Abstract
Lung cancer is the most common cause of cancer-associated death worldwide. Postoperative relapse and subsequent metastasis result in a high mortality rate, even in early stage lung cancer. MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression at the post-transcriptional level and are frequently dysregulated in various cancers. The aim of this study was to identify recurrence-associated miRNAs in early stage lung cancer. To screen for differentially expressed miRNAs related to postoperative recurrence, miRNA microarray data derived from stage I lung adenocarcinoma formalin-fixed paraffin-embedded (FFPE) tissue samples (n = 6) and publically available the Cancer Genome Atlas (TCGA) data were analyzed. An independent sample (n = 29) was used to validate candidate miRNAs by quantitative real-time polymerase chain reaction (qRT-PCR). In miRNA expression profiling, we identified 60 significantly dysregulated miRNAs in the relapsed group. Additionally, 20 dysregulated miRNAs were found using TCGA data set. Three miRNAs (let-7g-5p, miR-143-3p, and miR-374a-5p) were associated with postoperative recurrence in both microarray and TCGA data sets. All 3 candidate miRNAs were validated in the independent cohort of stage I adenocarcinoma by qRT-PCR. We discovered 3 recurrence-associated miRNAs of stage I lung adenocarcinoma samples using FFPE tissue, which showed possible clinical utility as biomarkers predicting recurrence after curative surgery. Further investigation of the functional properties of these miRNAs is needed.
Keywords: adenocarcinoma, lung cancer, microRNA, recurrence, stage
1. Introduction
Primary lung cancer is the leading cause of cancer mortality worldwide.[1] Death due to lung cancer is primarily due to recurrence or distant metastasis. After curative surgery of early non-small cell lung carcinoma, the possibility of recurrence and overall prognosis are evaluated primarily by assessment of histopathologic characteristics such as tumor size, histologic type, differentiation, and lymph-vascular or pleural invasion.[2] According to the National Comprehensive Cancer Network, patients with lung adenocarcinoma advanced beyond stage II should receive adjuvant chemotherapy and/or radiotherapy after curative surgery (https://www.nccn.org/). However, guidelines for patients with stage I adenocarcinoma are not clear yet, and only high-risk patients derive substantial benefit from adjuvant therapy. Therefore, biomarkers predicting recurrence in early lung cancer are needed to improve management.
MicroRNAs (miRNAs) are short (∼22 nucleotides) non-protein-coding RNAs first discovered in 1993.[3] MiRNAs negatively regulate gene expression either by suppressing mRNA translation or by causing mRNA degradation at the post-transcriptional level.[3] They have been found to be important in physiologic processes including inflammation, metabolism, differentiation, cell-cycle regulation, and apoptosis. High throughput analyses have shown that miRNA expression is commonly deregulated in cancer tissue compared to normal tissue.[4] This dysregulation is associated with the development and progression of malignant tumors with the miRNAs functioning as oncogenes or tumor suppressor genes in various human cancers.[5] Cancer-related miRNAs have a role in almost all hallmarks of cancer such as cell proliferation, apoptosis, genomic instability, angiogenesis, immune response, invasion, and metastasis.[6]
The RNA expression analysis using formalin-fixed paraffin-embedded (FFPE) samples is limited by the lower RNA quality than that obtained from fresh frozen samples.[7] However, miRNAs are not affected by formalin fixation-induced cross-linking, and this can result in FFPE miRNA expression levels similar to those found in fresh-frozen tissue even though FFPE samples exhibit near total degradation of mRNA.[8] Several other studies have shown that miRNAs are expressed at reliable levels in FFPE tissue compared with fresh frozen samples.[9–16] Tumor specimens are routinely stored as FFPE blocks, and these can be linked with clinical follow-up information. Therefore, a study can be conducted using FFPE tissue to evaluate miRNA as a new diagnostic, prognostic, and predictive biomarker.
In this study, we identified miRNAs associated with recurrence of early lung adenocarcinoma after curative surgery using archived FFPE specimens.
