Supplemental Digital Content is available in the text
Keywords: acute ischemic stroke, in-hospital delay, intravenous thrombolysis, organizational improvement, prehospital delay, stroke pathway, tissue plasminogen activator
Abstract
The generalization of successful efforts for reducing time delays in intravenous thrombolysis (IVT) could help facilitate its utility and benefits in acute ischemic stroke (AIS) patients.
We searched the PubMed and Embase databases for articles reporting interventions to reduce time delays in IVT, published between January 1995 and September 2017. The IVT rate was chosen as the primary outcome, while the compliance rates of onset-to-door time (prehospital delay) and door-to-needle time (in-hospital delay) within the targeted time frame were the secondary outcomes. Interventions designed to reduce prehospital, in-hospital, or total time delays were quantitatively described in meta-analyses. The efficacy of postintervention improvement was illustrated as odds ratios (ORs) and 95% confidence intervals (95% CIs).
In total, 86 papers (17 on prehospital, 56 on in-hospital, and 13 on total delay) encompassing 17,665 IVT cases were enrolled, including 28 American, 23 Asian, 30 European, and 5 Australian studies. The meta-analysis revealed statistically significant improvement in promoting IVT delivery after prehospital improvement interventions with an OR of 1.45 (95% CI, 1.23–1.71) for the new transportation protocol, 1.38 (95% CI, 1.11–1.73) for educational and training programs, and 1.83 (95% CI, 1.44–2.32) for comprehensive prehospital stroke code. The benefits of reducing in-hospital delay were much greater in developed western countries than in Asian countries, with ORs of 2.90 (95% CI, 2.51–3.34), 2.17 (95% CI, 1.95–2.41), and 1.89 (95% CI, 1.74–2.04) in American, European, and Asian countries, respectively. And telemedicine (OR, 2.26; 95% CI, 2.08–2.46) seemed to work better than pre-notification alone (OR, 1.94; 95% CI, 1.74–2.17) and in-hospital organizational improvement programs (OR, 2.10; 95% CI, 1.97–2.23). Mobile stroke treatment unit and use of a comprehensive stroke pathway in the pre- and in-hospital settings significantly increased IVT rates by reducing total time delay, with ORs of 2.01 (95% CI, 1.60–2.51) and 1.77 (95% CI, 1.55–2.03), respectively.
Optimization of the work flow with organizational improvement or novel technology could dramatically reduce pre- and in-hospital time delays of IVT in AIS. This study provided detailed information on the net and quantitative benefits of various programs for reducing time delays to facilitate the generalization of appropriate AIS management.
1. Introduction
Intravenous thrombolysis (IVT) has been a mainstream therapy for acute ischemic stroke (AIS) since the publication of National Institute of Neurological Disorders and Stroke (NINDS) rt-PA stroke trial in 1995.[1] The utility and benefits of IVT are largely limited by the narrow therapeutic time window in which the time delays in the stroke pathway due to health system factors are main obstacles to IVT in clinical practice.[2] Various interventions to reduce time delays in the stroke pathway were promoted to improve IVT administration and the clinical outcome of AIS patients. The TARGET: Stroke quality improvement initiative showed that an improved timeliness of IVT following AIS was associated with better functional and safety outcomes.[3] However, it is important to implement practical and efficient strategies to reduce time delays of IVT in each specific institute. Optimal interventions for time delays (classified as prehospital, in-hospital, and total time delays) remain unknown in the absence of quantitative evidence. Here, we aim to compare the efficacy of various interventions to reduce time delays through a quantitative meta-analysis and conduct a comprehensive literature review of this topic.
2. Materials and methods
2.1. Inclusion/exclusion criteria
As most studies on this topic were observational, the Meta-analysis Of Observational Studies in Epidemiology guidelines[4] were followed. Systematic literature searches were independently performed by 2 authors following the standard selection criteria. Inclusion criteria were as follows: studies focused on reducing time delays (prehospital, in-hospital, or total delay) of IVT in cases of AIS; cohort study, case-controlled study, registry study, or clinical randomized controlled trial published in English; and completed data on pre- (control) and postintervention (experimental) group. Exclusion criteria were as follows: case series or report, review, or commentary paper; study reporting incomplete data for mentioned subgroups or data unavailable even in supplemental materials; and study using data published more than once. At least 2 of the study authors agreed to include each of the identified articles in the analysis.
2.2. Literature search
We searched the PubMed and Embase databases for articles published between January 1, 1995, and September 30, 2017. The following free or MeSH search terms were used: stroke, ischemic, thrombolytic treatment, thombolysis, tissue plasminogen activator, tPA, alteplase, prehospital, public awareness, emergency medical service (EMS), in-hospital, door to needle time, registry, initiative, organizational model, implementation, and stroke pathway were used. We also manually searched the reference lists and citations of included articles for further articles. The detailed search process is reported in Supplemental Figure 1.
2.3. Data collection
Two authors (H.Q. and Z.J.) independently extracted data from all included papers using a standardized data collection form. A third consultation was made in cases of disagreement regarding inclusion eligibility. Report characteristics (first and corresponding authors, journal, and year of publication), study design (type, location, and period), intervention classification (pre- and/or in-hospital setting improvement), study sample and characteristics [numbers of subjects, age, sex, baseline National Institutes of Health Stroke Scale (NIHSS), IVT use rate, median onset to door time (ODT), median door to needle time (DNT), median onset to needle time (ONT), compliance rate of ODT (prehospital delay) and DNT (in-hospital delay) in pre- (control) and postintervention (experimental) groups], functional outcomes [measured on the modified Rankin Scale (mRS)], and safety outcomes (mortality and symptomatic intracranial hemorrhage (SICH)] were recorded. When reported, detailed information about the interventions, other time indicators, and clinical endpoint indicators were also recorded. Data of variables extracted from included papers followed preset criteria or definitions. When multiple papers drew on the same datasets, data were extracted only once from the most comprehensive available report. If the improvement interventions lasted for more than 1 time unit, the data from the last time unit before the interventions and the first time unit after the interventions were selected.
Stroke onset time was defined as the time when stroke symptoms first occurred or the last time known to be normal, door time as when the patient arrived at the emergency department of the hospital or mobile stroke treatment unit (MSTU), and needle time as when the administration of thrombolytic agent started. Pre-hospital delay was defined as ODT, in-hospital delay as DNT, and total time delay equal to ODT plus DNT.[5] The utilization rate of IVT (percentage of patients treated with IVT in all AIS cases) was chosen as primary outcome, while the compliance rates of ODT and DNT (the percentage of IVT patients achieving a qualified timeliness, e.g., ODT < 180 minutes and DNT < 60 minutes) were recorded as secondary outcomes. Clinical endpoint indicators such as favorable functional outcome at 3 months (defined as mRS 0–2), mortality, and SICH (defined as intracranial hemorrhage after IVT resulting in measurable neurological deterioration, e.g., NIHSS increased to ≥1[1]) were also included in the secondary analysis. When the preferred definitions for secondary outcomes and clinical endpoint indicators were not available, the authors’ definitions were adopted.
2.4. Data analysis
Statistical calculations were performed and graphics created using RevMan 5.1 software (Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration). When data were not calculable in the software, descriptive analysis was used. The Mantel–Haenszel method was implemented by the fixed- or random-effects analysis models based on included study heterogeneity. The primary analysis was to compare the utilization rates of IVT in the pre- (control) and postintervention (experimental) groups. The secondary analysis involved detecting the differences in ODT and DNT compliance rates and other clinical indicators between the 2 groups. The numeration data results were calculated as odds ratios (ORs) with 95% confidence intervals (CIs) considering 2-tailed P values < .05 statistically significant.
