Abstract
The present study is to compare the efficacy and adverse effects of intensity-modulated radiotherapy (IMRT) combined with endostar and IMRT combined with concurrent chemotherapy on locally advanced nasopharyngeal carcinoma (NPC).
A total of 23 patients with stage III-IVa NPC were included in the present study, and randomly divided into experimental group (10 cases treated with IMRT + endostar) and control group (13 cases treated with IMRT + chemotherapy of cis-dichlorodiamineplatinum). Endostar was intravenously administered from the first day of IMRT. The patients received a total of 2 cycles (14 days each) separating by a 7-day interval.
IMRT combined with endostar did not have significantly different recent efficacy compared with IMRT combined with chemotherapy. IMRT combined with endostar and IMRT combined with chemotherapy had 2-year overall survival (OS) rates of 100.0% and 69.6%, respectively, without significant difference between each other (χ2 = 1.446, P = .299). The 2-year local relapse-free survival (LRFS) of the 2 groups were 100.0% and 81.3%, respectively, without significant difference between each other (χ2 = 1.000, P = .317). The 2-year distant metastasis-free survival (DMFS) of the 2 groups were 100.0% and 73.5% (χ2 = 1.591, P = .207), respectively. The 2-year progression-free survival (PFS) of the 2 groups were 100.0% and 67.3% (χ2 = 2.164, P = .141), respectively. However, the cumulative survival curves of OS, LRFS, DMFS, and PFS were separated between the 2 groups. The result that IMRT combined with endostar did not have significantly different long-term efficacy than IMRT combined with chemotherapy probably due to limited case number and short follow-up time. IMRT combined with endostar resulted in significantly lower grades of leucopenia, nausea/vomiting, weight loss, and oral mucositis compared with IMRT combined with chemotherapy. The grades of late adverse reactions of IMRT combined with endostar were not different from those of IMRT combined with chemotherapy.
The present study demonstrates that, compared with IMRT combined with chemotherapy, IMRT combined with endostar has similar efficacy in the treatment of locally advanced NPC, but significantly weaker acute adverse reactions, which improve the life quality of NPC patients.
Keywords: chemotherapy, endostar, intensity-modulated radiotherapy, nasopharyngeal carcinoma, recombinant human endostatin
1. Introduction
Nasopharyngeal carcinoma (NPC) is one of the commonest malignant tumors in South China, with its incidence in Guangxi province ranking the second in the world.[1] Radiotherapy is the primary treatment method for NPC. As the improvement of technology, especially the development of intensity-modulated radiotherapy (IMRT), tumor local control rate has been dramatically enhanced, and overall 5-year survival has been significantly increased.[2–11] Studies show that concurrent chemotherapy fails to improve the treatment effect of IMRT on locally advanced NPC.[12–14] In addition, concurrent chemotherapy significantly increases III/IV hematological toxicity level and mucosal toxicity, and severely affects the treatment process and life quality of patients.[15] Therefore, drugs with high efficiency and low toxicity that can replace concurrent chemotherapy for locally advanced NPC are drawing more and more attentions from researchers.
Endostar, independently developed by Chinese scholars, is a recombinant human endostatin constructed through adding 9 amino acid residues to the N-terminus of endostatin [16]. Endostar has better stability against protease, acid and heat than endostatin [17]. It inhibits the growth of a variety of tumors by blocking tumor angiogenesis, normalizes the structure and function of vasoganglion, improves blood circulation and tissue hypoxia, and enhances the radiosensitivity of hypoxic cells.[18] Vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor-2 (VEGFR-2), and platelet-derived growth factor receptor (PDGFR) are the primary targets of the endostar. In clinical practice, endostar has efficacy in the treatment of non-small cell lung cancer, colorectal cancer, bone soft tissue sarcoma, metastatic malignant melanoma, gastric cancer, esophageal cancer, and cervical cancer.[11,12,19–23] However, there are few reports on the treatment of NPC using radiotherapy combined with antiangiogenic agents. The present study compares the efficacy and toxic side effects of IMRT combined with endostar and IMRT combined with concurrent chemotherapy on locally advanced NPC.
