Abstract
Background:
Anterior cruciate ligament (ACL) ruptures have become increasingly common in pediatric and adolescent athletes. While multiple methods exist, all-epiphyseal ACL reconstruction is a popular technique in the skeletally immature patient. Given the high rate of reruptures in this population and the increasing number of commercially available fixation devices, biomechanical testing is crucial to understand the performance of these devices in pediatric epiphyseal bone. To our knowledge, there has not been a biomechanical analysis of ACL fixation devices in skeletally immature bone.
Purpose:
To compare cortically based button fixation with interference screw and sheath fixation in skeletally immature femoral epiphyseal cadaveric bone. Our hypothesis was that there would be no difference in peak load to failure, stiffness, or cyclic displacement between these 2 fixation constructs.
Study Design:
Controlled laboratory study.
Methods:
Fresh-frozen matched-pair knees from 3 pediatric cadaveric specimens were obtained. A synthetic graft was fixed in an all-epiphyseal femoral tunnel. Both the lateral and medial condyles were utilized to increase the sample size. Specimens were randomized and assigned to receive either an interference screw and sheath construct designed for pediatric patients or an adjustable loop cortical button. Biomechanical testing was performed to obtain ultimate load to failure, stiffness, total displacement after 500 cycles, and the failure mode for each condyle.
Results:
Each medial and lateral condyle in 3 pairs of skeletally immature cadaveric knees (ages 7, 9, and 11 years) was utilized for testing. One specimen was excluded after it failed by having a transphyseal fracture. The median peak load to failure was 769.80 N (interquartile range [IQR], 628.50-930.41 N) for the screw and sheath group and 862.80 N (IQR, 692.34-872.65 N) for the button group (P = .893). The median displacement after 500 cycles for the screw and sheath group was 0.65 mm (IQR, 0.47-1.03 mm) and 1.13 mm (IQR, 0.96-1.25 mm) for the button group (P = .08). The median stiffness of the screw and sheath group was significantly higher than that of the button group (31.47 N/mm [IQR, 26.40-43.00 N/mm] vs 25.22 N/mm [IQR, 21.18-27.07 N/mm], respectively) (P = .043).
Conclusion:
When comparing femoral fixation with a screw and sheath construct developed for pediatric patients to an adjustable loop cortical button in skeletally immature bone, our results showed that fixation did not significantly differ with respect to cyclic displacement or peak load to failure. While the screw and sheath construct was significantly stiffer, its effect on clinical outcomes is not yet known.
Clinical Relevance:
With regard to femoral fixation, there is no significant biomechanical difference between the use of cortically based button fixation or interference screw and sheath fixation in pediatric epiphyseal cadaveric bone.
Keywords: anterior cruciate ligament, ACL, pediatric, adolescent, skeletally immature, all-epiphyseal, biomechanics
Once considered a rare diagnosis, a rupture of the anterior cruciate ligament (ACL) in the skeletally immature has been increasingly recognized.2,5 According to the Centers for Disease Control and Prevention, there are between 45 and 50 million children playing organized sports each year in the United States.23 The increased participation and intensity in cutting and contact sports, combined with increased physician awareness and improved diagnostic methods, are likely responsible for the increased recognition and incidence of pediatric ACL injuries.19,35
Historically, ACL reconstruction was delayed until skeletal maturity to avoid the risk of growth disturbance. However, nonsurgical treatment of the active, skeletally immature patient with ACL deficiency has poor prognosis. Nonoperative treatment in this particular population carries an increased risk for secondary meniscal tears, persistent instability, radiographic degenerative changes, and inability to return to sport at the prior activity level.3,13–15,24,25,35 In an effort to prevent these subsequent injuries, surgical reconstruction has been advocated in the large majority of cases.2,3,5,19
All-epiphyseal hamstring reconstruction has become a popular method; however, to date, there are currently no data on the biomechanical performance of ACL fixation devices in skeletally immature bone. Given the high rate of reruptures in this population and the increasing number of devices available for fixation on the market, testing of these devices in pediatric epiphyseal bone is crucial.8,11,28
The purpose of this study was to directly compare cortical button and interference screw and sheath fixation in skeletally immature cadaveric bone in a matched-pair analysis. Our null hypothesis was that there is no difference in peak load to failure, stiffness, or cyclic displacement between these 2 constructs.