2. Materials and methods
2.1. Patients and tissue specimens
For miRNA expression profiling, 6 patients with relapsed or recurrent-free stage I lung adenocarcinoma after curative surgical excision at Hanyang University Hospital in Korea were enrolled. To validate the miRNA profiling data, 29 independent patients with stage I lung adenocarcinoma who underwent primary surgical treatment were enrolled in the study. This study was approved by the Institutional Review Board of Hanyang University Hospital (IRB file no. 2016-07-038).
2.2. RNA extraction
Total RNA was extracted from FFPE tumor tissues with the miRNeasy FFPE kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. The concentration of RNA was evaluated using a NanoDrop 2000 (NanoDrop Technologies, Waltham, MA), and RNA quality was assessed by an Agilent 2100 bioanalyzer (Agilent Technologies, CA). Hematoxylin and eosin-stained slides were reviewed to identify areas of tumor with minimal necrosis and non-neoplastic lung parenchyme. Slides and blocks were aligned, and the non-neoplastic areas were trimmed out. Three or four 10-μm-thick tissue sections were obtained from each of the trimmed FFPE blocks.
2.3. MiRNA microarray and data analysis
The MiRNA expression profiling was performed using the miRCURY LNA microRNA array kit (Exiqon, Vedbaek, Denmark) according to the manufacturer's protocol. This array contains 3100 capture probes covering human miRNAs in miRBase v. 19.0. Slides were scanned using an Agilent C scanner (Agilent Technologies). Next, scanned images were imported into GenePix Pro 5.1 software (Axon Instruments, Foster City, CA) for grid alignment and data extraction. Expressed data were normalized using quantile normalization. After normalization, miRNAs that were significantly differentially expressed between the 2 groups were identified through fold changes (>1.5) and P-values (<.05). The sample number was limited; thus, we did not perform a multiple hypotheses testing, such as false discovery rate.
2.4. The Cancer Genome Atlas miRNA data set
Level 3 miRNA-seq isoform quantification data and corresponding clinical data for 450 lung adenocarcinoma specimens obtained using illumine HiSeq were downloaded from The Cancer Genome Atlas (TCGA) data portal (https://tcga-data.nci.nih.gov/tcga/tcgaHome2.jsp). We only selected patients with stage I lung adenocarcinoma with follow-up data, resulting in inclusion of 277 cases. The patients consisted of 216 Caucasians, 27 Black or African Americans, 6 Asians, and 28 unknowns. Mean miRNA expression more than 10 reads per kilobase per million mapped reads (RPKM) in each group was used to calculate fold changes and P-values.
2.5. Quantitative real-time polymerase chain reaction
To convert RNA into complimentary DNA, we used a Universal cDNA synthesis kit (Exiqon) according to the manufacturer's protocol. Expression of let-7g-5p, miRNA-143-3p, and miRNA-374a-5p was measured by quantitative real-time polymerase chain reaction (qRT-PCR) using a specific primer set (microRNA LNA PCR primer set; Exiqon) and ExiLENT SYBR Green Master mix (Exiqon). Primers used in the experiments are listed in Table 1. Amplification was performed using a CFX96 thermocycler (Bio-Rad, Hercules, CA). PCR parameters were as follows: 95°C for 15 minutes, followed by 45 cycles of 95°C for 10 seconds and 60°C for 1 minute. Melting curve analysis was performed at the end of the PCR cycle. The expression level of each miRNA was normalized using U6 small nuclear RNA (RNU6B), and relative expression was calculated with the 2-ΔΔCt method.
Table 1.
MicroRNA primer name, miRBase 19.0 ID, and sequence information.

2.6. Statistical analysis
Statistical analysis was performed using SPSS 21.0 software for Windows (SPSS Inc, Chicago, IL). Two groups were compared using Student t test. P-values <.05 were considered statistically significant.