3. Results
3.1. Study characteristics
A total of 86 papers (17 on prehospital delay, 56 on in-hospital delay, and 13 on total delay) encompassing 17,665 IVT cases were included in this analysis. All articles included were published between 2003 and 2017, and the study period ranged from 1996 and 2017. There were 28 studies from American countries, 23 from Asian countries, 5 from Australia, and 30 from European countries, of which 8.1% (7/86) were randomized controlled studies and 44.2% (38/86) were conducted within 5 years. Features of the included papers are listed in Table 1 . [6–90] The moderate risk of bias and the standard errors for included studies are depicted in the Supplemental Figures 2 to 8.
Table 1.
Features of included studies.
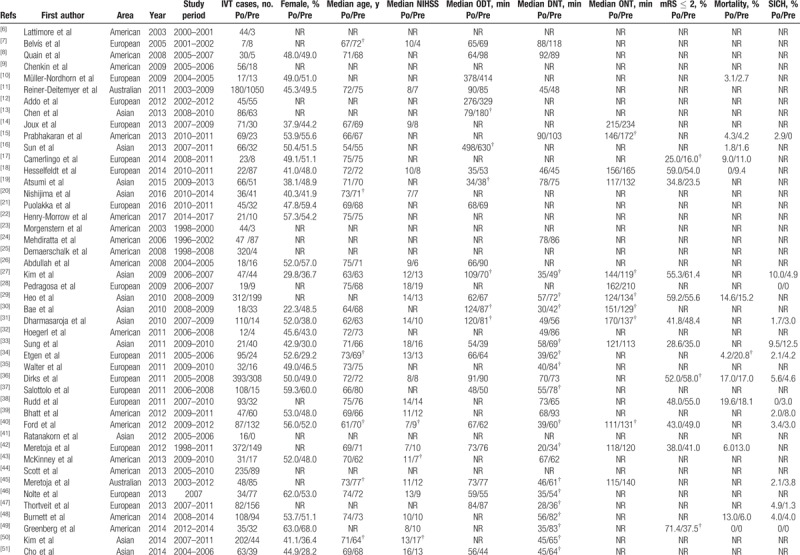
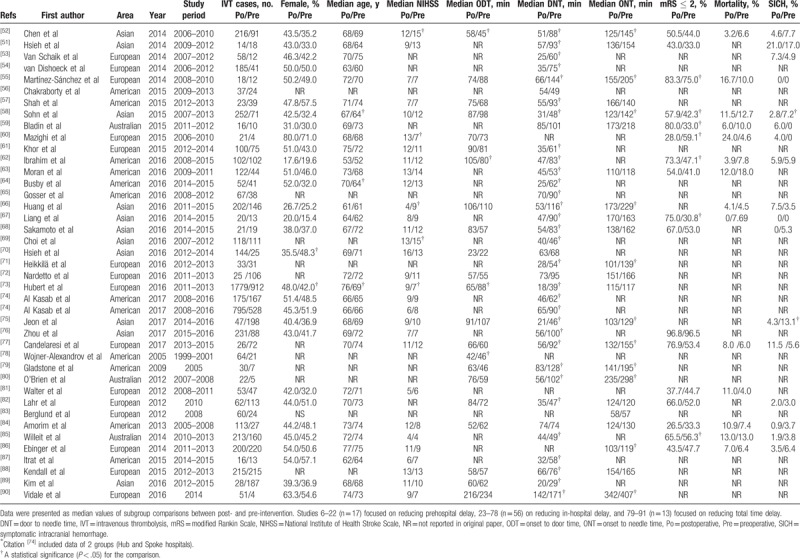
Table 1 (Continued).
Features of included studies.
3.2. Patient characteristics
A total of 17,665 IVT cases were enrolled in this review: 7491 in the preintervention (control) group and 10,174 in the postintervention (experimental) group. The difference of sex distribution of 5 studies, median age in 10 studies, and the median NIHSS in 10 studies were statistically significant between the 2 groups (Table 1 ). A statistically significant increase in favorable functional outcomes was observed in 10 studies, while a statistically significant decrease in mortality and the SICH rate was noted in 1 and 2 studies, respectively.
3.3. Interventions to reduce prehospital delay
In the analysis for reducing prehospital delay, 15 studies offered data of the use rate of IVT and 11 studies for the compliance rates of ODT (ODT < 180 minutes in 9 studies and < 120 minutes in other 2 studies). Using random-effect models, the meta-analysis revealed statistically significant improvement in IVT delivery after prehospital improving interventions, with the OR of 1.45 (95% CI, 1.23–1.71) for new transportation protocol, OR of 1.38 (95% CI, 1.11–1.73) for educational and training programs, and OR of 1.83 (95% CI, 1.44–2.32) for comprehensive prehospital stroke code, respectively. A significant increase in IVT rate was also observed in 2 subgroups: OR of 1.83 (95% CI, 1. 62–2.06) in the educational campaign and training protocol and OR of 1.49 (95% CI, 1.25–1.78) in the comprehensive improvement in the prehospital stroke code protocol but not in the new transportation method (OR, 1.22; 95% CI, 0.99–1.50; Fig. 1).
Figure 1.
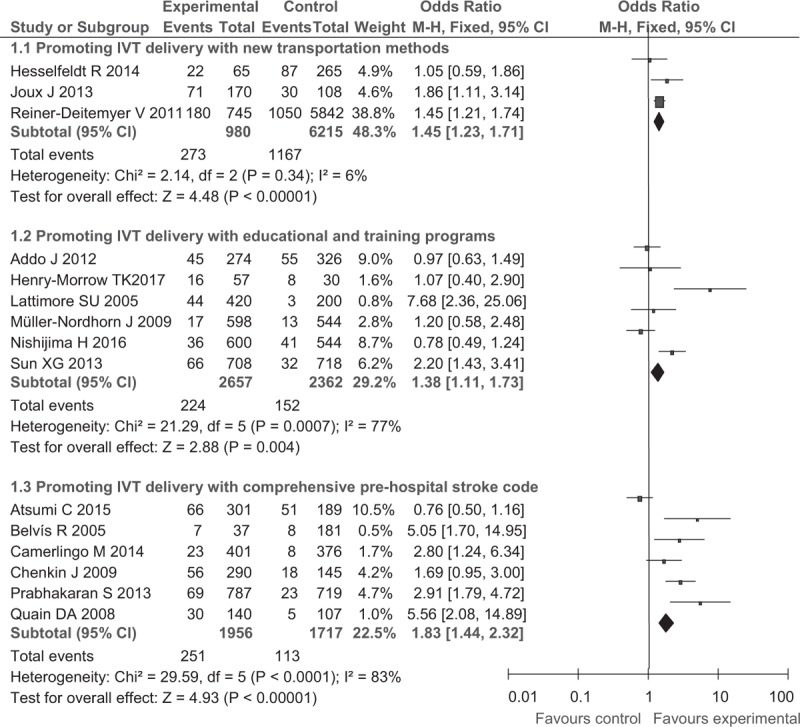
Post- versus pre-intervention in primary outcomes of reducing prehospital delay.
3.4. Interventions for reducing in-hospital delay
A total of 50 of the included studies focused on reducing in-hospital delay: 17 in American countries (America and Canada), 16 in Asia, 14 in Europe, and 3 in Australia. Details of the improving protocols were implemented via a telemedicine (telestroke or telephone consultation) system in 7 studies, using a pre-notification system alone by EMS in 4 studies, simply adding stroke team staff (emergency room nurse, pharmacist, or neurologist) in 4 studies, application of point-of-care laboratory platform based stroke management in 1 study, initiation of a comprehensive in-hospital organizational improvement program (which may include pre-notification, telemedicine system, or other above mentioned methods) in 29 studies.