2. Materials and methods
2.1. Patients
A total of 23 patients with stage III-IVa NPC admitted at the First Affiliated Hospital of Guangxi Medical University between January and November 2013 were included in the present study. The patients were randomly divided into experimental group (10 cases treated with IMRT + endostar) and control group (13 cases treated with IMRT + chemotherapy). The experimental group included 8 males and 2 females, who were aged between 40 and 77 years with a median of 59.2 years. Among the patients in experimental group, 4 cases had stage III NPC, and 6 cases had stage IV NPC. In addition, 2 cases out of the 10 patients had differentiated nonkeratinizing carcinoma, while the other 8 had undifferentiated nonkeratinizing carcinoma. The control group included 11 males and 2 females, who were aged between 41 and 64 years with a median of 50.5 years. Clinically, 4 out of the 13 patients in control group had stage III NPC and 9 had stage IV NPC. Moreover, 3 cases out of the 13 patients had differentiated nonkeratinizing carcinoma, while the other 10 had undifferentiated non-keratinizing carcinoma. All patients were diagnosed with NPC by histopathological examinations. The general data of the 2 groups were comparable (Table 1). All procedures were approved by the Ethics Committee of Guangxi Medical University. Written informed consents were obtained from all patients or their families.
Table 1.
Clinical characteristics of patients.

The inclusion criteria were: histopathological diagnosis of NPC; no distant metastasis by auxiliary examination; first diagnosis of NPC, without previous radiotherapy or chemotherapy; stage III-IVa according to the 7th Union for International Cancer Control staging standards; existence of NPC foci that were measurable according to Response Evaluation Criteria in Solid Tumors (RECIST) standards; serum creatinine ≤ 1.25 times of UNL, or creatinine clearance rate ≥ 60 mL/minute; serum bilirubin ≤ 1.5 times of UNL, AST (SGOT) and ALT (SGPT) ≤ 2.5 times of UNL, and alkaline phosphatase ≤ 5 times of UNL; serum hemoglobin ≥ 10 gm/dL, platelet count ≥ 100,000/μL, absolute neutrophil count ≥ 1,500/μL; Karnofsky scores > 70; x) estimated overall survival > 6 months. The exclusion criteria were: symptomatic cerebral metastasis, bone marrow metastasis, cognitive disorders, or any distant metastasis; pregnancy or lactation; history of malignant tumors or other diseases; history of radiotherapy, chemotherapy or immunotherapy; severe bone marrow dysfunction; hemorrhagic tendency; drug abuse or alcohol addiction.
During the research period, patients who failed to follow medication plan or complete more than 80% of the plan were also excluded from the study. Patients who did not complete the study due to side effects were not used for curative effect analysis, but were used for side effect analysis. In addition, other patients who had violation of the study protocol were also excluded. The test was terminated if any specific patient had disease progression, unacceptable toxicity, use of other drugs, or unwillingness to continue the study. Before the study, all patients underwent collection of disease history, physical examination, Karnofsky performance status (KPS) scoring, direct or indirect nasopharyngoscopy, nasopharyngeal and neck magnetic resonance imaging (MRI; plain and enhanced scans), chest x-ray or computed tomography (CT), abdominal color Doppler ultrasound/upper abdominal CT, whole body bone scanning, electrocardiogram, blood routine test, liver and kidney function test, and electrolyte examination.
2.2. IMRT
All patients underwent CT scan from the top of the head to 3 cm below clavicle, with a scanning thickness of 3 mm. After scanning, the image data were uploaded to Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA). Gross tumor volume of the nasopharynx (GTVnx), gross tumor volume of the positive cervical lymph node (GTVnd), clinical target volume-1 (CTV1), clinical target volume-2 (CTV2), and planned target volume (PTV; extension from CTV by 3 – 5 mm) were sketched layer by layer. Then, 7030 to 7400 CGy for PTVnx, 6800 to 7000 CGy for PTVnd, 6000 to 6600 CGy for PTV1 and 5000 to 5600 CGy for PTV2 were administered. Eclipse treatment planning system was used to make reverse IMRT plan. Before treatment, 0° and 90°/270° images were taken to confirm no metastasis. Then, 6MV-x-ray irradiation was performed 5 times a week (IX-SN4948; Varian Medical Systems, Palo Alto, CA). On the first day of IMRT, concurrent chemotherapy or endostar-targeted treatments also started.