3. Results
3.1. Identification of recurrence-associated miRNAs in early lung adenocarcinoma by microarray analysis
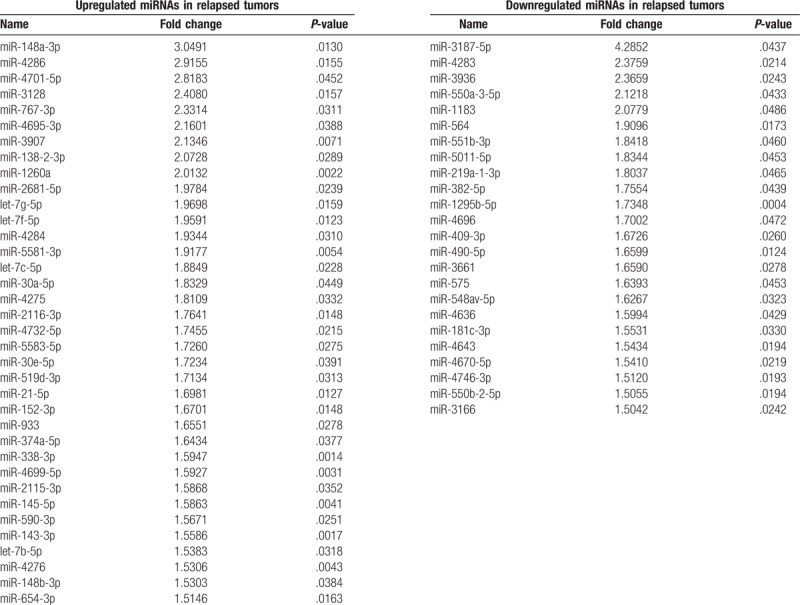
Microarray analysis was used to identify recurrence-associated miRNAs in lung adenocarcinoma. We compared the miRNA expression profiles of 3 stage I lung adenocarcinomas with recurrence within 2 years and 3 relapse-free stage I cases with a follow-up interval of more than 5 years after curative surgery. None of the 6 patients received adjuvant therapy after surgery. On histologic examination, none of the cases had pleural, lymphovascular, or perineural invasion (Table 2). The mean follow-up time of patients without recurrence was 95 months (range, 93–96 months). That of relapsed patients was 9.67 months (range, 5–14 months). Through comparison of these 2 profiles, we identified 36 upregulated and 24 downregulated miRNAs in relapsed tumors based on a fold change >1.5 and a P-value <.05 (Table 3).
Table 2.
Summary of clinical characteristics of the 6 cases for which microarray analyses were performed.

Table 3.
List of differentially expressed microRNAs (miRNAs) between recurrent and non-recurrent tumors based on microarray analysis (fold change >1.5, P-value <.05).
3.2. Identification of recurrence-associated miRNAs in early lung adenocarcinoma using TCGA data set
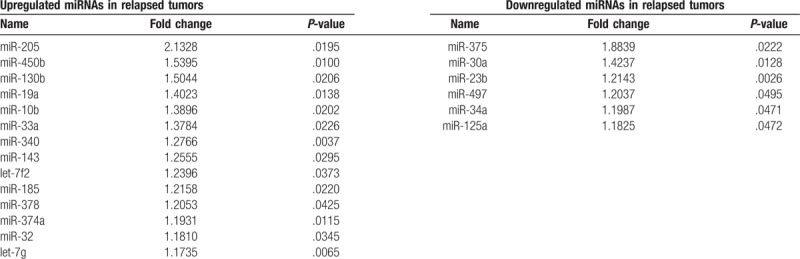
To further assess changes in miRNA expression profiles associated with recurrence, we used publically available miRNA expression and clinical data from TCGA. We identified 277 cases with stage I lung adenocarcinoma, including 68 cases with recurrence and 209 cases without recurrence (Table 4). The mean follow-up time of patients without recurrence was 462.66 days (range, 0–6812 days). That of relapsed patients was 964.72 days (range, 18–4961 days). Comparing these 2 groups, we identified 14 miRNAs that were significantly upregulated and 6 miRNAs that were significantly downregulated (Table 5) in the 68 cases with recurrence.
Table 4.
Summary of clinical characteristics of stage I lung adenocarcinoma in the The Cancer Genome Atlas data set.
