Abstract
Low vision has been defined by best-corrected visual acuity worse than 20/40 in the better eye, substantial visual field loss, or substantial loss of contrast sensitivity that cannot be corrected by refraction, medical treatment, or surgery. In the United States, low vision is most commonly caused by age-related macular degeneration, glaucoma, and diabetic retinopathy. Most patients with low vision are elderly, although patients of all ages – including pediatric patients – may be affected. Low vision may decrease a patient’s quality of life substantially, leading to emotional distress and possibly depression. Low vision specialists aim to maximize the remaining vision of a patient by providing optical aids, orientation and mobility training, psychosocial support, and other methods of rehabilitation. Innovations in technology and devices offer additional options in low vision rehabilitation. Clinicians should consider referral to low vision specialists when a patient has difficulty with reading, mobility, driving, recognizing faces, or suffers from emotional distress due to low vision. Early referral may lead to improved outcomes.
Keywords: age-related macular degeneration, diabetic retinopathy, glaucoma, low vision, quality of life
Low vision is a permanent visual impairment, broadly defined as a best-corrected visual acuity (BCVA) worse than 20/40 in the better eye, substantial visual field loss, or substantial loss of contrast sensitivity, that is not correctable by refraction, medical treatment, or surgery.1 In 2010, there were an estimated 2.9 million people in the United States aged 40 years or older with low vision. In the United States, low vision is most commonly caused by age-related macular degeneration (AMD), glaucoma, and diabetic retinopathy (DR). Low vision can also affect pediatric patients due to a variety of genetic or acquired diseases.
Patients with low vision may have difficulty with activities of daily living (ADLs), leading to a lower quality of life (QoL) and possibly loss of independence.2 A working understanding of low vision services is important so that appropriate patients may be recognized and referred promptly. Low vision aids may be defined broadly to include training, standard options (including magnifiers), electronic options, nonoptical options, and surgical options (Table 1).
Table 1.
Currently available low vision aids.
| Vision rehabilitation training | Low vision centers |
| Online training | |
| Support groups | |
| Standard options | Handheld magnifiers |
| Lighted magnifiers | |
| High-plus reading lenses | |
| Telescopic attachments to glasses | |
| Electronic options | Optical readers |
| Laptop electronic magnifiers | |
| Desktop computer programs | |
| Low vision websites | |
| Nonoptical options | Solar shields or filters |
| Adaptive equipment | |
| Surgical options | Implantable miniature telescope |
| Retinal prosthesis system |
Provision of low vision services is complex and beyond the scope of most practicing ophthalmologists and optometrists. This article is intended to provide a concise, practical guide for the practicing clinician. It is not intended to be a comprehensive or systematic review. It is the authors’ hope that this information will assist practicing clinicians to identify which patients would benefit from low vision referral and to offer guidance on when and where to refer these patients.
The impact of low vision
According to the Medical Expenditure Panel Survey (1996–2002), the total annual economic impact of visual impairment and blindness in the United States was more than US $5.5 billion, primarily due to home care costs. Furthermore, when including an estimate of US $50,000 lost per quality-adjusted life year, the total impact increased to nearly US $16 billion annually.3
Despite this impact, the efficacy of low vision rehabilitation has been relatively understudied. For example, a 2012 systematic review identified only 7 relevant randomized control trials (RCTs) investigating the effectiveness of low vision services.4
Assessment of the low vision patient
The impact of visual impairment on QoL may be assessed using a variety of instruments. Multiple questionnaires have been validated psychometrically, including the National Eye Institute Visual Function Questionnaire (NEI-VFQ),5 the Impact of Vision Impairment (IVI) Profile,6 the Short Form-36,7 and the more general EuroQol EQ-5D.8 Time trade-off utility analysis, in which patients with visual impairment are asked how many years of their life they would be willing to trade in return for visual recovery, has been reported for many ophthalmic diseases.9 Conjoint analysis, in which subjects are queried about relative preferences, has been used to evaluate patients with glaucoma10 and AMD.11 The Functional Reading Independence (FRI) index, a 7-item patient-reported index assessing patient independence in performing reading activities, was validated for use specifically in patients with geographic atrophy (GA).12
When to refer adult patients
The American Academy of Ophthalmology (AAO) Preferred Practice Patterns (PPP) considers patients with a visual acuity worse than 20/40, contrast sensitivity loss, scotoma, or visual field loss as potential candidates for low vision rehabilitation.1 However, the degree of contrast sensitivity or field loss is not specified nor is the type or location of scotoma. In a recent survey evaluating the referral patterns to low vision services among members of the American Glaucoma Society (AGS), only 22% of glaucoma specialists noted that they followed the AAO PPP guidelines. Even among those specialists who referred more than five patients to low vision services monthly, only 38% used the AAO-recommended criteria.13 In addition to the relatively nonspecific nature and limited use of the AAO PPP guidelines, another consideration is that patients who do not meet the AAO-recommended criteria may also have limitations in functional vision.