Methods
Fresh-frozen matched-pair knees from 3 pediatric cadaveric specimens (6 knees) were donated from a single source (AlloSource). Because of the paucity of pediatric specimens available for study, both lateral and medial condyles were used to serve as ACL femoral fixation sites (12 total testing sites) and were assigned in a random fashion. The specimens were previously used for unrelated patellofemoral and iliotibial band studies. The prior studies did involve cartilage sampling but did not involve any bony manipulation, cortical disruption, or other testing that would affect the biomechanical properties of the specimens.
Each matched-pair specimen was randomized to undergo cortical button fixation (TightRope RT; Arthrex) in one condyle and receive a screw and sheath construct (ShieldLoc; OrthoPediatrics) in the other condyle of one knee, with the opposite configuration of cortical button fixation and screw and sheath fixation in the matching contralateral knee. The specimens were 7, 9, and 11 years of age. All specimens were male and did not have any known metabolic or endocrine disorders.
All femoral condyles were potted in plaster of paris before biomechanical testing. Specimens were stored at –20°C, thawed at room temperature 24 hours before testing, and kept moist with saline irrigation throughout the preparation process and mechanical testing.
Surgical Technique
After the specimens had been randomized, they were inspected for mechanical damage or defects, and any residual cruciate ligament attachments on the femur were sharply excised. A small amount of remnant tissue was left on the condyle to act as a guide for femoral tunnel placement, as is typical in all-epiphyseal ACL reconstruction. To guide our placement of the tunnel on the medial femoral condyle, we referenced the posterior condyle and articular cartilage margin and placed the tunnel in a similar position to the corresponding tunnel in the lateral femoral condyle for that specimen. We elected to utilize a 4 mm–diameter, 550 lb–test paracord (Paracord Planet) as a synthetic ACL graft. Two pieces of the paracord were cut to a length of 300 mm. The loose inner core strands were then removed. The lengths of the paracord were then doubled back to construct a 150-mm quadrupled graft (Figure 1). Each limb was whipstitched with No. 2 FiberWire (Arthrex). The grafts were sized and easily fit through a 7-mm sizing block.
Figure 1.

Four-millimeter-diameter paracord was cut to 300 mm and doubled to serve as an anterior cruciate ligament graft.
Cortical Button Fixation
Two board-certified fellowship-trained sports medicine orthopaedic surgeons (C.W.N. and M.D.M.) performed both reconstruction construct techniques. First, a 2.4-mm guide pin was placed using an all-epiphyseal technique with the aid of an ACL guide set to 90°. Placement was confirmed distal to the distal femoral physis on mini C-arm fluoroscopy. The guide was centered over the native ACL footprint when the lateral condyle was used and set in the corresponding location of the medial notch when the medial condyle was utilized. The guide pin was then removed, and a 7-mm retrodrilling device (FlipCutter; Arthrex) was placed through the ACL guide in the same location to create a femoral socket. The drill guide sleeve was malleted down to the outer cortex before creation of the femoral socket to ensure a 7-mm bony bridge between the deepest portion of the femoral socket and the lateral cortex, as per the device design. A passing suture was used to pass the graft in an inside-out fashion. The button was flipped onto the lateral cortex; the adjustable loop was then cinched down to bring the graft into the femoral tunnel. Slight countertension was maintained throughout this process to simulate the operative environment (Figure 2).
Figure 2.

Cortical button being deployed and tensioned into the medial femoral condyle tunnel.