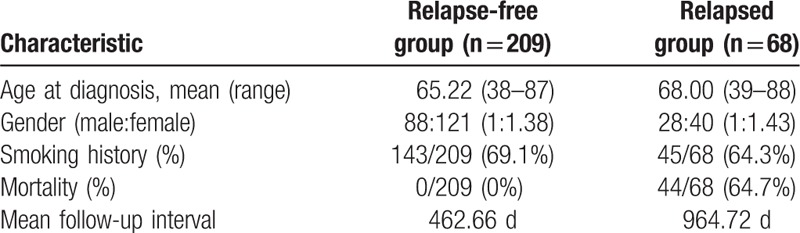
Table 5.
List of differentially expressed microRNAs (miRNAs) between recurrent and non-recurrent tumors in the The Cancer Genome Atlas data set (read per kilobase per million mapped reads >10, P-value <.05).
3.3. Validation of candidate miRNAs by qRT-PCR
We selected 3 candidate miRNAs (let-7g, miR-143, and miR-374a) identified in both our microarray and TCGA data sets. We performed qRT-PCR to validate the expression of the 3 candidate miRNAs in independent tumor tissues. Twenty-nine cases with stage I lung adenocarcinoma were used for validation. Thirteen of the 29 patients had local recurrence or distant metastasis, while the remaining 16 patients had no recurrence. None of the patients received neoadjuvant therapy (Table 6). The mean follow-up time of patients without recurrence was 1654.12 days. That of relapsed patients was 833.77 days. We found that the expression patterns of candidate miRNAs were consistent with the microarray data (Fig. 1). The relative expression of let-7g-5p, miR-143-3p, and miR-374-5p was significantly upregulated in relapsed tumors.
Table 6.
Summary of clinical characteristics of stage I lung adenocarcinoma samples used in the validation study.
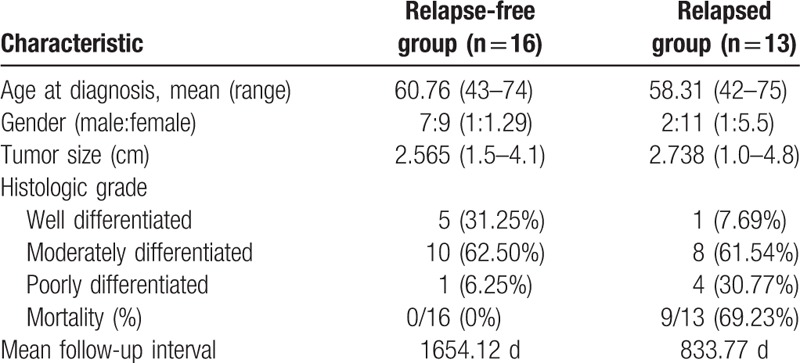
Figure 1.
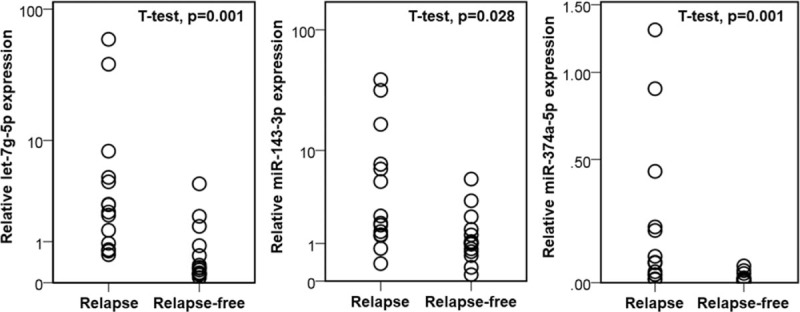
Validation of candidate microRNAs by real-time polymerase chain reaction. Candidate microRNAs (Let-7g-5p, miR-143-3p, and miR-374-5p) are significantly upregulated in stage I lung adenocarcinoma with recurrence compared to relapse-free tumors.
