Abstract
The aim of this study was to critically evaluate the quality of the models used in economic evaluations of screening strategies for cervical cancer prevention. We systematically searched multiple databases, selecting model-based full economic evaluations (cost-effectiveness analyses, cost-utility analyses, and cost-benefit analyses) of cervical cancer screening strategies. Two independent reviewers screened articles for relevance and performed data extraction. Methodological assessment of the quality of the models utilized formal checklists, and a qualitative narrative synthesis was performed. Thirty-eight articles were reviewed. The majority of the studies were conducted in high-income countries (82%, n=31). The Pap test was the most used screening strategy investigated, which was present in 86% (n=33) of the studies. Half of the studies (n=19) used a previously published Markov model. The deterministic sensitivity analysis was performed in 92% (n=35) of the studies. The mean number of properly reported checklist items was 9 out of the maximum possible 18. Items that were better reported included the statement of decision problem, the description of the strategies/comparators, the statement of time horizon, and information regarding the disease states. Compliance with some items of the checklist was poor. The Markov models for economic evaluation of screening strategies for cervical cancer varied in quality. The following points require improvement: 1) assessment of methodological, structural, heterogeneity, and parameter uncertainties; 2) model type and cycle length justification; 3) methods to account for heterogeneity; and 4) report of consistency evaluation (through calibration and validation methods).
Keywords: Uterine Cervical Neoplasms, Mass Screening, Decision Modeling, Markov Chains, Cost-benefit Analysis
INTRODUCTION
Cervical cancer continues to be an important public health problem, with an estimated 266,000 deaths from cervical cancer worldwide in 2012 (approximately 87% of cervical cancer deaths occur in less developed regions) 1. Screening programs have reduced the incidence and mortality of cervical cancer. However, substantial costs are involved in providing the infrastructure, training the manpower, buying consumables, elaborating surveillance mechanisms, and treating and following up with patients 2. Therefore, successful programs will require using evidence-based, cost-effective approaches and strengthening national health systems 3.
Decision-analytic modeling (DAM) has increasingly been used to assess cancer prevention and control strategies in terms of their cost-effectiveness and to inform public policies. DAM supports decision makers in making choices related to the evaluated screening strategies for cervical cancer options.
Cervical screening models vary considerably in their degree of complexity. The Markov model is the most common model used to simulate the natural history of progression to cervical pre-neoplastic and neoplastic disease. This popularity is likely due to the apparent simplicity of its implementation and use.
Previous reviews 4-8 have specifically discussed the use of DAM to evaluate the cost effectiveness of cervical cancer screening, and others have discussed models that also evaluate the impact of human papillomavirus (HPV) vaccination on screening programs. However, none of these reviews critically evaluated the quality of the Markov models used in economic evaluations of screening strategies for cervical cancer using formal checklists. These instruments may identify flaws that influence the cost-effectiveness results 9. Thus, critical evaluation can confirm the credibility and reliability of the results being used by decision makers 10.
The aim of this review, which was performed as part of a health technology assessment project funded by the Brazilian Public Health System, was to provide an overview of the quality of Markov models for economic evaluation of screening strategies for cervical cancer prevention. We identify some of the most important methodological issues, reflect on the reasons for the poor report and discuss implications for research standards.
MATERIALS AND METHODS
Protocol and registration
This methodological systematic review was conducted based on the Centre for Reviews and Dissemination (CRD) guidelines 9 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist 11. A protocol was developed prior to the initiation of this review but was not registered with International Prospective Register of Systematic Reviews (PROSPERO) because this review does not contain direct patient or clinically relevant outcomes.
Eligibility criteria
Studies were included if they reported on the use of a Markov model to evaluate the costs and health outcomes of cervical cancer screening. Eligibility criteria were defined based on the components of the PICOS approach:
Participants: Markov model for economic evaluation of cervical cancer screening.
Intervention: Cervical cancer screening in settings with or without an HPV immunization program.
Comparators: Screening tests: Papanicolaou smear (Pap test), liquid-based cytology (LBC), hybrid capture (HC2), HPV-DNA, visual inspection with acetic acid (VIA), visual inspection with Lugol’s iodine (VILI), and speculoscopy.
Outcome: Incremental cost-effectiveness ratio (ICER).
Study design: Model-based full economic evaluations (cost-effectiveness analyses, cost-utility analyses, and cost-benefit analyses).
This review included only English, Spanish, and German language publications. Editorials, abstracts of congress, review studies, studies that did not compare screening strategies in terms of costs and health consequences, and studies that exclusively analyzed vaccination strategies were excluded.
Electronic search
An electronic search was performed in the following databases: MEDLINE via PubMed (1946 to August (week 2) 2016), the NHS EED National Health Service Economic Evaluation Database (NHS EED) of the Centre for Reviews and Dissemination (CRD) (1994 to August week 2, 2016), EMBASE (1974 to August week 2, 2016) and Web of Science (1900 to August (week 2) 2016). The search included terms used in previous reviews 4,7,12,13 and relevant studies 14-23: “Markov model” AND (“Uterine Cervical Neoplasms” OR “Cervical Intraepithelial Neoplasia” OR “Squamous Intraepithelial Lesions of the Cervix”) AND “Human Papilloma Virus” AND “Screening” AND “Costs and Cost Analysis”. The electronic search strategies created specifically for each database are provided in Appendix 1.
Searching other sources
Additional relevant studies were identified by assessing the reference lists of major publications on the subject and the references of studies identified by electronic databases.
Study selection
This review included only Markov model-based full economic evaluations of cervical cancer screening in settings with or without an HPV immunization program. Two independent reviewers (JYKV and CGF) screened the titles and abstracts of the identified studies and selected them using specific inclusion and exclusion criteria. Any disagreements during this process were resolved by discussion or by a third reviewer (PCS).
Data collection process
Two reviewers (JYKV and CGF) independently extracted the data into a Microsoft Excel 2016 spreadsheet form tailored to this project. The data collection form was based on a prior publication 4 and was piloted in five studies.
The following data were extracted from all studies:
General study characteristics: authors, year of publication, country where the analysis was performed, screening tests for cervical cancer, target population, study type (cost-effectiveness analyses, cost-utility analyses), currency, year of reported costs, ICER, funding sources, conflicts of interest, health outcomes perspective and time horizon of analysis, cost-effectiveness thresholds, and HPV immunization program in place.
Model characteristics: use of own model, graphical representation, number of health states, cycle length, software used, calibration of parameters, model validation and types of sensitivity analysis.
Summary measures conversions
To enable comparisons across studies conducted in different countries and years and account for the effects of inflation over the designated period, the summary measures (ICERs) were updated to the year 2015. When the year of reported costs was not specified, the article publication year was used. Local currencies were initially inflated to 2015 values using specific consumer price indexes 24,25 and then converted into 2015 international dollars (I$) using purchasing power parity conversions provided by the World Bank (http://data.worldbank.org/indicator/PA.NUS.PPP) 26.
