Key Points
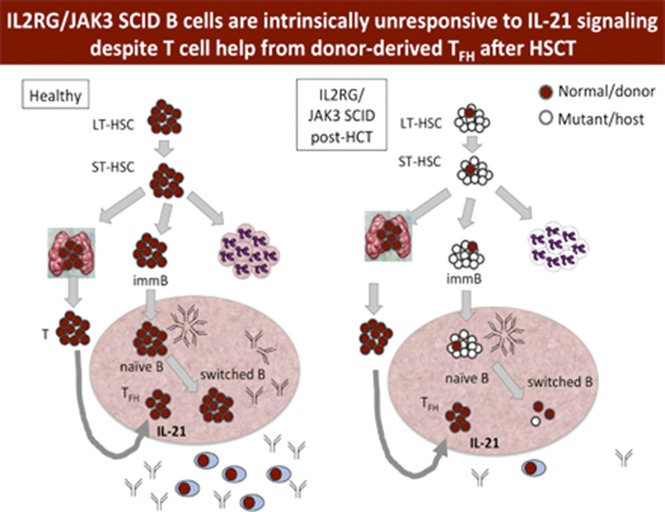
IL2RG/JAK3-deficient B cells remain intrinsically defective posttransplant despite follicular helper T-cell reconstitution.
In vitro response of B cells to IL-21 is a potential biomarker for humoral immunity in patients with IL2RG/JAK3 SCID after transplantation.
Abstract
Allogeneic hematopoietic stem cell transplant (HSCT) typically results in donor T-cell engraftment and function in patients with severe combined immunodeficiency (SCID), but humoral immunity, particularly when using donors other than matched siblings, is variable. B-cell function after HSCT for SCID depends on the genetic cause, the use of pre-HSCT conditioning, and whether donor B-cell chimerism is achieved. Patients with defects in IL2RG or JAK3 undergoing HSCT without conditioning often have poor B-cell function post-HSCT, perhaps as a result of impairment of IL-21 signaling in host-derived B cells. To investigate the effect of pre-HSCT conditioning on B-cell function, and the relationship of in vitro B-cell function to clinical humoral immune status, we analyzed 48 patients with IL2RG/JAK3 SCID who were older than 2 years after HSCT with donors other than matched siblings. T follicular helper cells (TFH) developed in these patients with kinetics similar to healthy young children; thus, poor B-cell function could not be attributed to a failure of TFH development. In vitro differentiation of B cells into plasmablasts and immunoglobulin secretion in response to IL-21 strongly correlated with the use of conditioning, donor B-cell engraftment, freedom from immunoglobulin replacement, and response to tetanus vaccine. Patients receiving immunoglobulin replacement who had normal serum immunoglobulin M showed poor response to IL-21 in vitro, similar to those with low serum IgM. In vitro response of B cells to IL-21 may predict clinically relevant humoral immune function in patients with IL2RG/JAK3 SCID after HSCT.
Visual Abstract
Introduction
Severe combined immunodeficiency (SCID) is a genetically heterogeneous group of disorders characterized by severe impairment of T-cell development, predisposing children to death in the first year of life as a result of opportunistic infection unless rescued by definitive cellular therapy. HSCT for SCID is successful in the majority of cases, with approximately 20% of patients requiring a second transplant or booster infusion for failure of T-cell reconstitution, and overall survival >80% in patients without active infection.1,2 The profound absence of T cells allows children with certain genetic variants of SCID to reconstitute T cells after allogeneic hematopoietic stem cell transplantation (HSCT) in the absence of stem cell ablative chemotherapy or medication to prevent immunologic rejection, using a variety of donors including matched sibling, haploidentical mismatched related (MMRD), unrelated adult volunteer donors, or umbilical cord blood donors.1-4 However, HSCT without pretransplant conditioning typically results in split chimerism, with donor-derived T cells and all other cells derived from the host.1,4-6 Furthermore, among those with successful T-cell reconstitution, humoral immune function is variable, and many patients, particularly those undergoing HSCT with donors other than matched siblings, remain dependent on immunoglobulin (Ig) replacement.1,2,4-6
