Abstract
Background:
Exercise has been shown to be of benefit in the general population and in patients with chronic diseases. Despite a lack of compelling evidence, patients with end-stage kidney disease (ESKD) treated with peritoneal dialysis (PD) are often discouraged from participating in exercise programs that include weight lifting due to concerns about the development of hernias and leaks. The actual effects of physical activity with or without structured exercise programs for patients on PD remain unclear.
Objective:
To determine the risks and benefits of physical activity in the ESKD population treated with PD.
Design:
Systematic review and meta-analysis.
Setting:
Included all studies that met our criteria regardless of country of origin.
Patients:
Adult patients with ESKD treated with PD.
Measurements:
Descriptive and quantitative analysis of our primary and secondary outcome variables.
Methods:
We searched MEDLINE, Embase, CINAHL, and Cochrane Central Register of Controlled Trials for observational and interventional studies examining the effects of physical activity in patients on PD. A systematic review and meta-analysis was conducted of the identified studies. The primary outcomes of interest included patient-centered outcomes of mental health, physical functioning, fatigue, quality of life, and adverse events. Secondary outcomes included nutritional measures, lipid profile, blood pressure changes, maximum heart rate, resting heart rate, maximal oxygen consumption, muscle development, cognitive function, and markers of inflammation.
Results:
Of 1828 studies identified by the literature search, 12 met the inclusion criteria including 6 interventional and 6 observational studies. There was limited information on the patient important outcomes. However, there is some evidence for improvements in burden of kidney disease, physical function, and some mental health measures with physical activity.
Limitations:
Lack of well-designed randomized controlled trials impaired our ability to determine the benefits and risks of increasing physical activity.
Conclusions:
There is limited evidence of benefit with increased levels of physical activity in PD patients. Further research is needed to define the exercise program that is likely to be of most benefit to patients treated with PD.
Keywords: peritoneal dialysis (PD), exercise, physical activity, systematic review, quality of life
Abrégé
Contexte :
Les bienfaits procurés par la pratique d’une activité physique sont démontrés tant dans la population générale que chez les patients souffrant de maladies chroniques. Pourtant, malgré un manque de données probantes à ce sujet, on déconseille souvent aux patients atteints d’insuffisance rénale terminale (IRT) et traités par dialyse péritonéale (DP) de prendre part à un programme d’entraînement incluant des exercices en résistance; on invoque notamment des préoccupations sur le développement d’une hernie ou de fuites. De plus, les effets réels de l’activité physique avec ou sans un programme structuré chez ces patients sont encore mal connus.
Objectif de l’étude :
L’étude visait à mieux définir les risques et les bienfaits de la pratique d’une activité physique chez les patients atteints d’IRT et traités par DP.
Type d’étude :
L’étude a été menée sous la forme d’une revue systématique de la littérature scientifique puis d’une méta-analyse.
Cadre :
Toutes les études satisfaisant nos critères, quel que soit leur pays d’origine, ont été incluses.
Patients :
Tous les patients atteints d’IRT et traités par DP.
Mesures :
Nous avons procédé à l’analyse descriptive et quantitative des variables de nos paramètres primaires et secondaires.
Méthodologie :
Les bases de données MEDLINE, Embase et CINAHL, de même que le registre central Cochrane des essais cliniques randomisés ont été passés en revue afin d’y répertorier les études observationnelles et interventionnelles traitant des effets de l’activité physique chez les patients sous dialyse péritonéale. Une revue systématique et une méta-analyse des études retenues ont été effectuées. On s’est d’abord intéressé aux observations concernant la santé mentale du patient, son bien-être général, son niveau de fatigue et sa qualité de vie, de même qu’aux événements indésirables rapportés. Les résultats secondaires auxquels nous nous sommes attardés incluaient les mesures nutritionnelles, le profil lipidique, les variations dans la pression artérielle, la fréquence cardiaque maximale, la fréquence cardiaque au repos, la consommation maximale d’oxygène, le développement de la masse musculaire, les fonctions cognitives et les marqueurs de l’inflammation.
Résultats :
Des 1 828 études recensées par la revue de la littérature, seule une douzaine satisfaisait nos critères d’inclusion, soit 6 études interventionnelles et 6 études observationnelles. Il y avait très peu d’information au sujet des principaux résultats des patients. Néanmoins, on a pu constater l’existence de preuves attestant de l’allègement du fardeau posé par les maladies rénales et de l’amélioration des fonctions physiques et de certains aspects de la santé mentale par la pratique d’une activité physique.
Limites de l’étude :
Le faible nombre d’essais contrôlés, randomisés et bien conçus traitant de notre sujet nous a empêchés de bien mesurer les bienfaits et les risques associés à l’augmentation de l’activité physique.
Conclusion :
À ce jour, il existe encore peu de preuves que l’accroissement de l’activité physique procure des bienfaits aux patients traités par dialyse péritonéale. D’autres recherches sont nécessaires pour mieux définir les programmes d’exercice susceptibles de procurer le plus de bienfaits sur la santé des patients atteints d’IRT et traités par dialyse péritonéale.
What was known before
Patients with other chronic diseases derive quality of life benefits from both aerobic and resistance exercise programs. An increase in physical activity has been associated with a reduced risk of death in patients with ESKD treated with hemodialysis.
What this adds
There appear to be some benefits to exercise for ESKD patients treated with PD. However, the lack of well-designed randomized controlled trials with a sufficient number of participants prevents us from being able to draw definitive conclusions especially about patient-oriented outcome measures.
Background
The prevalence of ESKD worldwide is quite high, with 2.6 million patients being treated with dialysis in 2010.1 A significant and growing proportion of those patients receive peritoneal dialysis (PD), with hemodialysis (HD) still being the predominant modality.2 The 2 treatment modalities are very different which may affect patient preferences for type and location of exercise programs. HD is typically completed 3 times per week for about 4 hours each session by trained personnel in a hospital or clinic environment; PD is usually done independently every day at home.3 The differences in treatment schedule result in altered risks of extracellular fluid volume expansion that may contribute to shortness of breath and postdialysis fatigue which are more likely to be experienced by HD patients.3 The prognosis for patients diagnosed with ESKD remains poor. They have an 18- to 20-fold increased risk of cardiovascular disease compared with age- and sex-matched controls from the general population.4 Furthermore, initiation of dialysis is associated with a significant decline in functional status. In a study of nursing home residents with ESKD, significant declines in ability to complete activities of daily living occurred as early as 3 months after dialysis start.5 One year after beginning dialysis, 58% of patients had died, and only 13% had managed to maintain their baseline functional capacity.5 The increased cardiovascular morbidity, mortality, and loss of function may be potentially amenable to interventions that are focused on increasing physical activity, with or without structured exercise programs.