Interference Screw and Sheath Fixation
The same surgeons performed fixation using the interference screw and sheath construct (ShieldLoc). First, the supplied ACL guide was set to 55° and positioned over the femoral footprint. An all-epiphyseal 2.4-mm guide pin was drilled into the center of the ACL footprint when utilizing the lateral femoral condyle or the medial notch when the medial condyle was used. Placement was confirmed on mini C-arm fluoroscopy. Per the technique guide, a cannulated 7-mm reamer was utilized to create the femoral tunnel. During creation of the tunnel, a hemostat was placed on the tip of the guide wire to protect the contralateral condylar notch. The corresponding counterbore for the 7-mm reamer was used to further open the lateral cortex. A 7-mm sheath was placed per the technique guide. The graft was passed in an outside-in manner as instructed in the technique guide. The whipstitched limbs of the graft were then held with manual countertension, with a supplied guide wire placed between the limbs, and the interference screw was placed in the sheath to complete the construct (Figure 3).
Figure 3.

Final interference screw and sheath construct.
Biomechanical Testing
Each specimen was tested after grafts were fixed to both condyles (Figure 4). The proximal portion of each femur was potted using plaster of paris in a 2 inch–diameter polyvinyl chloride pipe to allow fixation into the base fixture of the materials testing machine (Bionix II universal testing machine; MTS Systems). The distal portions of the graft were fixed to the MTS machine by a clamp and specimens positioned so that the force vector was collinear with the femoral tunnel (Figure 5). After a 10-N preload, each specimen was subjected to 500 cycles between 0 and 100 N with a crosshead speed of 100 mm/min, and the load displacement curve was recorded at a 20-Hz sampling rate (as described by Hapa et al16 and Tsukada et al34). Load (N) versus displacement (mm) was recorded until failure. The ultimate tensile load was considered the peak force recorded. The study endpoints for the different fixation devices include ultimate load to failure (N), stiffness (N/mm), total displacement after 500 cycles, and failure mode for each specimen.
Figure 4.

Screw and sheath construct in the lateral condyle and button in the medial condyle before MTS testing.
Figure 5.
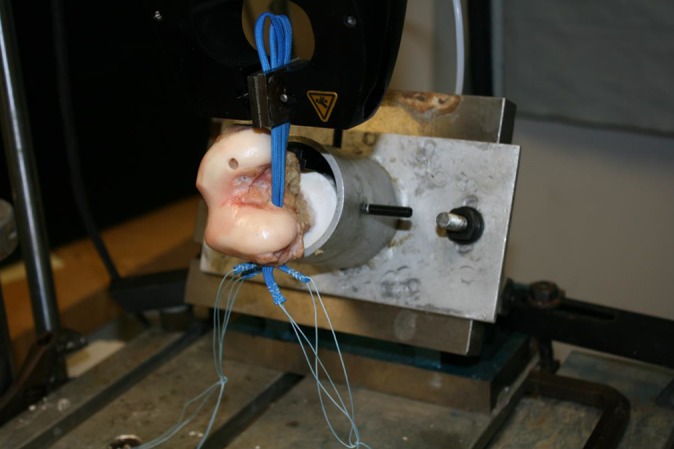
MTS setup: load is applied in line with the trajectory of the femoral tunnel.
Statistical Analysis
Statistical analyses were performed by a member of the research team with formal training in epidemiology and biostatistics (P.D.F.) using SPSS Statistics version 22 (IBM). Because of the rarity of skeletally immature cadaveric tissue and the resultant low sample size, all available tissue was utilized, and therefore, an a priori power calculation was not performed.18 Furthermore, to maximize statistical power, each specimen served as its own internal control in the matched-pair analysis. Additionally, nonparametric statistics were used to minimize the effect of outliers, which is appropriate for small sample sizes.18 To that end, descriptive statistics were reported as medians and interquartile ranges (IQRs), and comparative statistics were performed using the related-samples Wilcoxon signed-rank test. All comparative tests were 2-tailed, and P = .05 was set as the threshold for statistical significance.