We in the Primary Immune Deficiency Treatment Consortium (PIDTC)7 and others have shown that pre-HSCT conditioning with stem cell ablative agents such as busulfan or melphalan usually results in humoral reconstitution.2,8-11 Elimination of host-derived HSC promotes engraftment with donor-derived HSC, which in turn gives rise to fully functional donor-derived B cells post-HSCT. Among the variants of SCID, patients with genetic defects abrogating B-cell development (eg, B SCID caused by RAG1 or RAG2 mutation) who receive a transplant from a donor other than an HLA-matched sibling usually require pre-HSCT conditioning for B-cell reconstitution and function. Defects in the common γ chain (γc) encoded by IL2RG, which causes X-linked SCID, or in JAK3, which signals downstream of IL2RG, preserve B-cell development. However, even when T-cell help is restored after successful HSCT, host-derived IL2RG/JAK3-deficient B cells usually remain functionally defective, in part because of an inability to respond to the γc-dependent cytokine IL-21, which is critical for terminal B-cell maturation and differentiation into antibody-secreting plasmablasts.12-15 As a consequence, patients with IL2RG/JAK3 SCID who retain host B cells after transplant usually require Ig replacement. Conversely, patients who receive conditioning and have donor B-cell chimerism typically reconstitute B-cell function after HSCT.2,13,14,16 Whether the lack of humoral immune function in these patients is a result of failure of T follicular helper cell (TFH) development, persistent intrinsic B-cell dysfunction, or both has not been fully studied. To investigate T-cell and B-cell autonomous determinants of humoral immune function after HSCT, the PIDTC conducted a study of 48 patients with IL2RG/JAK3 SCID surviving more than 2 years after nonmatched sibling donor HSCT. We performed T- and B-cell phenotyping as well as assays of in vitro response to IL-21, using a 1-time blood sample, and correlated the results with retrospective clinical data of Ig replacement and vaccine response. We also explored the value of these measurements as potential biomarkers of clinical humoral immune function.
Methods
Patient data and blood samples
Blood samples and clinical data were collected from patients meeting criteria for enrollment on either of 2 PIDTC studies, protocol 6901 (NCT01186913) or protocol 6902 (NCT01346150), after institutional review board approval and signed informed consent from the patient and/or guardian. Patients had either IL2RG or JAK3 SCID and were at least 2 years post-HSCT with a donor other than a matched sibling donor. Peripheral blood was collected in EDTA, shipped overnight at room temperature to Boston Children’s Hospital, and compared with either a shipped blood sample from a healthy adult control or a sample drawn in Boston on the same day as the patient. Retrospective clinical data were collected by Data Management and Coordination Center at the University of South Florida, including patient age, gene defect, date of transplant, donor type, cell source (bone marrow, peripheral blood, or umbilical cord blood), conditioning regimen, cell lineage-specific chimerism, lymphocyte subset results at the time of blood draw, recent serum IgM level, Ig replacement status, and vaccine response. Patients were classified as having received no conditioning if there was no pre-HSCT conditioning or immunosuppression only (fludarabine, anti-thymocyte globulin, alemtuzumab, cyclophosphamide) was given, or as having received conditioning if a regimen containing an alkylating agent toxic to HSC (busulfan or melphalan) was used. Patients receiving reduced intensity (busulfan <12 mg/kg total or equivalent) or myeloablative conditioning (busulfan ≥12 mg/kg) were analyzed as 1 group. Humoral immune reconstitution was evaluated according to whether the patient was on or off Ig replacement or had demonstrated response to tetanus vaccine by the time of blood sampling. Responses to tetanus vaccination measured pre- and postvaccination, if available, were classified as no response, detectable, or protective response, as determined by criteria of the local laboratories. Additional samples were obtained from discarded samples of 33 pediatric control patients aged between 0.75 and 4 years (J.E.W.) or with informed consent from 7 healthy pediatric related bone marrow donors aged 3 to 11 years (J.S.W.).