Physical activity is defined as any bodily movement produced by skeletal muscles that results in energy expenditure.6 Exercise is a subset of physical activity that is planned, structured, and repetitive and is undertaken with the goal of improving or maintaining one or more components of physical fitness.6 Exercise can be further subdivided into resistance exercise (weights, bands) and aerobic exercise (running, cycling, swimming, dancing). Increases in activity levels, self-reported or measured with an accelerometer, have been associated with a reduced risk of death in ESKD patients treated with HD.7,8 Patients with other chronic diseases have been shown to benefit from participation in resistance exercise programs with increases in strength, muscle mass, and cardiovascular fitness.9-11 Aerobic exercise has been associated with improvements in self-esteem, quality of life, aerobic fitness, and percent body fat.12,13 Exercise has also been shown to decrease the risk of depression, a problem that exists for ~25% of patients with ESKD.7,14 Finally, exercise may also improve glycemic control, blood pressure, and serum triglyceride levels which could cumulatively lower cardiovascular disease risk.9
Despite guidelines recommending nephrologists encourage ESKD patients treated with dialysis to increase their activity levels, this is rarely done.15-17 In fact, PD patients have a weekly caloric expenditure from exercise that is comparable to that of community dwelling adults over the age of 70 years.18 This may be due to a number of real or perceived barriers but also uncertainty about the best exercise regimen for patients treated with PD. Although a recent Cochrane review suggested that there were benefits to exercise in patients with chronic kidney disease including patients with ESKD treated with HD and PD, the majority of evidence was from patients treated with HD.19 In a few studies, PD and HD patients were discussed together, or, in most cases, PD patients were not included.20 Extrapolation of these results to patients on PD is unreasonable. Patients on HD spend several hours each week receiving treatment, during which exercise programs can be tested, thus potentially enhancing participation rates that may not be the same for PD patients who are at home.21 PD patients undergo abdominal surgery and usually have dialysate in their peritoneal cavity.21,22 This last tenet underlies the rationale behind discouraging PD patients from participating in physical activity that involves lifting weights as the increased intra-abdominal pressure may precipitate the development of hernias and leaks.23-25
Given the potential equipoise about physical activity including aerobic and resistance exercise programs in the PD patient population, we undertook this systematic review and meta-analysis of the literature to more completely define the risks and benefits of physical activity in this patient population.
Methods/Design
Search Strategy
A comprehensive electronic search was conducted using Em-base (1947 to November 23, 2016), EBM Reviews—Cochrane Central Register of Controlled Trials (October 2016), Epub Ahead of Print, In-Process & Other Non-Indexed Citations, CINAHL, Ovid MEDLINE Daily, and Ovid MEDLINE (1946 to November 2016) with the assistance of a librarian experienced in systematic reviews. A structured search strategy was based on controlled vocabulary and relevant key terms and was broad to prioritize sensitivity (see Appendix A).
Study Screening and Inclusion
All titles and abstracts compiled from the search strategy were screened by 2 independent reviewers (T.T., R.I.). Each study was examined for appropriate inclusion and exclusion criteria. If the abstract was absent, the full text was examined, unless the title alone could be used to confidently exclude the study. Any disagreement between the reviewers at this stage resulted in the study proceeding to a full-text review.
Inclusion Criteria
We included studies in which adult (≥18 years) patients were (1) diagnosed with ESKD, (2) treated with PD (continuous cyclic peritoneal dialysis [CCPD], nocturnal intermittent peritoneal dialysis [NIPD], chronic ambulatory peritoneal dialysis [CAPD]), and (3) had some measure of physical activity/exercise (any type of physical activity or exercise was included using a broad search strategy with text words physical activity, motor activity, sports, yoga, running, swimming, jogging, skiing, bicycling/biking, walking; see Appendix A). We included all observational (prospective and retrospective cohorts, cross-sectional and case–control, and case-report) and interventional (randomized or nonrandomized) study designs. Only English language articles were included.
Exclusion Criteria
Studies that included both pediatric and adult patients and/or PD and HD patients in which the population of interest could not be assessed independently were excluded if we were unable to obtain patient-level data from the authors. Abstracts from conference proceedings were also excluded based on the nonuniformity of subsequent publication in peer-review journals raising concerns about potential study quality.26,27
Outcomes
The primary PD patient–centered outcomes included (1) depression (diagnosis via clinician structured interview or scales such as the Beck Depression Index, Hospital Anxiety and Depression Scale, Hamilton Depression Scale), (2) physical functioning (6-minute walk test, stair climbing capacity, activities of daily living, sit-stand test), (3) fatigue, (4) quality of life ([using validated questionnaires such as 36-Item Short Form Survey (SF-36), EuroQol, Kidney Disease Quality of Life Instrument [KDQOL] and their subscales) and, (5) adverse events (musculoskeletal injury, cardiovascular adverse events, hernias, leaks, mortality).Secondary outcomes included nutritional measures (albumin, pre-albumin, subjective global assessment [SGA], energy intake, protein intake, body mass indices), glucose, lipid profile, blood pressure changes, maximum heart rate, resting heart rate, maximal oxygen consumption (peak Vo2), muscle development (morphometrics), cognitive function, and markers of inflammation (serum interleukin 6, lymphocytes, protein catabolic rate).
Data Extraction
Each included study underwent standardized data extraction by 2 independent reviewers to minimize errors and bias (T.T., R.I.).
Extracted data included study identification (first author, year of publication), study design (type of study, sample size, inclusion and exclusion criteria, nature of physical activity/exercise regimen), and patient population (age, gender, duration of PD). The primary and secondary outcomes of interest were extracted from the studies as described under research objectives. We contacted the original authors for missing data and to obtain data separated by dialysis modality, if possible. Any difference of opinion was resolved by consensus and discussion with a third investigator (D.Z.).
Quality Assessment
Two review authors independently assessed the risk of bias in the included studies. All randomized controlled trials (RCTs) were evaluated using the Cochrane risk of bias assessment tool.28 Observational studies were evaluated with the Newcastle-Ottawa Scale.29
Analysis
The extracted data are presented through a series of tables and text. Most studies included reported variable definitions and measures of activity/exercise and outcomes; hence, we could perform a quantitative synthesis for certain quality of life and laboratory measures only. The outcomes were pooled in the form of weighted mean differences (WMD) using the random-effects model of DerSimonian and Laird.30 Heterogeneity across the studies was assessed using the Cochran Q statistic and I2 statistic.31 Publication bias was assessed by visual examination of the funnel plot and the Egger statistic.32 All reported P values are 2-tailed, and P < .05 was considered statistically significant. The analysis was performed using Comprehensive Meta-analysis software (version 2.2.064; Biostat Inc, Englewood, New Jersey). The protocol for this systematic review is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42016041695).
