.02% CaCl2·2H2O. The bands were visualized with the resorcinol-HCl spray, as described previously (Hauser et al., 2004). HPTLC of SN neutral lipids (b) and acidic lipids (c) in male control and PD subjects. The amount of neutral lipids and acidic lipids spotted per lane was equivalent to approximately 35 µg and 100 µg tissue dry weight, respectively. The plates were developed as we described previously (Baek et al., 2009). The second PD sample in (c) was moved to its position from another region on the same HPTLC, which explains the merge line seen on the plate. Neutral lipids include: CE = cholesteryl esters; TG = triglycerides; IS = internal standard; C = cholesterol; Cer = ceramide; CB = cerebrosides (doublet); PE = phosphatidylethanolamine; PC = phosphatidylcholine; SM = sphingomyelin. As the zwitterionic lipids (PC, PE, and SM) elute with the neutral lipids, they are included in this group. Acidic lipids include: FA = fatty acids; IS = internal standard; CL = cardiolipin; PA = phosphatidic acid; SULF = sulfatides (doublet); PS = phosphatidylserine; PI = phosphatidylinositol.
The distribution of the individual SN neutral lipids and acidic lipids is shown in Figure 1(b) and (c) and in Table 3. The levels of PC, sulfatides, and PS were significantly higher in male controls than in female controls (p = .0294, .0292, and .0017, respectively) and the levels of PE and PA trended lower in male controls than in females (p = .0522, .0545, respectively). The levels of PE, PC, and phosphatidylinositol (PI) were significantly lower, whereas the level of sphingomyelin was significantly higher in male PD subjects than in the control males (Table 3). The relative levels of the myelin-enriched cerebrosides and sulfatides were significantly higher in the PD males than in the control males (Table 3). No other statistically significant lipid abnormalities were found in distribution of neutral lipids or acidic lipids in the male PD subjects. No significant abnormalities were found for the distribution for any major neutral lipid or acidic lipid in the female PD subjects. Cholesterol content was similar in PD males and females and not different from the controls. No significant correlation was found between age and the content of any lipid in either control or PD subjects of either gender (data not shown).
Table 3.
Distribution of SN Neutral and Acidic Lipids in Male and Female Control and PD Subjects.
|
Males |
Females |
|||
|---|---|---|---|---|
| Lipidsa | Ctl, n=10 | PD, n=7 | Ctl, n=8 | PD, n=5 |
| Neutral | ||||
| Cholesterol | 23.3 ± 0.3 | 23.6 ± 0.3 | 23.7 ± 0.7 | 24.2 ± 0.4 |
| Ceramides | 8.1 ± 0.3 | 7.7 ± 0.2 | 7.3 ± 0.3 | 7.1 ± 0.5 |
| Cerebrosides | 25.6 ± 0.5 | 27.6 ± 0.5** | 27.0 ± 0.7 | 27.1 ± 0.4 |
| Phosphatidylethanolamine | 14.8 ± 0.1 | 13.9 ± 0.2** | 15.5 ± 0.3 | 15.7 ± 0.7 |
| Phosphatidylcholine | 17.6 ± 0.3 | 15.3 ± 0.6** | 14.9 ± 1.2 | 14.2 ± 0.7 |
| Sphingomyelin | 11.1 ± 0.2 | 12.0 ± 0.3* | 11.2 ± 0.5 | 11.7 ± 0.6 |
| Acidic | ||||
| Cardiolipin | 5.5 ± 0.7 | 5.8 ± 0.9 | 6.3 ± 0.6 | 6.4 ± 1.2 |
| Phosphatidic Acid | 1.0 ± 0.3 | 1.2 ± 0.4 | 2.8 ± 0.9 | 3.4 ± 1.1 |
| Sulfatides | 38.3 ± 0.9 | 41.7 ± 1.0* | 32.6 ± 2.4 | 27.3 ± 5.0 |
| Phosphatidylserine | 40.5 ± 0.5 | 39.8 ± 0.8 | 37.5 ± 0.6 | 38.7 ± 0.7 |
| Phosphatidylinositol | 14.8 ± 0.6 | 11.5 ± 0.8** | 13.2 ± 1.6 | 9.9 ± 1.4 |
aDetermined from densitometric scanning of HPTLC plates, as shown in Figure 1. All values are expressed as percents and represent the mean ± SEM. The asterisks indicate significant difference from controls of the same sex at *p < .05 and at **p < .01 as determined by the two-tailed t test.