4. Discussion
Screening for lung cancer with low-dose computed tomography has increased the detection of early stage lung cancer, which is feasible to completely resect.[17] However, the relapse rate of stage I non-small cell lung cancer (NSCLC) is nearly 35%, even though patients with stage I NSCLC have a better prognosis than those with higher stage NSCLCs, with a 5-year survival rate around 80%.[18] Recurrence is the most common cause of disease progression and mortality due to NSCLC after curative surgery. Previous studies have described the clinicopathologic and molecular parameters predicting recurrence in NSCLC after complete resection, which include histologic subtype, differentiation, lymphovascular invasion, serum carcinoembryonic antigen level, standard uptake value on positron emission tomography, and various cancer-specific molecular alterations.[19] These efforts to identify early stage patients at high risk of recurrence could allow stratification and selection of patients who need adjuvant therapy and closer surveillance after surgical treatment. We hypothesized that recurrent stage I lung adenocarcinomas have different miRNA expression patterns than relapse-free tumors and that differentially expressed miRNAs could be used as biomarkers to predict recurrence. Our microarray profiling data showed that 60 miRNAs were differentially expressed between relapsed and non-relapsed stage I lung adenocarcinoma tumors. In addition, we used the publicly available TCGA data set with miRNA sequencing results and found 24 recurrence-related miRNAs in stage I lung adenocarcinomas. Three candidate miRNAs (let-7g-5p, miR-143-3p, and miR-374a-5p) were observed in both the microarray and TCGA data sets, and these miRNAs were further validated by qRT-PCR in an independent sample set.
Several previous studies have reported specific miRNAs or expression signatures associated with recurrence of lung cancer. Patnaik et al evaluated the expression of 752 miRNAs in 77 cases of stage IA or IB NSCLC (51 adenocarcinomas and 26 squamous cell carcinomas).[20] In microarray profiling, 130 (47%) of the 279 expressed miRNAs were found to be differentially expressed, and 6 miRNA-based classifiers predicting recurrence with an accuracy of 70% were identified. The 6 miRNA classifiers were miR-200b, miR-30c-1, miR-510, miR-630, miR-657, and miR-146b-3p.[20] Lu et al used 357 FFPE tissues to correlate miRNA expression and prognosis in stage IA or IB NSCLCs.[21] They identified 2 miRNA expression signatures associated with prognosis. The first signature consisted of 34 miRNAs obtained from 357 stage I NSCLCs without regard to cancer subtype, whereas the second one consisted of 27 miRNAs obtained from 189 stage I lung adenocarcinomas. Then, using an independent cohort of 170 stage I NSCLCs, mostly adenocarcinomas, they validated both signatures.[21] For adenocarcinoma only, miRNAs predicting recurrence were miR-615-5p, miR-34b, miR-1248, miR-34c-3p, miR-512-3p, miR-34b∗, miR-934, miR-572, and miR-662.[21] Skrzypski et al performed microarray analysis and qRT-PCR to evaluate miRNA expression in 273 stage I-IIIA NSCLCs (184 squamous cell carcinomas and 89 adenocarcinomas).[22] Six miRNAs were differentially expressed in the not-relapsed vs relapsed groups: miR-10b, miR-662, miR-502-3p, miR-192∗, miR-192, and miR-128. Of these 6 miRNAs, miR-662, miR-192∗, and miR-192 were confirmed to be associated with relapse in the independent squamous cell carcinoma cohort; however, none of these miRNAs were prognostic in lung adenocarcinoma patients.[22] Edmonds and Eischen examined miRNA expression in fresh frozen tissue samples from stage IA or IB NSCLC patients with or without recurrence using a qPCR array and qRT-PCR.[23] The most significant differences in mRNA expression for recurrent tumors compared to non-recurrent tumors were decreases in miR-106b∗, miR-187, miR-205, miR-449b, and miR-774∗ and increases in miR-151-3p, let-7b, miR-215, miR-520b, and miR-512-3p.[23] MiRNAs differentially expressed between the recurrence and relapse-free groups varied among these previous studies, with only 2 miRNAs (miR-512-3p and miR-662) found in common; however, these 2 miRNAs were not identified in the current study. Possible explanations include different proportions of NSCLC histologic subtypes, stages, and different ethnicities. Compared with previously published studies, our discovery and validation studies only included patients with T1 tumors with an adenocarcinoma histology.