Results
One specimen experienced failure due to a transphyseal fracture during biomechanical testing, and both the lateral and medial condyle results for that specimen were excluded. This resulted in 10 femoral tunnels that were included in the final analysis. The median peak load to failure was 769.80 N (IQR, 628.50-930.41 N) for the screw and sheath group and 862.80 N (IQR, 692.34-872.65 N) for the button group (P = .893) (Appendix Figure A1). The median displacement after 500 cycles for the screw and sheath group was 0.65 mm (IQR, 0.47-1.03 mm) and 1.13 mm (IQR, 0.96-1.25 mm) for the button group (P = .08) (Appendix Figure A2). The median stiffness of the screw and sheath group was 31.47 N/mm (IQR, 26.40-43.00 N/mm) and 25.22 N/mm (IQR, 21.18-27.07 N/mm) for the button group, which was significantly different (P = .043) (Table 1 and Appendix Figure A3).
TABLE 1.
Descriptive and Biomechanical Data of Specimens
| Age, y | Side | Condyle | Fixation Method | Peak Load, N | Displacement, mm | Stiffness, N/mm | Mode of Failure |
|---|---|---|---|---|---|---|---|
| 7 | Left | Medial | Screw & sheath | 769.80 | 0.41 | 47.10 | Screw/sheath pull-through |
| 7 | Right | Lateral | Screw & sheath | 1070.10 | 0.53 | 38.83 | Screw/sheath pull-through |
| 9 | Left | Lateral | Screw & sheath | 631.10 | 0.73 | 25.84 | Fracture |
| 11 | Right | Medial | Screw & sheath | 790.72 | 0.65 | 26.97 | Graft disengaged from screw & sheath |
| 11 | Left | Lateral | Screw & sheath | 625.90 | 1.34 | 31.37 | Screw/sheath pull-through |
| 7 | Left | Lateral | Button | 862.80 | 1.29 | 27.99 | Button pull-through |
| 7 | Right | Medial | Button | 875.87 | 1.21 | 22.06 | Suture failure at button |
| 9 | Right | Lateral | Button | 625.24 | 0.97 | 25.22 | Fracture |
| 11 | Right | Lateral | Button | 759.43 | 0.95 | 20.30 | Button pull-through |
| 11 | Left | Medial | Button | 869.43 | 1.13 | 26.15 | Suture failure at button |
The mode of failure in the screw and sheath group was a tunnel fracture in 1 specimen, while the construct subsided without a tunnel fracture in 3 specimens. In 1 specimen, the synthetic paracord graft pulled through the interference screw and sheath construct. The mode of failure in the cortical button group was a direct result of a suture rupture at the cortical button in 2 specimens and button pull-through in the lateral cortex in 2 specimens (Table 1).
Discussion
Pediatric and adolescent ACL injuries are a serious, potentially disabling problem. The number of ACL reconstructions performed in the skeletally immature population is growing, and multiple methods of reconstruction exist.2,5,7,10,12,36 Many of the techniques used today are adapted from ACL reconstruction techniques in the adult population and utilize the same devices. However, there are inherent structural differences between pediatric and adult bone with regard to mineralization and bone strength, and the performance of these devices in pediatric bone has not been evaluated, to our knowledge.4,20 We compared 2 commonly used fixation devices for skeletally immature bone: an adjustable loop cortical button and an interference screw and sheath construct. Our results showed that fixation did not significantly differ with regard to cyclic displacement or peak load to failure. The screw and sheath construct was significantly stiffer, but the clinical relevance of this difference is not known.
Prior ACL fixation studies using porcine models have been criticized for overestimating displacement and yield load values when compared with human cadaveric specimens.21,27 However, the prohibitive cost and low availability associated with testing of human cadaveric specimens needs to be taken into consideration. Previous studies of human cadaveric specimens have typically used older donors, the results of which may not extend to skeletally immature tissue.
