Abstract
Introduction:
Synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome is an autoinflammatory disorder without standardized treatment. Janus kinase (JAK) inhibitors can block a range of cytokines and might possess significant anti-inflammatory activity. Here, we report the first case of efficacious treatment of refractory SAPHO syndrome with the JAK inhibitor tofacitinib.
Case presentation:
A 44-year-old woman presented with arthralgia in the right wrist and complained of having difficulty in doing housework. Symptoms were unresponsiveness to nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, and tumor necrosis factor inhibitors. A diagnosis of SAPHO syndrome was made based on previous dermatological and osteoarticular manifestations and bone scintigraphy findings. Oral treatment with tofacitinib at 5 mg twice daily in combination with the basic methotrexate treatment was initiated. After 4 weeks of using tofacitinib, the patient reported marked improvement of symptoms and also reported being competent in completing housework.
Conclusions:
The efficacy of JAK inhibitors in treating refractory SAPHO syndrome should be noted.
Keywords: inflammation, JAK inhibitor, SAPHO syndrome
1. Introduction
Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome, a constellation of clinical features including inflammatory osteoarticular lesions and dermatological disorders, has been described since 1987.[1,2] No evidence-based treatment recommendation has been proposed because of the rarity of SAPHO syndrome. The current treatment strategy varies from nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and disease-modifying antirheumatic drugs (DMARDs) to biologics, bisphosphonates, and antibiotics based on case reports, case series, and expert judgment.[1,3]
Here, we present a case of SAPHO syndrome with palmoplantar pustules and arthralgia. Although pustules remitted after use of DMARDs, arthralgia was refractory to NSAIDs, DMARDs, and tumor necrosis factor (TNF) inhibitors. Tofacitinib, a small-molecule inhibitor of Janus kinases (JAKs), showed good efficacy.
2. Case report
Written informed consent for publication of this case report was obtained from the patient, and the study was approved by the Ethics Committee of Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences.
A 44-year-old woman presented with arthralgia in the right wrist and complained of having difficulty in doing housework. Physical examination revealed a hot and swollen right wrist with tenderness and limited movement. No pustule was seen. Blood analysis showed erythrocyte sedimentation rate (ESR) 97 mm/h [reference range, 0–20 mm/h] and augmented hypersensitivity C-reactive protein (hsCRP) 5.50 mg/L [reference range, 0–3.00 mg/L]. Her past medical history was unremarkable. Her father developed osteoarthralgia as well as pustules on the palms of his hands and soles of his feet when he was a youth; the pustules disappeared spontaneously 10 years previously, but osteoarthralgia persisted.
The patient suffered from recurrent painless pustules on the palms of her hands and soles of her feet for 5 years before this admission. Palmoplantar pustulosis was diagnosed. Six months before this admission, she noticed pain in her anterior chest wall and right wrist. ESR and hsCRP were 19 mm/h and 3.80 mg/L, respectively. Complete blood count, liver, and renal function were all within the normal ranges. Rheumatoid factor (RF), antinuclear antibody (ANA), and human leukocyte antigen B27 (HLA-B27) measurements were negative. Whole-body bone scintigraphy (WBBS) using 99Tc-MDP revealed multiple lesions with increased tracer accumulation in the bilateral sternoclavicular joints, bilateral first anterior ribs, and right wrist (Fig. 1A). Magnetic resonance imaging (MRI) of the right wrist showed synovial inflammation, synovial hypertrophy, and joint effusion (Fig. 1B). A diagnosis of SAPHO syndrome was made according to the criteria proposed by Kahn and Khan.[4] Treatment with NSAIDs, methotrexate (MTX) (10 mg/wk), and Tripterygium wilfordii hook f (TwHF) (20 mg 3 times/d) was initiated. Pain in the anterior chest wall and pustules remitted, but the symptoms involving the right wrist remained.
Figure 1.