Results
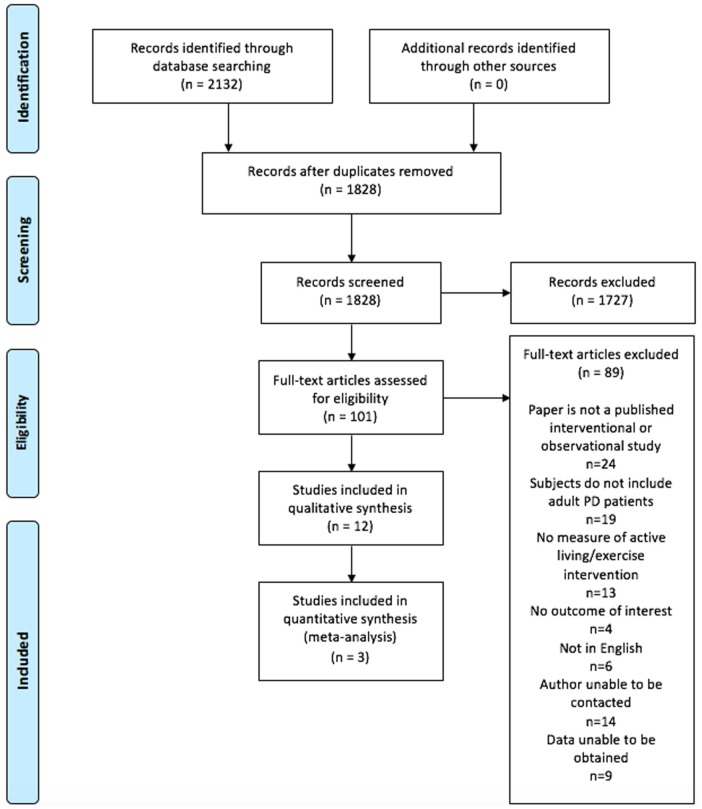
The search process and results are summarized in Figure 1. Prisma checklist is included in Supplementary file. From 1828 potential studies, 101 studies underwent full-text review. Reasons for exclusion included the following: not a primary research manuscript (n = 24), wrong study population (n = 19), no measure of physical activity or exercise (n = 13), no outcome of interest (n = 4), and non-English language (n = 6). Twenty-four studies included both HD and PD patients without separately reporting the results from these 2 groups. We were unable to contact the authors of 14 of these studies. Raw data were only available from 1 of 10 of the remaining studies and has been included in our review.33,34 Overall, 12 studies satisfied our inclusion criteria; 6 were observational and 6 included an exercise intervention. Of the observational studies, 3 measured physical activity level using a pedometer,35-37 1 used an accelerometer,38 1 inferred activity level from occupation,39 and 1 relied on self-reported activity levels.40 Of the interventional studies, traditional aerobic exercises (biking, running, walking),41-43 stationary cycling while lying down,44 resistance exercise,33,34 and tai chi45 were assessed in 3, 1, 1, and 1 studies, respectively. The characteristics of these studies are outlined in Tables 1 and 2.
Figure 1.
Flow diagram of study selection.
Table 1.
Noninterventional Studies of Physical Activity in PD Patients.
| First author (year), country | Population demographics |
Activity measurement |
Outcomes |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Age, y (SD) | Study groups (M = males, F = females) | Mean duration on PD, mo (SD) | Modality for data collection | Duration | Variable | Measurement (SD) | P value | |
| Matsuoka et al (1991), Japan | 15 | Activity maintained (AM): = 43-62 Activity restricted (AR): 40-64 |
AM (n = 7; 6 M,1
F) AR (n = 8; 5 M,3 F) |
Not provided | Chart review and personal interview (inferred by job description) | Not Applicable | Karnofsky score | AM = 82.7 (4.6) AR = 77.1 (4.9) |
<.05 |
| BP | AM = 158 (17)/86 (9) AR = 160 (11)/85 (7) |
NS | |||||||
| Change in blood pressure from supine to upright (mm Hg) [systolic/diastolic] | AM = 29 (25)/10 (7) AR = 43 (21)/17 (13) |
NS | |||||||
| Stack et al (2005), Ireland | 1203 | Not reported as PD alone | Patients grouped by restrictions in moderate (M) and vigorous (V) activities | Not provided | US Renal Data System | Not Applicable | Relative mortality risk | M = 1.87 (95% CI 1.44-2.43) | <.001 |
| V = 1.50 (95% CI 1.04-2.15) | <.05 | ||||||||
| Masuda et al (2009), Japan | 26 | 47.5 (14.2) | 14 M, 12 F | Minimum 3 | Digital pedometer (steps/day) | 7 days | HDL3-C | r = 0.505 | <.05 |
| HDL-C, HDL2-C, Apo A-I, LDL-C, Apo B, TC, log TG, LCAT | Not reported | NS | |||||||
| Oishi et al (2012), Japan | 38 | 63.9 (10.8) | 22 M, 16 F | 21.4 (15.1) | Digital pedometer (steps/day) | 1 month | Age | r = −0.33 | <.05 |
| CRP | r = −0.33 | <.05 | |||||||
| Serum Albumin | r = 0.45 | <.01 | |||||||
| Cobo et al (2015), Spain | 64 | 62 (14) | 45 M, 19 F (Low [n = 21], Middle [n = 22], High [n = 21]) |
9 | Digital pedometer (steps/day) – grouped by tertiles (L, M, H) | 7 days | MBP (mm Hg) | L = 92, M = 104, H = 98 | .077 |
| SGA > 1 (%) | L = 67%, M = 36%, H = 24% | .013 | |||||||
| Albumin (g/dL) | L = 2.8, M = 3.2, H = 3.5 | .005 | |||||||
| hsCRP (mg/L) | L = 9.7, M = 4.3, H = 1.8 | .003 | |||||||
| LBMI | L = 15.4, M = 16.5, H = 16.8 | .047 | |||||||
| LBM (%) | L = 44.3, M = 49.8, H = 52.4 | .064 | |||||||
| Wakamiya et al (2015), Japan | 44 | 64 | 28 M, 16 F | Not provided | Accelerometer (steps/day) | 7 days | GNRI | r = 0.38 | <.01 |
Note. PD = peritoneal dialysis; NS = not significant; HDL3-C = high-density lipoprotein 3 cholesterol; HDL-C = high-density lipoprotein cholesterol; HDL2-C = high-density lipoprotein 2 cholesterol; Apo A-I = apolipoprotein A-I; LDL-C = low-density lipoprotein cholesterol; Apo B = apolipoprotein B; TC = total cholesterol; TG = triglycerides; LCAT = lecithin:cholesterol acyltransferase; CRP = C-reactive protein; Hb = hemoglobin, CVD = cardiovascular disease; MBP = mean blood pressure; SGA = subjective global assessment; hsCRP = high-sensitivity CRP; LMBI = Lean Body Mass Index; LBM = lean body mass; GNRI = Geriatric Nutritional Risk Index.
Table 2.