It may be preferable to refer patients to low vision services based on an assessment of overall visual function, rather than a particular BCVA or visual field measurement. The following symptoms may warrant a referral for low vision rehabilitation:
Reading difficulty
Reading difficulty is reported frequently among patients with low vision. In a cross-sectional study of 357 patients with DR, ranging from mild nonproliferative DR to proliferative DR, it was reported that reading small print, completing lottery forms, and reading newspapers were the three most difficult vision-dependent activities. Difficulty in activities was assessed using the Vision-Specific Functioning Scale (VF-11), which includes 11 visual function questions to determine the level of difficulty in performing ADLs.14
As ophthalmic disease progresses, reading impairment may worsen. In a prospective study of 63 patients with glaucoma compared with 59 glaucoma suspect controls, reading ability and reading engagement were assessed over a total of 10 different reading activities. The patients with glaucoma reported significantly greater reading difficulty compared with controls in 9 of the 10 activities, with puzzles being the only exception. Worse reading ability, as measured by words read per minute, was associated with worse visual field loss, as measured by Humphrey 24-2 perimetry.15
Reading impairment may diminish QoL substantially, especially in elderly patients. A prospective study of 84 patients with glaucoma used the EQ-5D questionnaire, time trade-off utility analysis, and conjoint analysis and reported that reading was the most valued skill.16 Reading may be impaired by loss of visual acuity, visual field loss, or degradation of contrast sensitivity. Diminished reading ability has been associated with impaired emotional health, mobility, and participation in various activities.17
Low vision services that may improve a patient’s reading ability include environmental changes (such as better lighting), low vision optical aids, and eccentric viewing training.
Many patients report greater reading comfort with natural sunlight (when available) or with the OttLite (OttLite, Tampa, FL, USA). Similarly, many patients benefit from electronic reading devices, including the iPad (Apple, Cupertino, CA, USA), Kindle (Amazon, Seattle, WA, USA), or other devices. In a prospective study of 100 patients with low vision (aged 24–97 years), patients were tested reading standardized texts at baseline and with the iPad, the Clearview+ closed-circuit television (CCTV) (Optelec, Longueuil, QC, Canada), or a home magnification device such as a handheld magnifier. All of these devices were associated with significant improvements in reading speed.18
Low vision optical aids include high-plus reading lenses, magnifiers (including clip-on, handheld [Figure 1], wearable [Figure 2], and stand devices), and various electronic devices. The latter include video magnifiers (Ruby 7 HD, Freedom Scientific, St. Petersburg, FL, USA), assistive software (iZoom 6 USB; Issist Assistive Technologies, Georgetown, ON, Canada and ZoomText Fusion 11; Ai Squared, St. Petersburg, FL, USA), and wearable devices (Jordy; Enhanced Vision, Huntington Beach, CA, USA and OrCam, Jerusalem, Israel [Figure 3]).
Figure 1.

Demonstration of a patient using a handheld magnifier.
Figure 2.

Wearable magnifiers, similar to surgical loupes.
Figure 3.

Wearable electronic device (OrCam, Jerusalem, Israel).
Eccentric viewing training uses a patient’s preferred retinal locus (PRL) to view an object. The PRL is an undamaged area of the retina, other than the fovea, that allows patients to shift their vision so that they can see objects in the setting of a scotoma. In a prospective series of 20 patients with neovascular AMD, an absolute central scotoma and a mean BCVA of 20/475, 18 patients learned to use eccentric viewing. After an average training time of 5.2 hours, there was a significant improvement in reading speed from 9.0 ± 5.8 words per minute (wpm) to 68.3 ± 19.4 wpm.19 In a systematic review examining the effect of low vision strategies on reading speed, as measured by wpm, it was reported that compared with a microperimetric biofeedback and microscopes teaching program, eccentric viewing training demonstrated the greatest improvements in reading speed.20
Similarly, in a retrospective study of 530 patients with different stages of AMD, participants were given low vision aids, with appropriate training, based on their magnification requirements. In this series, 58% of patients used optical visual aids, whereas 42% of patients used electronic closed-circuit systems. Patients read two different texts of comparable difficulty with and without their low vision aids. A significant increase in average reading speed with a low vision aid was reported for the entire study group. It was also noted that only 16% of patients could read before the use of low vision aids, whereas 94% of patients could read after the use of low vision aids.21
Mobility
Difficulties with mobility, including walking, climbing stairs, maintaining balance, and exercise, are reported commonly among low vision patients. Such impairments in mobility are associated with an increased risk of falls,22 increased rates of morbidity and mortality, decreased physical activity, and a decreased QoL among patients with glaucoma.23
In a prospective observational study, investigators examined the mobility of 1078 patients through a variety of tasks including walking through an obstacle course. Of the 1078 patients, 74 had bilateral glaucoma, 76 had unilateral glaucoma, and 1064 had no or possible glaucoma. Glaucoma was defined using a combination of visual field defects and optic nerve appearance. Patients with bilateral glaucoma had a worse mean mobility speed, a higher mean number of ‘bumps’ (into obstacles), and a slower mean stair climbing speed than patients with unilateral or no glaucoma, and all of these differences were significant.24
Similarly, a prospective study compared 59 normal controls with 57 patients with AMD, defined as bilateral disease with either GA or choroidal neovascularization in at least one eye and drusen or pigment abnormalities in both eyes. Investigators examined mobility using accelerometers and cellular network–based tracking devices over 7 days of regular patient activity. The accelerometer was clipped to the subject’s waistband and recorded the patient’s steps during waking hours. The tracking device was also clipped to the waistband and recorded the subject’s longitude and latitude every 15 min between 7 a.m. and 11 p.m. The results demonstrated that patients with AMD spent significantly less time in moderate-to-vigorous physical activity and were significantly more likely to not leave their home on a given day.25
The degree of disease progression further affects an individual’s mobility. A greater degree of visual field loss, assessed using the Humphrey Field Analyzer II, and a thinner retinal nerve fiber layer, assessed by time-domain optical coherence tomography, was associated with greater postural sway. Postural sway was determined using a swaymeter, which is a rod attached to the subject’s waist that measures the amount of body displacement at waist height and provides a clinical measure of postural control.26
Training by a Certified Orientation and Mobility Specialist is a component of low vision rehabilitation which helps patients with basic movement, perceptual skills, physical capacity, and nonverbal communication, both indoors and outdoors. Assistive devices for mobility include canes, walking frames, electronic devices, guide dogs, and other devices. A retrospective, descriptive, cross-sectional study of a cohort of 617 individuals, all aged 85 years, in Sweden, reported visual impairment of about 20/30 or worse in 61%, the use of assistive devices in 77%, and a significant association between visual impairment and the use of assistive devices.27 White canes are distinct in the United States, Canada, and many European nations for their use by the visually impaired. The use of guide dogs is not as common.