It has been shown through simulated models that an ACL in an adult can experience 303 N of load during ground-level walking and up to 1294 N with single-leg landing from a running jump.31,32 Rowden et al30 performed a biomechanical study in relatively young but skeletally mature human cadaveric specimens. They showed that the pullout strength of a quadrupled hamstring graft fixed with a suture loop button and a bone–patellar tendon–bone (BPTB) graft fixed via an aperture interference screw produced an ultimate load to failure of 612 N and 416 N, respectively.30 The average age of the hamstring and BPTB groups was 30 and 24 years, respectively. Our results showed an ultimate load to failure that was greater, with values of 769.80 to 862.80 N. The Rowden et al30 study used cadaveric grafts, and the mode of failure in the BPTB group was graft failure in 3 of the 6 specimens and interference screw pullout in the other 3 specimens, while suture loop button failure occurred in all specimens in the hamstring group. Recently, Noonan et al26 showed that cortical loop fixation with no retensioning had an ultimate failure of 786 N, which is consistent with the findings of the current study. Ultimately, we have shown in this study that fixation with a cortical button or screw and sheath construct in skeletally immature bone performed at least as well as, if not better than, fixation devices previously tested in adult bone.
In addition to ultimate load to failure, displacement during cyclic loading has ramifications early in the postoperative period, as hamstring graft incorporation does not occur until roughly 12 weeks after surgical fixation.33 We demonstrated that with both modes of fixation, cyclic displacement was comparable with results from prior studies. After 500 cycles at 0 to 100 N, median displacement for the screw and sheath group was 0.65 mm (IQR, 0.47-1.03 mm) and 1.13 mm (IQR, 0.96-1.25 mm) for the button group (P = .08). Johnson et al17 recently performed cyclic testing on a variety of suture buttons and found the TightRope RT to displace 2.2 mm and 1.8 mm after not retensioning and retensioning, respectively. This is substantially more than the cyclic displacement found in our study; however, their parameters were different, as they cycled between 100 and 400 N for 1000 cycles to simulate peak loads. Our results are comparable with those of Petre et al,29 who found that adjustable loop buttons displaced 1.1 mm after 1000 cycles. Noonan et al26 performed a similar but more comprehensive evaluation of adjustable loop cortical fixation in a variety of scenarios and found that in conditions similar to those of our study, grafts were displaced a distance between 1.2 and 2.7 mm.
There are no prior cyclic loading studies evaluating the ShieldLoc screw and sheath system used in our study. However, Aga et al1 tested various screw and sheath devices for tibial fixation of hamstring grafts and found that graft displacement after 100 cycles ranged between 1.38 and 1.92 mm. While our findings revealed a displacement of 0.65 mm for the screw and sheath construct, it was not significantly different from the button. In terms of displacement compared with prior studies, these fixation devices performed similarly, if not slightly better, in skeletally immature bone than devices tested using adult tissue.
While load to failure and cyclic displacement were not significantly different in this study, stiffness was greater in the screw and sheath group. Construct stiffness is determined by the rigidity of fixation and the elastic properties of the graft material.38 Because of the standardized use of the paracord as a graft substitute, any observed differences in our study are likely because of the 2 different fixation methods, as the paracord is uniform throughout while soft tissue grafts may have variability between specimens. This finding is consistent with prior studies that have demonstrated superior stiffness when using interference screw fixation compared with cortical button fixation in ACL reconstruction.6,22 Interestingly, the values that we obtained for stiffness were much lower compared with previous reports. Woo et al37,38 performed biomechanical testing on intact ACLs in young human cadaveric knees (ages 22-35 years) and found mean stiffness to be 242 ± 28 N/mm. The stiffness of the constructs in our study ranged from 25.22 to 31.47 N/mm, which are below the values reported in the literature; however, the clinical significance of these differences in stiffness is unknown. This may have been the result of the introduction of the paracord, implant differences, and/or the inherent mechanical properties of skeletally immature bone as it has been shown that skeletally immature bone is inherently less stiff than adult bone.9 Further investigation is needed to fully understand these differences.