Dysregulation of the candidate miRNAs identified in our study (let-7g-5p, miR-143-3p, and miR-374a-5p) has been investigated in various human cancers. Overexpression of let-7g was associated with poor prognosis in non-small lung cancer.[24] However, another study using lung cancer cell lines revealed that let-7g repressed migration and acted as a tumor suppressor.[25] In gastric cancer cells, let-7g was reported to induce sensitivity to oxidative stress by indirect repression of DNA damage responsive and served as a tumor suppressor gene.[26] In breast cancer tissues and cell lines, low expression of let-7g was associated with invasion and metastasis, further supporting its role as a tumor suppressor gene.[27] MiR-143 levels have been reported to be lower in bladder cancer tissue than normal tissue.[28,29] However, upregulation of miR-143 is associated with advanced T category and high histologic grade.[28] Several studies using ovarian epithelial cancer tissues, a colorectal cancer cell line, non-small lung cancer cell lines, and prostate cancer tissue and cell lines have revealed that miR-143 inhibits migration, invasion, and cell growth; induces apoptosis; and serves as a tumor suppressor.[30–33] Upregulation of miR-374a inhibited lung adenocarcinoma cell proliferation and invasion by targeting transforming growth factor alpha (TGF-α).[34] However, another series of studies reported the opposite result. Xu et al found that miR-374a contributed to cell proliferation, migration, and invasion in gastric cancer by targeting SRC kinase signaling inhibitor 1.[35] He et al and Lu et al reported that miR-374a promoted the proliferation of human osteosarcomas by targeting forkhead box protein O1 (FoxO1) and axis inhibition protein 2 (Axin2).[36,37] Given that a single miRNA has multiple different mRNA targets, the overall effect of miRNA dysregulation on cancer can be determined by the dominant target pathway in a given cancer subtype.
However, the results of our study should be interpreted with caution for some limitations. First, the number of total FFPE samples was not enough to make a strong association for validation. Second, the patients from TCGA data were mainly composed of Caucasian, whereas those of our series were Asian, specifically Korean. Thus, we could not rule out the external factors, especially race/ethnicity. Thus, the further studies with larger sample size are required to clarify the role of these miRNAs in cancer.
5. Conclusion
We identified differentially expressed miRNAs predictive of recurrence in stage I lung adenocarcinoma through microarray profiling, TCGA data set analysis, and qRT-PCR validation using FFPE specimens. Further studies are needed to determine the cancer-specific functions of the candidate miRNAs identified in this study, including their target pathways.
Acknowledgment
The authors thank Jisook Kim and Jeong Yun Eom (Department of Pathology, Hanyang University Hospital) for excellent technical assistance.
Author contributions
Conceptualization: Kiseok Jang.
Data curation: Jongmin Sim, Yeseul Kim, Hyunsung Kim, Kiseok Jang.
Formal analysis: Kiseok Jang.
Funding acquisition: Seung Sam Paik, Kiseok Jang.
Methodology: Jongmin Sim, Su-Jin Shin, Dong-Hoon Kim.
Resources: Kiseok Jang.
Supervision: Su-Jin Shin, Dong-Hoon Kim, Seung Sam Paik, Kiseok Jang.
Validation: Su-Jin Shin.
Writing – original draft: Jongmin Sim.
Writing – review & editing: Kiseok Jang.
Footnotes
Abbreviations: Axin2 = axis inhibition protein 2, FFPE = formalin-fixed paraffin-embedded, FoxO1 = forkhead box protein O1, miRNAs = microRNAs, NSCLC = non-small cell lung cancer, qRT-PCR = quantitative real-time polymerase chain reaction, RPKM = reads per kilobase per million mapped reads, TCGA = The Cancer Genome Atlas, TGF-α = transforming growth factor alpha.