Regarding IVT delivery, the benefits after interventions were much larger in developed countries (western countries) than in Asian countries with an OR of 2.90 (95% CI, 2.51–3.34) in American countries, OR of 2.17 (95% CI, 1.95–2.41) in European countries and Australia, and an OR of 1.89 (95% CI, 1.74–2.04) in Asian countries (Fig. 2). Regarding detailed methods of promoting IVT delivery, telemedicine (OR, 2.26; 95% CI, 2.08–2.46) seemed to work better than pre-notification alone (OR, 1.94; 95% CI, 1.74–2.17) and organizational improvement programs (OR, 2.10; 95% CI, 1.97–2.23) (Fig. 3). In the analysis of secondary outcomes, the compliance rates of DNT were improved to a greater degree in western countries (OR, 6.21; 95% CI, 4.45–8.67 in European countries and Australia and OR, 5.61; 95% CI, 4.41–7.13 in American countries) than in Asian countries (OR, 3.10; 95% CI, 2.45–3.92) (Fig. 4), while the pre-notification program served as a better way of increasing the rate of DNT < 60 minutes (OR, 14.44; 95% CI, 9.97–20.90) than the telemedicine protocol (OR, 6.19; 95% CI, 3.34–11.48) and the organizational improvement program (OR, 4.15; 95% CI, 3.50–4.93) (Fig. 5).
Figure 2.
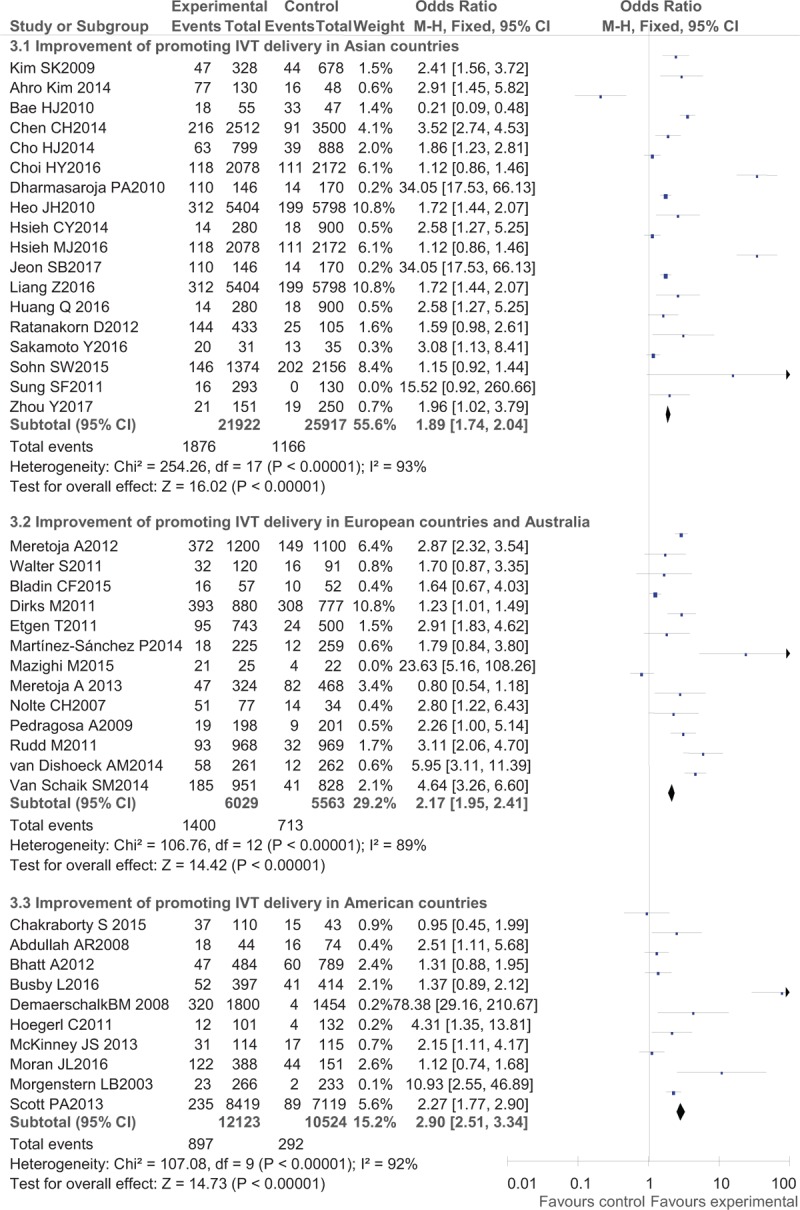
Post- versus pre-intervention in primary outcome of reducing in-hospital delay in different areas.
Figure 3.
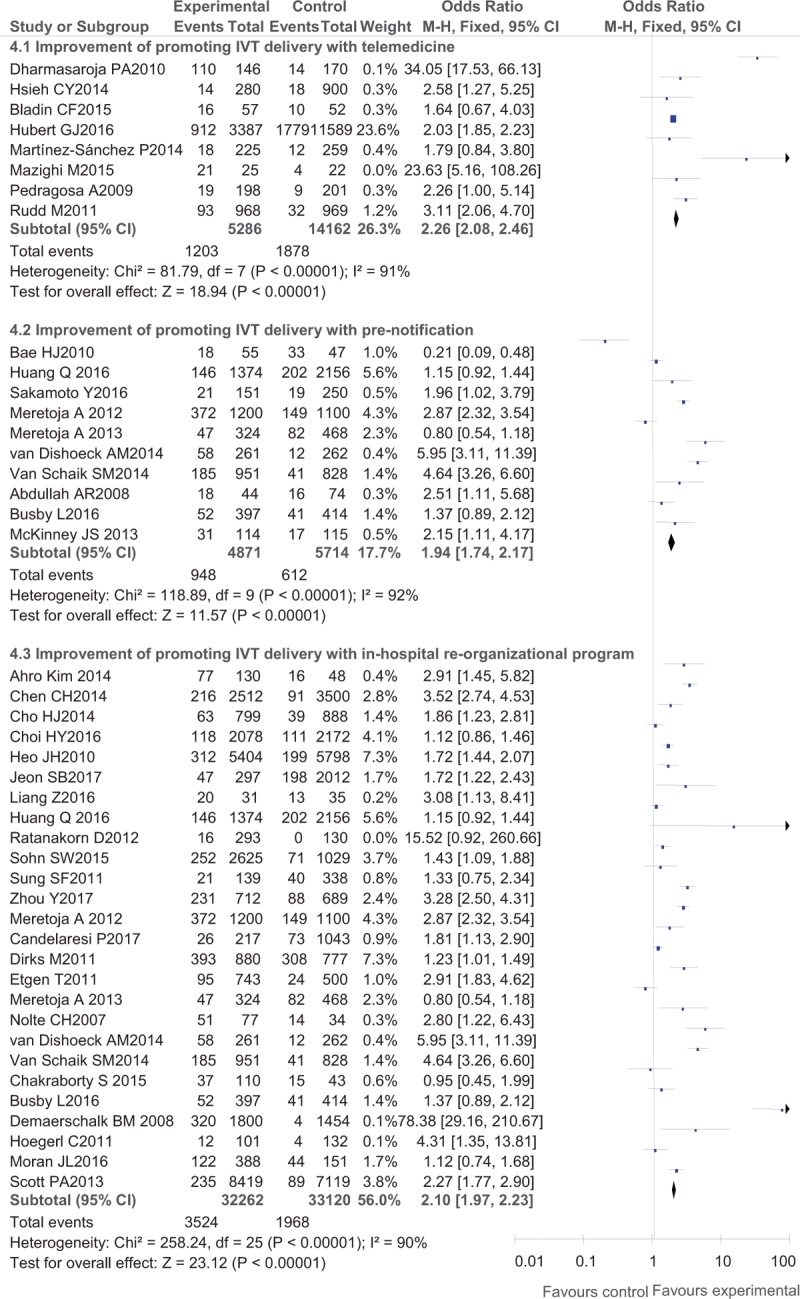
Post- versus pre-intervention in primary outcome of various methods for reducing in-hospital delay.