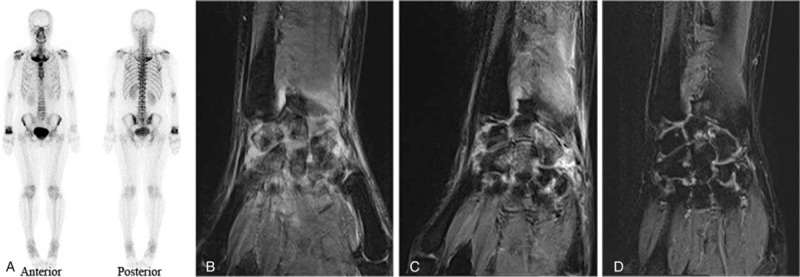
Imaging examination results. (A) 99Tc-MDP whole-body bone scintigraphy demonstrated lesions in bilateral sternoclavicular joints, bilateral first anterior ribs, and the right wrist. (B) MRI of the right wrist (T2WI sequence) was obtained when the patient was diagnosed with SAPHO syndrome. Synovial inflammation, synovial hypertrophy, and joint effusion were revealed. (C) MRI of the right wrist (T2WI sequence) obtained after treatment with a TNF inhibitor but before treatment with tofacitinib. (D) MRI of the right wrist (T2WI sequence) obtained after 12-week use of tofacitinib. Synovial inflammation, synovial hypertrophy, and joint effusion were improved. MRI = magnetic resonance imaging, SAPHO = synovitis, acne, pustulosis, hyperostosis, and osteitis, TNF = tumor necrosis factor.
On admission, one intramuscular injection of glucocorticoids (Diprospan, 1 mL) provided transient relief of arthralgia. A combination of MTX (10 mg/wk), TwHF (20 mg 3 times/d), and hydroxychloroquine (0.2 g twice/d) was initiated.
Seven months later, symptoms in the right wrist recurred. ESR and hsCRP were 29 mm/h and 13.59 mg/L, respectively. Biologics were considered. The TNF inhibitor etanercept was administered at 50 mg/wk for 2 weeks in combination with MTX (10 mg/wk). Nevertheless, expectative remission of symptoms, serum inflammatory parameters, and imaging findings did not appear (Fig. 1C). The dosage, frequency, and duration of different treatments after diagnosis of SAPHO syndrome are further illustrated in Figure 2.
Figure 2.
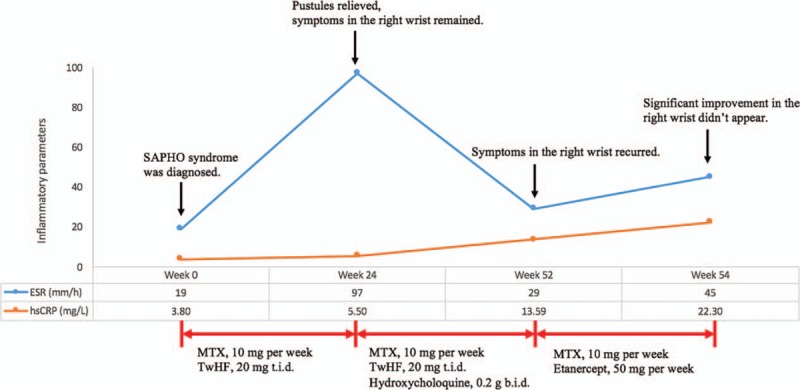
Dosage, frequency, and duration of different treatments before initiation of tofacitinib treatment. NSAIDs were used as needed. One dose of glucocorticoids (Diprospan, 1 mL) was injected intramuscularly in week 24. ESR, reference range 0 to 20 mm/h; hsCRP, reference range 0 to 3.00 mg/L. ESR = sedimentation rate, hsCRP = augmented hypersensitivity C-reactive protein, SAPHO = synovitis, acne, pustulosis, hyperostosis, and osteitis, NSAIDs = nonsteroid anti-inflammatory drugs, MTX = methotrexate, TwHF = Tripterygium wilfordii hook f, t.i.d. = 3 times per day; b.i.d. = twice per day.
Therefore, in combination with MTX treatment, oral administration of tofacitinib, a small-molecule inhibitor of JAKs, was tentatively initiated at 5 mg twice daily. After 4 weeks of using tofacitinib, the patient reported marked improvement of symptoms and being competent in completing housework. The patient's score on the visual analogue scale (VAS) decreased (Fig. 3). ESR and hsCRP, IL-6 and TNFα levels decreased to nearly normal ranges (Fig. 3). After 12 weeks of using tofacitinib, MRI of the right wrist demonstrated amelioration (Fig. 1D). No adverse effects, such as infection, anemia, or leukopenia, were noticed.
Figure 3.