Discussion
Gangliosides are sensitive biochemical indicators of the functional integrity of neuronal plasma membranes (Seyfried et al., 1982; Seyfried et al., 1984; Ledeen, 1985; Seyfried and Yu, 1985; Schnaar, 2016). The total ganglioside content found in the SN of the combined male and female controls is in general agreement with that reported previously in this brain region in humans (Kracun et al., 1992). The significant reduction in total ganglioside content in the PD males coupled with the significant reductions in the neuronal- or synaptic-enriched gangliosides GD1a and GT1b are consistent with significant neuronal loss in the SN of these subjects. Loss of dopaminergic neurons in the SN is the hallmark pathological feature of PD, regardless of sex (Braak et al., 2003). Thus, considering the significant changes in ganglioside expression in male PD subjects compared with male controls, the lack of change in ganglioside expression in PD females was surprising.
Significant reductions of GM1 in PD SN have been observed in tissue sections from subjects of both sexes, with no mention of any possible differences in the number of cells expressing GM1 between males and females (Wu et al., 2012; Hadaczek et al., 2015). Significant reductions in GM1 and GD1a in PD subjects of both sexes (again with no mention of any sex-related differences in ganglioside expression) were also observed in occipital cortex, a brain region not known to show any significant neurodegeneration in PD (Hadaczek et al., 2015). In the current study, we observed a significant decrease in GD1a and GT1b and a smaller decrease in GM1 in males with PD. The lack of change in ganglioside levels in female PD subjects is surprising and difficult to explain at this point. It is interesting, however, that disease duration before death was noticeably less in the female patients than in the male patients. It is unclear if the shortened disease duration could explain in part the failure to detect significant changes in SN lipids of the female PD patients whom we evaluated. Although previous studies suggested that substantia nigra cell loss in PD appeared to be correlated with disease duration and severity regardless of sex (Fearnley and Lees, 1991; Hughes et al., 1993; Braak et al., 2003), no significant correlation was found between SN ganglioside content and disease duration either within or between sexes in our study. Future studies will be needed to determine if lipid abnormalities differ between early- and late-stage PD males, and whether early stage males might also differ from early stage females.
No significant change in GD3 distribution was seen in the SN in male or female PD subjects studied. In normal adult human brain, GM1 and GD1b, and to a lesser extent GM2 and GD3, are expressed on astrocytes (Marconi et al., 2005). Ganglioside GD3 becomes elevated in association with the reactive astrocytosis and the neurodegeneration seen in Alzheimer’s disease, in Huntington’s disease, and in murine cerebellar mutants (Seyfried and Yu, 1985; Goldman and Reynolds, 1996; Desplats et al., 2007). In many, but not all cases of PD, there is a mild increase in the number of astrocytes with a relative lack of reactive astrocytosis in PD SN (Forno et al., 1992). Astrocytes have been suggested to have a potential neuroprotective role in PD, with density of GFAP-positive astrocytes inversely related to the magnitude of SN dopamine cell loss (Teismann and Schulz, 2004). The absence of significant changes in GD3 is consistent with the absence of reactive astrocytosis, as previously reported in the SN of PD subjects and animal models (Mirza et al., 2000; Song et al., 2009).
The reductions that we observed for the levels of phosphatidylethanolamine and phosphatidylcholine in the male PD subjects are consistent with findings previously reported for these lipids in the SN of PD subjects (Riekkinen et al., 1975). The reductions in these phospholipids were considered reflections of the deficiency of dopamine and loss of dopaminergic neurons in the SN of subjects with PD (Riekkinen et al., 1975). The elevation we observed for the distribution of sphingomyelin in the male PD subjects is also consistent with data previously reported for this lipid in the SN of PD subjects. The elevation of sphingomyelin in the SN of PD subjects has been attributed to its enrichment in Lewy bodies and potentially to α-synuclein production (den Jager, 1969; Kim and Halliday, 2012). The reason for the lack of increase in sphingomyelin in female PD subjects remains unclear.