Low vision rehabilitation programs have been reported to be effective in improving patient mobility. In a multicenter RCT of 128 patients using Veterans Affairs services and with a BCVA between 20/100 and 20/500 in the better-seeing eye, 64 received low vision intervention, whereas 62 patients served as a control group. The low vision intervention targeted common patient goals including seeing better at all distances, long-duration reading and distance viewing, glare control, and spot-checking (i.e. reading price tags). This intervention consisted of five weekly sessions with a low vision therapist who taught effective use of low vision aids and patients’ remaining vision, 5 hours of weekly homework to practice using the low vision devices, and one visit to set up low vision devices in the home. The most common low vision aids used were CCTV-viewing systems, stand magnifiers, outdoor filters for glare control, and pocket magnifiers. The Veterans Affairs Low-Vision Visual Functioning Questionnaire (VA LV VFQ-48) was used to compare visual functioning at baseline and at a 4-month follow-up. The VA LV VFQ-48 consists of 48 items representing four functional domains, of which one is mobility. Although the primary outcome was change in visual reading ability, patients in the treatment group reported significant improvement in mobility compared with the control group.28 To date, few RCTs have been performed to investigate the effectiveness of low vision services.29
Driving
Visual impairment can have a profound impact on an individual’s ability to drive. In patients with AMD, reduced central vision may decrease the ability to see road signs or cars, and in patients with glaucoma, a gradual constriction of the peripheral visual field can make it difficult to see cars or pedestrians approaching from the side.30
In a comparative cross-sectional study, 92 patients with early or intermediate AMD (defined as one or more large drusen or focal hyperpigmentation in at least one eye without GA or choroidal neovascularization in either eye) and a BCVA of 20/60 or better self-reported their visual difficulties with specific ADLs on a scale of 0 (extreme difficulty) to 100 (no difficulty). Activities were divided into subsections that included day driving, night driving, near vision, fine vision, and lack of glare. All 92 patients with AMD reported significantly more concern with night driving compared with age-matched controls with a BCVA of 20/35 or better and a normal retinal examination. Among those patients with AMD with a fellow (better) eye worse than 20/60, day driving was significantly worse compared with both the controls and patients with AMD with a fellow (better) eye vision of 20/60 or better.31
Difficulty with driving due to visual impairment can pose substantial risks to the health of the patient and to the public. In a retrospective series of 48 patients with glaucoma and 47 age-matched controls (defined as having a normal eye examination and BCVA better than 20/40 in each eye) using both self-reported and police-reported motor vehicle collisions as a main outcome measure, patients with glaucoma were six times more likely to have been involved in one or more collisions within the previous 5 years.32
For patients with visual impairment that may affect driving, it is important to consider a referral to low vision services even if the patients still meet their jurisdiction’s requirements for legal driving. In elderly patients, loss of driving privileges is associated with a lower QoL and higher rates of depression.23
Vision rehabilitation can educate patients on restrictions they can undertake to reduce risk such as limited driving at night or driving only in familiar surroundings.33 Bioptic telescopic spectacles, which are legal in some jurisdictions for driving, are also an option that help improve visual acuity while driving and help patients see key targets; however, information on whether or not these spectacles reduce the risk of motor vehicle collisions is limited.34 Patients with AMD in particular can also be taught to use scanning eye movements while driving so that objects do not become lost in their scotomas.
