Abstract
Background:
Vascular-derived progenitor and endothelial cell populations (CD31, CD34, CD146) are capable of multipotent differentiation at the site of injured ligamentous tissue to aid in the intrinsic healing response. Proximal ulnar collateral ligament (UCL) tears have been reported to have better healing capability when compared with distal UCL tears.
Purpose:
To compare the vascular composition of the proximal and distal insertions of the anterior bundle of the UCL of the elbow via known markers of endothelial and vascular-derived progenitor cells (CD31, CD34, CD146).
Study Design:
Descriptive laboratory study.
Methods:
UCLs were harvested from 10 nonpaired fresh-frozen human cadaveric elbows and transected into proximal and distal portions. Endothelial and vascular-derived progenitor cell densities were assessed with 4 staining groups: CD31 (immunohistochemistry) and CD31/α-smooth muscle actin (α-SMA), CD34/α-SMA, and CD146/α-SMA (immunofluorescence). CD31 immunohistochemistry identified endothelial progenitor cells in the UCL. Later staining of the same slides with α-SMA demonstrated the relationship of progenitor cells to the surrounding vasculature. Fluorescent staining was quantified by calculating the proportion of positively stained nuclei versus the total number of nuclei in the proximal and distal UCL.
Results:
CD31+ cells were present in the proximal and distal sections of all 10 UCLs. Fluorescent staining revealed no significant differences in the ratio of CD31 to total nuclei between the distal (median, 36% [range, 23%-53%]) and proximal UCL (39% [22%-56%]) (P = .432, Wilcoxon signed-rank test). Similarly, no differences were seen between CD34 distal (39% [24%-64%]) and proximal regions (46% [28%-63%]) (P = .846, Wilcoxon signed-rank test) or CD146 distal (40% [12%-65%]) and proximal regions (40% [22%-51%]) (P ≥ .999, Wilcoxon signed-rank test).
Conclusion:
Analysis of UCL tissues demonstrated equal distributions of vascular endothelial and vascular-derived progenitor cell markers throughout the proximal and distal UCL. Unlike that of the medial collateral ligament of the knee, the microvascular composition of the proximal and distal UCL insertions was not different, suggesting a well-vascularized ligament throughout its course.
Clinical Relevance:
These findings investigate one of the possible contributors to UCL healing after injury, which may provide insight into operative and nonoperative management of UCL injuries in the future. This study also indicates that reasons other than differences in progenitor cell density alone may explain the clinical healing differences seen between proximal and distal UCL tears. A better understanding of the microvascular environment and associated blood supply is warranted to understand the healing capability of the UCL.
Keywords: vascularity, UCL, healing, ligament biology
The anterior bundle of the medial ulnar collateral ligament (UCL) of the elbow is the primary static stabilizer to valgus stress in the elbow, and instability can result in significant disability among overhead athletes.5,11,12 Surgical intervention is indicated for the majority of acute complete tears, but less definitive evidence remains for the treatment of partial tears.2–4,13,15–17,19 Given the increased recognition of symptomatic partial UCL injuries and the paucity of definitive data, a more focused analysis of the location and pattern of injury within the anterior band of the UCL may prove critical in establishing a treatment algorithm for partial tears.
Frangiamore et al4 examined magnetic resonance imaging characteristics of the failure of nonoperative treatment of UCL tears and found that professional baseball players with distal tears failed nonoperative treatment more frequently than those with proximal tears. Although ligament tissues are relatively hypovascular, the vascular endothelial and progenitor cell response to injury or reconstruction is critical for its healing potential.1 Previous studies have demonstrated the importance of angiogenesis and vascular-derived progenitor cells on the ligament healing potential in the anterior cruciate ligament (ACL) and medial collateral ligament (MCL) of the knee.8,10
While the biomechanical properties of the UCL have been studied, little is known about its vascular topography and regenerative capabilities.6,9,12,18 Vascular-derived progenitor and endothelial cell populations (CD31, CD34, CD146) are capable of multipotent differentiation and are recruited to the rupture site of injured ligamentous tissue to aid in the intrinsic healing response.8,10 Thus, the purpose of this study was to quantify and compare the microvascular composition of proximal and distal UCL insertions to improve our understanding of UCL injury patterns and healing potential. We hypothesized that differences in vascular-derived progenitor and endothelial cell populations of the proximal UCL would be significantly higher than in the distal aspect of the ligament.