Quality assessment
We evaluated the reporting quality of the structuring and development of Markov models using items of the framework for quality assessment of DAM 27 and a previously described instrument 28. The adapted checklist is an 18-item measure of the overall quality assessment of a DAM and contains three dimensions: 1) structure, 2) data, and 3) consistency (see Appendix 2). We chose these instruments as they are widely accepted as a scientific standard for the reporting of DAM studies, and they can be applied to quality assessment of DAMs for health technology assessment (HTA). The response options for each item include ‘yes’, ‘no’ and ‘not applicable’. Each reviewed study was evaluated individually, and we counted each properly reported item (answer = ‘yes’) and summed responses based on a maximum possible count of 18.
Synthesis of results
The more relevant results were summarized as a narrative synthesis. The study characteristics are presented in tables and figures. Due to study heterogeneity, meta-analysis or statistical pooling of the extracted summary measure (ICER) was not performed, given that this was neither feasible nor meaningful 29.
RESULTS
Search results
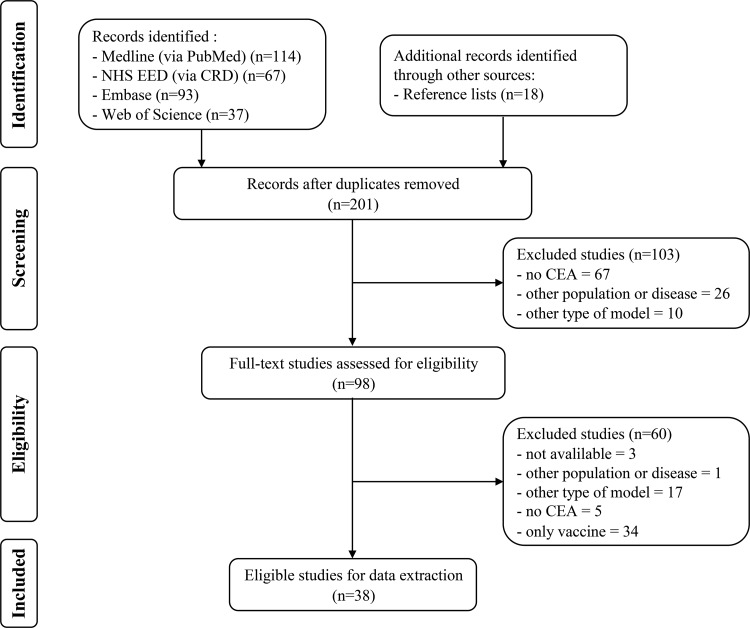
After the removal of duplicates, a total of 201 potentially relevant articles were identified. After assessment of the eligibility criteria, 38 studies 30-66 met these review inclusion criteria. Figure 1 presents the flowchart of the selection process.
Figure 1.
Flowchart of systematic review selection process.
HEE: health economic evaluation
Study characteristics
Table 1 presents the economic evaluation of included Markov model-based studies. The majority of the studies were conducted in high-income countries (82%, n=31). Greater than half of the studies were set in three high-income countries (USA=13, GBR=5, and CAN=3). Sixteen percent (n=6) of these studies were conducted in upper-middle income countries, and only one study included lower-middle and low-income countries 48.
Table 1.
Characteristics of the economic evaluation of the included Markov model-based studies.
| Study | Country 1 | Screening tests 2 | Target pop 3 | Study type 4 | Currency (year) 5 | ICER (I$) 6 |
|---|---|---|---|---|---|---|
| (McCrory et al. 1999) | USA | PAP, NewCyto | 15-85 years | CEA | USD (1997) | 32,503.08/LY |
| (Hutchinson et al. 2000) | USA | PAP, AutoPap, LBC | 20-65 years | CEA | USD (1997) | 73,837.07/LY |
| (Myers et al. 2000) | USA | PAP, NewCyto | 15-85 years | CEA | USD (1997) | 4,310.60/LY |
| (Taylor et al. 2000) | USA | PAP, PAP+speculoscopy | 18-65 years | CEA | USD (2001) | not calculated |
| (Montz et al. 2001) | USA | PAP, LBC | 20-80 years | CEA | USD (1997) | 26,532.61/LY |
| (Mandelblatt et al. 2002) | USA | PAP, LBC, HC2 | ≥20 years | CUA | USD (2000) | 96,826.00/QALY |
| (Kulasingam and Myers 2003) | USA | PAP | 12-85 years | CEA | USD (2001) | 60,076.00/LY |
| (Goldie et al. 2004a) | USA | PAP, LBC, HC2, HC2+Cyto | ≥30 years | CEA | USD (2001) | 41,488.03/LY |
| (Goldie et al. 2004b) | USA | PAP, LBC, HC2 | ≥12 years | CUA | USD (2002) | 77,073.34/QALY |
| (Karnon et al. 2004) | GBR | PAP, LBC | 15-95 years | CEA | GBP (NI) | 16,782.16/LY |
| (Kim et al. 2004) | HKG | PAP, LBC | ≥15 years | CEA | USD (2000) | 12,821.96/LY |
| (Sherlaw-Johnson and Philips 2004) | GBR | PAP, LBC, HPV test | ≥15 years | CEA | GBP (2001) | 6,010.34/LY |
| (Kim et al. 2005) | GBR, NLD, FRA, ITA | PAP, HC2, PAP+HC2 | NI | CEA | USD (2004) | 23,590.95 - 106,985.77/LY |
| (Anderson et al. 2008) | AUS | PAP | 15-85 years | CEA | USD (NI) | 252,485.84/LY |
| (Andres-Gamboa et al. 2008) | COL | PAP, HPV test | 15-76 years | CEA | USD (2006) | 262,302.32/LY |
| (Bistoletti et al. 2008) | SWE | PAP, PAP+HPV test | ≥32 years | CEA | USD (2005) | 7,132.05/LY |
| (Gutierrez-Delgado et al. 2008) | MEX | PAP, HC2, PAP+HC2 | 12-64 years | CUA | MXN (2006) | 209,410.00/DALY |
| (Rogoza et al. 2008) | CAN, NLD, TWN, GBR, USA | PAP | ≥12 years | CEA and CUA | CAD,EUR,NTD,GBP,USD (2006) | 9,203.22 - 1,173,080.66/QALY |
| (Coupe et al. 2009) | NLD | PAP, HPV test | 12-100 years | CUA | EUR (2006) | 11,810.39/QALY |
| (Ginsberg et al. 2009) | WHO 14 regions | PAP, HPV test, PAP+HPV test, VIA | NI | CUA | I (2000) | 156.91 - 63,616.09/DALY |
| (Kulasingam et al. 2009) | CAN | PAP, HC2, PAP+HC2 | NI | CEA and CUA | CAD (2006) | 7,060.14/LY |
| (Reynales-Shigematsu et al. 2009) | MEX | PAP | 12-85 years | CEA | USD (2004) | 2,075.00/LY |
| (Balasubramanian et al. 2010) | USA | LBC, HPV test, Self-colletion | 12-85 years | CUA | USD (2007) | 15,142.00/QALY |
| (Chuck 2010) | CAN | PAP, LBC, HC2 | 12-80 years | CUA | CAD (2007) | 16,528.54/QALY |
| (Creighton et al. 2010) | AUS | PAP | 10-84 years | CEA | AUD (NI) | 30,583.80/LY |