Emotional distress
Low vision can have a profound impact on an individual’s psychosocial functioning. A prospective study examined QoL in 86 individuals with AMD who had a BCVA worse than 20/60 in both eyes with at least one eye 20/200 or worse. Investigators used multiple assessments including the Quality of Well-Being Scale, Instrumental Activities of Daily Living Index, and self-rated general health status and profile of mood states. It was reported that the emotional distress experienced by patients with AMD was comparable with adults living with chronic illnesses such as AIDS.35 In patients with AMD who experience emotional distress, it has been estimated that depression develops in almost one-third of patients.36,37
The severity of visual impairment is also associated with increased emotional impact. A prospective observational study stratified 360 patients with neovascular AMD into four different groups based on visual acuity level and asked them to rate themselves on the Hospital Anxiety and Depression Scale (HADS). This scale contains seven questions related to anxiety and seven related to depression. Patients rated each question on a scale of 0 to 3. Scores of 8 or higher, out of a total of 21, signify anxiety or depression needing further assessment. It was reported that a worse visual acuity was associated with an increased prevalence of depression.38 Another prospective study reported similar findings among patients with glaucoma. Using the Glaucoma Quality of Life-15 Questionnaire, 121 patients self-reported a significantly worse QoL compared with 31 patients without glaucoma. Patients with glaucoma were further divided into subgroups of mild, moderate, or severe disease using visual field criteria. It was reported that the QoL questionnaire results correlated with disease severity.39
Vision rehabilitation can be important in improving psychosocial function. Low vision services have been associated with improvement in depressive symptoms, emotional well-being, and overall QoL in low vision patients.40-42 A prospective study of 438 elderly patients with visual impairment who applied for low vision rehabilitation examined the impact of low vision services on disability and depression. Patients were interviewed before rehabilitation and 6 months after rehabilitation, and depressive symptoms were analyzed using the 20-item Center for Epidemiologic Studies Depression Scale. Patients rated how often they experienced depressive symptoms, such as feelings of worthlessness, on a scale of 1 to 4 with 1 being symptoms that last a day or less and 4 being symptoms occurring 5 to 7 days in a week. At 6 months of follow-up, the use of optical aids, including magnifiers, telescopes, and specialized sunglasses, significantly reduced the prevalence of depressive symptoms.42
The integration of low vision rehabilitation with mental health services can provide an even greater improvement in emotional well-being. A randomized clinical trial of 188 low vision patients with subthreshold depression compared the effectiveness of behavioral activation (BA) with low vision therapy versus standard outpatient low vision rehabilitation with respect to preventing depression. Behavioral activation is a behavioral treatment that focuses on the association of action, mood, and mastery with promoted self-efficacy and social connection as a method to improve mood and function. Subthreshold depression was defined as a depressed mood several days a week or a score of >5 on the Patient Health Questionnaire-9, which consists of nine criteria that define the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, DSM-IV) diagnoses of depression. In both treatment groups, patients received six 1-hour sessions over 8 weeks. After 4 months of follow-up, the incidence of depression was 12.6% in the BA+ rehabilitation group, compared with 23.4% in the standard therapy group.43
Facial recognition
Low vision patients may have difficulty in identifying faces or facial expressions. A prospective study compared the ability of 54 patients with glaucoma to correctly identify faces using the Cambridge Face Memory Test against 41 visually healthy controls. The patients with glaucoma were further classified based on the severity of the visual field defect in their better-seeing eye (early, moderate, or advanced visual field defects). Patients with advanced visual field defects were reported to identify fewer faces correctly compared with controls and those with early or moderate defects.44 The patients with AMD were also reported to be significantly worse at recognizing if a face is expressing an emotion, and then subsequently classifying that emotion, compared with age-matched controls.45 Use of magnification alone improved participant performance, but it was still worse than that of the age-matched controls.45 Trouble with facial recognition may impair social relationships, which can further deteriorate QoL.
Low vision rehabilitation offers coping methods, group support, optical aids, and bioptic telescopic low vision devices to aid facial recognition. In a prospective study of 30 patients with bilateral AMD and a visual acuity in the better eye between 20/50 and 20/500, the efficacy of the bioptic telescopic device for facial recognition was investigated. The use of the bioptic telescopic device significantly improved both familiar face recognition, measured by identifying images of famous people, and the ability to discriminate between different facial expressions, measured by a patient’s ability to associate an image with a facial expression.46 New downloadable apps for mobile devices offer inexpensive and readily available reading tools for everyday use.
Pediatric low vision
Statistics on pediatric visual loss are relatively limited, but in 2010, there were an estimated 5 million children worldwide with low vision.47 Low vision in children and teenagers can result from many different conditions, both congenital and acquired, such as pediatric cataracts, pediatric glaucoma, nystagmus, and retinal abnormalities.