Figure 4.
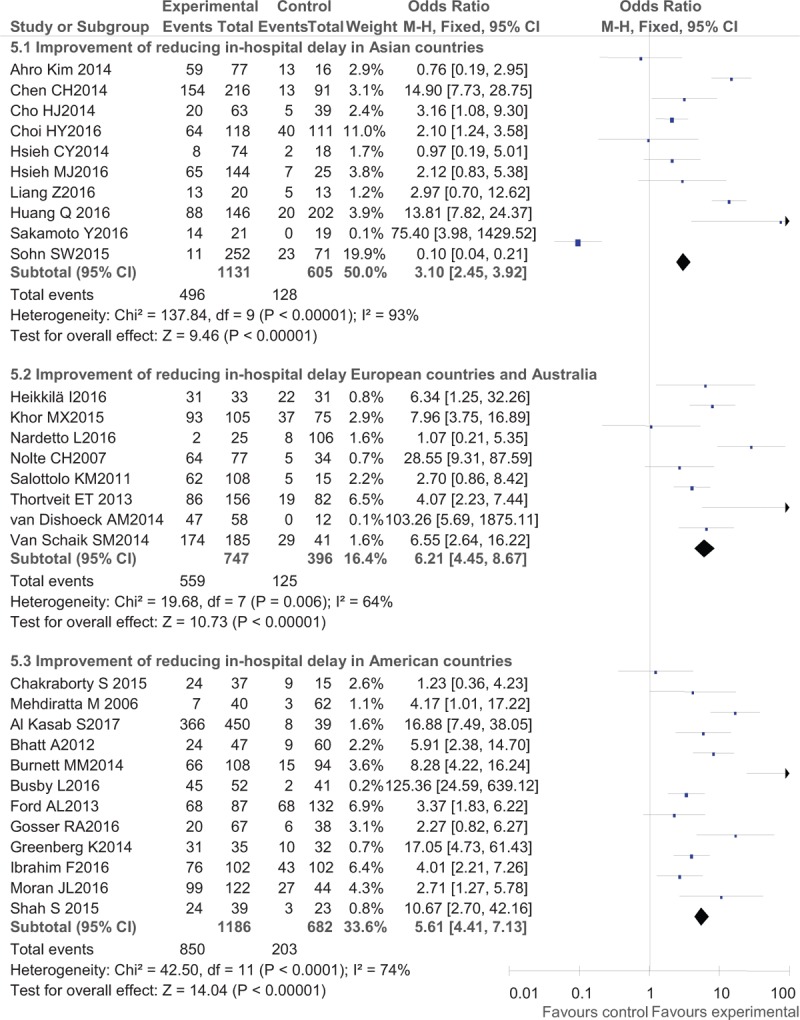
Post- versus pre-intervention in secondary outcome of reducing in-hospital delay in different areas.
Figure 5.
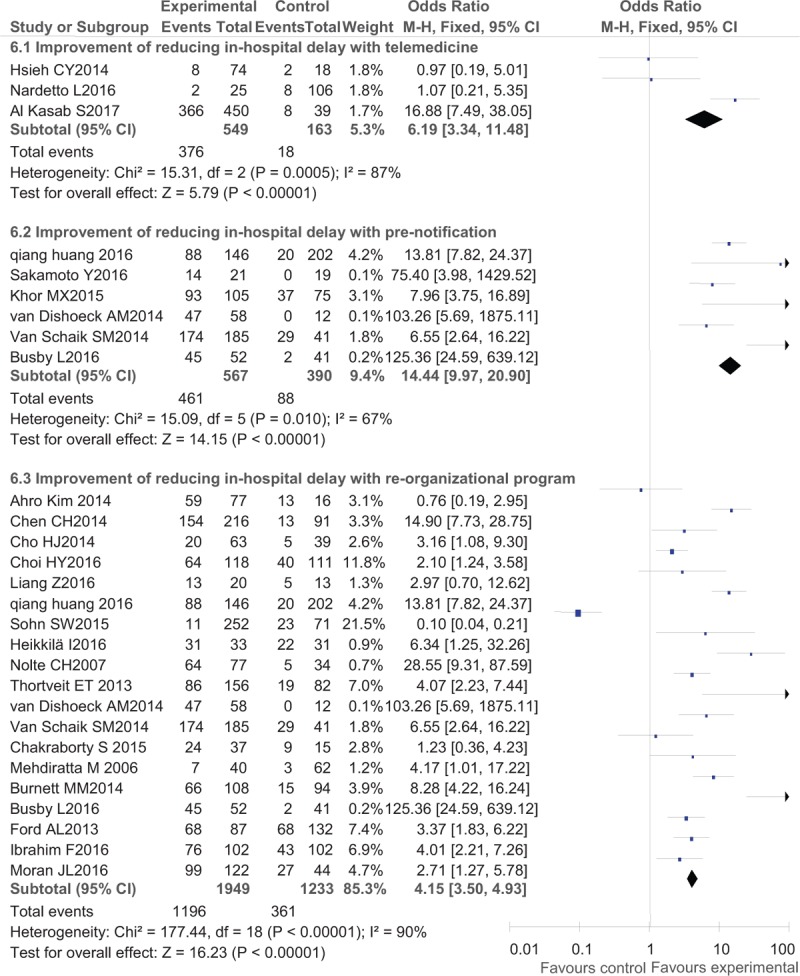
Post- versus pre-intervention in secondary outcome of various methods to reduce in-hospital delay.
3.5. Interventions for reducing total time delay
Interventions aiming at reducing total time delay of IVT included using MSTU, and implementation of comprehensive improving stroke pathway in both the pre-hospital and in-hospital settings. For the 2 subgroups (Fig. 6), the rates of IVT were both significantly increased after the application of MSTU or the comprehensive improving stroke pathway, with the OR of 2.01 (95% CI: 1.60–2.51) and OR of 1.77 (95% CI: 1.55–2.03), respectively.
Figure 6.
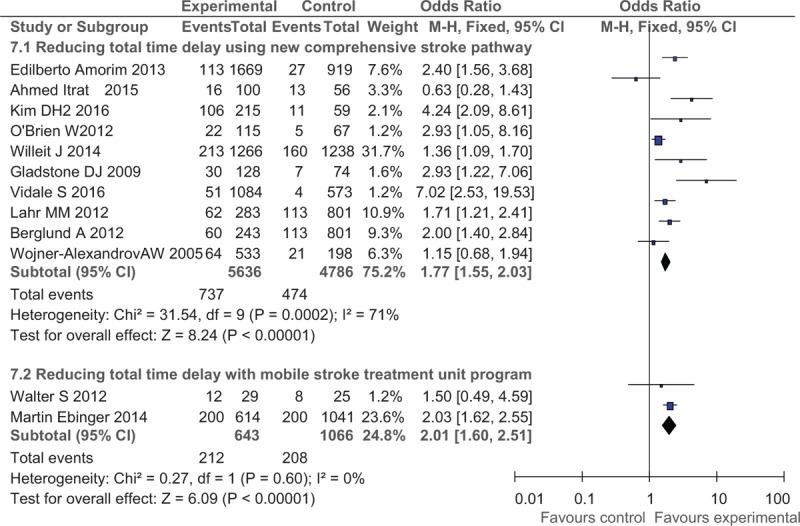
Post- versus pre-intervention in primary outcome of reducing total time delay.
4. Discussion
Various factors contributing to pre- and/or in-hospital delays for IVT in AIS have been detected and solutions addressing these factors proposed as our results showed. An optimal and continuous gain in thrombolysis administration for AIS involved multifaceted interventions, including reorganization of in-hospital and prehospital systems, the application of new technologies and facilities, and targeted training and educational programs. A detailed analysis demonstrated that streamline workflow for reducing in-hospital delays serves as the most efficient way to deliver IVT, of which the telestroke program was likely to be most successful and beneficial improving models.