| (Sroczynski et al. 2010) | DEU | PAP, PAP+HC2 | ≥15 years | CEA | EUR (2007) | 107,285.43/LY |
| (Chen et al. 2011) | TWN | PAP, HPV test | NI | CEA | USD (NI) | 64,249.86/LY |
| (Kitchener et al. 2011) | GBR | LBC, HC2, Self-colletion | 10-84 years | CEA and CUA | GBP (2007) | 49,193.51/LY |
| (Praditsitthikorn et al. 2011) | THA | PAP, VIA | ≥15 years | CUA | THB (2007) | 543,574.08/QALY |
| (Shi et al. 2011) | CHN Rural | VIA, VIA/VILI, HPV test | NI | CEA and CUA | USD (2009) | 30,206.17/LY |
| (Sopina and Ashton 2011) | NZL | LBC, HPV test | 12-85 years | CEA and CUA | NZD (2009) | 9,164.45/QALY |
| (Sroczynski et al. 2011) | DEU | PAP, HC2, PAP+HC2 | ≥15 years | CEA | EUR (2007) | 177,473.23/LY |
| (Vokó et al. 2012) | HUN | PAP | ≥25 years | CUA | HUF (NI) | 33,339.15/QALY |
| (Yamamoto et al. 2012) | JPN | PAP | ≥11 years | CUA | JPY (2010) | 28,865.17/QALY |
| (Fonseca et al. 2013) | BRA Amazon | PAP | ≥12 years | CUA | USD (NI) | 1,341.06/QALY |
| (Ostensson et al. 2013) | SWE | PAP, HPV test | ≥15 years | CEA | EUR (2011) | 8,795.19/LY |
| (Huh et al. 2015) | USA | PAP, HPV test, PAP+HPV test | 30-70 years | CUA | USD (2013) | 7,800.62/QALY |
| (Nghiem et al. 2015) | USA | LBC, HPV test | ≥12 years | CUA | USD (2012) | 18,854.49/QALY |
Countries classified according to the list of country names and 3-letter codes abbreviations by the United Nations (http://unstats.un.org/unsd/methods/m49/m49alpha.htm).
Screening tests: PAP = conventional cytological test also known as Papanicolaou (pap) smear test; New Cyto = hypothetical new cytological test; AutoPap = automated read cytological test; LBC = liquid-based cytology test; HC2 = Digene high-risk HPV Hybrid Capture© 2 test (Qiagen); HPVtest = Human Papillomavirus (HPV) DNA detection with genotyping high-risk types by polymerase chain reaction (PCR); Self-collection = high-risk HPV DNA testing of self-collected vaginal samples; VIA = visual inspection with acetic acid; VIA/VILI = VIA in combination with Lugol’s iodine.
Target population: women within the age range indicated.
Economic study type: CEA = cost-effectiveness analysis; CUA = cost-utility analysis.
Currencies classified according to the International Organization for Standardization, ISO 4217:2015 (http://www.iso.org/iso/home/standards/currency_codes.htm).
ICER = incremental cost-effectiveness ratio; I$: Geary-Khamis dollar, more commonly known as the international dollar; LY = life years; QALY = quality-adjusted life years; DALY = disability-adjusted life years; NI = Not informed.
The Pap test was the most commonly used screening strategy investigated and was employed in 86% (n=33) of the studies. The LBC, HC2 and HPV-DNA test were employed in 34% (n=13), 29% (n=11) and 24% (n=9) of the studies, respectively. Combined tests, such as Pap + HC2, Pap + HPV-DNA, Pap + speculum and HC2 + cytology, were employed in 26% (n=10) of the studies. Other technologies, such as VIA, VILI and self-collection, were also investigated (16%, n=6). Thirteen studies (34%) considered the effect of an HPV immunization program on the analysis.
The majority of the studies (53%, n=20) were cost-effectiveness analyses, followed by cost-utility analyses (34%, n=13) and a combination of both (13%, n=5). The lowest ICER estimate (I$156.91) was obtained in the African region 48, and the highest (I$1,173,080.66) was noted in Taiwan 46. Most of the calculated ICERs (67%, n=24) could be considered cost-effective strategies.
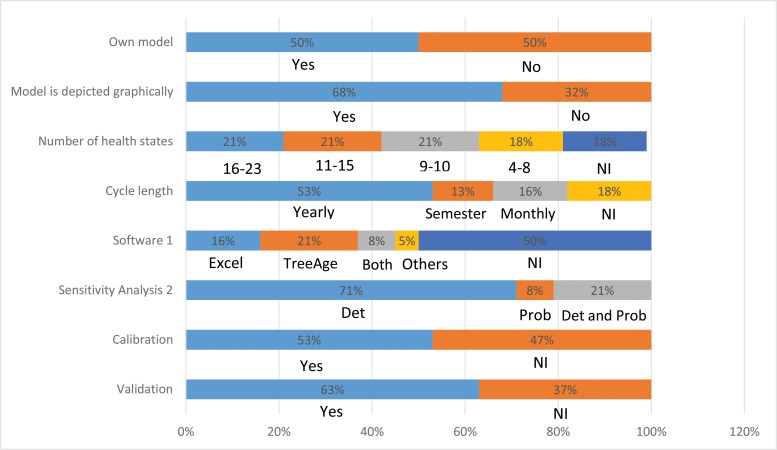
Half of the studies (n=19) used a previously published Markov model. In particular, five studies 36,43,49,51,66 used the model developed by Myers et al. 67. A graphical representation was presented in 68% (n=26) of the studies. The number of health states considered when stated (n=31, 82%) ranged from 4 to 23 states (mean of 12). Among the studies that reported the duration of the Markovian cycle used (n=31, 82%), the majority (n=20, 65%) considered annual cycles. Among the studies that reported (n=19, 50%) the use of some software, most studies (n=11, 58%) used TreeAge (TreeAge Software Inc., Williamstown, MA), whereas Excel (Microsoft Corp., Redmond, WA) was used by 47% (n=9) of the studies. One study used software developed by the WHO, PopMod 68, and one study implemented the model using the C ++ programming language 35 (Figure 2).
Figure 2.
Decision-model characteristics of included studies.
1 Software: Others = WHO PopMod or C++ Program.
2 Sensitivity analysis: Det = deterministic, Prob = probabilistic.
NI = not informed.
Deterministic sensitivity analysis was performed in 92% (n=35) of the studies, of which 23% (n=8) also performed probabilistic analysis. The validation of the model was informed by 24 (63%) studies, whereas 53% (n=20) of the studies mentioned that model parameters were calibrated (Figure 2).