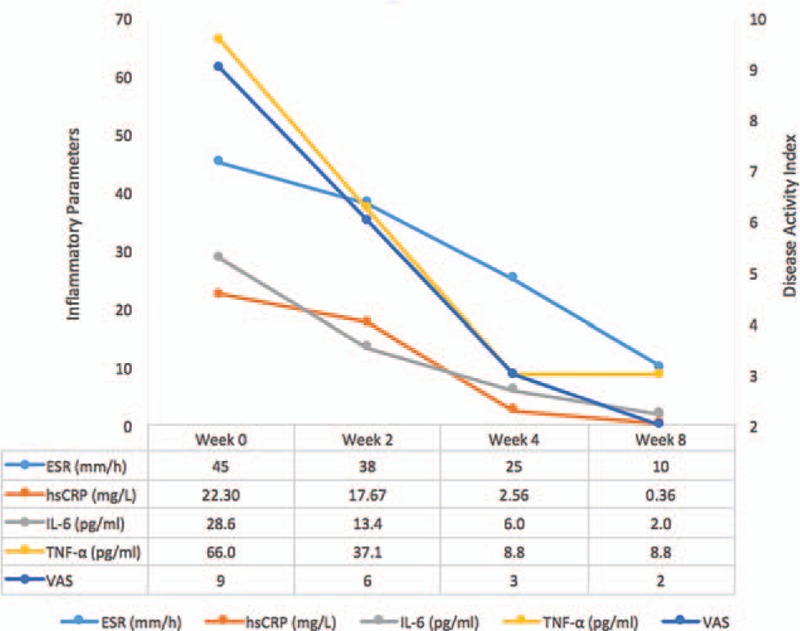
Serum inflammatory parameters and disease activity index decreased after administration of tofacitinib in combination with MTX treatment. ESR, reference range 0 to 20 mm/h; hsCRP, reference range, 0 to 3.00 mg/L; IL-6, reference range, 0 to 5.9 pg/mL; TNFα, reference range 0 to 8.1 pg/mL. ESR = sedimentation rate, hsCRP = augmented hypersensitivity C-reactive protein, IL-6 = interleukin-6; TNF = tumor necrosis factor, VAS = visual analogue scale.
3. Discussion
SAPHO syndrome is recognized as an autoinflammatory disorder that is associated with increased release of multiple inflammatory cytokines and global neutrophil activition[5,6]; among these changes, the overexpression of the proinflammatory cytokines TNFα, IL-8, IL-17, and IL-1β was well documented.[3]
Treatment mainly aims at relief of symptoms and protection from disease exacerbation.[1] To the best of our knowledge, no randomized controlled trials have been conducted to assess the efficacy of different therapeutic methods.[1,3] Treatment was experience-based and expert-based, learning from psoriatic arthritis (PsA) and rheumatoid arthritis (RA). NSAIDs and analgesics were the first-line treatment for pain management.[3] In patients who are unresponsive to NSAIDs and analgesics, short-term use of corticosteroids during flare-ups can be temporarily effective and allow slow-active agents, such as DMARDs, to be adjusted.[3,7] The long-term use of DMARDs has been commonly accepted and reported to be beneficial in some patients.[1,3] For SAPHO cases refractory to conventional drugs, biotherapy, such as TNF inhibitor treatment, has been the next therapeutic option.[1,3] Use of anti-IL-1 agents[3,8] and IL-23- or IL-17-targeted therapies[9] has also been reported. There were also treatments with bisphosphonates or antibiotics.[1,3]
In our case, the patient was primarily bothered by the predominant and recurrent symptoms of a single peripheral joint, as well as the elevation of serum inflammatory parameters. Treatment was referred to the therapeutic strategies of SAPHO syndrome and RA.[7,10–12] However, in the reported patient, after sequential administration of NSAIDs, DMARDs, glucocorticoids, and TNF inhibitors, there was no substantial amelioration of symptomatic, laboratory, or imaging parameters. Then, treatment with the small-molecule inhibitor of JAKs was attempted.