The efficacy and safety of IVT with rt-PA in AIS is highly time-dependent, and the narrow therapeutic time window and time delays contributed to the most common of barriers of generalization of this therapy.[91] A previous systematic review by Evenson et al[5] observed that prehospital delay comprised the majority of time delays and the median prehospital delay was in the range of 3 to 6 hours. However, only a few studies showing a moderate effect on increasing the rate of IVT implemented detailed interventions to reduce time delays in the prehospital period, and the interventions included, for example, mass media and public awareness campaigns, professional education programs, and streamlined ambulance protocols.[16,20,92–94] Noted that the effect of comprehensive improving prehospital stroke code (OR, 1.83) was better than new transportation method (OR, 1.45) or educational program (OR, 1.38) alone (Fig. 7), which implied that the efforts made in this area called for multifaceted departments other than the hospital side alone and the role of EMS in stroke symptom recognition, patient transportation, and communication with hospital staff deserved the most attention for reducing prehospital delay. However, given the huge gap in the structures of EMS systems between countries or even districts within a single country, experience achieved in other places might not easily be copied. The cost-effectiveness of prehospital educational programs and EMS improvement remains to be demonstrated (which is mainly due to a larger number of emergency department visits for stroke mimics[92] or alternative diagnoses other than stroke[82]), and the positive effects could be decreased soon after the interventions.[78,92]
Figure 7.
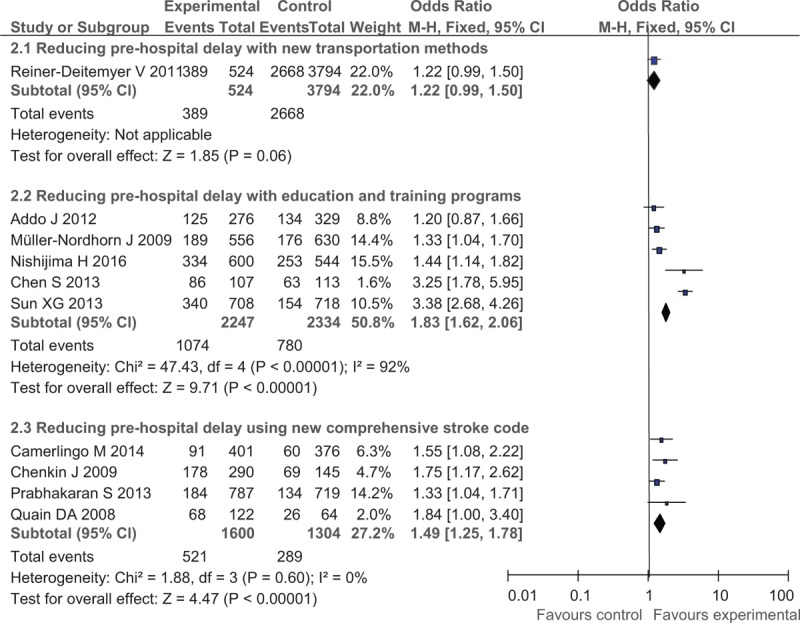
Post- versus pre-intervention in secondary outcomes of reducing prehospital delay.
Interventions to reduce in-hospital delays seemed to have made much greater progress than the former mentioned above and worked much better in developed areas (western countries) than in Asian countries (Fig. 3). One of the reasons for this could have been the initiative of national projects like the Safe Implementation of Thrombolysis in Stroke Monitoring Study),[95] the stroke registry in Australia,[96] and Target: Stroke in America [3] enable monitoring of therapeutic actions in IVT and teach many hospital staff how to improve their health care systems by reducing time delays. AS the time consumed by noncritical tasks was saved (lean principle), the median DTN could be made short to <20 minutes in 1 advanced European hospital.[42] Due to the detailed methods of promoting IVT delivery, telemedicine seemed to work better than pre-notification alone and organizational improvement programs (Fig. 4). That is, the population benefits of IVT were limited in rural areas and underdeveloped countries resulting from the restricted availability of stroke expertise and excellent medical resource, while the application of telemedicine could not only spread the excellent experience but also promote IVT use.[73,84,97] Previous studies have also demonstrated IVT delivery in spoke hospitals through telestroke networks is as effective and safe as that in hub institutions[98] and serves as a cost-saving protocol for remote practitioners.[99] Therefore, telestroke is a promising modern strategy to overcome the practical limitations and extend existing progress of reducing in-hospital delays.
Comprehensively improving stroke pathways that aim to integrate and improve prehospital and in-hospital settings could cover almost all aspects of acute stroke care. A significant increase in IVT administration was noted in our analysis (Fig. 6) and accompanied by a sustained increase in the likelihood of favorable outcomes.[85] Improvements in EMS including the centralization of stroke care (as in MSTU [81,87]) and infrastructure advancement (such as pre-notification or consultation using telemedicine technology platforms [43,87]) contributed the most to reducing total delays and tackling the problem of IVT undertreatment (Fig. 6). In a word, smooth coordination and timely communication between departments or disciplines (such as EMS staff, health authorities, and stroke physicians) are the intersections at which stroke can be managed most effectively.
Study limitations include the following. Use of the IVT rate as a performance measure to compare between centers and ethnic groups can be confounding because it is subject to selection and referral bias. For example, in developed countries (e.g., the United States), advanced medical resources could be available and more patients with AIS would be administrated rt-PA; thus, the progress from organizational and technological reforms could be more difficult to achieve than those in developing countries or underserved regions. However, IVT with rt-PA has long been a worldwide mainstream treatment of AIS since the publication of the NINDS results 22 years prior, which has made the process more normalized and generalized even without large gaps among countries.
5. Conclusion
Optimization in the work flow with organizational improvement or novel technology (e.g., MSTU) could dramatically reduce pre- and in-hospital time delays of IVT in AIS. Our study provided detail information on the net and quantitative benefits of various programs for promoting the delivery and reducing time delays of IVT, which could help the generalization of appropriate AIS management programs.
Author contributions
Conceptualization: Qiang Huang, Jian Wu.
Data curation: Qiang Huang, Jing-ze Zhang, Wen-deng Xu.
Formal analysis: Qiang Huang, Jian Wu.
Funding acquisition: Jian Wu.
Investigation: Qiang Huang, Wen-deng Xu, Jian Wu.
Methodology: Qiang Huang.
Project administration: Qiang Huang.
Resources: Qiang Huang.
Software: Qiang Huang.
Supervision: Jian Wu.
Validation: Qiang Huang.
Visualization: Qiang Huang.
Writing – original draft: Qiang Huang.
Writing – review & editing: Jing-ze Zhang, Wen-deng Xu, Jian Wu.
Supplementary Material
Footnotes
Abbreviations: 95%CI = 95% confidence interval, AIS = acute ischemic stroke, DNT = door to needle time, EMS = emergency medical service, IVT = intravenous thrombolysis, mRS = modified Rankin Scale, MSTU = mobile stroke treatment unit, NIHSS = National Institutes of Health Stroke Scale, NINDS = National Institute of Neurological Disorders and Stroke rt-PA stroke trial, ODT = onset to door time, ONT = onset to needle time, OR = odds ratio, SICH = symptomatic intracranial hemorrhage.
Funding/support: This study is funded by Tsinghua University Initiative Scientific Research Program (20161080076) and supported by Beijing Hospital Authority “DengFeng Project (DFL20152201).”