2.3. Endostar-targeted treatment and chemotherapy
Endostar (15 mg/day; Simcere, Yantai, China) was dissolved in 500 mL saline and infused intravenously for 3 to 4 hours from the first day of chemotherapy. Days 1 to 14 were the first cycle, followed by 7 days of suspension. The patients received a total of 2 cycles. For chemotherapy, cis-dichlorodiamineplatinum (80 mg/m2, d1–2) was administered for 3 to 4 cycles of 21 days. During chemotherapy, antiemetic, live-protective, and stomach-protective measures and hydration were carried out. All patients in experimental group completed 2 cycles of endostar treatment, and 13 patients in control group completed 3 cycles of concurrent chemotherapy.
2.4. Recent efficacy evaluation
Three months after radiotherapy, the recent efficacy of treatment was evaluated using physical examination, nasopharyngeal fiberscope, and MRI. According to RECIST, the efficacy of treatment was classified into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The response rate (RR) was calculated using the rates of CR and PR.
2.5. Adverse reaction evaluation
To evaluate acute adverse reactions every week, National Cancer Institute-Common Toxicity Criteria (NCI-CTC) was employed. Early and late radiation reactions were evaluated according to Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC) standards.
2.6. Follow-ups
Tumor regression and acute adverse reactions were recorded every week during treatment. Three months after radiotherapy, the recent efficacy was evaluated. Within 2 years after treatment, the patients were examined every 3 months. From the 3rd to the 5th years after treatment, the patients were examined every 6 months. After 5 years, the patients were examined annually. During each follow-up, the patients received physical examination, chest radiography, abdominal ultrasound, nasopharyngeal fiberscopy, and laboratory examinations. Late radiation injury was examined using RTOG/EORTC staging standards. Head and neck MRI was performed every 6 months. Biopsy was performed on patients suspected with recurrence. Chest and abdominal CT and bone isotope scan were carried out on patients suspected with distant metastasis. The follow-ups were terminated on April 1, 2016, with a median duration of 36 months, ranging from 27 to 38 months. The parameters for analysis included overall survival (OS), disease-free survival (DFS), local relapse-free survival (LRFS), nodal relapse-free survival (NRFS), distant metastasis-free survival (DMFS), and progression-free survival (PFS). Survival time calculation started on the first day of diagnosis.
2.7. Statistical analysis
All results were analyzed using SPSS 18.0 software (IBM, Armonk, NY). Kaplan–Meier method was used to calculate survival rates. Log-rank test was employed to examine the significance of survival rate differences. COX proportional hazard model was used for univariate and multivariate analyses, as well as the calculation of relative hazard ratio. Multivariate analysis was performed using the backward method. Hematological toxicity and nonhematologic toxicity of the 2 groups underwent χ2 test. Differences with P < .05 were considered statistically significant.
3. Results
3.1. IMRT combined with endostar does not have significantly different recent efficacy compared with IMRT combined with chemotherapy
To evaluate recent efficacy, physical examination, nasopharyngeal fiberscopy, and MRI were performed 3 months after treatments. The data showed that IMRT combined with endostar resulted in a CR rate of 60% (6/10) and a PR rate of 40% (4/10). In addition, IMRT combined with chemotherapy led to a CR rate of 61.5% (8/13) and a PR rate of 38.5% (5/13). There was no statistically significant difference between the 2 groups (χ2 = 0.006, P = .94) (Table 2). The result suggests that IMRT combined with endostar does not have significantly different recent efficacy compared with IMRT combined with chemotherapy.
Table 2.
Recent treatment efficacy [No. of cases (%)].