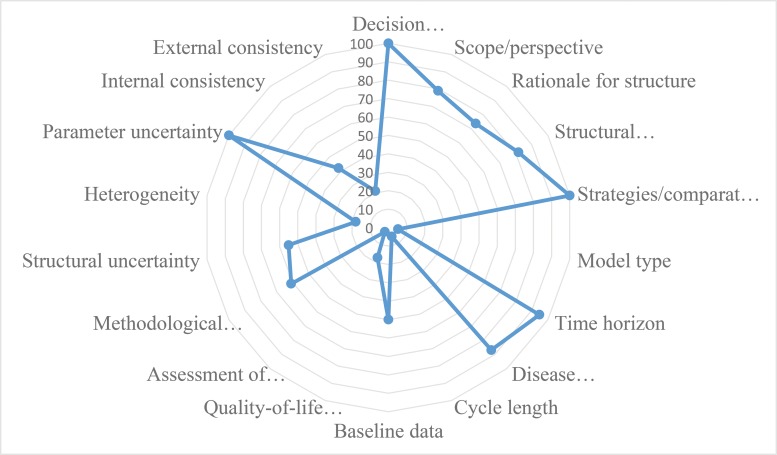
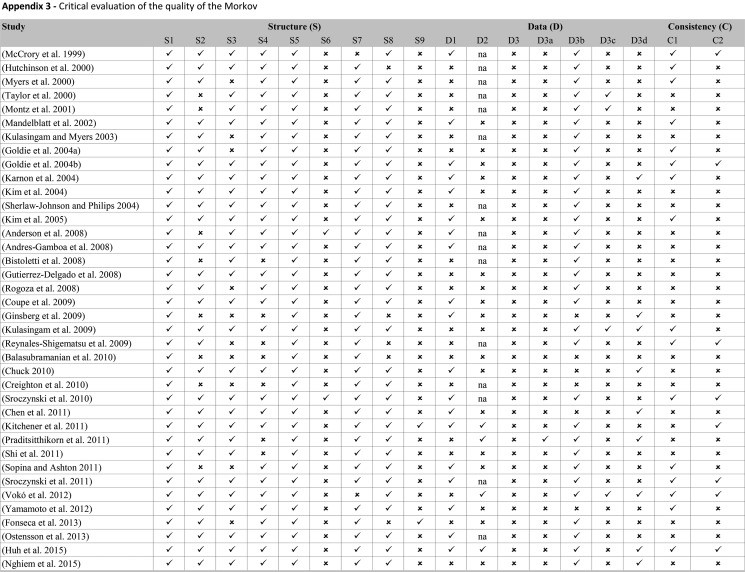
Figure 3 presents the proportion of economic evaluation studies (n=38) that properly complied with the 18 items of the checklist domains. The detailed assessment is reported in Appendix 3. The mean number of properly reported checklist items was 9 (SD 2.0) out of the maximum possible 18. Items that were better reported than others were the statement of decision problem (item 1, 100%), the description of the strategies/comparators (item 5, 100%), the statement of the time horizon (item 7, 95%) and informing the disease states (item 8, 87%). Only one study simultaneously assessed the methodological, structural, heterogeneity, and parameter uncertainties (item 12) 61. Compliance was poor for the assessment of structural uncertainty (55%, n=21) and extremely poor for the justification of model type (5%, n=2), cycle length (5%, n=2), assessment of heterogeneity (18%, n=4), the appropriateness of utilities (17%, n=4), and assessment of external consistency (21%, n=8).
Figure 3.
Proportion of economic evaluation studies adequately reporting checklist items (n=38).
DISCUSSION
This systematic review was the first study to comprehensively assess the methodological quality of the models of previously published studies using items of formal checklists. We evaluated 38 decision-analytic cost-effectiveness models, and the results demonstrated poor compliance with these checklists.
As noted in a previous review 12, only one study has been conducted in lower-middle and low-income countries 48, which exhibit the greatest cervical cancer burden. Approximately 84% of cervical cancer cases occur in less developed countries, with the highest incidences of cervical cancer noted in Africa, Latin America and the Caribbean. This finding reflects a lack of technical expertise and shortage of trained health economists in these regions. This finding also highlights the importance of local studies and enforces the need for strengthening the local modeling capacity.
Model structure
Half of the included studies (n=19) used a previously published Markov model. Only two studies justified the choice of model type 43,54, and the overwhelming majority did not provide reasons or explain why the use of a Markov model was appropriate. The choice of model type should be appropriate for the problem. In the case of cervical cancer, a Markov model may be suitable if the objective of the study is to assess alternative screening strategies in a setting in which disease prevalence is constant. The Markov model will simulate disease progression for a particular cohort of patients, assigning a probability of progression and regression between each of the classifications of dysplasia and invasive cancer 69. One limitation of the closed population model (such as a Markov cohort model) is that it may predict an increased cancer incidence compared with an open model. If the analysis incorporates the effect of an HPV immunization program, the ideal model would be a dynamic model that follows an entire population, allowing for evaluation of the impact of herd immunity (i.e., indirect protection of susceptible individuals by a significant proportion of immune individuals in the population) 69. Thirteen studies 36,38,45-48,50,55,57,59,62,63,66 reported that the effect of an HPV immunization program was considered in the analysis but did not explain how herd immunity was incorporated using a static cohort model.
The Markov model can be more transparent and easy to understand and provides more conservative estimates than dynamic models. In contrast, because the latter model type allows for the inclusion of more detail, it can generate several uncertainties in the evaluation process in addition to requiring more input and computational resources that may not be available in all settings. The direct and indirect effects of vaccination may not be observed in surveillance data for many years. Thus, although dynamic models are still developed by a small group of modelers 70, the development of these models will become increasingly important to explore the impact on screening as the first vaccinated cohorts approach the age of cervical cancer screening 12. Previous studies have reported an increased screening rate among vaccinated women and the lowest proportion of cervical abnormalities compared with those not vaccinated 71,72. Future model-based economic evaluations will need to take into account the continuum interaction between screening and vaccination to predict the effects of vaccination on screening programs 6.
Only two studies justified the choice of cycle length 56,64. The cycle length should reflect the clinical problem and be the shortest interval at which the pathologies and/or diagnosis typically occurs 73, and its justification should be based on the natural history of the disease 74. In the case of cervical cancer, often the only source of information regarding cases is the clinical examination results. However, this information may be under-reported given that HPV infections and precursor lesions may regress in less than a year 75 and screening is typically performed annually. Therefore, ideally, the definition of the cycle should not be based on the intervals between exams 74. However, occasionally, these are the only available data. The other option would be to use data from another setting, and both approaches would impact the analysis results.
Model data
Although half of the included studies presented the transition probabilities, none of them explained how the probabilities were calculated or whether the cycle correction was used. Concern has been raised in the DAM literature regarding confusion about the appropriate use of rates and probabilities. Depending on the model, this misconception may introduce important errors, impacting the validity of the model results 76,77. Various approaches can be used to estimate transition probabilities for the natural history of cervical cancer in Markov models, including a literature review of HPV and cervical intraepithelial neoplasia (CIN) progression and regression rates, data from observational studies, and fitting approaches 78. Although some relevant publications exist, no formal guidelines are available for the estimation of transition probabilities for use in Markov models 79. The understanding of the difference between rates and probabilities and how to transform them correctly is essential for those developing Markov models.