Interventional Studies of Physical Activity in PD Patients (Shortened - full table available in supplementary file).
| Author | Population Demographics |
Exercise intervention |
Outcomes |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Study Groups (M=males, F=females) | Age [years] (SD) | Mean duration on PD [years] (SD) | Prescription | Duration | Variable | Measurement (SD) | p value | |
| Chi-yuen Lo et al, (1998) China | 20 | Intervention (I) = 6 M, 7 F Control (C) = 4 M, 3 F |
I = 46.5 (12.8) C = 44.1 (7.4) |
I = 4.8 (3.8) C=5.1 (3.8) |
Treadmill, ski training machine or cycle ergometer for 45-60 mins, 3 x wk | 12 wks | MET peak | Ipre=6.4(2.1), Ipost=8.4(1.9) | 0.005 |
| V02peak (mL/kg/min) | Ipre=17.2(5.2), Ipost=20(6.4) | 0.004 | |||||||
| % of pred. max HR | Ipre=77(16), Ipost=83(16) | 0.05 | |||||||
| Albumin (mg/dL) | Ipre=3.6(0.5), Ipost=3.8(0.5) | NS | |||||||
| FBG (mg/dL) | Ipre=95.4(12.6), Ipost=90(10.8) | 0.01 | |||||||
| Amb Whole day SBP | Ipre=139(27), Ipost=154(21) | 0.02 | |||||||
| Amb Whole day MAP | Ipre= 100(13), Ipost=108(10) | 0.02 | |||||||
| Amb Daytime SBP | Ipre=142(26), Ipost=157(22) | 0.003 | |||||||
| Amb Daytime MAP | Ipre=101(13), Ipost=110(11) | 0.004 | |||||||
| ESRD-target scores: | |||||||||
| Burden of CKD | Ipre=27.1(26.0),Ipost=42.7(27.5) | 0.02 | |||||||
| SF-36 scores: | |||||||||
| Physical function | Ipre=74.2(15.1), Ipost=84.5(8.31) | 0.03 | |||||||
| * C = no change pre/post | |||||||||
| Derici et al, (2005) Turkey | 3 | 3 F with dialysate leakage – No control group | 52.6 (6.43) | 16.3 (9.07) | Lying down cycling for 10 min with 10-15 min intervals for 1 hr, daily | 1 month | Complications | None reported | N/A |
| Leakage Status | Resolved in 7 to 10 days | N/A | |||||||
| Recurrence of leakage | No recurrence 3 mo, 6 mo, 1 yr | N/A | |||||||
| Mustata et al, (2005) Canada | 6 | 2 F 4 M – No control group | 53 (13) | 2(1) | Elements of Tai Chi Wu-style adapted for PD patients | 3 months | Albumin | Ipre=37(1.9), Ipost=37(1.6) | NS |
| SF-36 scores: | |||||||||
| Social functioning | Ipre=56(8), Ipost=65(11) | 0.05 | |||||||
| Role emotional | Ipre=33(17), Ipost=61(18) | 0.05 | |||||||
| Total mental health score | Ipre=48(9), Ipost=58(10) | 0.05 | |||||||
| Straub et al, (2005) USA | 10 | I = 5 (4 M, 1 F) C = 5 (3 M, 2 F) |
I = 35-50 n=1, 50-65 n=2, 65+ n=2 C = 35-50 n=3, 50-65 n=1, 65+ n=1 |
I = <1 n=1 1-5 n=3 >5 n=1 C = <1 n=2 1-5 n=3 >5 n=0 |
Cycle ergometer, walking or swimming, 30 mins, 3-4 x wk | 8 wks | Total Fatigue Score | Ipre=2.482(1.02), Ipost=3.16(1.81) | NS |
| Physical Performance Battery Score | Ipre=8.8(2.39), Ipost=9.4(1.67) | NS | |||||||
| * C = no change pre/post | |||||||||
| Molsted et al, (2013 & 2014) Denmark | 5 | 2 F 3 M – No control group | 62 (9.19) | 3.0 (0.77) | 5 min cycle ergometer and up to 5 sets of dynamic leg press, dynamic leg extension, and dynamic leg curl, 3 x wk | 16 wks | Albumin | Ipre=38 (34,39.5), Ipost=38.5(33.8,41.8) | NS |
| CRP | Ipre=4(3,26.5), Ipost=13.5(4,26) | NS | |||||||
| *Outcomes reported as median (IQR) | |||||||||
| Shahgholian et al, (2015) Iran | 22 | I = 11 (9 M, 2 F) C = 11 (8 M, 3 F) |
I = 50.5 (14.3) C = 52.3 (10.3) |
I = 1.01 (0.675) C = 1.08 (0.742) |
Cycle ergometer, 40 min, 2 x wk | 8 wks, 16 wks | FBG (mg/dL) | At 16 wks: C=117(15.3), I=93.6(12.5) | 0.001 |
| 2h PPBS (mg/dL) | At 16 wks: C=182 (18.6), I=162 (12.5) | 0.01 | |||||||
MET = metabolic equivalent, V02peak = maximal rate of oxygen consumption, FBG = Fasting blood glucose, TC = Total Cholesterol, LDL = Low-density Lipoprotein, HDL = High-density Lipoprotein, TG = Triglycerides, SBP = Systolic Blood Pressure, MAP = Mean Arterial Pressure, ESRD = End Stage Renal Disease, SF-36 = Short From 36 – Health Survey, PBBS = Post-prandial Blood Sugar
Assessment of Quality
Quality assessment of the noninterventional studies was performed using the Newcastle-Ottawa Quality Assessment Scale.29 The mean (SD) score was 4.38 (±1.47) out of a possible 10 points. Quality assessment of the interventional studies was performed using the Cochrane risk of bias tool.28 All 6 interventional studies were found to have a high risk of bias particularly in the following categories: sequence generation, allocation concealment, blinding of participants and personnel, and small sample size. The detailed results of the quality assessment are shown in Table 3 and Figure 2.
Table 3.
Results of Newcastle-Ottawa Quality Assessment Scale Adapted for Noninterventional Studies Included in the Systematic Review.
| First author (year), country | Total score (/10) |
|---|---|
| Matsuoka et al (1991), Japan | 2 |
| Stack et al (2005), Ireland | 6 |
| Masuda et al (2009), Japan | 5 |
| Oishi et al (2012), Japan | 5 |
| Cobo et al (2015), Spain | 5 |
| Wakamiya (2015), Japan | 6 |
Figure 2.
Graph showing the results of the quality assessment of interventional studies included in systematic review done using the Cochrane risk of bias tool (Modified).
Primary Outcomes
Depression, Physical Function, Fatigue, and Quality of life
Overall, 2 interventional studies43,45 involving a total of 30 patients (18 intervention and 12 control) assessed changes in several domains of the SF-36 scores, specifically physical function, role physical, pain, general health, role emotional, and social function. The pooled analysis demonstrated a small improvement in all domains of SF-36 analyzed, which was significant for physical function, pain, general health, and role emotional, but not significant for role physical and social function (see Table 4 for details). The heterogeneity was not significant for any of these domains (I2 = 0, P = .66; I2 = 0, P = .89; I2 = 0, P = .84; I2 = 0, P = .66; I2 = 0, P = .06; I2 = 73, P = .66; I2 = 41, P = .19, respectively). Depression was not assessed independently of overall mental health. After 3 months of Tai Chi, SF-36 scores improved in the domain of total mental health.45 There was also a significant improvement in burden of kidney disease as measured with the Chinese version of the KDQOL after a 3-month aerobic exercise program.43 However, in 2 smaller studies of aerobic exercise for 16 and 8 weeks, respectively, no improvement in leg strength or the short physical performance battery was shown.33,34,42 This last study also failed to demonstrate an improvement in fatigue as assessed by the Piper Fatigue Scale.46 In one observational study, physical activity was inferred from an individual’s occupation and divided into 2 categories; “free/good” and “restricted.”39 Functional impairment was assessed with the Karnofsky score.47 Patients with a “free/good” activity level reported significantly higher Karnofsky performance scores indicative of less functional impairment.