3.2. IMRT combined with endostar does not have significantly different long-term efficacy than IMRT combined with chemotherapy
To evaluate long-term efficacy, the patients were followed-up for 27 to 38 months with a median of 36 months. For all patients, the 2-year OS rate was 78.9%, with 2 case of local recurrence, 3 cases of distant metastasis and 1 case being dead. The 2-year LRFS, DMFS, and PFS for all patients were 87.3%, 82.1%, and 77.7%, respectively. IMRT combined with endostar and IMRT combined with chemotherapy had 2-year OS rates of 100.0% and 69.6%, respectively, without significant difference between each other (χ2 = 1.446, P = .299). The 2-year LRFS of the 2 groups were 100.0% and 81.3%, respectively, without significant difference between each other (χ2 = 1.000, P = .317). The 2-year DMFS of the 2 groups were 100.0% and 73.5% (χ2 = 1.591, P = .207), respectively. The 2-year PFS of the 2 groups were 100.0% and 67.3% (χ2 = 2.164, P = .141), respectively. However, the cumulative survival curves of OS, LRFS, DMFS, and PFS were separated between the 2 groups (Fig. 1). The results indicate that IMRT combined with endostar does not have significantly different long-term efficacy than radiotherapy combined with chemotherapy.
Figure 1.

Cumulative survival curves after treatment with IMRT combined with endostar compared with radiotherapy combined with chemotherapy. The follow-ups lasted for 27 to 38 months, with a median duration of 36 months. (A) OS curves; (B) LRFS curves; (C) DMFS curves; and (D) PFS curves of the 2 groups. DMFS = distant metastasis-free survival, IMRT = intensity-modulated radiotherapy, LRFS = local relapse-free survival, OS = overall survival, PFS = progression-free survival.
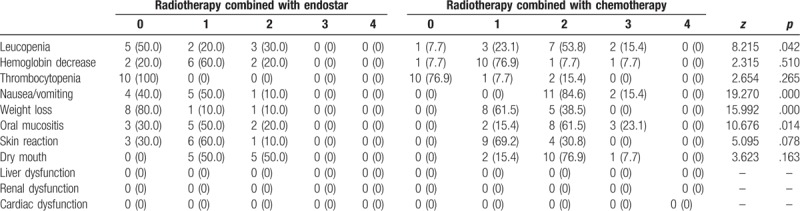
3.3. IMRT combined with endostar results in significantly lower grades of leucopenia, nausea/vomiting, weight loss, and oral mucositis compared with IMRT combined with chemotherapy
To evaluate acute adverse reactions, NCI-CTC was used. The common acute adverse reactions included leucopenia, neutropenia, hemoglobin decrease, nausea/vomiting, weight loss, and acute oral mucositis. No dysfunction of liver, kidney, or heart was observed in either group. In the group of IMRT combined with endostar, the occurrence rates of leucopenia with grades 0, 1 and 2 were 50.0%, 20.0%, and 30.0%, respectively. In the group of IMRT combined with chemotherapy, the occurrence rates of leucopenia with grades 0, 1, 2, and 3 were 7.7%, 23.1%, 53.8%, and 15.4%, respectively. The 2 groups had significant difference in leucopenia rate (Z = 8.215, P = .042). In the group of radiotherapy combined with endostar, the occurrence rates of nausea/vomiting with grades 0, 1, and 2 were 40.0%, 50.0%, and 10.0%, respectively. In the group of IMRT combined with chemotherapy, the occurrence rates of nausea/vomiting with grades 2 and 3 were 84.6% and 15.4%, respectively. The 2 groups had significant difference in nausea/vomiting rate (Z = 19.270, P < .001). In the group of IMRT combined with endostar, the occurrence rates of weight loss with grades 0, 1 and 2 were 80.0%, 10.0%, and 10.0%, respectively. In the group of IMRT combined with chemotherapy, the occurrence rates of weight loss with grades 1 and 2 were 61.5%, and 38.5%, respectively. The 2 groups had significant difference in weight loss rate (Z = 15.992, P < .001). In the group of IMRT combined with endostar, the occurrence rates of oral mucositis with grades 0, 1 and 2 were 30.0%, 50.0% and 20.0%, respectively. In the group of IMRT combined with chemotherapy, the occurrence rates of oral mucositis with grades 1, 2 and 3 were 15.4%, 61.5% and 23.1%, respectively. The 2 groups had significant difference in oral mucositis rate (Z = 10.676, P = .014). In addition, no significant differences were observed in hemoglobin decrease, thrombocytopenia, dry mouth or skin reactions between the 2 groups (P > .05) (Table 3). These results suggest that IMRT combined with endostar results in significantly lower grades of leucopenia, nausea / vomiting, weight loss and oral mucositis compared with IMRT combined with chemotherapy.