This work was funded by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (no. 2015R1C1A1A01056091).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was waived for individual participants included in the study given the retrospective nature of this work.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung adenocarcinoma in resected specimens: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:685–705. [DOI] [PubMed] [Google Scholar]
- [3].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- [4].Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 2009;27:5848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Ann Rev Pathol 2014;9:287–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett 2009;285:116–26. [DOI] [PubMed] [Google Scholar]
- [7].Abdueva D, Wing M, Schaub B, et al. Quantitative expression profiling in formalin-fixed paraffin-embedded samples by affymetrix microarrays. J Mol Diagn 2010;12:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hall JS, Taylor J, Valentine HR, et al. Enhanced stability of microRNA expression facilitates classification of FFPE tumour samples exhibiting near total mRNA degradation. Br J Cancer 2012;107:684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Klopfleisch R, Weiss AT, Gruber AD. Excavation of a buried treasure – DNA, mRNA, miRNA and protein analysis in formalin fixed, paraffin embedded tissues. Histol Histopathol 2011;26:797–810. [DOI] [PubMed] [Google Scholar]
- [10].Leite KR, Canavez JM, Reis ST, et al. miRNA analysis of prostate cancer by quantitative real time PCR: comparison between formalin-fixed paraffin embedded and fresh-frozen tissue. Urol Oncol 2011;29:533–7. [DOI] [PubMed] [Google Scholar]
- [11].Li J, Smyth P, Flavin R, et al. Comparison of miRNA expression patterns using total RNA extracted from matched samples of formalin-fixed paraffin-embedded (FFPE) cells and snap frozen cells. BMC Biotechnol 2007;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Meng W, McElroy JP, Volinia S, et al. Comparison of microRNA deep sequencing of matched formalin-fixed paraffin-embedded and fresh frozen cancer tissues. PLoS One 2013;8:e64393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vojtechova Z, Zavadil J, Klozar J, et al. Comparison of the miRNA expression profiles in fresh frozen and formalin-fixed paraffin-embedded tonsillar tumors. PLoS One 2017;12:e0179645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Biase D, Visani M, Morandi L, et al. miRNAs expression analysis in paired fresh/frozen and dissected formalin fixed and paraffin embedded glioblastoma using real-time pCR. PLoS One 2012;7:e35596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kolbert CP, Feddersen RM, Rakhshan F, et al. Multi-platform analysis of microRNA expression measurements in RNA from fresh frozen and FFPE tissues. PLoS One 2013;8:e52517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhu M, Zhang N, He S. Similarly up-regulated microRNA-106a in matched formalin-fixed paraffin-embedded and fresh frozen samples and the dynamic changes during gastric carcinogenesis and development. Pathol Res Pract 2014;210:909–15. [DOI] [PubMed] [Google Scholar]
- [17].Sanchez-Salcedo P, Berto J, de-Torres JP, et al. Lung cancer screening: fourteen year experience of the Pamplona early detection program (P-IELCAP). Arch Bronconeumol 2015;51:169–76. [DOI] [PubMed] [Google Scholar]
- [18].Maeda R, Yoshida J, Ishii G, et al. Risk factors for tumor recurrence in patients with early-stage (stage I and II) non-small cell lung cancer: patient selection criteria for adjuvant chemotherapy according to the seventh edition TNM classification. Chest 2011;140:1494–502. [DOI] [PubMed] [Google Scholar]
- [19].Uramoto H, Tanaka F. Prediction of recurrence after complete resection in patients with NSCLC. Anticancer Res 2012;32:3953–60. [PubMed] [Google Scholar]