This study does not require ethical approval and patient consent because the study was a systematic review of previous studies and does not involve patients.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].National Institute of Neurological Disorders and Strole rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333:1581–1587. [DOI] [PubMed] [Google Scholar]
- [2].Paul CL, Ryan A, Rose S, et al. How can we improve stroke thrombolysis rates? A review of health system factors and approaches associated with thrombolysis administration rates in acute stroke care. Implement Sci 2016;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014;311:1632–40. [DOI] [PubMed] [Google Scholar]
- [4].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [5].Evenson KR, Rosamond WD, Morris DL. Prehospital and in-hospital delays in acute stroke care. Neuroepidemiology 2001;20:65–76. [DOI] [PubMed] [Google Scholar]
- [6].Lattimore SU, Chalela J, Davis L, et al. Impact of establishing a primary stroke center at a community hospital on the use of thrombolytic therapy: the NINDS Suburban Hospital Stroke Center experience. Stroke 2003;34:e55–7. [DOI] [PubMed] [Google Scholar]
- [7].Belvis R, Cocho D, Marti-Fabregas J, et al. Benefits of a prehospital stroke code system. Feasibility and efficacy in the first year of clinical practice in Barcelona, Spain. Cerebrovasc Dis 2005;19:96–101. [DOI] [PubMed] [Google Scholar]
- [8].Quain DA, Parsons MW, Loudfoot AR, et al. Improving access to acute stroke therapies: a controlled trial of organised pre-hospital and emergency care. Med J Aust 2008;189:429–33. [DOI] [PubMed] [Google Scholar]
- [9].Chenkin J, Gladstone DJ, Verbeek PR, et al. Predictive value of the Ontario prehospital stroke screening tool for the identification of patients with acute stroke. Prehosp Emerg Care 2009;13:153–9. [DOI] [PubMed] [Google Scholar]
- [10].Muller-Nordhorn J, Wegscheider K, Nolte CH, et al. Population-based intervention to reduce prehospital delays in patients with cerebrovascular events. Arch Intern Med 2009;169:1484–90. [DOI] [PubMed] [Google Scholar]
- [11].Reiner-Deitemyer V, Teuschl Y, Matz K, et al. Helicopter transport of stroke patients and its influence on thrombolysis rates: data from the Austrian Stroke Unit Registry. Stroke 2011;42:1295–300. [DOI] [PubMed] [Google Scholar]
- [12].Addo J, Ayis S, Leon J, et al. Delay in presentation after an acute stroke in a multiethnic population in South London: the South London stroke register. J Am Heart Assoc 2012;1:e1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen S, Sun H, Zhao X, et al. Effects of comprehensive education protocol in decreasing pre-hospital stroke delay among Chinese urban community population. Neurol Res 2013;35:522–8. [DOI] [PubMed] [Google Scholar]
- [14].Joux J, Olindo S, Girard-Claudon A, et al. Prehospital transfer medicalization increases thrombolysis rate in acute ischemic stroke. A French stroke unit experience. Clin Neurol Neurosurg 2013;115:1583–5. [DOI] [PubMed] [Google Scholar]
- [15].Prabhakaran S, O’Neill K, Stein-Spencer L, et al. Prehospital triage to primary stroke centers and rate of stroke thrombolysis. JAMA Neurol 2013;70:1126–32. [DOI] [PubMed] [Google Scholar]
- [16].Sun XG, Zhang N, Wang T, et al. Public and professional education on urgent therapy for acute ischemic stroke: a community-based intervention in Changsha. Neurol Sci 2013;34:2131–5. [DOI] [PubMed] [Google Scholar]
- [17].Camerlingo M, D’Asero S, Perego L, et al. How to improve access to appropriate therapy and outcome of the acute ischemic stroke: a 24-month survey of a specific pre-hospital planning in Northern Italy. Neurol Sci 2014;35:1359–63. [DOI] [PubMed] [Google Scholar]
- [18].Hesselfeldt R, Gyllenborg J, Steinmetz J, et al. Is air transport of stroke patients faster than ground transport? A prospective controlled observational study. Emerg Med J 2014;31:268–72. [DOI] [PubMed] [Google Scholar]
- [19].Atsumi C, Hasegawa Y, Tsumura K, et al. Quality assurance monitoring of a citywide transportation protocol improves clinical indicators of intravenous tissue plasminogen activator therapy: a community-based, longitudinal study. J Stroke Cerebrovasc Dis 2015;24:183–8. [DOI] [PubMed] [Google Scholar]
- [20].Nishijima H, Kon T, Ueno T, et al. Effect of educational television commercial on pre-hospital delay in patients with ischemic stroke. Neurol Sci 2016;37:105–9. [DOI] [PubMed] [Google Scholar]
- [21].Puolakka T, Vayrynen T, Erkkila EP, et al. Fire engine support and on-scene time in prehospital stroke care: a prospective observational study. Prehosp Disaster Med 2016;31:278–81. [DOI] [PubMed] [Google Scholar]
- [22].Henry-Morrow TK, Nelson BD, Conahan E, et al. An educational intervention allows for greater prehospital recognition of acute stroke. Am J Emerg Med 2017;35:1959–61. [DOI] [PubMed] [Google Scholar]
- [23].Morgenstern LB, Bartholomew LK, Grotta JC, et al. Sustained benefit of a community and professional intervention to increase acute stroke therapy. Arch Intern Med 2003;163:2198–202. [DOI] [PubMed] [Google Scholar]
- [24].Mehdiratta M, Woolfenden AR, Chapman KM, et al. Reduction in IV t-PA door to needle times using an Acute Stroke Triage Pathway. Can J Neurol Sci 2006;33:214–6. [DOI] [PubMed] [Google Scholar]
- [25].Demaerschalk BM, Bobrow BJ, Paulsen M. Development of a metropolitan matrix of primary stroke centers: the Phoenix experience. Stroke 2008;39:1246–53. [DOI] [PubMed] [Google Scholar]
- [26].Abdullah AR, Smith EE, Biddinger PD, et al. Advance hospital notification by EMS in acute stroke is associated with shorter door-to-computed tomography time and increased likelihood of administration of tissue-plasminogen activator. Prehosp Emerg Care 2008;12:426–31. [DOI] [PubMed] [Google Scholar]
- [27].Kim SK, Lee SY, Bae HJ, et al. Pre-hospital notification reduced the door-to-needle time for iv t-PA in acute ischaemic stroke. Eur J Neurol 2009;16:1331–5. [DOI] [PubMed] [Google Scholar]
- [28].Pedragosa A, Alvarez-Sabin J, Molina CA, et al. Impact of a telemedicine system on acute stroke care in a community hospital. J Telemed Telecare 2009;15:260–3. [DOI] [PubMed] [Google Scholar]
- [29].Heo JH, Kim YD, Nam HS, et al. A computerized in-hospital alert system for thrombolysis in acute stroke. Stroke 2010;41:1978–83. [DOI] [PubMed] [Google Scholar]
- [30].Bae HJ, Kim DH, Yoo NT, et al. Prehospital notification from the emergency medical service reduces the transfer and intra-hospital processing times for acute stroke patients. J Clin Neurol 2010;6:138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dharmasaroja PA, Muengtaweepongsa S, Kommarkg U. Implementation of Telemedicine and Stroke Network in thrombolytic administration: comparison between walk-in and referred patients. Neurocrit Care 2010;13:62–6. [DOI] [PubMed] [Google Scholar]
- [32].Hoegerl C, Goldstein FJ, Sartorius J. Implementation of a stroke alert protocol in the emergency department: a pilot study. J Am Osteopath Assoc 2011;111:21–7. [PubMed] [Google Scholar]