Table 3.
Acute adverse reactions in the 2 groups [No. of cases (%)].
3.4. The grades of late adverse reactions of IMRT combined with endostar are not different from those of IMRT combined with chemotherapy
To evaluate late adverse reactions, the parameters such as dry mouth, subcutaneous soft tissue fibrosis, hearing loss, limitation of mouth opening, cranial nerve paralysis, temporal lobe necrosis, and decreased vision were studied 2 years after IMRT. For both groups, no adverse reactions with grades 3 or 4 were observed. There was no significant difference between the 2 groups (P > .05) (Table 4). The result indicates that the grades of late adverse reactions of IMRT combined with endostar are not different from those of IMRT combined with chemotherapy.
Table 4.
Late adverse reactions in the 2 groups [No. of cases (%)].

4. Discussion
It has been reported that tumor growth is related with angiogenesis in 1971.[23,24] O’Reilly et al[25] demonstrate that endostatin inhibits the growth of tumors by inhibiting the proliferation of vascular endothelial cells. Later, Wang et al[26] have shown good efficacy of endostar combined with vinorelbine and cisplatin in the treatment of advanced non-small cell lung cancer. Around 22 patients were analyzed with stage rIII-IVb locally recurrent NPC and found endostar combined with chemoradiotherapy may be effective in decreasing both the incidence of nasopharyngeal mucosal necrosis. Zhou et al[27] report that endostar combined with IMRT significantly inhibits the growth of NPC xenografts in nude mice, probably because endostar reduces vascular endothelial growth factor expression that is necessary for the repair of damaged tumor vascular endothelial cells. Other studies demonstrate that endostar reduces metastatic ability of HNE-1 cells and inhibits vasculogenic mimicry formation in vitro,[28] and that endostar inhibits the formation of tumor vascular endothelial cells of CNE-2 xenograft tumor and tumor growth in nude mice.[29–31]
The results of the present study show that IMRT combined with endostar does not have significantly different recent efficacy compared with IMRT combined with chemotherapy. Although 2-year OS, LRFS, DMFS, and PFS are not significantly different between the 2 groups, but the cumulative survival curves were separated between the 2 groups, indicating that IMRT combined with endostar has better long-term efficacy compared with radiotherapy combined with chemotherapy. This might be due to the limited sample size and short follow-up duration. The mechanisms by which endostar enhances treatment efficacy may be the following: endostar blocks vascular endothelial growth factor that stimulates vascular endothelial cell proliferation, inhibits its apoptosis, promotes blood vessel construction, and facilitates lymphangiogenesis and lymphatic metastasis; endostar improves disordered vascular network, blood circulation, and hypoxia, and enhances the radiation sensitivity of hypoxic cells; endostar facilitates cell cycle redistribution, promoting the effects of endostar and radiotherapy in different cell cycle phases.
The acute adverse reactions during the treatment include leukopenia, neutropenia, hemoglobin decrease, nausea/vomiting, weight loss, and oral mucositis. The most significant acute adverse reaction is nausea/vomiting. For the group treated with IMRT combined with chemotherapy, 5-hydroxytryptamine-3 receptor antagonist was used to stop nausea/vomiting. By contrast, the group treated with IMRT combined with endostar received no antiemetic drugs, but still had reduced degree of nausea/vomiting compared with the other group, suggesting that IMRT combined with endostar had milder acute toxic side effects. In addition, the group treated with IMRT combined with chemotherapy had more severe oral mucositis, and led to eating difficulties that, together with severe nausea/vomiting, caused more severe weight loss. The grade of leucopenia in the group treated with IMRT combined with endostar is significantly different from that in the other group, indicating that IMRT combined with chemotherapy is more likely to cause myelosuppression. After treatment with recombinant human granulocyte colony stimulating factor, the white blood cells in patients with myelosuppression returned to normal. In addition, none of the patients in the study had liver, heart, or kidney dysfunction. The group treated with IMRT combined with chemotherapy used liver protective drugs, which might have alleviated the liver toxicity effect of chemotherapy drugs. In summary, antiangiogenic therapy has been playing important roles in the treatment of malignant tumors. Compared with IMRT combined with chemotherapy, IMRT combined with endostar has similar efficacy but lower acute toxic reactions, which may be helpful in improving the life quality of patients with locally advanced NPC.