According to international guidelines, if health benefits are measured through utility measures, the methods used (e.g., time trade-off, standard gamble, specific questionnaires) and the subjects in whom the assessments were performed (e.g., patients, members of the general public, health professionals) need to be reported 80,81. Only 17% of the reviewed studies reported the applied instruments, methods of measurement and the sources of utilities employed. Inadequate reporting of utility measurement methods leads first to difficulties in comparing different assessments, given that discrepancies between these measures using different measurement instruments and methods were previously observed in other studies 82-84. In addition, in relation to the lack of reporting of sources of utility measures (populations used to derive these measures), if the ultimate objective of the evaluation is to influence the allocation of resources to decisions based on social interests, it would be important that health state evaluations were based on utility weights representative of the preferences of the general population 85.
Specifically in relation to economic evaluations of cervical cancer screening, differences in utility values for CIN lesions, presence of cervical cancer and genital warts may partially explain the differences in the analysis results. In addition, considering the limited data available on the utility values associated with these states 7, it is fundamental that sensitivity analyses performed in future studies consider a wide range of variation, including all plausible utility values.
Uncertainty
Uncertainty is present in all HTA models 74. DMA researchers distinguish among parameter, structural and methodological uncertainties, all of which require assessment 27. Parameter uncertainty can be addressed by deterministic or probabilistic sensitivity analysis. Structural uncertainty can be managed through alternative model structures, which involves re-running the model under alternative structural assumptions and presenting the results of each scenario. Methodological uncertainty can be addressed with a similar method. Only one study simultaneously assessed methodological, structural, heterogeneity, and parameter uncertainties 61. Approximately half of the included studies failed to account for structural uncertainty, reflecting the gap between guidelines and applied research. This finding was also highlighted in a previous review 28, where many published models failed to account correctly for the major sources of uncertainty, particularly structural uncertainty. Most studies (92%) addressed only parameter uncertainty through deterministic sensitivity analysis. In addition to the standard considerations of uncertainty about parameter estimates, it is important to assess the implications of model uncertainty on results 28.
Most models (89%, n=34) simulated aggregate groups of women at risk of cervical cancer over time without accounting for other aspects of population heterogeneity in screening behavior. Heterogeneity (i.e., the extent to which variability between patients can be explained as a function of their characteristics) 86 reflects differences in outcomes that may in principle be explained by variations among subgroups of patients, including characteristics such as age, sex, level of risk and severity of the disease, or the relative effects of treatment 87. Given the natural history of cervical cancer, women less than 30 years of age have more HPV infections than older women, while older women may experience the progression of this virus 116-fold more frequently than younger women. Therefore, HPV-DNA screening after the age of 30 years seems to be more effective than before the age of 30 88. Thus, not considering "heterogeneity" during the analysis, which could be performed by executing the model for different subgroups of patients, may lead to errors in the results obtained 89. To capture heterogeneity in screening and vaccination behavior, it would be ideal to use individual-based models (microsimulation).
Model consistency
Model consistency refers to the quality of the model overall. This parameter tests the internal logic of the modeling practice, changing model inputs and examining the direction of results (internal consistency). Model consistency also compares the model’s result with the best available evidence or with the results of previously developed models (external consistency, also known as calibration). For instance, the model consistency of cervical precancerous lesions predicted by cytology can be compared with observed CIN-related outcomes. However, it is generally not clear whether these outcomes are predicted by cytological results or histologically confirmed lesions 8. Only 8 studies (21%) reported the use of some calibration method. This low value can be explained by the lack of standards in calibrating disease models in economic evaluation, especially cancer screening models 90,91. There is no consensus in the literature regarding an acceptable minimum specification for the fitting targets that should be reported 78. Another potential barrier to calibration is insufficient local data to estimate parameters associated with organized screening.
The Markov models for economic evaluation of screening strategies for cervical cancer varied in quality. Items that were generally well reported were the statement of the decision problem, the description of the strategies/comparators, the statement of time horizon, and informing disease states. One limitation of the present study is that most models did not adequately assess methodological, structural, heterogeneity, and parameter uncertainties. Moreover, the minority justified the model type and cycle length, assessed heterogeneity and the appropriateness of utilities, and evaluated external consistency. Future studies should evaluate the appropriateness of the different methods to account for uncertainty (through sensitivity analysis and alternative model structures), heterogeneity, consistency (through calibration and validation techniques), and the relevance of reporting guidelines for Markov models to improve their transparency.
AUTHOR CONTRIBUTIONS
Viscondi JY and De Soárez PC designed the research. Viscondi JY, Faustino CG and Itria A performed the research. Viscondi JY, Faustino CG, Campolina AG and De Soárez PC analyzed the data. Viscondi JY, Campolina AG and De Soárez PC wrote the manuscript.
ACKNOWLEGMENTS
This review is part of the health technology assessment project “Cost-effectiveness of cervical cancer screening strategies in Brazil” coordinated by Professor Patrícia Coelho de Soárez and funded by the Research Program for the Unified Health System – São Paulo (Programa de Pesquisa para o Sistema Único de Saúde - São Paulo, PPSUS-SP, 2013) and research grant number FAPESP: 2014/50042-4; the funding sources pose no conflicts of interest related to this study. Juliana Y. K. Viscondi received a doctoral scholarship (CNPq no. 153505/2016-8).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.World Health Organization IARC GLOBACAN 2012: Estimaded Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx [Access 2017 Sep 29]. Available from.