Methods
Specimen Selection and Paraffin Processing, Embedding, and Sectioning
Ten nonpaired fresh-frozen human cadaveric elbows (mean age, 54.1 years; range, 42-64 years; all male) without prior surgery or evidence of previous injury were utilized for this study. The cadaveric specimens were donated to a tissue bank for medical research and then purchased by our institution. The use of cadaveric specimens is exempt at the senior authors’ institution, so institutional review board approval was not required. Specimens were dissected to harvest the anterior bundle of the UCL along its entire length, and the harvested UCL was transected into proximal and distal portions. All UCLs were fixed in 10% neutral buffered formalin at room temperature for 72 hours, rinsed in phosphate-buffered saline, and stored in 70% ethanol (EtOH) at 4°C before paraffin processing. The ligaments were then paraffin processed by hand. Specifically, specimens were dehydrated from 75% EtOH through 100% EtOH, cleared through 3 changes of xylene, and paraffin infiltrated with 3 changes of paraffin at 60°C while being shaken. Specimens were embedded in paraffin, solidified in cassettes on ice, and sectioned at 7 µm.
Immunohistochemistry and Immunofluorescence Staining
UCL vascularity was assessed with 4 staining groups: CD31 immunohistochemistry, CD31/α-smooth muscle actin (α-SMA) fluorescence, CD34/α-SMA fluorescence, and CD146/α-SMA fluorescence. CD31 staining provided a visualization of vascular endothelial cells, while CD34 (vascular endothelial progenitor cell) and CD146 (pericyte) staining marked the presence of 2 populations of vascular-derived progenitor cells.8,10 The α-SMA costaining served to locate smooth muscle surrounding the arterioles.8 Two rounds of staining (ie, 2 total slides per specimen) were performed for each group to account for variability between cross sections. For immunohistochemistry and immunofluorescence, slides were dried in a 60°C oven for 2 hours, deparaffinized with 2 changes of xylene, and rehydrated from 100% EtOH to Tris-buffered saline (CD31 immunohistochemistry) or deionized water (immunofluorescent staining). Heat-activated antigen retrieval was performed in 10 mM sodium citrate buffer (pH 6.0) in a 90°C water bath for 20 minutes.
Immunofluorescent staining of CD31 (Abcam ab28364, 1:50), CD34 (Abcam ab8535, 1:250), or CD146 (Abcam ab24577, 1:200) was completed. All fluorescent staining of CD markers was costained with α-SMA (Abcam ab32575, 1:300), with 0.1% phosphate-buffered saline containing Tween 20 used throughout staining. Following antigen retrieval, slides were blocked in 5% donkey serum (DS) for 1 hour and incubated overnight at 4°C with CD31, CD34, or CD146 supplemented with 5% DS. Secondary antibodies diluted 1:500 were applied the next day for 1 hour at room temperature (or 30 minutes for α-SMA). Concurrent primary staining, followed by successive secondary staining, was performed for CD146/α-SMA. Sequential primary staining was performed for CD31/α-SMA and CD34/α-SMA because these antibodies were all made in rabbit. Thus, in these cases, primary and secondary staining for CD31 and CD34 were conducted, and slides were washed in 0.1% phosphate-buffered saline with Tween 20, blocked again in 5% DS, and then stained completely for α-SMA. After secondary staining, nuclear stain DAPI (4′,6-diamidino-2-phenylindole) was applied for 15 minutes at room temperature. All slides were then washed 3 times in phosphate-buffered saline and rinsed once in deionized water. Fluorescent slides were coverslipped with aqueous mounting medium.