We also found that SN water content was significantly lower in the PD males than in the control males. Water content is tightly regulated in the brain, is critical for brain function, and is higher in neuron-rich gray matter than in neuron-poor white matter, which contains the relatively dehydrated myelin (Seyfried et al., 1979; Seyfried and Yu, 1980; Benjamins et al., 2012). We also found that SN ganglioside content was highly correlated with SN water content both within and across sexes. The higher is the water content, the higher is the ganglioside content. Dopaminergic neuron loss, without myelin loss, will reduce gray matter thus altering the ratio of gray matter to white matter in the SN. Consequently, lipids enriched in myelin (cerebrosides and sulfatides) will become differentially elevated in tissue samples containing neuronal loss (Baek et al., 2009). This is consistent with elevated levels of cerebrosides and sulfatides seen in the SN of the PD males. The sparing of myelin in the SN of PD males could also explain in part the failure to see a significant loss of ganglioside GM1 in these subjects, as GM1 ganglioside is also enriched in myelin (Seyfried and Yu, 1980). The enrichment of GM1 in myelin could mask the reduction of GM1 that would be present in dopaminergic neurons.
In contrast to the abnormalities that we found in SN water content and lipids in the male PD subjects, no significant abnormalities were found in the female PD subjects for SN water content or for any major SN lipid. Although no differences were found between control females and PD females for SN gangliosides and water content, ganglioside content was correlated with SN water content across all females. Neuronal loss, however, is not uniform throughout the SN of PD subjects and neuronal loss is greater in posterior and ventrolateral regions of the SNc, with the medially adjacent ventral tegmental area relatively intact (Fearnley and Lees, 1991; Gibb and Lees, 1991; Braak et al., 2003). As we were working with postmortem samples received from brain banks, there was no control over the nature of samples received, and the samples were not uniform in regard to SN anatomy. However, that alone cannot explain the differences in results obtained from male and female PD samples, as it is unlikely that all of the female PD samples were obtained from parts of the SN that are less vulnerable to the PD disease process. Although we do not have details of the clinical presentations of the disease in the cases we have examined, it is possible that the female PD cases had significantly less severe disease than the male cases. Considering the absence of reductions in SN water content and in the content of the neuronal- or synaptic-enriched gangliosides GM1, GD1a, and GT1b in the female PD patients, we cannot rule out the possibility that neuronal loss, of an extent similar to that occurring in the male patients and of an extent sufficient to produce the observed changes in tissue water content and gangliosides, did not occur in the five female PD patients that we studied. Further studies will be needed to correlate severity of clinical disease and numbers of residual neurons and glial cell expression with lipid composition in male and female PD SNc samples.
Numerous studies have shown a strong sex effect on PD. The higher level of estrogen expression in females than in males and the potential neuroprotective effects of estrogen are thought to at least partly underlie the differences in disease prevalence in males and females. Although the female samples were obtained from women several years past the age of menopause, a neuroprotective effect of estrogen could have occurred at an earlier age. Previous studies have shown that estrogen can influence the content and composition of gangliosides and other lipids in various tissues (Islam et al., 1986; Dahiya et al., 1988). Estrogen administration increased the levels of gangliosides in rat kidney and in rabbit hippocampus, amygdala, and olfactory bulbs (Islam et al., 1986; Dahiya et al., 1988). As at least some gangliosides are neuroprotective and may be upregulated in neurons in response to injury (Park et al., 2016), it is possible that estrogen effects may at least partially underlie the expression of normal ganglioside levels in residual neurons in the female SN despite the loss of dopaminergic neurons. Further studies will be needed to determine the effects of estrogen on gangliosides, phospholipids, and dopaminergic neurons in the SN of PD males and females and in animal models with PD. In summary, we present evidence showing significant abnormalities in gangliosides, phospholipids, and water content in the SN of male but not female subjects with PD. Our conclusions must be tempered somewhat due to the small sample sizes, especially for the females. Additional research and larger sample sizes will be necessary in order to confirm the findings.
Acknowledgments
The authors thank Amanda Lynn and Sonia Iosim for their technical assistance.
Author Contributions
J. S. S. and T. N. S.: Conception, organization, execution of project; and manuscript writing and review; H. C., A. C., D. H., Z. A., A. L., and S. I.: execution of project, data analysis, and manuscript review.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Cure Tay-Sachs Disease Foundation and the Boston College Research Expense Fund (T. N. S.) and a grant from Qilu Pharmaceuticals, Inc. (J. S. S.).