Low vision devices used for children include handheld magnifiers, stand magnifiers, and CCTV, among others. In a prospective study examining the clinical characteristics of 150 children who presented for low vision rehabilitation, it was reported that telescopic lenses were most frequently used for distance vision, and magnifiers and tele-microscopic systems were most frequently used for near vision.47
A prospective study reported the benefits of low vision rehabilitation in a group of 35 low vision children from the age of 6 to 16. These children self-reported their functional vision using the Low-Vision Prasad-Functional Vision Questionnaire (LVP-FVQ) before and after low vision rehabilitation. This questionnaire consists of 19 questions to assess functional vision and each question is rated from 0 to 5, with 5 being ‘unable to perform activity due to visual reasons’. The most common difficulties in low vision children were related to academic activities including copying from a blackboard, reading a textbook at arm’s length, and writing along a straight line. After 2 months of low vision rehabilitation, which included telescopes and nonoptical devices such as reading stands and large printed books, there was a significant improvement in the performance of all three of these activities as measured by the participant response to the LVP-FVQ.48
In an RCT of 33 children in the Netherlands with a BCVA of 20/50 or worse in the better-seeing eye, the benefits of stand magnifiers were studied. A total of 18 children were given low vision rehabilitation training with a magnifier and were compared against 15 children with low vision rehabilitation training without a magnifier. Children were asked to follow different trails on a large cardboard and identify a hidden picture both before and after training. After twelve 30-min sessions over 6 weeks, both study groups could follow more trails in the posttest compared with the pretest. Although both groups improved, the posttest performance of the children using magnifiers was found to be significantly better than the group that did not use magnifiers.49 Other RCTs have reported benefits in reading skills and reading volume after using visual analysis training50 and in using large-print textbooks.51
Importance of early referral
Clinicians should consider referral to low vision specialists early in the visual impairment process. An earlier referral provides more time for patients to develop a relationship with the low vision team, which can lead to a better understanding of which interventions are best for the patient.
Patients with relatively milder degrees of visual loss may require less intensive interventions, making them more likely to accept the assistance. The need for less intensive intervention was demonstrated in an RCT of 323 Veterans Affairs patients with any macular disease and a BCVA in the better-seeing eye of 20/50 to 20/200. A total of 160 patients were assigned to receive basic low vision services, which consisted of low vision devices without therapy or homework, and 163 patients were assigned to receive low vision rehabilitation, which included low vision devices with one to three therapy sessions. These therapy sessions included instruction in eccentric viewing, use of low vision devices, environmental modification, homework, and integration of devices into lifestyle. Patients were assessed at baseline and after 4 months using the self-reported VA LV VFQ-48, Short Form-36, and EuroQuol-5D. Using the VA LV VFQ-48, patients in the low vision rehabilitation group demonstrated significantly more improvement in reading ability, visual information processing, visual motor skill, and overall visual ability than patients in the basic low vision services group. When these results were stratified by visual acuity, there was no significant difference between the two groups in any domain among those with a visual acuity of 20/50 to 20/63. However, in those with a visual acuity of 20/63 to 20/200, there was a significantly greater improvement in the low vision rehabilitation group compared with the basic low vision group in reading ability, visual motor skill, and overall visual ability.52
The importance of early referral is also important in children. Visual impairment can interfere with learning in an academic environment, and early referral may increase children’s comfort with optical aids and improve their functional vision, allowing them to perform better in school.48
Clinicians should not let active ophthalmologic treatment, such as with anti-vascular endothelial growth factor therapy, delay referral to Certified Low Vision Therapists and Occupational Low Vision Therapists. A retrospective study compared the referral patterns of a retina practice to low vision services in the year before the Food and Drug Administration approved ranibizumab (Lucentis; Genentech, South San Francisco, CA, USA) (year 1) against the year after ranibizumab was approved (year 2). In year 1, before the use of ranibizumab, 24 patients with neovascular AMD were referred to low vision services. In year 2 of the study, during which patients received ranibizumab, only 12 patients with neovascular AMD were referred to low vision services. At the time of referral, the patients referred in year 2 still had a worse visual acuity and contrast sensitivity compared with those patients referred in year 1. A possible reason cited for this decreased referral was waiting to see what the final visual acuity of the patient would be once therapy was complete.53 Because patients may struggle with functional vision even while receiving therapy, referral to low vision professionals for evaluation should not be delayed.
Surgical options
Two currently available surgical interventions may provide benefit to patients with otherwise uncorrectable low vision. These include the Implantable Miniature Telescope (IMT; VisionCare Ophthalmic Technologies, Saratoga, CA, USA) and the retinal prosthesis system (Argus II; Second Sight, Sylmar, CA, USA).
The IMT (Figure 4) is a surgically implantable visual prosthesis that is approved for use in patients 65 years and older with end-stage AMD, defined as bilateral AMD due to GA, disciform scar associated with choroidal neovascularization, or both.54,55 The retinal prosthesis system (Figure 5) is approved for use in patients 25 years and older with retinitis pigmentosa causing bare to no light perception.56 The preoperative evaluation and postoperative care of patients treated with both of these devices requires close collaboration between the ophthalmologists and the low vision specialists.
Figure 4.
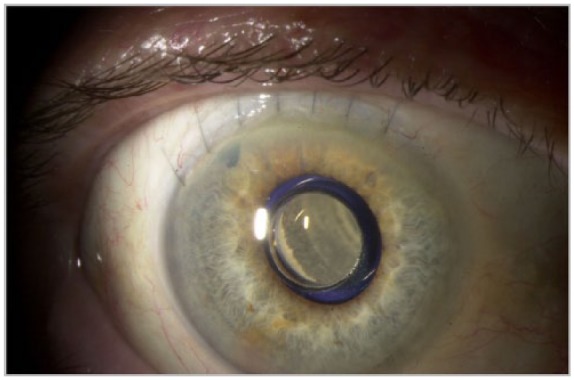
Slit-lamp photograph, right eye, of an implantable miniature telescope (VisionCare Ophthalmic Technologies, Saratoga, CA, USA).