- 2.Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low-and middle-income developing countries. Bull World Health Organ. 2001;79((10)):954–62. [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO guidance note: comprehensive cervical cancer prevention and control: a healthier future for girls and women. WHO Press; 2013. p. 12. [Google Scholar]
- 4.Kim JJ, Brisson M, Edmunds WJ, Goldie SJ. Modeling cervical cancer prevention in developed countries. Vaccine. 2008;26(Suppl 10):K76–86. doi: 10.1016/j.vaccine.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jit M, Demarteau N, Elbasha E, Ginsberg G, Kim J, Praditsitthikorn N, et al. Human papillomavirus vaccine introduction in low-income and middle-income countries: guidance on the use of cost-effectiveness models. BMC Med. 2011;9:54. doi: 10.1186/1741-7015-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canfell K, Chesson H, Kulasingam SL, Berkhof J, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine. 2012;30(Suppl 5):F157–67. doi: 10.1016/j.vaccine.2012.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fesenfeld M, Hutubessy R, Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: a systematic review. Vaccine. 2013;31((37)):3786–804. doi: 10.1016/j.vaccine.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 8.Simonella L, Canfell K. Development of a quality framework for models of cervical screening and its application to evaluations of the cost-effectiveness of HPV vaccination in developed countries. Vaccine. 2015;33((1)):34–51. doi: 10.1016/j.vaccine.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 9.Centre for Reviews and Dissemination . University of York. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: York Publishing Services Ltd; 2009. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf Available from. [Google Scholar]
- 10.Olson BM, Armstrong EP, Grizzle AJ, Nichter MA. Industry’s perception of presenting pharmacoeconomic models to managed care organizations. J Manag Care Pharm. 2003;9((2)):159–67. doi: 10.18553/jmcp.2003.9.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6((7)):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendes D, Bains I, Vanni T, Jit M. Systematic review of model-based cervical screening evaluations. BMC Cancer. 2015;15:334. doi: 10.1186/s12885-015-1332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novaes HMD, Silva GA e, Ayres AR, Rama C, Padovan J, Sartori AM, et al. Avaliação tecnológica de vacinas para a prevenção de infecção por papilomavírus humano (HPV): estudo de custo-efetividade da incorporação de vacina contra HPV no Programa Nacional de Imunizações/PNI do Brasil. Relatóriotécnico-científico. [Internet] São Paulo:: Ministério da Saúde;; 2012. p. 154. Available from: ///C:/Users/Patrícia Soárez/Downloads/AVE HPV (3).pdf. [Google Scholar]
- 14.Colantonio L, Gomez J, Demarteau N, Standaert BA, Pichon-Riviere A, Augustovski F, et al. Cost-effectiveness analysis of a cervical cancer vaccine in five Latin American countries. Value Health. 2009;12((7)):A484. doi: 10.1016/S1098-3015(10)75286-4. [DOI] [PubMed] [Google Scholar]
- 15.Eluf J, Neto, Booth M, Muãoz N, Bosch FX, Meijer CJ, Walboomers JM. Human papillomavirus and invasive cervical cancer in Brazil. Br J Cancer. 1994;69((1)):114–9. doi: 10.1038/bjc.1994.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103((5)):368–83. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldie SJ, Kim JJ, Kobus K, Goldhaber-Fiebert JD, Salomon J, O’shea MK, et al. Cost-effectiveness of HPV 16, 18 vaccination in Brazil. Vaccine. 2007;25((33)):6257–70. doi: 10.1016/j.vaccine.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--3. Value Health. 2012;15((6)):812–20. doi: 10.1016/j.jval.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Vanni T, Luz PM, Foss A, Mesa-Frias M, Legood R. Economic modelling assessment of the HPV quadrivalent vaccine in Brazil: a dynamic individual-based approach. Vaccine. 2012;30((32)):4866–71. doi: 10.1016/j.vaccine.2012.04.087. [DOI] [PubMed] [Google Scholar]
- 20.Caetano R, Vianna CM, Thuler LC, Girianelli VR. Custo-efetividade no diagnóstico precoce do câncer de colo uterino no Brasil. Physis: Rev Saúde Coletiva. 2006;16((1)):99–118. [Google Scholar]
- 21.Vanni T, Legood R, Franco EL, Villa LL, Luz PM, Schwartsmann G. Economic evaluation of strategies for managing women with equivocal cytological results in Brazil. Int J Cancer. 2011;129((3)):671–9. doi: 10.1002/ijc.25708. [DOI] [PubMed] [Google Scholar]
- 22.Moyer VA, U.S. Preventive Services Task Force Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156((12)):880–91. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 23.Cox JT, Castle PE, Behrens CM, Sharma A, Wright TC, Jr, Cuzick J, et al. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: results from the ATHENA HPV study. Am J Obstet Gynecol. 2013;208((3)):184.e1–184.e11. doi: 10.1016/j.ajog.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 24.CPI Inflation Calculator [Internet] https://www.bls.gov/data/inflation_calculator.htm [Acced 2016 Jun 16]. Available from.
- 25.Inflation calculator and change of price between 2 dates [Internet] http://fxtop.com/en/inflation-calculator.php [Acced 2016 Jun 16]. Available from.
- 26.PPP conversion factor, GDP (LCU per international $) [Internet] http://data.worldbank.org/indicator/PA.NUS.PPP [Acced 2016 Jun 16]. Available from.
- 27.Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics. 2006;24((4)):355–71. doi: 10.2165/00019053-200624040-00006. [DOI] [PubMed] [Google Scholar]
- 28.Ramos MC, Barton P, Jowett S, Sutton AJ. A Systematic Review of Research Guidelines in Decision-Analytic Modeling. Value Health. 2015;18((4)):512–29. doi: 10.1016/j.jval.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Anderson R. Systematic reviews of economic evaluations: utility or futility? Health Econ. 2010;19((3)):350–64. doi: 10.1002/hec.1486. [DOI] [PubMed] [Google Scholar]
- 30.McCrory DC, Matchar DB, Bastian L, Datta S, Hasselblad V, Hickey J, et al. Evaluation of Cervical Cytology. Evidence Report/Technology Assessment No. 5. (Prepared by Duke University under Contract No. 290-97-0014.) [Internet] Rockville, MD: Agency for Health Care Policy and Research; 1999. https://www.ncbi.nlm.nih.gov/books/NBK32961/ AHCPR Publication No. 99-E010. Available from. [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchinson ML, Berger BM, Farber FL. Clinical and cost implications of new technologies for cervical cancer screening: the impact of test sensitivity. Am J Manag Care. 2000;6((7)):766–80. [PubMed] [Google Scholar]
- 32.Myers ER, McCrory DC, Subramanian S, McCall N, Nanda K, Datta S, et al. Setting the target for a better cervical screening test: characteristics of a cost-effective test for cervical neoplasia screening. Obstet Gynecol. 2000;96((5 Pt 1)):645–52. doi: 10.1016/S0029-7844(00)00979-0. [DOI] [PubMed] [Google Scholar]
- 33.Taylor LA, Sorensen S V, Ray NF, Halpern MT, Harper DM. Cost-effectiveness of the conventional papanicolaou test with a new adjunct to cytological screening for squamous cell carcinoma of the uterine cervix and its precursors. Arch Fam Med [Internet] 2000;9((8)):713–21. doi: 10.1001/archfami.9.8.713. http://www.ncbi.nlm.nih.gov/pubmed/10927709 Available from. [DOI] [PubMed] [Google Scholar]