- [33].Sung SF, Huang YC, Ong CT, et al. A parallel thrombolysis protocol with nurse practitioners as coordinators minimized door-to-needle time for acute ischemic stroke. Stroke Res Treat 2011;2011:198518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Etgen T, Freudenberger T, Schwahn M, et al. Multimodal strategy in the successful implementation of a stroke unit in a community hospital. Acta Neurol Scand 2011;123:390–5. [DOI] [PubMed] [Google Scholar]
- [35].Walter S, Kostopoulos P, Haass A, et al. Point-of-care laboratory halves door-to-therapy-decision time in acute stroke. Ann Neurol 2011;69:581–6. [DOI] [PubMed] [Google Scholar]
- [36].Dirks M, Niessen LW, van Wijngaarden JD, et al. Promoting thrombolysis in acute ischemic stroke. Stroke 2011;42:1325–30. [DOI] [PubMed] [Google Scholar]
- [37].Salottolo KM, Fanale CV, Leonard KA, et al. Multimodal imaging does not delay intravenous thrombolytic therapy in acute stroke. AJNR Am J Neuroradiol 2011;32:864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rudd M, Rodgers H, Curless R, et al. Remote specialist assessment for intravenous thrombolysis of acute ischaemic stroke by telephone. Emerg Med J 2012;29:704–8. [DOI] [PubMed] [Google Scholar]
- [39].Bhatt A, Shatila A. Neurohospitalists improve door-to-needle times for patients with ischemic stroke receiving intravenous tPA. Neurohospitalist 2012;2:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ford AL, Williams JA, Spencer M, et al. Reducing door-to-needle times using Toyota's lean manufacturing principles and value stream analysis. Stroke 2012;43:3395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ratanakorn D, Keandoungchun J, Sittichanbuncha Y, et al. Stroke fast track reduces time delay to neuroimaging and increases use of thrombolysis in an academic medical center in Thailand. J Neuroimaging 2012;22:53–7. [DOI] [PubMed] [Google Scholar]
- [42].Meretoja A, Strbian D, Mustanoja S, et al. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology 2012;79:306–13. [DOI] [PubMed] [Google Scholar]
- [43].McKinney JS, Mylavarapu K, Lane J, et al. Hospital prenotification of stroke patients by emergency medical services improves stroke time targets. J Stroke Cerebrovasc Dis 2013;22:113–8. [DOI] [PubMed] [Google Scholar]
- [44].Scott PA, Meurer WJ, Frederiksen SM, et al. A multilevel intervention to increase community hospital use of alteplase for acute stroke (INSTINCT): a cluster-randomised controlled trial. Lancet Neurol 2013;12:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Meretoja A, Weir L, Ugalde M, et al. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology 2013;81:1071–6. [DOI] [PubMed] [Google Scholar]
- [46].Nolte CH, Malzahn U, Kuhnle Y, et al. Improvement of door-to-imaging time in acute stroke patients by implementation of an all-points alarm. J Stroke Cerebrovasc Dis 2013;22:149–53. [DOI] [PubMed] [Google Scholar]
- [47].Thortveit ET, Boe MG, Ljostad U, et al. Organizational changes aiming to reduce iv tPA door-to-needle time. Acta Neurol Scand 2014;130:248–52. [DOI] [PubMed] [Google Scholar]
- [48].Burnett MM, Zimmermann L, Coralic Z, et al. Simple text-messaging intervention is associated with improved door-to-needle times for acute ischemic stroke. Stroke 2014;45:3714–6. [DOI] [PubMed] [Google Scholar]
- [49].Greenberg K, Maxwell CR, Moore KD, et al. Improved door-to-needle times and neurologic outcomes when IV tissue plasminogen activator is administered by emergency physicians with advanced neuroscience training. Am J Emerg Med 2015;33:234–7. [DOI] [PubMed] [Google Scholar]
- [50].Kim A, Lee JS, Kim JE, et al. Trends in yield of a code stroke program for enhancing thrombolysis. J Clin Neurosci 2015;22:73–8. [DOI] [PubMed] [Google Scholar]
- [51].Hsieh CY, Chen WF, Chen CH, et al. Efforts to reduce the door-to-needle time of thrombolysis in acute ischemic stroke: video-assisted therapeutic risk communication. J Formos Med Assoc 2014;113:929–33. [DOI] [PubMed] [Google Scholar]
- [52].Chen CH, Tang SC, Tsai LK, et al. Stroke code improves intravenous thrombolysis administration in acute ischemic stroke. PLoS One 2014;9:e104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Van Schaik SM, Van der Veen B, Van den Berg-Vos RM, et al. Achieving a door-to-needle time of 25 minutes in thrombolysis for acute ischemic stroke: a quality improvement project. J Stroke Cerebrovasc Dis 2014;23:2900–6. [DOI] [PubMed] [Google Scholar]
- [54].van Dishoeck AM, Dippel DW, Dirks M, et al. Measuring quality improvement in acute ischemic stroke care: interrupted time series analysis of door-to-needle time. Cerebrovasc Dis Extra 2014;4:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Martinez-Sanchez P, Miralles A, Sanz DBR, et al. The effect of telestroke systems among neighboring hospitals: more and better? The Madrid Telestroke Project. J Neurol 2014;261:1768–73. [DOI] [PubMed] [Google Scholar]
- [56].Chakraborty S, Ross J, Hogan MJ, et al. Beating the clock: time delays to thrombolytic therapy with advanced imaging and impact of optimized workflow. J Stroke Cerebrovasc Dis 2015;24:1270–5. [DOI] [PubMed] [Google Scholar]
- [57].Shah S, Luby M, Poole K, et al. Screening with MRI for accurate and rapid stroke treatment: SMART. Neurology 2015;84:2438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sohn SW, Park HS, Cha JK, et al. A systemized stroke code significantly reduced time intervals for using intravenous tissue plasminogen activator under magnetic resonance imaging screening. J Stroke Cerebrovasc Dis 2015;24:465–72. [DOI] [PubMed] [Google Scholar]
- [59].Bladin CF, Molocijz N, Ermel S, et al. Victorian Stroke Telemedicine Project: implementation of a new model of translational stroke care for Australia. Intern Med J 2015;45:951–6. [DOI] [PubMed] [Google Scholar]
- [60].Mazighi M, Meseguer E, Labreuche J, et al. TRUST-tPA trial: telemedicine for remote collaboration with urgentists for stroke-tPA treatment. J Telemed Telecare 2017;23:174–80. [DOI] [PubMed] [Google Scholar]
- [61].Khor MX, Bown A, Barrett A, et al. Pre-hospital notification is associated with improved stroke thrombolysis timing. J R Coll Physicians Edinb 2015;45:190–5. [DOI] [PubMed] [Google Scholar]
- [62].Ibrahim F, Akhtar N, Salam A, et al. Stroke thrombolysis protocol shortens “door-to-needle time” and improves outcomes-experience at a tertiary care center in Qatar. J Stroke Cerebrovasc Dis 2016;25:2043–6. [DOI] [PubMed] [Google Scholar]
- [63].Moran JL, Nakagawa K, Asai SM, et al. 24/7 neurocritical care nurse practitioner coverage reduced door-to-needle time in stroke patients treated with tissue plasminogen activator. J Stroke Cerebrovasc Dis 2016;25:1148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Busby L, Owada K, Dhungana S, et al. CODE FAST: a quality improvement initiative to reduce door-to-needle times. J Neurointerv Surg 2016;8:661–4. [DOI] [PubMed] [Google Scholar]
- [65].Gosser RA, Arndt RF, Schaafsma K, et al. Pharmacist impact on ischemic stroke care in the emergency department. J Emerg Med 2016;50:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Huang Q, Song HQ, Ji XM, et al. Generalization of the right acute stroke prevention strategies in reducing in-hospital delays. PLoS One 2016;11:e154972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Liang Z, Ren L, Wang T, et al. Effective management of patients with acute ischemic stroke based on lean production on thrombolytic flow optimization. Australas Phys Eng Sci Med 2016;39:987–96. [DOI] [PubMed] [Google Scholar]