- [20].Patnaik SK, Kannisto E, Knudsen S, et al. Evaluation of microRNA expression profiles that may predict recurrence of localized stage I non-small cell lung cancer after surgical resection. Cancer Res 2010;70:36–45. [DOI] [PubMed] [Google Scholar]
- [21].Lu Y, Govindan R, Wang L, et al. MicroRNA profiling and prediction of recurrence/relapse-free survival in stage I lung cancer. Carcinogenesis 2012;33:1046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Skrzypski M, Czapiewski P, Goryca K, et al. Prognostic value of microRNA expression in operable non-small cell lung cancer patients. Br J Cancer 2014;110:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Edmonds MD, Eischen CM. Differences in miRNA expression in early stage lung adenocarcinomas that did and did not relapse. PLoS One 2014;9:e101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Capodanno A, Boldrini L, Proietti A, et al. Let-7g and miR-21 expression in non-small cell lung cancer: correlation with clinicopathological and molecular features. Int J Oncol 2013;43:765–74. [DOI] [PubMed] [Google Scholar]
- [25].Park S, Minai-Tehrani A, Xu CX, et al. Suppression of A549 lung cancer cell migration by precursor let-7g microRNA. Mol Med Rep 2010;3:1007–13. [DOI] [PubMed] [Google Scholar]
- [26].Hu H, Zhao X, Jin Z, et al. Hsa-let-7g miRNA regulates the anti-tumor effects of gastric cancer cells under oxidative stress through the expression of DDR genes. J Toxicol Sci 2015;40:329–38. [DOI] [PubMed] [Google Scholar]
- [27].Qian P, Zuo Z, Wu Z, et al. Pivotal role of reduced let-7g expression in breast cancer invasion and metastasis. Cancer Res 2011;71:6463–74. [DOI] [PubMed] [Google Scholar]
- [28].Avgeris M, Mavridis K, Tokas T, et al. Uncovering the clinical utility of miR-143, miR-145 and miR-224 for predicting the survival of bladder cancer patients following treatment. Carcinogenesis 2015;36:528–37. [DOI] [PubMed] [Google Scholar]
- [29].Motawi TK, Rizk SM, Ibrahim TM, et al. Circulating microRNAs, miR-92a, miR-100 and miR-143, as non-invasive biomarkers for bladder cancer diagnosis. Cell Biochem Funct 2016;34:142–8. [DOI] [PubMed] [Google Scholar]
- [30].Wang L, He J, Xu H, et al. MiR-143 targets CTGF and exerts tumor-suppressing functions in epithelial ovarian cancer. Am J Transl Res 2016;8:2716–26. [PMC free article] [PubMed] [Google Scholar]
- [31].Yang F, Xie YQ, Tang SQ, et al. miR-143 regulates proliferation and apoptosis of colorectal cancer cells and exhibits altered expression in colorectal cancer tissue. Int J Clin Exp Med 2015;8:15308–12. [PMC free article] [PubMed] [Google Scholar]
- [32].Wei J, Ma Z, Li Y, et al. miR-143 inhibits cell proliferation by targeting autophagy-related 2B in non-small cell lung cancer H1299 cells. Mol Med Rep 2015;11:571–6. [DOI] [PubMed] [Google Scholar]
- [33].Chu H, Zhong D, Tang J, et al. A functional variant in miR-143 promoter contributes to prostate cancer risk. Arch Toxicol 2016;90:403–14. [DOI] [PubMed] [Google Scholar]
- [34].Wu H, Liu Y, Shu XO, et al. MiR-374a suppresses lung adenocarcinoma cell proliferation and invasion by targeting TGFA gene expression. Carcinogenesis 2016;37:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xu X, Wang W, Su N, et al. miR-374a promotes cell proliferation, migration and invasion by targeting SRCIN1 in gastric cancer. FEBS Lett 2015;589:407–13. [DOI] [PubMed] [Google Scholar]
- [36].He W, Feng L, Xia D, et al. MiR-374a promotes the proliferation of human osteosarcoma by downregulating FOXO1 expression. Int J Clin Exp Med 2015;8:3482–9. [PMC free article] [PubMed] [Google Scholar]
- [37].Lu T, Zhang C, Chai MX, et al. MiR-374a promotes the proliferation of osteosarcoma cell proliferation by targeting Axin2. Int J Clin Exp Pathol 2015;8:10776–83. [PMC free article] [PubMed] [Google Scholar]