Image courtesy of Marc H Levy, MD.
Figure 5.

Fundus photograph, right eye, of the retinal prosthesis system (Argus II, Second Sight, Sylmar, CA, USA) in a patient with retinitis pigmentosa.
Image courtesy of Ninel Z Gregori, MD.
Barriers to low vision services
Even when low vision services are available, patients may not use them. In a cross-sectional study, 702 patients with a BCVA in the better-seeing eye of worse than 20/60 or a visual field worse than 60° in either the horizontal or vertical meridian underwent structured interviews aimed at assessing patient demographics. Among these patients, only 54% had used low vision services, 33% of patients had never heard of vision rehabilitation or were never referred, and 13% knew of the services but did not use them.57 In this series, highly educated patients were significantly more likely to be aware of low vision services and use them.57 In a global survey also analyzing the barriers to low vision services, it was reported that availability, funding, and awareness were the three main obstacles to using low vision services worldwide.58
Where to refer patients
Many organizations provide low vision services (Table 2). At the federal level, the US Department of Veterans Affairs offers a Visual Impairment Services Team (VIST) in many locations for eligible veterans. More locally, many academic centers provide low vision services. These include departments of ophthalmology and optometry, such as the Lions Low Vision and Vision Rehabilitation Center at Johns Hopkins’ Wilmer Eye Institute and the Low Vision Clinic at the University of California Berkeley School of Optometry. Many nonprofit organizations offer these services to their local communities. Some of these organizations use the name Lighthouse, although the various Lighthouse organizations around the US (such as the Miami Lighthouse for the Blind and visually impaired, Lighthouse Guild in New York, and Chicago Lighthouse) are not typically related at the organizational level. Finally, many private practices offer low vision services; these practices may be independent or affiliated with ophthalmology or optometry practices.
Table 2.
Providers of low vision services.
| Federal | US Department of Veterans Affairs |
| Academic centers | Wilmer Eye Institute |
| University of California Berkeley | |
| Nonprofit organizations | Lighthouses |
| Private organizations |
Summary
Low vision in both adult and pediatric patients can impair QoL and ADLs. New therapies, such as voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics, Philadelphia, PA),59 have the potential to treat some formerly untreatable diseases, but for many patients low vision remains the best option. Ophthalmologists may underrecognize the role that timely referral to low vision professionals may play in optimizing patients’ vision-related functioning. Through multiple different approaches, of which optical aids are only one, low vision rehabilitation can help patients maximize their vision-related functioning to optimize their QoL and ADLs.
Footnotes
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.G.S. reports consulting fees from Alimera and Welch Allyn. The other authors have no interests to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is partially supported by NIH Center Core Grant P30EY014801 and an Unrestricted Grant from Research to Prevent Blindness.
ORCID iD: Stephen G Schwartz  https://orcid.org/0000-0002-1441-9473
https://orcid.org/0000-0002-1441-9473
Contributor Information
Parth Shah, Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Naples, FL, USA.
Stephen G. Schwartz, Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Naples, FL, USA.
Scott Gartner, Miami Lighthouse for the Blind and Visually Impaired, Miami, FL, USA Lighthouse for the Blind of the Palm Beaches, West Palm Beach, FL, USA.
Ingrid U. Scott, Departments of Ophthalmology and Public Health Sciences, Penn State College of Medicine, Hershey, PA, USA
Harry W. Flynn, Jr, Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, FL, USA.
References
- 1. American Academy of Ophthalmology Vision Rehabilitation Committee. Preferred practice pattern guidelines. Vision rehabilitation. San Francisco, CA: American Academy of Ophthalmology, 2013, www.aao.org/ppp (accessed 24 July 2017). [Google Scholar]
- 2. Scott IU, Smiddy WE, Schiffman J, et al. Quality of life of low-vision patients and the impact of low-vision services. Am J Ophthalmol 1999; 128: 54–62. [DOI] [PubMed] [Google Scholar]
- 3. Frick KD, Gower EW, Kempen JH, et al. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol 2007; 125: 544–550. [DOI] [PubMed] [Google Scholar]
- 4. Binns AM, Bunce C, Dickinson C, et al. How effective is low vision service provision? A systematic review. Surv Ophthalmol 2012; 57: 34–65. [DOI] [PubMed] [Google Scholar]
- 5. Mangione CM, Lee PP, Pitts J, et al. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ field test investigators. Arch Ophthalmol 1998; 116: 1496–1504. [DOI] [PubMed] [Google Scholar]
- 6. Weih LM, Hassell JB, Keeffe J. Assessment of the impact of vision impairment. Invest Ophthalmol Vis Sci 2002; 43: 927–935. [PubMed] [Google Scholar]