JAKs are crucial intracellular signaling mediators from the type I and type II cytokine receptor superfamily.[12] This superfamily contains different receptors bound by >50 cytokines, interleukins, interferons, colony-stimulating factors, and hormones.[12] Blocking the downstream intracellular JAKs can block the upstream action of a range of cytokines, including IL-2, IL-4, IL-7, IL-9, IL-15, IL-21, and IL-6.[10,12,13] Therefore, JAK inhibitors might demonstrate greater anti-inflammatory activity than biologics that can block only one kind of cytokine.[10,12,13] Recently, JAK inhibitors have shown great efficacy in the treatment of inflammation-derived diseases such as RA, psoriasis, and inflammatory bowel disease.[12]
In our case, inspired by the efficacy seen in pilot therapy of RA[12] and PsA,[14] tofacitinib, a first-generation small-molecule nonspecific JAK inhibitor that can inhibit JAK1 and JAK3, as well as JAK2 to a lesser degree, was selected.[12,15]
Precautions should be taken when using JAK inhibitors, based on their biological functions. The greatest concerns regarding adverse effects of using JAK inhibitors are increased risk of infection, anemia, and leukopenia.[12] Some other concerns are still under investigation, for example, risk of cardiovascular disease, gastrointestinal perforation, and cancer.[12]
4. Conclusion
To our knowledge, this is the first case report of treating SAPHO syndrome with a JAK inhibitor. The efficacy of JAK inhibitors in treating a refractory SAPHO case indicated a complicated inflammatory process. JAK inhibitors could be an option for SAPHO syndrome, especially in refractory cases.
Author contributions
Conceptualization: Qiao Yang, Yumo Zhao, Chen Li, Wen Zhang.
Formal analysis: Qiao Yang.
Writing – original draft: Qiao Yang, Yaping Luo.
Writing – review and editing: Qiao Yang, Yumo Zhao, Chen Li, Weixin Hao, Wen Zhang.
Footnotes
Abbreviations: ANA = antinuclear antibody, DMARDs = disease-modifying antirheumatic drugs, ESR = sedimentation rate, HLA-B27 = human leukocyte antigen B27, hsCRP = augmented hypersensitivity C-reactive protein, JAK = Janus kinase, MRI = magnetic resonance imaging, MTX = methotrexate, NSAIDs = nonsteroid anti-inflammatory drugs, PsA = psoriatic arthritis, RA = rheumatoid arthritis, RF = rheumatoid factor, SAPHO = synovitis, acne, pustulosis, hyperostosis, and osteitis, TNF = tumor necrosis factor, TwHF = Tripterygium wilfordii hook f, VAS = visual analogue scales, WBBS = whole-body bone scintigraphy.
QY, YZ, and CL equally contributed to this study.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Nguyen MT, Borchers A, Selmi C, et al. The SAPHO syndrome. Semin Arthritis Rheum 2012;42:254–65. [DOI] [PubMed] [Google Scholar]
- [2].Chamot AM, Benhamou CL, Kahn MF, et al. [Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases]. Rev Rhum Mal Osteoartic 1987;54:187–96. [PubMed] [Google Scholar]
- [3].Firinu D, Garcia-Larsen V, Manconi PE, et al. SAPHO syndrome: current developments and approaches to clinical treatment. Curr Rheumatol Rep 2016;18:35. [DOI] [PubMed] [Google Scholar]
- [4].Kahn MF, Khan MA. The SAPHO syndrome. Baillieres Clin Rheumatol 1994;8:333–62. [DOI] [PubMed] [Google Scholar]
- [5].Stern SM, Ferguson PJ. Autoinflammatory bone diseases. Rheum Dis Clin North Am 2013;39:735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hurtado-Nedelec M, Chollet-Martin S, Nicaise-Roland P, et al. Characterization of the immune response in the synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. Rheumatology (Oxford) 2008;47:1160–7. [DOI] [PubMed] [Google Scholar]
- [7].Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- [8].Wendling D, Prati C, Aubin F. Anakinra treatment of SAPHO syndrome: short-term results of an open study. Ann Rheum Dis 2012;71:1098–100. [DOI] [PubMed] [Google Scholar]
- [9].Wendling D, Aubin F, Verhoeven F, et al. IL-23/Th17 targeted therapies in SAPHO syndrome. A case series. Joint Bone Spine 2017;84:733–5. [DOI] [PubMed] [Google Scholar]
- [10].Mocsai A, Kovacs L, Gergely P. What is the future of targeted therapy in rheumatology: biologics or small molecules? BMC Med 2014;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van Vollenhoven RF. Rheumatoid arthritis in 2012: progress in RA genetics, pathology and therapy. Nat Rev Rheumatol 2013;9:70–2. [DOI] [PubMed] [Google Scholar]
- [12].Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 2017;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev 2009;228:273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017;377:1525–36. [DOI] [PubMed] [Google Scholar]
- [15].Riese RJ, Krishnaswami S, Kremer J. Inhibition of JAK kinases in patients with rheumatoid arthritis: scientific rationale and clinical outcomes. Best Pract Res Clin Rheumatol 2010;24:513–26. [DOI] [PubMed] [Google Scholar]