Table 4.
Summary of the Meta-analysis Results.
| Outcome | No. of studies | Summary WMD | 95% CI | P value | Heterogeneity |
||
|---|---|---|---|---|---|---|---|
| Q | I 2 | P value | |||||
| Glucose | 3 | −15.7 | (–30.9 to −0.3) | 0.045 | 5.7 | 65.1 | .06 |
| Albumin | 3 | 0.08 | (–0.09 to 0.25) | 0.37 | 0.23 | 0 | .89 |
| SF-36 Physical Function | 2 | 0.50 | (0.15 to 0.85) | 0.005 | 0.19 | 0 | .66 |
| SF-36 Role Physical | 2 | 0.21 | (–0.13 to 0.55) | 0.22 | 0.02 | 0 | .89 |
| SF-36 Pain | 2 | 0.45 | (0.11 to 0.80) | 0.01 | 0.19 | 0 | .84 |
| SF-36 General Health | 2 | 0.54 | (0.19 to 0.90) | 0.003 | 0.04 | 0 | .66 |
| SF-36 Role Emotional | 2 | 1.13 | (0.10 to 2.16) | 0.03 | 3.7 | 73 | .06 |
| SF-36 Social Function | 2 | 0.58 | (–0.03 to 1.18) | 0.06 | 1.7 | 41 | .19 |
Note. WMD = weighted mean differences; CI = confidence interval.
Adverse Events
Three PD patients with a pericatheter dialysate leak performed daily recumbent cycling for 1 hour. There were no reported complications from the exercise program; the leaks resolved over 7 to 10 days with no recurrences in 3, 6, and 12 months of follow-up.44 We were unable to find any other reports of hernias or leaks in association with exercise. In a large observational study, mortality risk was higher in the patients who reported limitations in moderate (Relative Risk (RR) 1.87, p < 0.001) and vigorous activity (RR = 1.50, P < .05) relative to those who reported minimal or no limitations.40
Secondary Outcomes
Inflammatory and nutritional markers were assessed with C-reactive protein (CRP), albumin, SGA, and/or Geriatric Nutritional Risk Index (GNRI). An inverse correlation was found between number of steps per day and serum CRP concentration in 2 observational studies.35,36 However, this trend was not seen in the interventional study by Molsted et al.33 Greater evidence of malnutrition as assessed by the SGA was reported for patients in the lowest tertile of physical activity assessed by steps per day.35 This observation was consistent with another study in which there was a positive correlation between physical activity and GNRI scores.38 Changes in albumin were reported in 3 interventional studies33,34,43,45 involving 35 patients (23 intervention and 12 control). The WMD with exercise was not statistically significant (95% confidence interval [CI] = −0.09 to −0.25, P = .37; I2 = 0, P = .89).
Glucose, lipids, and body composition were assessed in 3,34,41,43 2,37,43 and 234,35 studies, respectively. Changes in fasting blood glucose following exercise interventions were reported in 3 interventional studies34,41,43 involving 47 patients (29 intervention and 18 control). The pooled analysis demonstrated a significant decrease in fasting glucose after exercise (WMD: –15.7 mg/dL,95% CI = −30.9 to −0.3, P = .045; I2 = 65.1, P = .06).
A positive correlation was reported between average steps per day over 1 week and levels of high-density lipoprotein 3 cholesterol (HDL3-C) (r = 0.505, P < .05) in 1 study.37 None of the other measured lipids were associated with activity. A similar observation was reported after a 12-week aerobic exercise program.43 Higher lean body mass index (P = .047) and percentage lean body mass (P = .064) were associated with increasing tertiles of physical activity.35 However, muscle biopsies in a small number of patients after a 16-week strength training program did not show evidence of muscle hypertrophy or change in muscle fiber type composition.34
Blood Pressure, Vo2 Peak, and Peak Heart Rate
The relationship between blood pressure and physical activity was assessed in 3 studies (2 observational35,39 and 1 experimental43). In both observational studies, blood pressure was not associated with physical activity. In the only interventional study that examined the impact of exercise on blood pressure in PD patients, ambulatory whole day systolic blood pressure (139(27) to 154(21), P = .02), ambulatory whole day mean blood pressure (100(13) to 108(10), P = .02), ambulatory daytime systolic blood pressure (142(26) to 157(22), P = .003), and ambulatory daytime mean blood pressure (101(13) to 110(11), P = .004) all increased with exercise. Diastolic blood pressure was unaffected by exercise.42 In a 12-week aerobic exercise program, the intervention group had an increase in the Vo2 peak (17.2-20 mL/kg/min, P = .004) and peak heart rate (77%-83% of the age predicted maximum value, P = .05).43
Discussion
To our knowledge, this is the first systematic review of the literature examining the effects of activity and exercise in the PD-specific patient population. There were a limited number of studies that included a small number of patients. The studies were of lower quality and had a high risk of bias. With these caveats in mind, there appear to be some benefits to exercise with respect to quality of life, Vo2 max, peak heart rate, muscle mass, serum glucose, and markers of inflammation. These results are similar to studies in HD patients.48 Furthermore, the included studies do not identify any risks associated with exercise in PD patients.
Patients with ESKD have a reduced quality of life compared with people in the general population.49 They experience difficulties with functional limitations, fatigue, and depression.50,51 In patients with other chronic diseases, exercise has been associated with an improved QOL.52 Similarly in PD patients, QOL appears to improve with physical activity. A decrease in burden of kidney disease, increased physical and social functioning, and improved emotional and mental health have all been reported. The current evidence does not support the use of exercise to improve fatigue. However, we only found one small study that used a nonvalidated fatigue assessment scale. These findings are in contrast with other studies in which fatigue was improved with exercise in other chronically ill patients.52-54 These contradictory results highlight the need for further research to better characterize the effects of physical activity and exercise on PD patient fatigue.
Leaks and hernias have both been reported in literature as potential concerns for PD patients engaging in resistance exercise.23-25 One interventional study examined the impact of supine exercise following dialysate leakage.44 The authors hypothesized that increasing abdominal musculature may actually decrease hernias and leaks. There were no complications or recurrence, with complete resolution of leakage in 7 days. We were unable to find any other studies which examined adverse events with increased physical activity and exercise in PD patients. Mortality was lower in patients who are able to participate in moderate or vigorous physical activities but residual confounding is likely in this observational study.40
Poor nutrition and inflammation have been associated with an increased risk of death in dialysis patients.55-57 Exercise has had variable effects on CRP levels in other patient populations.58-61 In our study, lower levels of physical activity were associated with malnutrition and inflammation. However, the serum concentration of albumin and CRP did not improve with exercise interventions. These results may be secondary to study design (small participant numbers, type of intervention) or a noncausal relationship.