We recognize that our study has limitations. The study was performed on a small number of cadaveric specimens because of the extreme rarity of available pediatric specimens for study use and, as a result of this, is likely underpowered. We also used both medial and lateral condyles to perform femoral ACL fixation in an effort to maximize the utility of a limited number of specimens; however, our data did not significantly differ from medial to lateral condyle fixation so we do not have reason to believe that this introduced any inaccurate results. With the introduction of the medial condyle for testing, we do recognize that this is nonanatomic and may introduce an element of variability from structural differences such as bone density. However, we are unaware of any literature to support significant differences. Fixation devices were tested with the force vector applied to the graft in line with the tunnel, which may be nonphysiological when compared with in vivo graft loading of a native or reconstructed ACL. Finally, we elected to utilize synthetic material for our graft, as cadaveric tissue was not available at the time of the study. In addition, the use of a synthetic graft would conceivably eliminate any inherent variability that biological tissue could introduce between specimens. This in turn maximized the comparability of testing environments and isolated fixation device as the variable of interest. However, the use of a synthetic graft may have different fixation strength characteristics in the screw and sheath construct compared with a soft tissue graft, as the construct relies on friction fit, which may be different between different materials.
Conclusion
The current study is the first to biomechanically test and directly compare graft fixation constructs in skeletally immature epiphyseal tissue. Our results indicated that cortical button fixation and interference screw and sheath fixation were no different with regard to peak load to failure and cyclic displacement for all-epiphyseal ACL reconstruction. Biomechanical stiffness was significantly greater with screw and sheath fixation; however, the clinical relevance of the absolute difference observed here is unknown and remains a subject of interest for future study.
Acknowledgment
The authors acknowledge AlloSource, Arthrex, and OrthoPediatrics for their assistance, contribution, and support of this project. They also extend very special thanks to the families and their tremendous sacrifice of pediatric tissue, which in large part made this project possible.
Appendix
Figure A1.
Box plot displaying median and interquartile ranges of peak load to failure for the screw and sheath group and for the button group, which did not reach statistical significance. Minimum and maximum Newtons are connoted by whisker bars.
Figure A2.
Box plot displaying median and interquartile ranges of cyclic displacement for the screw and sheath group and for the button group, which did not reach statistical significance. Minimum and maximum displacement are connoted by whisker bars.
Figure A3.
Box plot displaying median and interquartile ranges of stiffness for the screw and sheath group and for the button group, which reached statistical significance (P = .043). Minimum and maximum stiffness values are connoted by whisker bars.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: K.G.S. has received educational support from DePuy and Sanofi-Aventis. P.D.F. has received educational support from Smith & Nephew. M.D.M. receives royalties from Elsevier. K.G.S., C.W.N., P.D.F., and M.D.M. are members of the Research in Osteochondritis Dissecans of the Knee (ROCK) study group, which receives research funding from AlloSource and Vericel.
Ethical approval for this study was waived by the University of Connecticut Health Center.