To histologically determine the presence of vascular endothelial cells within blood vessels, immunohistochemical staining for CD31+ cells was completed according to Millipore’s Blood Vessel Staining Kit protocol (Millipore ECM590, CD31, 1°C, 1:200). Slides were dehydrated and coverslipped with xylene-based mounting medium.
Data Collection and Processing
All images were taken with a Nikon Eclipse Ni-U upright microscope. CD31 immunohistochemistry images were taken at 10× and 20× for qualitative purposes. Fluorescent images were taken at 20× for quantitation. Six images per slide were taken of tissue regions with the highest blood vessel density based on α-SMA staining, and nuclei were manually counted with the count function in Nikon NIS-Elements microscope imaging software. Quantification of fluorescent staining for each marker was determined by calculating the proportion of positively stained nuclei versus total number of nuclei.
Statistical Analysis
To address the primary hypothesis of this study, comparisons of progenitor cell distribution were made between distal and proximal UCL locations. To obtain a more accurate representation of the quantities of interest, measurements were averaged across the 6 images taken per slide and then averaged across the 2 slides prior to analysis. Wilcoxon signed-rank (WSR) tests were used to compare central tendency between locations. Summary statistics are presented as medians with ranges, and P values <.05 were deemed statistically significant. Based on the assumption of 2-tailed matched pairs testing and an alpha of 0.05, 10 specimens are sufficient to detect an effect size (d) of 1 with 80% statistical power. The statistical computing software R was used for all analyses.14
Results
Qualitative Analysis
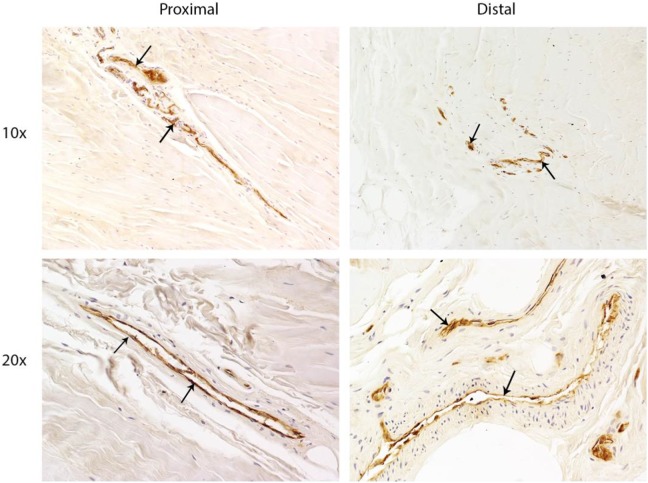
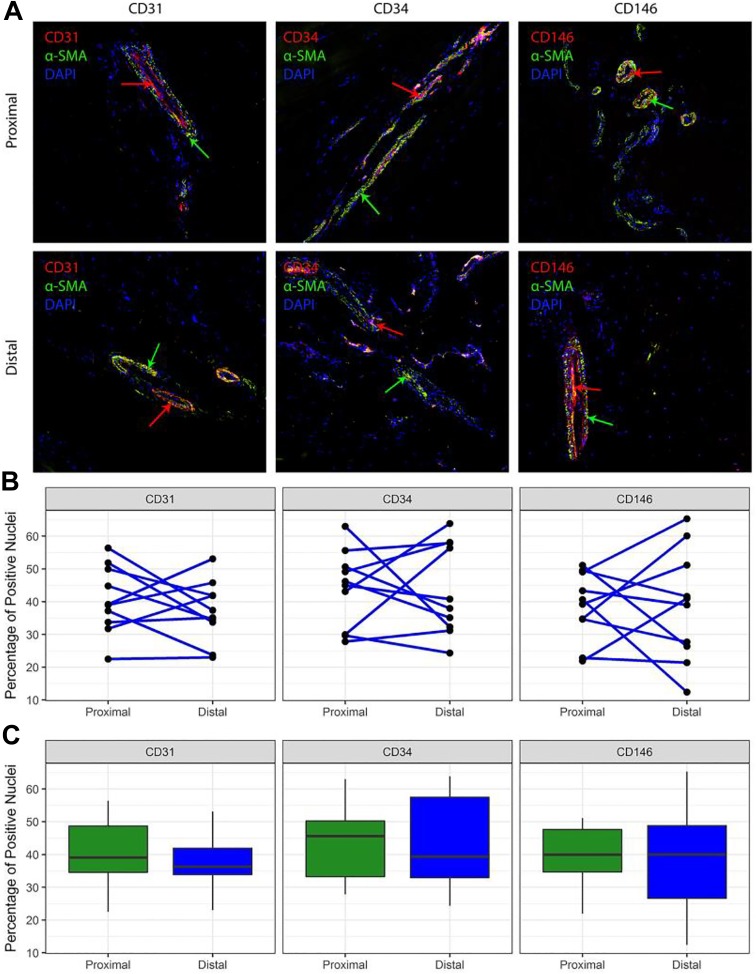
Immunohistochemistry staining revealed CD31+ blood vessels in the proximal and distal sections of the UCL in all ligament tissue test samples (Figure 1); α-SMA expression was frequently observed adjacent to CD31-expressing cells, indicating specific staining of the larger vasculature surrounding the progenitor cells (Figure 2, A and B).
Figure 1.
Immunohistochemistry of tissue samples demonstrates that immature blood vessels (arrows) are present in proximal and distal portions of the ulnar collateral ligament, as shown by brown CD31 staining.
Figure 2.