Cardiovascular disease is the most common cause of mortality in the ESKD population.62 Much of this increased risk is secondary to traditional risk factors including diabetes mellitus, dyslipidemia, and hypertension. Exercise has been shown to improve insulin resistance 63 and lower glucose levels in patients either at risk for or diagnosed with type 2 diabetes mellitus.64 Exercise also improves fasting blood glucose (FBG) in patients treated with PD. Although exercise also has beneficial effects on cholesterol in the general population,65 the effects are less clear in PD patients. Although there was a correlation between physical activity and HDL-3C, lipid levels were not improved by exercise.37,43 Similarly, exercise improves blood pressure in the general population66 but not in patients treated with PD.39,43 It may be that the very complex nature of hypertension in ESKD is not amenable to a single intervention. Alternatively, the observational nature and/or small number of patients included in these studies may have obscured a potential benefit.
This study has several limitations that require further consideration. The studies were heterogeneous with respect to patient characteristics, study design, and exercise regimens implemented which limited our ability to pool data. The lack of well-designed randomized controlled trials with a sufficient number of participants prevents us from being able to draw definitive conclusions about the potential positive impact of increasing PD patient physical activity. Defining attributable risks is even more problematic with the currently available studies, lack of surveillance data, and probable publication bias. Finally, we were unable to comment on certain outcomes of interest due to an absence of studies examining their relationship in context of PD and exercise.
Conclusions
Overall, our systematic review and meta-analysis suggests that there may be benefits to increasing physical activity in PD patients. However, before any clinical guidelines for exercise prescriptions can be established, there is a strong need for future high-quality studies to better characterize these benefits in addition to any potential risks.
Supplemental Material
Supplemental material, PD_and_Exercise_SR_CJKHD_Supplementary_File for Physical Activity in Patients Treated With Peritoneal Dialysis: A Systematic Review and Meta-analysis by Tharshika Thangarasa, Rameez Imtiaz, Swapnil Hiremath and Deborah Zimmerman in Canadian Journal of Kidney Health and Disease
Supplemental Material
Supplemental material, Supplementary_File_PRISMA_2009_checklist for Physical Activity in Patients Treated With Peritoneal Dialysis: A Systematic Review and Meta-analysis by Tharshika Thangarasa, Rameez Imtiaz, Swapnil Hiremath and Deborah Zimmerman in Canadian Journal of Kidney Health and Disease
Supplemental Material
Supplemental material, Supplementary_file__table_2_(long) for Physical Activity in Patients Treated With Peritoneal Dialysis: A Systematic Review and Meta-analysis by Tharshika Thangarasa, Rameez Imtiaz, Swapnil Hiremath and Deborah Zimmerman in Canadian Journal of Kidney Health and Disease
Acknowledgments
We thank the Kidney Research Center at the Ottawa Hospital, as well as Risa Shorr, the staff librarian who assisted with the literature search.
Appendix A
Database: Embase Classic+Embase <1947 to 2016 November 23>, EBM Reviews—Cochrane Central Register of Controlled Trials <October 2016>, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily, and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
--------------------------------------------------------------------------------
1 exp Peritoneal Dialysis/ (55794)
(peritoneal adj2 dialysis).tw. (53061)
CAPD.tw. (14990)
*Kidney Failure, Chronic/ (80042)
(end stage renal disease* or esrd).tw. (72283)
or/1-5 (191425)
exp Exercise/ (497969)
(running or swimming or jogging or skiing or bicycling or biking or walking or exercis*).tw. (872482)
exp exercise movement techniques/ or exp exercise therapy/ (124612)
yoga.tw. (8612)
exp physical endurance/ (59838)
(resistance train* or combination train*).tw. (15337)
exp sports/ (324127)
Motor Activity/ (150661)
physical activit*.tw. (204217)
or/7-15 (1433877)
6 and 16 (2592)
hemodialysis/ not (exp peritoneal dialysis/ or Kidney Failure, Chronic/) (129751)
17 not 18 (2317)
19 use ppez (1291) Medline
exp Peritoneal Dialysis/ (55794)
(peritoneal adj2 dialysis).tw,kw. (53734)
CAPD.tw,kw. (15197)
*Kidney Failure, Chronic/ (80042)
(end stage renal disease* or esrd).tw,kw. (75097)
or/21-25 (193800)
exp Exercise/ (497969)
(running or swimming or jogging or skiing or bicycling or biking or walking or exercis*).tw,kw. (886804)
exp exercise movement techniques/ or exp exercise therapy/ (124612)
yoga.tw,kw. (8803)
exp physical endurance/ (59838)
(resistance train* or combination train*).tw,kw. (16520)
motor activity/ or physical activit*.tw,kw. (330806)
exp sports/ (324127)
or/27-34 (1439835)
26 and 35 (2686)
hemodialysis/ not (exp peritoneal dialysis/ or Kidney Failure, Chronic/) (129751)
36 not 37 (2367)
38 use cctr (80) Cochrane
peritoneal dialysis/ (47162)
continuous ambulatory peritoneal dialysis/ (22704)
(peritoneal adj2 dialysis).tw. (53061)
CAPD.tw. (14990)
*end stage renal disease/ (66144)
(end stage renal disease* or esrd).tw. (72283)
or/40-45 (178921)
exp *exercise/ (258213)
(running or swimming or jogging or skiing or bicycling or biking or walking or exercis*).tw. (872482)
exp *kinesiotherapy/ (29126)
exp *sport/ (185076)
yoga.tw. (8612)
(resistance train* or combination train*).tw. (15337)
exp physical activity/ or physical activit*.tw. (724206)
or/47-53 (1440963)
46 and 54 (2519)
hemodialysis/ not (continuous ambulatory peritoneal dialysis/ or peritoneal dialysis/ or end stage renal disease/) (128113)
55 not 56 (2313)
57 use emczd (1008) Embase
20 or 39 or 58 (2379)
remove duplicates from 59 (1718)
60 use ppez (1177) Medline
60 use emczd (528) Embase
60 use cctr (13) Cochrane
CINAHL Complete—November 28, 2016.
| No. | Query | Results |
| S1 | Peritoneal N2 dialysis | 3403 |
| S2 | CAPD | 400 |
| S3 | (MM “Kidney Failure, Chronic”) | 11 948 |
| S4 | end stage renal disease* OR esrd | 5683 |
| S5 | S1 OR S2 OR S3 OR S4 | 17 060 |
| S6 | (MH “Exercise+”) | 76 389 |
| S7 | TI ( running or swimming or jogging or skiing or bicycling or biking or walking or exercis* ) OR AB ( running or swimming or jogging or skiing or bicycling or biking or walking or exercis* ) | 95 106 |
| S8 | (MH “Therapeutic Exercise+”) | 37 469 |
| S9 | (MH “Yoga”) | 4967 |
| S10 | TI yoga OR AB yoga | 2955 |
| S11 | (MH “Physical Endurance+”) | 9280 |
| S12 | (MH “Sports+”) | 55 150 |
| S13 | (resistance train* or combination train*) | 4010 |
| S14 | (MH “Physical Activity”) | 25 514 |
| S15 | TI physical activit* OR AB physical activit* | 33 126 |
| S16 | S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 | 214 503 |
| S17 | S5 AND S16 | 416 |
Footnotes
Ethics Approval and Consent to Participate: Not Applicable for this type of study.