Author contributions
Conceptualization: Rensheng Wang.
Data curation: Fangfang Wang, Pingting Zhou.
Formal analysis: Xueyin Liao.
Project administration: Rensheng Wang.
Writing – original draft: Min Kang.
Footnotes
Abbreviations: CR = complete response, CT = computed Tomography, CTV1 = clinical target volume-1, CTV2 = clinical target volume-2, DFS = disease-free survival, DMFS = distant metastasis-free survival, EORTC = European Organization for Research and Treatment of Cancer, GTVnd = gross tumor volume of the positive cervical lymph node, GTVnx = gross tumor volume of the nasopharynx, IMRT = intensity-modulated radiotherapy, KPS = Karnofsky Performance Status, LRFS = local relapse-free survival, NCI-CTC = National Cancer Institute-Common Toxicity Criteria, NPC = nasopharyngeal carcinoma, NRFS = nodal relapse-free survival, OS = overall survival, PD = progressive disease, PDGFR = platelet-derived growth factor receptor, PFS = progression-free survival, PR = partial response, PTV = planned target volume, RR = response rate, RTOG = Radiation Therapy Oncology Group, SD = stable disease, VEGF = vascular endothelial growth factor, VEGFR-2 = vascular endothelial growth factor receptor-2.
This work was supported by the Projects for Research and Development of Medical and Health Appropriate Technology of Guangxi Zhuang Autonomous Region (No. S201415-07), the National Natural Science Foundation of China (No. 81460460, 81360405, 81760542), China Postdoctoral Science Foundation (No. 2016M602918XB), and the Research Foundation of the Science and Technology Department of Guangxi Province, China (No. 2016GXNSFAA380252, No. 2014GXNSFBA118144), Guangxi Medical University Training Program for Distinguished Young Scholars (2017), the Central Government Guide Local Science and Technology Development Projects (ZY18057006), and the Medical Excellence Award Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University.
Disclosures: All authors declare no financial competing interests.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Deng W, Li J, Yu J, et al. Epidemiological characteristics and trends of nasopharyngeal carcinoma deaths in Guangxi. J Appl Prevent Med 2011;17:73–6. [Google Scholar]
- [2].Cooper JS, Lee H, Torrey M, et al. Improved outcome secondary to concurrent chemoradiotherapy for advanced carcinoma of the nasopharynx: preliminary corroboration of the intergroup experience. Int J Radiat Oncol Biol Phys 2000;47:861–6. [DOI] [PubMed] [Google Scholar]
- [3].Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 2005;23:6966–75. [DOI] [PubMed] [Google Scholar]
- [4].Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005;23:6730–8. [DOI] [PubMed] [Google Scholar]
- [5].Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 2003;21:631–7. [DOI] [PubMed] [Google Scholar]
- [6].Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol 2002;20:2038–44. [DOI] [PubMed] [Google Scholar]
- [7].Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2010;102:1188–98. [DOI] [PubMed] [Google Scholar]
- [8].Lin JC, Liang WM, Jan JS, et al. Another way to estimate outcome of advanced nasopharyngeal carcinoma--is concurrent chemoradiotherapy adequate? Int J Radiat Oncol Biol Phys 2004;60:156–64. [DOI] [PubMed] [Google Scholar]
- [9].Kang M, Zhou P, Wei T. The new T staging system for nasopharyngeal carcinoma based on intensity modulated radiation therapy: results of a prospective multicentric clinical study. Int J Radiat Oncol Biol Phys 2016;96:E343. [Google Scholar]
- [10].Zhao H, Zhu C, Gu L. Efficacy of three dimensional conformal radiotherapy and chemotherapy plus adjuvant chemotherapy for locally advanced nasopharyngeal carcinoma. Chin J Radiat Oncol 2011;20:8–9. [Google Scholar]