References
- 1. Aga C, Rasmussen MT, Smith SD, et al. Biomechanical comparison of interference screws and combination screw and sheath devices for soft tissue anterior cruciate ligament reconstruction on the tibial side. Am J Sports Med. 2013;41(4):841–848. [DOI] [PubMed] [Google Scholar]
- 2. Al-Hadithy N, Dodds AL, Akhtar KSN, Gupte CM. Current concepts of the management of anterior cruciate ligament injuries in children. Bone Joint J. 2013;95B(11):1562–1569. [DOI] [PubMed] [Google Scholar]
- 3. Anderson AF, Anderson CN. Correlation of meniscal and articular cartilage injuries in children and adolescents with timing of anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43(2):275–281. [DOI] [PubMed] [Google Scholar]
- 4. Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15(11):2245–2250. [DOI] [PubMed] [Google Scholar]
- 5. Beck NA, Patel NM, Ganley TJ. The pediatric knee: current concepts in sports medicine. J Pediatr Orthop B. 2014;23(1):59–66. [DOI] [PubMed] [Google Scholar]
- 6. Brown CH, Wilson DR, Hecker AT, Ferragamo M. Graft-bone motion and tensile properties of hamstring and patellar tendon anterior cruciate ligament femoral graft fixation under cyclic loading. Arthroscopy. 2004;20(9):922–935. [DOI] [PubMed] [Google Scholar]
- 7. Buller LT, Best MJ, Baraga MG, Kaplan LD. Trends in anterior cruciate ligament reconstruction in the United States. Orthop J Sports Med. 2015;3(1):2325967114563664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cassard X, Cavaignac E, Maubisson L, Bowen M. Anterior cruciate ligament reconstruction in children with a quadrupled semitendinosus graft: preliminary results with minimum 2 years of follow-up. J Pediatr Orthop. 2014;34(1):70–77. [DOI] [PubMed] [Google Scholar]
- 9. Chotel F, Braillon P, Sailhan F, et al. Bone stiffness in children. J Pediatr Orthop. 2008;28(5):534–537. [DOI] [PubMed] [Google Scholar]
- 10. Dodwell ER, LaMont LE, Green DW, Pan TJ, Marx RG, Lyman S. 20 years of pediatric anterior cruciate ligament reconstruction in New York State. Am J Sports Med. 2014;42(3):675–680. [DOI] [PubMed] [Google Scholar]
- 11. Engelman GH, Carry PM, Hitt KG, Polousky JD, Vidal AF. Comparison of allograft versus autograft anterior cruciate ligament reconstruction graft survival in an active adolescent cohort. Am J Sports Med. 2014;42(10):2311–2318. [DOI] [PubMed] [Google Scholar]
- 12. Fabricant PD, Kocher MS. Management of ACL injuries in children and adolescents. J Bone Joint Surg Am. 2017;99(7):600–612. [DOI] [PubMed] [Google Scholar]
- 13. Fabricant PD, Lakomkin N, Cruz AI, Spitzer E, Lawrence JTR, Marx RG. Early ACL reconstruction in children leads to less meniscal and articular cartilage damage when compared with conservative or delayed treatment. J ISAKOS. 2016;1(1):10–15. [Google Scholar]
- 14. Fabricant PD, Lakomkin N, Cruz AI, Spitzer E, Marx RG. ACL reconstruction in youth athletes results in an improved rate of return to athletic activity when compared with non-operative treatment: a systematic review of the literature. J ISAKOS. 2016;1:1–8. [Google Scholar]
- 15. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229–233. [DOI] [PubMed] [Google Scholar]
- 16. Hapa O, Barber FA, Süner G, et al. Biomechanical comparison of tibial eminence fracture fixation with high-strength suture, endobutton, and suture anchor. Arthroscopy. 2012;28(5):681–687. [DOI] [PubMed] [Google Scholar]
- 17. Johnson JS, Smith SD, LaPrade CM, Turnbull TL, LaPrade RF, Wijdicks CA. A biomechanical comparison of femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction under high loads. Am J Sports Med. 2015;43(1):154–160. [DOI] [PubMed] [Google Scholar]
- 18. Kocher MS, Zurakowski D. Clinical epidemiology and biostatistics: a primer for orthopaedic surgeons. J Bone Joint Surg Am. 2004;86(3):607–620. [PubMed] [Google Scholar]