- 34.Montz FJ, Farber FL, Bristow RE, Cornelison T. Impact of increasing Papanicolaou test sensitivity and compliance: a modeled cost and outcomes analysis. Obstet Gynecol. 2001;97((5 Pt 1)):781–8. doi: 10.1016/S0029-7844(01)01322-9. [DOI] [PubMed] [Google Scholar]
- 35.Mandelblatt JS, Lawrence WF, Womack SM, Jacobson D, Yi B, Hwang Y, et al. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA. 2002;287((18)):2372–81. doi: 10.1001/jama.287.18.2372. [DOI] [PubMed] [Google Scholar]
- 36.Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. JAMA. 2003;290((6)):781–9. doi: 10.1001/jama.290.6.781. [DOI] [PubMed] [Google Scholar]
- 37.Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol. 2004;103((4)):619–31. doi: 10.1097/01.AOG.0000120143.50098.c7. [DOI] [PubMed] [Google Scholar]
- 38.Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, Bosch FX, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst. 2004;96((8)):604–15. doi: 10.1093/jnci/djh104. [DOI] [PubMed] [Google Scholar]
- 39.Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N. Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis. Health Technol Assess. 2004;8((20)):1–78. doi: 10.3310/hta8200. [DOI] [PubMed] [Google Scholar]
- 40.Kim JJ, Leung GM, Woo PP, Goldie SJ. Cost-effectiveness of organized versus opportunistic cervical cytology screening in Hong Kong. J Public Health. 2004;26((2)):130–7. doi: 10.1093/pubmed/fdh138. [DOI] [PubMed] [Google Scholar]
- 41.Sherlaw-Johnson C, Philips Z. An evaluation of liquid-based cytology and human papillomavirus testing within the UK cervical cancer screening programme. Br J Cancer. 2004;91((1)):84–91. doi: 10.1038/sj.bjc.6601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of human papillomavirus DNA testing in the United Kingdom, The Netherlands, France, and Italy. J Natl Cancer Inst. 2005;97((12)):888–95. doi: 10.1093/jnci/dji162. [DOI] [PubMed] [Google Scholar]
- 43.Anderson R, Haas M, Shanahan M. The cost-effectiveness of cervical screening in Australia: what is the impact of screening at different intervals or over a different age range? Aust N Z J Public Health. 2008;32((1)):43–52. doi: 10.1111/j.1753-6405.2008.00165.x. [DOI] [PubMed] [Google Scholar]
- 44.Andres-Gamboa O, Chicaiza L, Garcia-Molina M, Diaz J, Gonzalez M, Murillo R, et al. Cost-effectiveness of conventional cytology and HPV DNA testing for cervical cancer screening in Colombia. Salud Publica Mex. 2008;50((4)):276–85. doi: 10.1590/S0036-36342008000400005. [DOI] [PubMed] [Google Scholar]
- 45.Gutierrez-Delgado C, Baez-Mendoza C, Gonzalez-Pier E, de la Rosa AP, Witlen R. Generalized cost-effectiveness of preventive interventions against cervical cancer in Mexican women: results of a Markov model from the public sector perspective. Salud Publica Mex. 2008;50((2)):107–18. doi: 10.1590/S0036-36342008000200004. [DOI] [PubMed] [Google Scholar]
- 46.Rogoza RM, Ferko N, Bentley J, Meijer CJ, Berkhof J, Wang KL, et al. Optimization of primary and secondary cervical cancer prevention strategies in an era of cervical cancer vaccination: a multi-regional health economic analysis. Vaccine. 2008;26(Suppl 5):F46–58. doi: 10.1016/j.vaccine.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 47.Coupe VM, de Melker HE, Snijders PJ, Meijer CJ, Berkhof J. How to screen for cervical cancer after HPV16/18 vaccination in The Netherlands. Vaccine. 2009;27((37)):5111–9. doi: 10.1016/j.vaccine.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 48.Ginsberg GM, Edejer TT, Lauer JA, Sepulveda C. Screening, prevention and treatment of cervical cancer -- a global and regional generalized cost-effectiveness analysis. Vaccine. 2009;27((43)):6060–79. doi: 10.1016/j.vaccine.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 49.Kulasingam SL, Rajan R, St Pierre Y, Atwood CV, Myers ER, Franco EL. Human papillomavirus testing with Pap triage for cervical cancer prevention in Canada: a cost-effectiveness analysis. BMC Med. 2009;7:69. doi: 10.1186/1741-7015-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynales-Shigematsu LM, Rodrigues ER, Lazcano-Ponce E. Cost-effectiveness analysis of a quadrivalent human papilloma virus vaccine in Mexico. Arch Med Re. 2009;40((6)):503–13. doi: 10.1016/j.arcmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Balasubramanian A, Kulasingam SL, Baer A, Hughes JP, Myers ER, Mao C, et al. Accuracy and cost-effectiveness of cervical cancer screening by high-risk human papillomavirus DNA testing of self-collected vaginal samples. J Low Genit Tract Dis. 2010;14((3)):185–95. doi: 10.1097/LGT.0b013e3181cd6d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chuck A. Cost-effectiveness of 21 alternative cervical cancer screening strategies. Value Health. 2010;13((2)):169–79. doi: 10.1111/j.1524-4733.2009.00611.x. [DOI] [PubMed] [Google Scholar]
- 53.Creighton P, Lew JB, Clements M, Smith M, Howard K, Dyer S, et al. Cervical cancer screening in Australia: modelled evaluation of the impact of changing the recommended interval from two to three years. BMC Public Health. 2010;10:734. doi: 10.1186/1471-2458-10-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sroczynski G, Schnell-Inderst P, Muhlberger N, Lang K, Aidelsburger P, Wasem J, et al. Decision-analytic modeling to evaluate the long-term effectiveness and cost-effectiveness of HPV-DNA testing in primary cervical cancer screening in Germany. GMS Health Technol Assess. 2010;6:Doc05. doi: 10.3205/hta000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen MK, Hung HF, Duffy S, Yen AM, Chen HH. Cost-effectiveness analysis for Pap smear screening and human papillomavirus DNA testing and vaccination. J Eval Clin Pract. 2011;17((6)):1050–8. doi: 10.1111/j.1365-2753.2010.01453.x. [DOI] [PubMed] [Google Scholar]
- 56.Kitchener HC, Blanks R, Cubie H, Desai M, Dunn G, Legood R, et al. MAVARIC - a comparison of automation-assisted and manual cervical screening: a randomised controlled trial. Health Technol Assess. 2011;15((3)) doi: 10.3310/hta15030. iii-iv, ix-xi, 1, 170. [DOI] [PubMed] [Google Scholar]
- 57.Praditsitthikorn N, Teerawattananon Y, Tantivess S, Limwattananon S, Riewpaiboon A, Chichareon S, et al. Economic evaluation of policy options for prevention and control of cervical cancer in Thailand. Pharmacoeconomics. 2011;29((9)):781–806. doi: 10.2165/11586560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Shi JF, Canfell K, Lew JB, Zhao FH, Legood R, Ning Y, et al. Evaluation of primary HPV-DNA testing in relation to visual inspection methods for cervical cancer screening in rural China: an epidemiologic and cost-effectiveness modelling study. BMC Cancer. 2011;11:239. doi: 10.1186/1471-2407-11-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sopina E, Ashton T. Cost-effectiveness of a cervical screening program with human papillomavirus vaccine. Int J Technol Assess Health Care. 2011;27((4)):290–7. doi: 10.1017/S0266462311000456. [DOI] [PubMed] [Google Scholar]