(A) Immunofluorescent staining of ulnar collateral ligament (UCL) tissue. CD (31, 34, 146) markers demonstrate the presence of vascular progenitor cells (red arrows), while α-smooth muscle actin (α-SMA) demonstrates the presence of mature blood vessels (green arrows). Nuclei were stained with DAPI (blue). (B) Line plots demonstrating individual variation in UCL vascularity among specimens and between proximal and distal regions. Each dot represents the mean frequency for 1 specimen of each vascularity marker in the given portion of the UCL; lines connect the observations for each specimen at proximal and distal locations. (C) Box plots indicating quartiles for each CD marker. No statistically significant differences in the percentage of positively stained nuclei were found when proximal and distal portions of the anterior bundle of the UCL were compared. Values are presented as median (horizontal line), interquartile range (box), and 95% CI (vertical line).
Quantitative Results
Immunofluorescent staining of CD31, CD34, and CD146 revealed no significant differences in vascular endothelial or vascular-derived progenitor cell markers between the proximal and distal regions in any of the samples tested (Table 1). When images were compared within sections, no significant differences were detected in the ratio of CD31 to total nuclei between the distal (median, 36% [range, 23%-53%]) and proximal UCL (39% [22%-56%]) (P = .432, WSR). Similarly, no differences were seen in CD34 staining (distal = 39% [24%-64%], proximal = 46% [28%-63%]) (P = .846, WSR) or CD146 (distal = 40% [12%-65%], proximal = 40% [22%-51%]) (P ≥ .999, WSR).
TABLE 1.
Percentage-Positive Microvasculature Markers in the Proximal and Distal Regions of the Anterior Bundle of the Ulnar Collateral Ligament
| CD31 | CD34 | CD146 | |
|---|---|---|---|
| Proximal | 40.62 ± 10.28 | 44.01 ± 11.71 | 38.71 ± 10.37 |
| Distal | 37.00 ± 9.29 | 43.78 ± 13.95 | 38.59 ± 16.99 |
Discussion
The most important finding of this study was the lack of disparity in the comparison of the microvascular density between the proximal and distal insertions of the UCL. This finding suggests that the increased nonoperative failure rates reported with distal versus proximal UCL tears clinically may be due to alternative differences, such as injury chronicity, anatomic relationships of the dynamic stabilizers, or other mechanical factors, rather than a difference in the number of blood vessels demonstrating vascularity and vascular-derived progenitor cells, which would affect the UCL healing rates. A better understanding of the microvascular environment and associated blood supply is warranted to further validate that vascularity does not play a role in the differences in the healing capacity observed between proximal and distal UCL tears.