References
- Akgoc Z., Sena-Esteves M., Martin D. R., Han X., d'Azzo A., Seyfried T. N. (2015). Bis(monoacylglycero)phosphate: A secondary storage lipid in the gangliosidoses. J Lipid Res, 56, 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S., Chang N. C., Yu R. K. (1978). High-performance thin-layer chromatography and densitometric determination of brain ganglioside compositions of several species. Anal Biochem, 89, 437–450. [DOI] [PubMed] [Google Scholar]
- Arthur J. R., Wilson M. W., Larsen S. D., Rockwell H. E., Shayman J. A., Seyfried T. N. (2013). Ethylenedioxy-PIP2 oxalate reduces ganglioside storage in juvenile Sandhoff disease mice. Neurochem Res, 38, 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y., Putzke J. D., Whaley N. R., Wszolek Z. K., Uitti R. J. (2005). Gender and the Parkinson's disease phenotype. J Neurol, 252, 1201–1205. [DOI] [PubMed] [Google Scholar]
- Baek R. C., Martin D. R., Cox N. R., Seyfried T. N. (2009). Comparative analysis of brain lipids in mice, cats, and humans with Sandhoff disease. Lipids, 44, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldereschi M., Di Carlo A., Rocca W. A., Vanni P., Maggi S., Perissinotto E., Grigoletto F., Amaducci L., Inzitari D. (2000). Parkinson's disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. ILSA Working Group. Italian longitudinal study on aging. Neurology, 55, 1358–1363. [DOI] [PubMed] [Google Scholar]
- Behari M., Srivastava A. K., Pandey R. M. (2005). Quality of life in patients with Parkinson's disease. Parkinsonism Relat Disord, 11, 221–226. [DOI] [PubMed] [Google Scholar]
- Benjamins J. A., Murphy E. J., Seyfried T. N. (2012). Lipids In: Brady S. T., Siegel G. J., Albers R. W., Price D. L. (Eds), Basic neurochemistry: Principles of molecular, cellular, and medical neurobiology (8th ed., pp 81–100). Burlington, NJ: Elsevier Academic Press. [Google Scholar]
- Braak H., Del Tredici K., Rub U., de Vos R. A., Jansen Steur E. N., Braak E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging, 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I., Keller-McGandy C., Bouzou B., Asteris G., Clark T. W., Frosch M. P., Standaert D. G. (2007). Effects of gender on nigral gene expression and Parkinson disease. Neurobiol Dis, 26, 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya R., Dudeja P. K., Brasitus T. A. (1988). Estrogen-induced alterations of the acidic and neutral glycosphingolipids of rat kidney. Biochim Biophys Acta, 962, 390–395. [DOI] [PubMed] [Google Scholar]
- den Jager W. A. (1969). Sphingomyelin in Lewy inclusion bodies in Parkinson's disease. Arch Neurol, 21, 615–619. [DOI] [PubMed] [Google Scholar]
- Denny C. A., Kasperzyk J. L., Gorham K. N., Bronson R. T., Seyfried T. N. (2006). Influence of caloric restriction on motor behavior, longevity, and brain lipid composition in Sandhoff disease mice. J Neurosci Res, 83, 1028–1038. [DOI] [PubMed] [Google Scholar]
- Derry D. M., Wolfe L. S. (1967). Gangliosides in isolated neurons and glial cells. Science, 158, 1450–1452. [DOI] [PubMed] [Google Scholar]
- Desplats P. A., Denny C. A., Kass K. E., Gilmartin T., Head S. R., Sutcliffe J. G., Seyfried T. N., &, Thomas E. A. (2007). Glycolipid and ganglioside metabolism imbalances in Huntington's disease. Neurobiol Dis, 27, 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria M., Maugest L., Moreau T., Lizard G., Vejux A. (2016). Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson's disease. Free Radic Biol Med, 101, 393–400. [DOI] [PubMed] [Google Scholar]
- Elbaz A., Bower J. H., Maraganore D. M., McDonnell S. K., Peterson B. J., Ahlskog J. E., Schaid D. J., Rocca W. A. (2002). Risk tables for parkinsonism and Parkinson's disease. J Clin Epidemiol, 55, 25–31. [DOI] [PubMed] [Google Scholar]
- Fearnley J. M., Lees A. J. (1991). Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain, 114(Pt 5), 2283–2301. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane-Stanley G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem, 226, 497–509. [PubMed] [Google Scholar]