- [11].Kang M, Long J, Li G. A new staging system for nasopharyngeal carcinoma based on intensity-modulated radiation therapy: results of a prospective multicentric clinical study. Oncotarget 2016;7:15252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee A, Tung SY, Chua DT. Final report of NPC-9901trial on therapeutic gain and late toxicities attributed to concurrent-adjuvant chemotherapy for T1-4N2-3M0 nasopharyngeal carcinoma. Eur J Cancer Suppl 2009;7:471. [Google Scholar]
- [13].Lee A, Tung SY, Chan AT. Final report of NPC-9902 trial on therapeutic gain and late toxicities by concurrent-adjuvant chemotherapy and/or accelerated fractionation for T3-4N0-1M0 nasopharyngeal carcinoma. Eur J Cancer Suppl 2009;7:22. [Google Scholar]
- [14].Lin S, Lu JJ, Han L, et al. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer 2010;10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kwong DL, Sham JS, Au GK, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J Clin Oncol 2004;22:2643–53. [DOI] [PubMed] [Google Scholar]
- [16].Guan Y, Li A, Xiao W, et al. The efficacy and safety of endostar combined with chemoradiotherapy for patients with advanced, locally recurrent nasopharyngeal carcinoma. Oncotarget 2015;6:33926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ling Y, Yang Y, Lu N, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun 2007;361:79–84. [DOI] [PubMed] [Google Scholar]
- [18].Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 2011;91:1071–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peng F, Xu Z, Wang J, et al. Recombinant human endostatin normalizes tumor vasculature and enhances radiation response in xenografted human naso-pharyngeal carcinoma models. PLoS One 2012;7:e34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun Y, Wang JW, Liu Y, et al. Results of phase III trial of rh-endostatin (YH-16) in advanced non-small lung cancer (NSCLC) patients. J Clin Oncol 2005;23:7138. [Google Scholar]
- [21].Han B, Xiu Q, Wang H, et al. A multicenter, randomized,double-blind, placebo-controlled study to evaluate the efficacy of paclitaxel-carboplatin alone or with endostar for advanced non-small cell lung cancer. J Thorac Oncol 2011;6:1104–9. [DOI] [PubMed] [Google Scholar]
- [22].Zhou JF, Bai CM, Wang YZ, et al. Endostar combined with chemotherapy for treatment of metastatic colorectal and gastric cancer:a pilot study. Chin Med J (Engl) 2011;124:4299–303. [PubMed] [Google Scholar]
- [23].Folkman J. Tumor angiogenesis and tissue factor. Nat Med 1996;2:167–8. [DOI] [PubMed] [Google Scholar]
- [24].Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–31. [DOI] [PubMed] [Google Scholar]
- [25].O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997;88:277–85. [DOI] [PubMed] [Google Scholar]
- [26].Wang J, Sun Y, Liu Y, et al. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi 2005;8:283–90. [DOI] [PubMed] [Google Scholar]
- [27].Zhou J, Wang L, Xu X, et al. Antitumor activity of Endostar combined with radiation against human nasopharyngeal carcinoma in mouse xenograft models. Oncol Lett 2012;4:976–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zeng D, Lin Y, Wang H, et al. Inhibitory effect of rh-endostatin on vasculogenic mimicry in nasopharyngeal cancer cell line HNE-1 in vitro. Chin Clin Oncol 2008;6:481–4. [Google Scholar]
- [29].Wen QL, Meng MB, Yang B, et al. Endostar, a recombined humanized endostatin, enhances the radioresponse for human nasopharyngeal carcinoma and human lung adenocarcinoma xenografts in mice. Cancer Sci 2009;100:1510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang Z, Du Y, Xie Y. Inhibition of nasopharyngeal carcinoma CNE-2 xenograft in nude mice by recombinant human endostatin combined with radiotherapy and analysis of its mechanism. Chin J Can Prevention Treatment 2012;4:259–62. [Google Scholar]
- [31].Keyes KA, Mann L, Sherman M, et al. LY317615 decreases plasma VEGF levels in human tumor xenograft-bearing mice. Cancer Chemother Pharmacol 2004;53:133–40. [DOI] [PubMed] [Google Scholar]