Surgical reconstruction of the UCL has recently increased in frequency, as evidenced by findings of a 343% increase between 2003 and 2014 from a database study by Mahure et al.7 Despite this increasing trend toward reconstructing the UCL, the treatment algorithm for management remains unclear, particularly in the setting of incomplete tears. A study by Rettig et al15 in 2001 reported that only 42% (13 of 31) of patients recovered with nonoperative treatment, although the degree of injury to the UCL was not reported. In 2016, Ford et al3 reported that 93% (26 of 28) of professional baseball players returned to the same level of play or higher with nonoperative treatment of incomplete (partial) UCL tears, intimating that the UCL has some intrinsic regenerative potential. Subsequently, magnetic resonance imaging characteristics associated with failure of nonoperative treatment of professional pitchers revealed an increased failure rate with distal UCL tears versus proximal ones.4
We sought to establish a baseline understanding of the vascularity of the UCL by comparing markers of vascular endothelial cells and vascular-derived progenitor cells in the proximal versus distal UCL to determine if this differential biological environment for ligamentous healing exists. We found no difference in endothelial or vascular progenitor cell density between the proximal and distal sections for any of the markers evaluated (P = .432, P = .846, and P ≥ .999 for CD31, CD34, and CD146, respectively), suggesting that vascular density alone may not explain the difference in healing rates between the proximal and distal UCL.
Over the past decade, the intrinsic healing potential of commonly injured ligaments, particularly the ACL and MCL of the knee, has been studied.1,8,10,20,21 In a study investigating the therapeutic role of vascular endothelial growth factor in MCL healing, angiogenesis was established to play a critical role in ligament healing.10 In an immunohistochemical study of 6 fetal and 8 adult ACLs, Matsumoto et al8 demonstrated that the ACL septum contains a population of vascular-derived progenitor cells (CD34+ and CD146+) that may contribute to ligament regeneration and repair at the site of rupture, contributing to primary ligament healing or tendon-bone healing. Furthermore, the ACL septum region was demonstrated to have richer vascularization than the midsubstance region.10 Unlike the ACL model, our study demonstrated that UCL fibers demonstrated no significant differences in the presence of endothelial progenitor cells and pericytes when the proximal insertion on the medial epicondyle was compared with the distal insertion onto the sublime tubercle of the ulna.
Limitations
Our study has some limitations. Because this was a cadaveric study, the age range of the studied samples was greater than that of the population for which UCL injuries are reported. However, a search performed by our network of national tissue banks found only 1 male elbow specimen in the 18- to 35-year-old age group over the past 2 years, so obtaining a younger specimen population was not possible. Additionally, only 2 zones were selected for the analysis: proximal and distal. However, the preserved lack of relative difference between proximal and distal specimens and overall uniform values of total cells and progenitor density among the samples support this methodology as an effective estimate of progenitor cell density.
Conclusion
Analysis of UCL tissues demonstrated equal distribution of vascular endothelial and vascular-derived progenitor cell markers throughout the proximal and distal UCL. Unlike the MCL of the knee, the microvascular composition of the UCL insertions was not different between the proximal and distal portions.
Acknowledgment
The authors acknowledge Grant Dornan, MS, for conducting the statistical analysis.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was internally funded by the Steadman Philippon Research Institute. G.M. has received research grants from the South-Eastern Norway Health Authorities and Arthrex. M.T.P. receives royalties from Arthrex and SLACK Inc and is a paid consultant for Arthrex and the Joint Restoration Foundation (Allosource). T.R.H. is a paid consultant for Arthrex. R.F.L. receives royalties from Arthrex, Ossur, and Smith & Nephew; is a paid consultant for Arthrex, Ossur, and Smith & Nephew; and receives research support from Arthrex, Linvatec, Ossur, and Smith & Nephew.
Ethical approval was not sought for the present study.