- Forno L. S., DeLanney L. E., Irwin I., Di Monte D., Langston J. W. (1992). Astrocytes and Parkinson's disease. Prog Brain Res, 94, 429–436. [DOI] [PubMed] [Google Scholar]
- Gibb W. R., Lees A. J. (1991). Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry, 54, 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies G. E., Pienaar I. S., Vohra S., Qamhawi Z. (2014). Sex differences in Parkinson's disease. Front Neuroendocrinol, 35, 370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. E., Reynolds R. (1996). A reappraisal of ganglioside GD3 expression in the CNS. Glia, 16, 291–295. [DOI] [PubMed] [Google Scholar]
- Haaxma C. A., Bloem B. R., Borm G. F., Oyen W. J., Leenders K. L., Eshuis S., Booij J., Dluzen D. E., Horstink M. W. (2007). Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry, 78, 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P., Wu G., Sharma N., Ciesielska A., Bankiewicz K., Davidow A. L., Lu Z. H., Forsayeth J., Ledeen R. W. (2015). GDNF signaling implemented by GM1 ganglioside; failure in Parkinson's disease and GM1-deficient murine model. Exp Neurol, 263, 177–189. [DOI] [PubMed] [Google Scholar]
- Hauser E. C., Kasperzyk J. L., d'Azzo A., Seyfried T. N. (2004). Inheritance of lysosomal acid beta-galactosidase activity and gangliosides in crosses of DBA/2J and knockout mice. Biochem Genet, 42, 241–257. [DOI] [PubMed] [Google Scholar]
- Hristova D. R., Hristov J. I., Mateva N. G., Papathanasiou J. V. (2009). Quality of life in patients with Parkinson's disease. Folia Med, 51, 58–64. [PubMed] [Google Scholar]
- Hughes A. J., Daniel S. E., Lees A. J. (1993). The clinical features of Parkinson's disease in 100 histologically proven cases. Adv Neurol, 60, 595–599. [PubMed] [Google Scholar]
- Islam F., Hasan M., Saxena K. (1986). Isolation and estimation of gangliosides in discrete regions of the forebrain: Effects of estrogen on regional lipid profiles. Exp Pathol, 29, 159–164. [DOI] [PubMed] [Google Scholar]
- Jellinger K. A. (1991). Pathology of Parkinson's disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol, 14, 153–197. [DOI] [PubMed] [Google Scholar]
- Kasperzyk J. L., d'Azzo A., Platt F. M., Alroy J., Seyfried T. N. (2005). Substrate reduction reduces gangliosides in postnatal cerebrum-brainstem and cerebellum in GM1 gangliosidosis mice. J Lipid Res, 46, 744–751. [DOI] [PubMed] [Google Scholar]
- Kim W. S., Halliday G. M. (2012). Changes in sphingomyelin level affect alpha-synuclein and ABCA5 expression. J Parkinsons Dis, 2, 41–46. [DOI] [PubMed] [Google Scholar]
- Kracun I., Rosner H., Drnovsek V., Vukelic Z., Cosovic C., Trbojevic-Cepe M., Kubat M. (1992). Gangliosides in the human brain development and aging. Neurochem Int, 20, 421–431. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W. (1985). Gangliosides of the neuron. Trends Neurosci, 8, 169–174. [Google Scholar]
- Macala L. J., Yu R. K., Ando S. (1983). Analysis of brain lipids by high performance thin-layer chromatography and densitometry. J Lipid Res, 24, 1243–1250. [PubMed] [Google Scholar]
- Marconi S., De Toni L., Lovato L., Tedeschi E., Gaetti L., Acler M., Bonetti B. (2005). Expression of gangliosides on glial and neuronal cells in normal and pathological adult human brain. J Neuroimmunol, 170, 115–121. [DOI] [PubMed] [Google Scholar]
- Miettinen T., Takki-Luukkainen I. T. (1959). Use of butyl acetate in the determination of sialic acid. Acta Chem Scand, 13, 856–858. [Google Scholar]
- Mirza B., Hadberg H., Thomsen P., Moos T. (2000). The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson's disease. Neuroscience, 95, 425–432. [DOI] [PubMed] [Google Scholar]
- Park D. H., Wang L., Pittock P., Lajoie G., Whitehead S. N. (2016). Increased expression of GM1 detected by electrospray mass spectrometry in rat primary embryonic cortical neurons exposed to glutamate toxicity. Anal Chem, 88, 7844–7852. [DOI] [PubMed] [Google Scholar]