Consent for Publication: We have the authors consent for publication.
Availability of Data and Materials: All data available on request.
Author Contributions: TT, RI, and DZ participated in research design, the literature search and analysis of the data. SH conducted the meta-analysis. All authors contributed to the writing of the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Drs Hiremath and Zimmerman receive salary support from the Department of Medicine at the Ottawa Hospital.
ORCID iD: Tharshika Thangarasa  https://orcid.org/0000-0002-3900-7045
https://orcid.org/0000-0002-3900-7045
References
- 1. Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975-1982. [DOI] [PubMed] [Google Scholar]
- 2. Li PK, Chow KM, Van de Luijtgaarden MW, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13(2):90-103. [DOI] [PubMed] [Google Scholar]
- 3. Ramapriya S, Jean LH. Peritoneal dialysis versus hemodialysis: risks, benefits, and access issues. Adv Chronic Kidney Dis. 2011;18:428-432. [DOI] [PubMed] [Google Scholar]
- 4. 2015 annual data report: end stage renal disease (ESRD) in the United States. United States Renal Data System. https://www.usrds.org/2015/view/Default.aspx. Accessed July 19, 2016. [Google Scholar]
- 5. Manjula T, Kenneth EC, Glenn MC, Kristine Y, Seth L, Charles EM. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;110(2):126-131. [PMC free article] [PubMed] [Google Scholar]
- 7. Tentori F, Elder SJ, Thumma J, et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant. 2010;25:3050-3062. [DOI] [PubMed] [Google Scholar]
- 8. Matsuzawa R, Matsunaga A, Wang G, et al. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:2010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hurley BF, Hanson ED, Sheaff AK. Strength training as a continuous measure to aging muscle and chronic disease. Sports Med. 2011;41(4):289-306. [DOI] [PubMed] [Google Scholar]
- 10. Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc. 2013;45:2080-2090. [DOI] [PubMed] [Google Scholar]
- 11. Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized controlled trial. J Am Soc Nephrol. 2006;17:2307-2314. [DOI] [PubMed] [Google Scholar]
- 12. Murtezani A, Ibraimi Z, Bakalli A, Krasniqi S, Disha ED, Kurtishi I. The effect of aerobic exercise on quality of life among breast cancer survivors: a randomized controlled trial. J Can Res Ther. 2014;10:658-664. [DOI] [PubMed] [Google Scholar]
- 13. Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a randomized controlled trial. J Clin Oncol. 2007;25:4396-4404. [DOI] [PubMed] [Google Scholar]
- 14. Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney int. 2013;84:179-191. [DOI] [PubMed] [Google Scholar]
- 15. Johansen KL, Sakkas GK, Doyle J, Shubert T, Dudley RA. Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis. 2003;41:171-178. [DOI] [PubMed] [Google Scholar]
- 16. Cynthia D, Kirsten LJ. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant. 2012;27:1152-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johansen Kirsten L. Exercise in the end-stage renal disease population. J Am Soc Nephrol. 2007;18:1845-1854. [DOI] [PubMed] [Google Scholar]
- 18. Painter P, Agarwal A, Drummond M. Physical function and physical activity in peritoneal dialysis patients. Perit Dial Int. 2017;37:598-604. [DOI] [PubMed] [Google Scholar]
- 19. Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;(10):CD003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johansen KL. Exercise in end-stage renal disease population. J Am Soc Nephrol. 2007;18:1845-1854. [DOI] [PubMed] [Google Scholar]
- 21. Ellam T, Wilkie M. Peritoneal dialysis. Medicine. 2007;35:466-469. [Google Scholar]
- 22. Mehrotra R, Boeschoten EW. Current status of peritoneal dialysis. In: Khanna R, Kredit RT. eds. Nolph and Gokal’s Textbook of Peritoneal Dialysis. New York, NY: Springer; 2009:19-38. [Google Scholar]
- 23. Bargman JM. Hernias in peritoneal dialysis patients: limiting occurrence and recurrence. Perit Dial Int. 2008;28:349-351. [PubMed] [Google Scholar]
- 24. Albee BJ. Precautions for sports-minded patients on peritoneal dialysis. ANNA J. 1995;22:332-333. [PubMed] [Google Scholar]
- 25. Twardowski ZJ, Khanna R, Nolph KD, et al. Intra abdominal pressures during natural activities in patients with treated continuous ambulatory peritoneal dialysis. Nephron. 1986;44:11-16. [DOI] [PubMed] [Google Scholar]
- 26. Harel Z, Wald R, Juda A, Bell CM. Frequency and factors influencing publication of abstracts presented at three major nephrology meetings. Int Arch Med. 2011;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glick N, MacDonald I, Knoll G, Brabant A, Gourishankar S. Factors associated with publication following presentation at a transplantation meeting. Am J Transplant. 2006;6(3):552-556. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital. 2016. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 19, 2016.