References
- 1. Bray RC, Rangayyan RM, Frank CB. Normal and healing ligament vascularity: a quantitative histological assessment in the adult rabbit medial collateral ligament. J Anat. 1996;188(pt 1):87–95. [PMC free article] [PubMed] [Google Scholar]
- 2. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67–83. [PubMed] [Google Scholar]
- 3. Ford GM, Genuario J, Kinkartz J, Githens T, Noonan T. Return-to-play outcomes in professional baseball players after medial ulnar collateral ligament injuries: comparison of operative versus nonoperative treatment based on magnetic resonance imaging findings. Am J Sports Med. 2016;44(3):723–728. [DOI] [PubMed] [Google Scholar]
- 4. Frangiamore SJ, Lynch TS, Vaughn MD, et al. Magnetic resonance imaging predictors of failure in the nonoperative management of ulnar collateral ligament injuries in professional baseball pitchers. Am J Sports Med. 2017;45(8):1783–1789. [DOI] [PubMed] [Google Scholar]
- 5. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158–1163. [PubMed] [Google Scholar]
- 6. Lin F, Kohli N, Perlmutter S, Lim D, Nuber GW, Makhsous M. Muscle contribution to elbow joint valgus stability. J Shoulder Elbow Surg. 2007;16(6):795–802. [DOI] [PubMed] [Google Scholar]
- 7. Mahure SA, Mollon B, Shamah SD, Kwon YW, Rokito AS. Disproportionate trends in ulnar collateral ligament reconstruction: projections through 2025 and a literature review. J Shoulder Elbow Surg. 2016;25(6):1005–1012. [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto T, Ingham SM, Mifune Y, et al. Isolation and characterization of human anterior cruciate ligament-derived vascular stem cells. Stem Cells Dev. 2012;21(6):859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319. [DOI] [PubMed] [Google Scholar]
- 10. Nishimori M, Matsumoto T, Ota S, et al. Role of angiogenesis after muscle derived stem cell transplantation in injured medial collateral ligament. J Orthop Res. 2012;30(4):627–633. [DOI] [PubMed] [Google Scholar]
- 11. Otoshi K, Kikuchi S, Shishido H, Konno S. Ultrasonographic assessment of the flexor pronator muscles as a dynamic stabilizer of the elbow against valgus force. Fukushima J Med Sci. 2014;60(2):123–128. [DOI] [PubMed] [Google Scholar]
- 12. Park MC, Ahmad CS. Dynamic contributions of the flexor-pronator mass to elbow valgus stability. J Bone Joint Surg Am. 2004;86(10):2268–2274. [DOI] [PubMed] [Google Scholar]
- 13. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689–1694. [DOI] [PubMed] [Google Scholar]
- 14. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.r-project.org/.
- 15. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15–17. [DOI] [PubMed] [Google Scholar]
- 16. Savoie FH, 3rd, Morgan C, Yaste J, Hurt J, Field L. Medial ulnar collateral ligament reconstruction using hamstring allograft in overhead throwing athletes. J Bone Joint Surg Am. 2013;95(12):1062–1066. [DOI] [PubMed] [Google Scholar]
- 17. Savoie FH, 3rd, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066–1072. [DOI] [PubMed] [Google Scholar]
- 18. Sojbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186–190. [PubMed] [Google Scholar]
- 19. Walters BL, Cain EL, Emblom BA, Frantz JT, Dugas JR. Ulnar collateral ligament repair with internal brace augmentation: a novel UCL repair technique in the young adolescent athlete. Orthop J Sports Med. 2016;4(3 suppl 3):2325967116S00071. [Google Scholar]
- 20. Zhang J, Pan T, Im HJ, Fu FH, Wang JH. Differential properties of human ACL and MCL stem cells may be responsible for their differential healing capacity. BMC Med. 2011;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang S, Matsumoto T, Uefuji A, et al. Anterior cruciate ligament remnant tissue harvested within 3-months after injury predicts higher healing potential. BMC Musculoskelet Disord. 2015;16:390. [DOI] [PMC free article] [PubMed] [Google Scholar]