- Riekkinen P., Rinne U. K., Pelliniemi T. T., Sonninen V. (1975). Interaction between dopamine and phospholipids. Studies of the substantia nigra in Parkinson disease patients. Arch Neurol, 32, 25–27. [DOI] [PubMed] [Google Scholar]
- Schnaar R. L. (2016). Gangliosides of the vertebrate nervous system. J Mol Biol, 428, 3325–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N. W., Macleod A. D., Counsell C. E. (2016). Motor complications in an incident Parkinson's disease cohort. Eur J Neurol, 23, 304–312. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Yu R. K. (1980). Heterosis for brain myelin content in mice. Biochem Genet, 18, 1229–1238. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Yu R. K. (1985). Ganglioside GD3: Structure, cellular distribution, and possible function. Mol Cell Biochem, 68, 3–10. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Glaser G. H., Yu R. K. (1978). Cerebral, cerebellar, and brain stem gangliosides in mice susceptible to audiogenic seizures. J Neurochem, 31, 21–27. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Glaser G. H., Yu R. K. (1979). Genetic variability for regional brain gangliosides in five strains of young mice. Biochem Genet, 17, 43–55. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Yu R. K., Miyazawa N. (1982). Differential cellular enrichment of gangliosides in the mouse cerebellum: Analysis using neurological mutants. J Neurochem, 38, 551–559. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Bernard D. J., Yu R. K. (1984). Cellular distribution of gangliosides in the developing mouse cerebellum: Analysis using the staggerer mutant. J Neurochem, 43, 1152–1162. [DOI] [PubMed] [Google Scholar]
- Shulman L. M., Bhat V. (2006). Gender disparities in Parkinson's disease. Expert Rev Neurother, 6, 407–416. [DOI] [PubMed] [Google Scholar]
- Song Y. J., Halliday G. M., Holton J. L., Lashley T., O'Sullivan S. S., McCann H., Lees A. J., Ozawa T., Williams D. R., Lockhart P. J., Revesz T. R. (2009). Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol, 68, 1073–1083. [DOI] [PubMed] [Google Scholar]
- Svennerholm L. (1957). Quantitative estimation of sialic acids II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta, 24, 604–611. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Bostrom K., Fredman P., Mansson J. E., Rosengren B., Rynmark B. M. (1989). Human brain gangliosides: Developmental changes from early fetal stage to advanced age. Biochim Biophys Acta, 1005, 109–117. [DOI] [PubMed] [Google Scholar]
- Teismann P., Schulz J. B. (2004). Cellular pathology of Parkinson's disease: Astrocytes, microglia and inflammation. Cell Tissue Res, 318, 149–161. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden S. K., Tanner C. M., Bernstein A. L., Fross R. D., Leimpeter A., Bloch D. A., Nelson L. M. (2003). Incidence of Parkinson's disease: Variation by age, gender, and race/ethnicity. Am J Epidemiol, 157, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Warren Olanow C., Kieburtz K., Rascol O., Poewe W., Schapira A. H., Emre M., Nissinen H., Leinonen M., Stocchi F., & Stalevo Reduction in Dyskinesia Evaluation in Parkinson's Disease Investigators. (2013). Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov Disord, 28, 1064–1071. [DOI] [PubMed] [Google Scholar]
- Waters C. M., Peck R., Rossor M., Reynolds G. P., Hunt S. P. (1988). Immunocytochemical studies on the basal ganglia and substantia nigra in Parkinson's disease and Huntington's chorea. Neuroscience, 25, 419–438. [DOI] [PubMed] [Google Scholar]
- Williams M. A., McCluer R. H. (1980). The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J Neurochem, 35, 266–269. [DOI] [PubMed] [Google Scholar]
- Wooten G. F., Currie L. J., Bovbjerg V. E., Lee J. K., Patrie J. (2004). Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry, 75, 637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Lu Z. H., Kulkarni N., Ledeen R. W. (2012). Deficiency of ganglioside GM1 correlates with Parkinson's disease in mice and humans. J Neurosci Res, 90, 1997–2008. [DOI] [PubMed] [Google Scholar]