- 7. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 8. Devlin NJ, Brooks R. EQ-5D and the EuroQol Group: past, present and future. Appl Health Econ Health Policy 2017; 15: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown MM, Brown GC, Sharma S, et al. Quality of life associated with visual loss: a time tradeoff utility analysis comparison with medical health states. Ophthalmology 2003; 110: 1076–1081. [DOI] [PubMed] [Google Scholar]
- 10. Aspinall PA, Hill AR, Nelson P. Quality of life in patients with glaucoma: a conjoint analysis approach. Vis Impair Res 2005; 7: 13–26. [Google Scholar]
- 11. Aspinall PA, Hill AR, Dhillon B, et al. Quality of life and relative importance: a comparison of time trade-off and conjoint analysis methods in patients with age-related macular degeneration. Br J Ophthalmol 2007; 91: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kimel M, Leidy NK, Tschosik E, et al. Functional Reading Independence (FRI) index: a new patient-reported outcome measure for patients with geographic atrophy. Invest Ophthalmol Vis Sci 2016; 57: 6298–6304. [DOI] [PubMed] [Google Scholar]
- 13. Kaleem MA, West SK, Im LT, et al. Referral to low vision services for glaucoma patients: referral patterns and characteristics of those who refer. J Glaucoma 2017; 26: e115–e120. [DOI] [PubMed] [Google Scholar]
- 14. Lamoureux EL, Tai ES, Thumboo J, et al. Impact of diabetic retinopathy on vision-specific function. Ophthalmology 2010; 117: 757–765. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen AM, van Landingham SW, Massof RW, et al. Reading ability and reading engagement in older adults with glaucoma. Invest Ophthalmol Vis Sci 2014; 55: 5284–5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aspinall PA, Johnson ZK, Azuara-Blanco A, et al. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci 2008; 49: 1907–1915. [DOI] [PubMed] [Google Scholar]
- 17. Hassell JB, Lamoureux EL, Keeffe JE. Impact of age related macular degeneration on quality of life. Br J Ophthalmol 2006; 90: 593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morrice E, Johnson AP, Marinier JA, et al. Assessment of the Apple iPad as a low-vision reading aid. Eye 2017; 31: 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nilsson UL, Frennesson C, Nilsson SE. Patients with AMD and a large absolute central scotoma can be trained successfully to use eccentric viewing, as demonstrated in a scanning laser ophthalmoscope. Vision Res 2003; 43: 1777–1787. [DOI] [PubMed] [Google Scholar]
- 20. Hamade N, Hodge WG, Rakibuz-Zaman M, et al. The effects of low-vision rehabilitation on reading speed and depression in age related macular degeneration: a meta-analysis. PLoS ONE 2016; 11: e0159254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen NX, Weismann M, Trauzettel-Klosinski S. Improvement of reading speed after providing of low vision aids in patients with age-related macular degeneration. Acta Ophthalmol 2009; 87: 849–853. [DOI] [PubMed] [Google Scholar]
- 22. Ivers RQ, Cumming RG, Mitchell P, et al. Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc 1998; 46: 58–64. [DOI] [PubMed] [Google Scholar]
- 23. Ramulu P. Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol 2009; 20: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friedman DS, Freeman E, Munoz B, et al. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology 2007; 114: 2232–2237. [DOI] [PubMed] [Google Scholar]
- 25. Sengupta S, Nguyen AM, van Landingham SW, et al. Evaluation of real-world mobility in age-related macular degeneration. BMC Ophthalmol 2015; 15: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Black AA, Wood JM, Lovie-Kitchin JE, et al. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci 2008; 85: 489–497. [DOI] [PubMed] [Google Scholar]
- 27. Dahlin-Ivanoff S, Sonn U. Use of assistive devices in daily activities among 85-year-olds living at home focusing especially on the visually impaired. Disabil Rehabil 2004; 26: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 28. Stelmack JA, Tang XC, Reda DJ, et al. Outcomes of the Veterans Affairs Low Vision Intervention Trial (LOVIT). Arch Ophthalmol 2008; 126: 608–617. [DOI] [PubMed] [Google Scholar]
- 29. Rubin GS. Demonstrating the effectiveness of low-vision rehabilitation with outcomes of the Veterans Affairs Low Vision Intervention Trial II (LOVIT II). JAMA Ophthalmol. Epub ahead of print 15 December 2016. DOI: 10.1001/jamaophthalmol.2016.4778. [DOI] [PubMed] [Google Scholar]
- 30. Klein R. Age-related eye disease, visual impairment, and driving in the elderly. Hum Factors 1991; 33: 521–525. [DOI] [PubMed] [Google Scholar]
- 31. Scilley K, Jackson GR, Cideciyan AV, et al. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology 2002; 109: 1235–1242. [DOI] [PubMed] [Google Scholar]
- 32. Haymes SA, Leblanc RP, Nicolela MT, et al. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci 2007; 48: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 33. Colenbrander A, Goodwin L, Fletcher DC. Vision rehabilitation and AMD. Int Ophthalmol Clin 2007; 47: 139–148. [DOI] [PubMed] [Google Scholar]