- 60.Sroczynski G, Schnell-Inderst P, Muhlberger N, Lang K, Aidelsburger P, Wasem J, et al. Cost-effectiveness of primary HPV screening for cervical cancer in Germany--a decision analysis. Eur J Cancer. 2011;47((11)):1633–46. doi: 10.1016/j.ejca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Vokó Z, Nagyjánosi L, Margitai B, Kövi R, Tóth Z, László D, et al. Modeling cost-effectiveness of cervical cancer screening in Hungary. Value Health. 2012;15((1)):39–45. doi: 10.1016/j.jval.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto N, Mori R, Jacklin P, Osuga Y, Kawana K, Shibuya K, et al. Introducing HPV vaccine and scaling up screening procedures to prevent deaths from cervical cancer in Japan: a cost-effectiveness analysis. BJOG. 2012;119((2)):177–86. doi: 10.1111/j.1471-0528.2011.03036.x. [DOI] [PubMed] [Google Scholar]
- 63.Fonseca AJ, Ferreira LC, Neto GB. Cost-effectiveness of the vaccine against human papillomavirus in the Brazilian Amazon region. Rev Assoc Med Bras. 2013;59((5)):442–51. doi: 10.1016/j.ramb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Ostensson E, Hellstrom AC, Hellman K, Gustavsson I, Gyllensten U, Wilander E, et al. Projected cost-effectiveness of repeat high-risk human papillomavirus testing using self-collected vaginal samples in the Swedish cervical cancer screening program. Acta Obstet Gynecol Scand. 2013;92((7)):830–40. doi: 10.1111/aogs.12143. [DOI] [PubMed] [Google Scholar]
- 65.Huh WK, Williams E, Huang J, Bramley T, Poulios N. Cost effectiveness of human papillomavirus-16/18 genotyping in cervical cancer screening. Appl Health Econ Health Policy. 2015;13((1)):95–107. doi: 10.1007/s40258-014-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nghiem VT, Davies KR, Beck JR, Follen M, MacAulay C, Guillaud M, et al. Economic evaluation of DNA ploidy analysis vs liquid-based cytology for cervical screening. Br J Cancer. 2015;112((12)):1951–7. doi: 10.1038/bjc.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myers ER, McCrory DC, Nanda K, Bastian L, Matchar DB. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151((12)):1158–71. doi: 10.1093/oxfordjournals.aje.a010166. [DOI] [PubMed] [Google Scholar]
- 68.Lauer JA, Röhrich K, Wirth H, Charette C, Gribble S, Murray CJ. PopMod: a longitudinal population model with two interacting disease states. Cost Eff Resour Alloc. 2003;1((1)):6. doi: 10.1186/1478-7547-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esselen KM, Feldman S. Cost-effectiveness of cervical cancer prevention. Clin Obstet Gynecol. 2013;56((1)):55–64. doi: 10.1097/GRF.0b013e3182823797. [DOI] [PubMed] [Google Scholar]
- 70.Jit M, Brisson M, Portnoy A, Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet Glob Health. 2014;2((7)):e406–14. doi: 10.1016/S2214-109X(14)70237-2. [DOI] [PubMed] [Google Scholar]
- 71.Beer H, Hibbitts S, Brophy S, Rahman MA, Waller J, Paranjothy S. Does the HPV vaccination programme have implications for cervical screening programmes in the UK? Vaccine. 2014;32((16)):1828–33. doi: 10.1016/j.vaccine.2014.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim J, Bell C, Sun M, Kliewer G, Xu L, Mclnerney M, et al. Effect of human papillomavirus vaccination on cervical cancer screening in Alberta. CMAJ. 2016;188((12)):E281–8. doi: 10.1503/cmaj.151528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13((4)):322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 74.Sculpher M, Fenwick E, Claxton K. Assessing quality in decision analytic cost-effectiveness models. A suggested framework and example of application. Pharmacoeconomics. 2000;17((5)):461–77. doi: 10.2165/00019053-200017050-00005. [DOI] [PubMed] [Google Scholar]
- 75.Castle PE, Rodríguez AC, Burk RD, Herrero R, Wacholder S, Alfaro M, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller DK, Homan SM. Determining transition probabilities: confusion and suggestions. Med Decis Making. 1994;14((1)):52–8. doi: 10.1177/0272989X9401400107. [DOI] [PubMed] [Google Scholar]
- 77.Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25((1)):3–6. doi: 10.2165/00019053-200725010-00002. [DOI] [PubMed] [Google Scholar]
- 78.Canfell K. Models of cervical screening in the era of human papillomavirus vaccination. Sex Health. 2010;7((3)):359–67. doi: 10.1071/SH10016. [DOI] [PubMed] [Google Scholar]
- 79.Olariu E, Cadwell K, Hancock E, Trueman D, Chevrou-Severac H. Current recommendations on the estimation of transition probabilities in Markov cohort models for use in health care decision-making: a targeted literature review. Clinicoecon Outcomes Res. 2017;9:537–546. doi: 10.2147/CEOR.S135445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313((7052)):275–83. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16((2)):231–50. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Huang IC, Willke RJ, Atkinson MJ, Lenderking WR, Frangakis C, Wu AW. US and UK versions of the EQ-5D preference weights: does choice of preference weights make a difference? Qual Life Res. 2007;16((6)):1065–72. doi: 10.1007/s11136-007-9206-4. [DOI] [PubMed] [Google Scholar]
- 83.Johnson JA, Luo N, Shaw JW, Kind P, Coons SJ. Valuations of EQ-5D health states: are the United States and United Kingdom different? Med Care. 2005;43((3)):221–8. doi: 10.1097/00005650-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 84.Badia X, Roset M, Herdman M, Kind P. A comparison of United Kingdom and Spanish general population time trade-off values for EQ-5D health states. Med Decis Making. 2001;21((1)):7–16. doi: 10.1177/0272989X0102100102. [DOI] [PubMed] [Google Scholar]
- 85.Ferko N, Postma M, Gallivan S, Kruzikas D, Drummond M. Evolution of the health economics of cervical cancer vaccination. Vaccine. 2008;26(Suppl 5):F3–15. doi: 10.1016/j.vaccine.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 86.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--6. Value Health. 2012;15((6)):835–42. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 87.Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ. 2011;342:d1766. doi: 10.1136/bmj.d1766. [DOI] [PubMed] [Google Scholar]
- 88.Nahvijou A, Hadji M, Marnani AB, Tourang F, Bayat N, Weiderpass E, et al. A systematic review of economic aspects of cervical cancer screening strategies worldwide: discrepancy between economic analysis and policymaking. Asian Pac J Cancer Prev. 2014;15((19)):8229–37. doi: 10.7314/APJCP.2014.15.19.8229. [DOI] [PubMed] [Google Scholar]
- 89.Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices--Modeling Studies. Value Health. 2003;6((1)):9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 90.Vanni T, Karnon J, Madan J, White RG, Edmunds WJ, Foss AM, et al. Calibrating models in economic evaluation: a seven-step approach. Pharmacoeconomics. 2011;29((1)):35–49. doi: 10.2165/11584600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 91.Stout NK, Knudsen AB, Kong CY, McMahon PM, Gazelle GS. Calibration methods used in cancer simulation models and suggested reporting guidelines. Pharmacoeconomics. 2009;27((7)):533–45. doi: 10.2165/11314830-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]