- [68].Sakamoto Y, Tanabe M, Masuda K, et al. Feasibility of using magnetic resonance imaging as a screening tool for acute stroke thrombolysis. J Neurol Sci 2016;368:168–72. [DOI] [PubMed] [Google Scholar]
- [69].Choi HY, Kim EH, Yoo J, et al. Decision-making support using a standardized script and visual decision aid to reduce door-to-needle time in stroke. J Stroke 2016;18:239–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hsieh MJ, Tang SC, Chiang WC, et al. Effect of prehospital notification on acute stroke care: a multicenter study. Scand J Trauma Resusc Emerg Med 2016;24:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Heikkila I, Kuusisto H, Stolberg A, et al. Stroke thrombolysis given by emergency physicians cuts in-hospital delays significantly immediately after implementing a new treatment protocol. Scand J Trauma Resusc Emerg Med 2016;24:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nardetto L, Dario C, Tonello S, et al. A one-to-one telestroke network: the first Italian study of a web-based telemedicine system for thrombolysis delivery and patient monitoring. Neurol Sci 2016;37:725–30. [DOI] [PubMed] [Google Scholar]
- [73].Hubert GJ, Meretoja A, Audebert HJ, et al. Stroke thrombolysis in a centralized and a decentralized system (Helsinki and Telemedical Project for Integrative Stroke Care Network). Stroke 2016;47:2999–3004. [DOI] [PubMed] [Google Scholar]
- [74].Al KS, Harvey JB, Debenham E, et al. Door to needle time over telestroke: a comprehensive stroke center experience. Telemed J E Health 2018;24:111–5. [DOI] [PubMed] [Google Scholar]
- [75].Jeon SB, Ryoo SM, Lee DH, et al. Multidisciplinary approach to decrease in-hospital delay for stroke thrombolysis. J Stroke 2017;19:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhou Y, Xu Z, Liao J, et al. New standardized nursing cooperation workflow to reduce stroke thrombolysis delays in patients with acute ischemic stroke. Neuropsychiatr Dis Treat 2017;13:1215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Candelaresi P, Lattuada P, Uggetti C, et al. A high-urgency stroke code reduces in-hospital delays in acute ischemic stroke: a single-centre experience. Neurol Sci 2017;38:1671–6. [DOI] [PubMed] [Google Scholar]
- [78].Wojner-Alexandrov AW, Alexandrov AV, Rodriguez D, et al. Houston paramedic and emergency stroke treatment and outcomes study (HoPSTO). Stroke 2005;36:1512–8. [DOI] [PubMed] [Google Scholar]
- [79].Gladstone DJ, Rodan LH, Sahlas DJ, et al. A citywide prehospital protocol increases access to stroke thrombolysis in Toronto. Stroke 2009;40:3841–4. [DOI] [PubMed] [Google Scholar]
- [80].O’Brien W, Crimmins D, Donaldson W, et al. FASTER (Face, Arm, Speech, Time, Emergency Response): experience of Central Coast Stroke Services implementation of a pre-hospital notification system for expedient management of acute stroke. J Clin Neurosci 2012;19:241–5. [DOI] [PubMed] [Google Scholar]
- [81].Walter S, Kostopoulos P, Haass A, et al. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol 2012;11:397–404. [DOI] [PubMed] [Google Scholar]
- [82].Lahr MM, Vroomen PC, Luijckx GJ, et al. Prehospital factors determining regional variation in thrombolytic therapy in acute ischemic stroke. Int J Stroke 2014;9(suppl A100):31–5. [DOI] [PubMed] [Google Scholar]
- [83].Berglund A, Svensson L, Sjostrand C, et al. Higher prehospital priority level of stroke improves thrombolysis frequency and time to stroke unit: the Hyper Acute STroke Alarm (HASTA) study. Stroke 2012;43:2666–70. [DOI] [PubMed] [Google Scholar]
- [84].Amorim E, Shih MM, Koehler SA, et al. Impact of telemedicine implementation in thrombolytic use for acute ischemic stroke: the University of Pittsburgh Medical Center telestroke network experience. J Stroke Cerebrovasc Dis 2013;22:527–31. [DOI] [PubMed] [Google Scholar]
- [85].Willeit J, Geley T, Schoch J, et al. Thrombolysis and clinical outcome in patients with stroke after implementation of the Tyrol Stroke Pathway: a retrospective observational study. Lancet Neurol 2015;14:48–56. [DOI] [PubMed] [Google Scholar]
- [86].Ebinger M, Winter B, Wendt M, et al. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA 2014;311:1622–31. [DOI] [PubMed] [Google Scholar]
- [87].Itrat A, Taqui A, Cerejo R, et al. Telemedicine in prehospital stroke evaluation and thrombolysis: taking stroke treatment to the doorstep. JAMA Neurol 2016;73:162–8. [DOI] [PubMed] [Google Scholar]
- [88].Kendall J, Dutta D, Brown E. Reducing delay to stroke thrombolysis: lessons learnt from the Stroke 90 Project. Emerg Med J 2015;32:100–4. [DOI] [PubMed] [Google Scholar]
- [89].Kim DH, Nah HW, Park HS, et al. Impact of prehospital intervention on delay time to thrombolytic therapy in a stroke center with a systemized stroke code program. J Stroke Cerebrovasc Dis 2016;25:1665–70. [DOI] [PubMed] [Google Scholar]
- [90].Vidale S, Arnaboldi M, Bezzi G, et al. Reducing time delays in the management of ischemic stroke patients in Northern Italy. Int J Cardiol 2016;215:431–4. [DOI] [PubMed] [Google Scholar]
- [91].Fassbender K, Balucani C, Walter S, et al. Streamlining of prehospital stroke management: the golden hour. Lancet Neurol 2013;12:585–96. [DOI] [PubMed] [Google Scholar]
- [92].Hodgson C, Lindsay P, Rubini F. Can mass media influence emergency department visits for stroke? Stroke 2007;38:2115–22. [DOI] [PubMed] [Google Scholar]
- [93].Soulleihet V, Nicoli F, Trouve J, et al. Optimized acute stroke pathway using medical advanced regulation for stroke and repeated public awareness campaigns. Am J Emerg Med 2014;32:225–32. [DOI] [PubMed] [Google Scholar]
- [94].Nishijima H, Ueno T, Kon T, et al. Effects of educational television commercial on pre-hospital delay in patients with ischemic stroke wore off after the end of the campaign. J Neurol Sci 2017;381:117–8. [DOI] [PubMed] [Google Scholar]
- [95].Basic KV, Zavoreo I, Vargek-Solter V, et al. Quantitative and qualitative evaluation tool in planning stroke treatment strategies: the “Safe implementation of treatments in stroke Monitoring Study (SITS MOST)” registry. Acta Neurol Belg 2014;114:95–106. [DOI] [PubMed] [Google Scholar]
- [96].Ferrari J, Seyfang L, Lang W. Can online benchmarking increase rates of thrombolysis? Data from the Austrian stroke unit registry. J Neurol 2013;260:2271–8. [DOI] [PubMed] [Google Scholar]
- [97].Chalouhi N, Dressler JA, Kunkel ES, et al. Intravenous tissue plasminogen activator administration in community hospitals facilitated by telestroke service. Neurosurgery 2013;73:667–71. [DOI] [PubMed] [Google Scholar]
- [98].Kepplinger J, Barlinn K, Deckert S, et al. Safety and efficacy of thrombolysis in telestroke: a systematic review and meta-analysis. Neurology 2016;87:1344–51. [DOI] [PubMed] [Google Scholar]
- [99].Switzer JA, Demaerschalk BM, Xie J, et al. Cost-effectiveness of hub-and-spoke telestroke networks for the management of acute ischemic stroke from the hospitals’ perspectives. Circ Cardiovasc Qual Outcomes 2013;6:18–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