- 34. Owsley C, McGwin G. Driving and age-related macular degeneration. J Vis Impair Blind 2008; 102: 621–635. [PMC free article] [PubMed] [Google Scholar]
- 35. Williams RA, Brody BL, Thomas RG, et al. The psychosocial impact of macular degeneration. Arch Ophthalmol 1998; 116: 514–520. [DOI] [PubMed] [Google Scholar]
- 36. Rovner BW, Casten RJ, Tasman WS. Effect of depression on vision function in age-related macular degeneration. Arch Ophthalmol 2002; 120: 1041–1044. [DOI] [PubMed] [Google Scholar]
- 37. Brody BL, Gamst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology 2001; 108: 1893–1900; discussion 1891–1900. [DOI] [PubMed] [Google Scholar]
- 38. Augustin A, Sahel JA, Bandello F, et al. Anxiety and depression prevalence rates in age-related macular degeneration. Invest Ophthalmol Vis Sci 2007; 48: 1498–1503. [DOI] [PubMed] [Google Scholar]
- 39. Goldberg I, Clement CI, Chiang TH, et al. Assessing quality of life in patients with glaucoma using the Glaucoma Quality of Life-15 (GQL-15) Questionnaire. J Glaucoma 2009; 18: 6–12. [DOI] [PubMed] [Google Scholar]
- 40. Vingolo E, De Rosa V, Domanico D, et al. Low vision rehabilitation: current perspectives. Clin Optomet 2015; 7: 53–58. [Google Scholar]
- 41. Lamoureux EL, Pallant JF, Pesudovs K, et al. The effectiveness of low-vision rehabilitation on participation in daily living and quality of life. Invest Ophthalmol Vis Sci 2007; 48: 1476–1482. [DOI] [PubMed] [Google Scholar]
- 42. Horowitz A, Brennan M, Reinhardt JP, et al. The impact of assistive device use on disability and depression among older adults with age-related vision impairments. J Gerontol B Psychol Sci Soc Sci 2006; 61: S274–S280. [DOI] [PubMed] [Google Scholar]
- 43. Rovner BW, Casten RJ, Hegel MT, et al. Low vision depression prevention trial in age-related macular degeneration: a randomized clinical trial. Ophthalmology 2014; 121: 2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Glen FC, Crabb DP, Smith ND, et al. Do patients with glaucoma have difficulty recognizing faces? Invest Ophthalmol Vis Sci 2012; 53: 3629–3637. [DOI] [PubMed] [Google Scholar]
- 45. Johnson AP, Woods-Fry H, Wittich W. Effects of magnification on emotion perception in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 2017; 58: 2520–2526. [DOI] [PubMed] [Google Scholar]
- 46. Tejeria L, Harper RA, Artes PH, et al. Face recognition in age related macular degeneration: perceived disability, measured disability, and performance with a bioptic device. Br J Ophthalmol 2002; 86: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ozen Tunay Z, Caliskan D, Idil A, et al. Clinical characteristics and low vision rehabilitation methods for partially sighted school-age children. Turk J Ophthalmol 2016; 46: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ganesh S, Sethi S, Srivastav S, et al. Impact of low vision rehabilitation on functional vision performance of children with visual impairment. Oman J Ophthalmol 2013; 6: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cox RF, Reimer AM, Verezen CA, et al. Young children’s use of a visual aid: an experimental study of the effectiveness of training. Dev Med Child Neurol 2009; 51: 460–467. [DOI] [PubMed] [Google Scholar]
- 50. Bieger E. Effectiveness of visual training on letters and words on reading skills of non-readers. J Educ Res 1978; 71: 157–161. [Google Scholar]
- 51. Lackey G, Efron M, Rowls M. For more reading: large print books or the Visolett? Educ Visual Handicap 1982; 14: 87–94. [Google Scholar]
- 52. Stelmack JA, Tang XC, Wei Y, et al. Outcomes of the Veterans Affairs Low Vision Intervention Trial II (LOVIT II): a randomized clinical trial. JAMA Ophthalmol. Epub ahead of print 15 December 2016. DOI: 10.1001/jamaophthalmol.2016.4742. [DOI] [PubMed] [Google Scholar]
- 53. Sunness JS, Schartz RB, Thompson JT, et al. Patterns of referral of retinal patients for low vision intervention in the anti-VEGF era. Retina 2009; 29: 1036–1039. [DOI] [PubMed] [Google Scholar]
- 54. Boyer D, Freund KB, Regillo C, et al. Long-term (60-month) results for the implantable miniature telescope: efficacy and safety outcomes stratified by age in patients with end-stage age-related macular degeneration. Clin Ophthalmol 2015; 9: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hudson HL, Lane SS, Heier JS, et al. Implantable miniature telescope for the treatment of visual acuity loss resulting from end-stage age-related macular degeneration: 1-year results. Ophthalmology 2006; 113: 1987–2001. [DOI] [PubMed] [Google Scholar]
- 56. Humayun MS, Dorn JD, da Cruz L, et al. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology 2012; 119: 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Overbury O, Wittich W. Barriers to low vision rehabilitation: the Montreal Barriers Study. Invest Ophthalmol Vis Sci 2011; 52: 8933–8938. [DOI] [PubMed] [Google Scholar]
- 58. Chiang PP, O’Connor PM, Le Mesurier RT, et al. A global survey of low vision service provision. Ophthalmic Epidemiol 2011; 18: 109–121. [DOI] [PubMed] [Google Scholar]
- 59. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 2017; 390: 849-860. [DOI] [PMC free article] [PubMed] [Google Scholar]