- 19. LaBella CR, Hennrikus W, Hewett TE. Anterior cruciate ligament injuries: diagnosis, treatment, and prevention. Pediatrics. 2014;133(5):e1437–e1450. [DOI] [PubMed] [Google Scholar]
- 20. Levine MA. Assessing bone health in children and adolescents. Indian J Endocrinol Metab. 2012;16(suppl 2):S205–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35–43. [DOI] [PubMed] [Google Scholar]
- 22. Mayr R, Heinrichs CH, Eichinger M, Coppola C, Schmoelz W, Attal R. Biomechanical comparison of 2 anterior cruciate ligament graft preparation techniques for tibial fixation: adjustable-length loop cortical button or interference screw. Am J Sports Med. 2015;43(6):1380–1385. [DOI] [PubMed] [Google Scholar]
- 23. Merkel D. Youth sport: positive and negative impact on young athletes. Open Access J Sports Med. 2013;4:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Millett PJ, Willis AA, Warren RF. Associated injuries in pediatric and adolescent anterior cruciate ligament tears: does a delay in treatment increase the risk of meniscal tear? Arthroscopy. 2002;18(9):955–959. [DOI] [PubMed] [Google Scholar]
- 25. Mizuta H, Kubota K, Shiraishi M, Otsuka Y, Nagamoto N, Takagi K. The conservative treatment of complete tears of the anterior cruciate ligament in skeletally immature patients. J Bone Joint Surg Br. 1995;77(6):890–894. [PubMed] [Google Scholar]
- 26. Noonan BC, Dines JS, Allen AA, Altchek DW, Bedi A. Biomechanical evaluation of an adjustable loop suspensory anterior cruciate ligament reconstruction fixation device: the value of retensioning and knot tying. Arthroscopy. 2016;32(10):2050–2059. [DOI] [PubMed] [Google Scholar]
- 27. Nurmi JT. Porcine tibia is a poor substitute for human cadaver tibia for evaluating interference screw fixation. Am J Sports Med. 2004;32(3):765–771. [DOI] [PubMed] [Google Scholar]
- 28. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petre BM, Smith SD, Jansson KS, et al. Femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction: a comparative biomechanical study. Am J Sports Med. 2013;41(2):416–422. [DOI] [PubMed] [Google Scholar]
- 30. Rowden NJ, Sher D, Rogers GJ, Schindhelm K. Anterior cruciate ligament graft fixation: initial comparison of patellar tendon and semitendinosus autografts in young fresh cadavers. Am J Sports Med. 1997;25:472–478. [DOI] [PubMed] [Google Scholar]
- 31. Shelburne KB, Torry MR, Pandy MG. Muscle, ligament, and joint-contact forces at the knee during walking. Med Sci Sports Exerc. 2005;37(11):1948–1956. [DOI] [PubMed] [Google Scholar]
- 32. Shin CS, Chaudhari AM, Andriacchi TP. The influence of deceleration forces on ACL strain during single-leg landing: a simulation study. J Biomech. 2007;40(5):1145–1152. [DOI] [PubMed] [Google Scholar]
- 33. Tomita F, Yasuda K, Mikami S, Sakai T, Yamazaki S, Tohyama H. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(5):461–476. [DOI] [PubMed] [Google Scholar]
- 34. Tsukada H, Ishibashi Y, Tsuda E, Hiraga Y, Toh S. A biomechanical comparison of repair techniques for anterior cruciate ligament tibial avulsion fracture under cyclic loading. Arthroscopy. 2005;21(10):1197–1201. [DOI] [PubMed] [Google Scholar]
- 35. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Werner BC, Yang S, Looney AM, Gwathmey FW. Trends in pediatric and adolescent anterior cruciate ligament injury and reconstruction. J Pediatr Orthop. 2016;36(5):447–452. [DOI] [PubMed] [Google Scholar]
- 37. Woo SL, Debski RE, Withrow JD, Janaushek MA. Biomechanics of knee ligaments. Am J Sports Med. 1999;27(4):533–543. [DOI] [PubMed] [Google Scholar]
- 38. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217–225. [DOI] [PubMed] [Google Scholar]