- 30. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 31. Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37(3/4):256-266. [PubMed] [Google Scholar]
- 32. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Molsted S, Eiken P, Andersen JL, Eidemak I, Harrison AP. Interleukin-6 and vitamin D status during high-intensity resistance training in patients with chronic kidney disease. Biomed Res Int. 2014;2014:Article ID 176790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Molsted S, Harrison AP, Eidemak I, Dela F, Andersen JL. Improved glucose tolerance after high-load strength training in patients undergoing dialysis. Nephron Clin Pract. 2013;123(1-2):134-141. [DOI] [PubMed] [Google Scholar]
- 35. Cobo G, Gallar P, Gama-Axelsson T, et al. Clinical determinants of reduced physical activity in hemodialysis and peritoneal dialysis patients. J Nephrol. 2015;28(4):503-510. [DOI] [PubMed] [Google Scholar]
- 36. Oishi D, Koitabashi K, Hiraki K, et al. Physical activity is associated with serum albumin in peritoneal dialysis patients. Adv Perit Dial. 2012;28:148-152. [PubMed] [Google Scholar]
- 37. Masuda R, Imamura H, Mizuuchi K, Miyahara K, Kumagai H, Hirakata H. Physical activity, high-density lipoprotein cholesterol subfractions and lecithin:cholesterol acyltransferase in dialysis patients. Nephron Clin Pract. 2009;111(4):c253-c259. [DOI] [PubMed] [Google Scholar]
- 38. Wakamiya A, Hiraki K, Hotta C, et al. Poor nutritional status is associated with low physical activity in patients undergoing peritoneal dialysis. Int J Cardiol. 2015;187:648-650. [DOI] [PubMed] [Google Scholar]
- 39. Matsuoka K, Nakao T, Atsumi Y, Takekoshi H. Exercise regimen for patients with diabetic nephropathy. J Diabet Complications. 1991;5(2-3):98-100. [DOI] [PubMed] [Google Scholar]
- 40. Stack AG, Molony DA, Rives T, Tyson J, Murthy BV. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis. 2005;45(4):690-701. [DOI] [PubMed] [Google Scholar]
- 41. Shahgholian N, KarimiFard O, Shahidi S. Effects of aerobic exercise on blood glucose in continuous ambulatory peritoneal dialysis patients. Iran J Nurs Midwifery Res. 2015;20(2):165. [PMC free article] [PubMed] [Google Scholar]
- 42. Straub CK, Murphy SO, Rosenblum R. Exercise in the management of fatigue in patients on peritoneal dialysis. Nephrol Nurs J. 2008;35(5):469. [PubMed] [Google Scholar]
- 43. Lo CY, Li L, Lo WK, et al. Benefits of exercise training in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1998;32(6):1011-1018. [DOI] [PubMed] [Google Scholar]
- 44. Derici U, Canseven N, Sindel S. Dialysate leakage in CAPD patients. EDTNA ERCA J. 2005;31(1):13-14. [DOI] [PubMed] [Google Scholar]
- 45. Mustata S, Cooper L, Langrick N, Simon N, Jassal SV, Oreopoulos DG. The effect of a Tai Chi exercise program on quality of life in patients on peritoneal dialysis: a pilot study. Perit Dial Int. 2005;25(3):291-294. [PubMed] [Google Scholar]
- 46. Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677-684. [PubMed] [Google Scholar]
- 47. Grieco A, Long CJ. Investigation of the Karnofsky Performance Status as a measure of quality of life. Health Psychol. 1984;3(2):129-142. [DOI] [PubMed] [Google Scholar]
- 48. Sheng K, Zhang P, Chen L, Cheng J, Wu C, Chen J. Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol. 2014;40(5):478-490. [DOI] [PubMed] [Google Scholar]
- 49. Okaka EI, Naidoo S, Ahmed MM, Davies M, Naicker S. Quality of life in patients on continuous ambulatory peritoneal dialysis in an African setting. Saudi J Kidney Dis Transpl. 2015;26(3):631. [DOI] [PubMed] [Google Scholar]
- 50. Karakan S, Sezer S, Ozdemir FN. Factors related to fatigue and subgroups of fatigue in patients with end-stage renal disease. Clin Nephrol. 2011;76(5):358-364. [DOI] [PubMed] [Google Scholar]
- 51. Bujang MA, Musa R, Liu WJ, Chew TF, Lim CT, Morad Z. Depression, anxiety and stress among patients with dialysis and the association with quality of life. Asian J Psychiatr. 2015;18:49-52. [DOI] [PubMed] [Google Scholar]
- 52. Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 2015;137(2):471-480. [DOI] [PubMed] [Google Scholar]
- 53. Ericsson A, Bremell T, Cider Å, Mannerkorpi K. Effects of exercise on fatigue and physical capacity in men with chronic widespread pain-a pilot study. BMC Sports Sci Med Rehabil. 2016;8(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Voet N, Bleijenberg G, Hendriks J, et al. Both aerobic exercise and cognitive-behavioral therapy reduce chronic fatigue in FSHD: an RCT. Neurology. 2014;83(21):1914-1922. [DOI] [PubMed] [Google Scholar]
- 55. Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391-398. [DOI] [PubMed] [Google Scholar]
- 56. Kwon YE, Kee YK, Yoon CY, et al. Change of nutritional status assessed using subjective global assessment is associated with all-cause mortality in incident dialysis patients. Medicine. 2016;95(7):e2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jovanovic DB, Stosović MD, Gojakovic BM, et al. Inflammatory markers as mortality predictors in continuous ambulatory peritoneal dialysis patients. Ren Fail. 2015;37(2):230-236. [DOI] [PubMed] [Google Scholar]
- 58. Neefkes-Zonneveld CR, Bakkum AJ, Bishop NC, van Tulder MW, Janssen TW. Effect of long-term physical activity and acute exercise on markers of systemic inflammation in persons with chronic spinal cord injury: a systematic review. Arch Phys Med Rehabil. 2015;96(1):30-42. [DOI] [PubMed] [Google Scholar]
- 59. Monteiro-Junior RS, de Tarso Maciel-Pinheiro P, da Matta Mello Portugal E, da Silva Figueiredo LF, Terra R, Carneiro LS, et al. Effect of exercise on inflammatory profile of older persons: systematic review and meta-analyses. J Phys Act Health. 2017;15(1):64-71. [DOI] [PubMed] [Google Scholar]
- 60. Ryan AS, Ge S, Blumenthal JB, Serra MC, Prior SJ, Goldberg AP. Aerobic exercise and weight loss reduce vascular markers of inflammation and improve insulin sensitivity in obese women. J Am Geriatr Soc. 2014;62(4):607-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ahmad T, Fiuzat M, Mark DB, et al. The effects of exercise on cardiovascular biomarkers in patients with chronic heart failure. Am Heart J. 2014;167(2):193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sakhuja R, Shah AJ, Hiremath S, Thakur RK. End-stage renal disease and sudden cardiac death. Card Electrophysiol Clin. 2009;1(1):61-77. [DOI] [PubMed] [Google Scholar]
- 63. Fedewa MV, Gist NH, Evans EM, Dishman RK. Exercise and insulin resistance in youth: a meta-analysis. Pediatrics. 2014;133(1):e163-e174. [DOI] [PubMed] [Google Scholar]
- 64. Nelson AG, Kokkonen J, Arnall DA. Twenty minutes of passive stretching lowers glucose levels in an at-risk population: an experimental study. J Physiother. 2011;57(3):173-178. [DOI] [PubMed] [Google Scholar]
- 65. Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44(2):211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fagard RH. Effects of exercise, diet and their combination on blood pressure. J Hum Hypertens. 2005;19(suppl 3):S20-S24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PD_and_Exercise_SR_CJKHD_Supplementary_File for Physical Activity in Patients Treated With Peritoneal Dialysis: A Systematic Review and Meta-analysis by Tharshika Thangarasa, Rameez Imtiaz, Swapnil Hiremath and Deborah Zimmerman in Canadian Journal of Kidney Health and Disease
Supplemental material, Supplementary_File_PRISMA_2009_checklist for Physical Activity in Patients Treated With Peritoneal Dialysis: A Systematic Review and Meta-analysis by Tharshika Thangarasa, Rameez Imtiaz, Swapnil Hiremath and Deborah Zimmerman in Canadian Journal of Kidney Health and Disease
Supplemental material, Supplementary_file__table_2_(long) for Physical Activity in Patients Treated With Peritoneal Dialysis: A Systematic Review and Meta-analysis by Tharshika Thangarasa, Rameez Imtiaz, Swapnil Hiremath and Deborah Zimmerman in Canadian Journal of Kidney Health and Disease