B-cell and TFH T-cell phenotyping
Staining panels were validated on both fresh blood and thawed peripheral blood mononuclear cells (PBMC), with most analyses performed on thawed cells. Frozen PBMC isolated by gradient cell separation (Ficoll Paque Plus, GE Healthcare, Boston, MA) were thawed and analyzed the same day (B-cell subsets) or after overnight incubation at 37°C in complete medium, if needed, to enhance antigen expression (TFH staining), which consisted of RPMI (Life Technologies), 10% fetal bovine serum (Omega Scientific, FB-12), 1% penicillin-streptomycin (Sigma), 1% l-glutamine (Sigma), and 2.5% N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Fisher Scientific). Cells were incubated with fluorescently labeled monoclonal antibodies, washed, and subjected to flow cytometry (LSR Fortessa, BD Biosciences), and data were analyzed using FlowJo version 8.8.7 (Treestar). When whole blood was used, red blood cells were lysed with 1× FACS Lysis Buffer (BD Biosciences) according to the manufacturer’s instructions. The following antibodies were used for flow cytometry: CD19-PECy7 (HIB19, Biolegend), CD27-APC (O323, eBioscience), IgD-FITC (1D6-2, BD Biosciences), CXCR5-Alexa488 (RF8B2, BD Biosciences), CD4-PECy7 (RPA-T4, BD Biosciences), CD45RA-APC (HI100, Biolegend), CCR6-PE (G034E3, Biolegend), CXCR3-BV421 (G025H7, Biolegend), CD24-PE (ML5, Biolegend), and CD38-BV421 (HIT2, BD Biosciences). Representative plots and gating are shown in supplemental Figure 1, available on the Blood Web site.
In vitro plasmablast differentiation and antibody secretion
In vitro plasmablast differentiation was performed largely as described13,14,17-19 and validated on both freshly isolated and frozen PBMC. For frozen samples, thawed PBMC were incubated overnight at 37°C to ensure optimal cell recovery and function, at a concentration of 1 million cells/mL, and then plated on day 0 at a concentration of 200 000 cells/well in medium containing soluble CD40L and IL-21-Fc at concentrations determined by bioassay,17-19 restimulated between day 2 and 4, and harvested between day 5 and 7. Cells were incubated with monoclonal antibodies, washed, and subjected to flow cytometry (LSR Fortessa, BD Biosciences). Data were analyzed using FlowJo version 8.8.7 (Treestar). Plasmablasts were defined as the percentage of CD19+ cells that were CD27+CD38high, identified with CD19-PECy5 (HIB19, BD Biosciences), CD27-PE (O323, Biolegend), and CD38-BV421 (HIT2, BD Biosciences). Representative plots and gating are shown in supplemental Figure 1. Supernatants from the plasmablast cultures were collected and frozen at −80°C until analysis for IgG and IgM by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (human IgG and IgM Ready-SET-Go kit, eBioscience). Samples were tested in duplicate and compared with standard curve to generate concentrations.
Statistical analysis
Normal distribution could not be assumed for any of the variables. Therefore, the Mann-Whitney U test was employed when 2 groups were compared. When analysis was performed using 3 or more groups, the Kruskal-Wallis test was performed. If a statistically significant difference was observed between any of the groups, the Dunn test was executed to determine between which groups this difference occurred. Adjustment for multiple comparisons was included in these analyses. Fisher’s exact test was performed to compare categorical data. A P-value of less than .05 indicated statistical significance. Use of potential biomarkers to predict humoral immune reconstitution, defined as either independence from Ig substitution or detectable or protective response to tetanus vaccination, were summarized using area under the receiver operating characteristics curves (plot of sensitivity vs 1 minus the specificity of biomarker), and curves were compared nonparametrically, using a published method.20
Results
Patient characteristics
A total of 48 samples from patients older than 2 years post-HSCT were analyzed. Mutations were available for all except 1 patient, who had documented absence of JAK3 protein expression21 (supplemental Table 1). Of 50 mutations, 28 mutations in 27 patients were likely to abrogate protein expression (12 nonsense, 3 frameshift with stop, 1 large deletion, 12 splice site mutations); among the other 20 patients, 18 of 19 mutations were missense. Within the group, 29 had not received conditioning, and 19 had (Table 1). Nearly all patients who had not received conditioning had undergone MMRD transplant (27/29), whereas in the conditioned group, 9 patients and 10 patients had undergone MMRD and unrelated adult volunteer donor HSCT, respectively. Unconditioned patients had a higher median age at the time of sampling than those who had received conditioning (P = .0034), reflecting the greater use of MMRD at centers with the longest experience in transplant for SCID. In both categories, most patients were male and had mutation in IL2RG. We gathered retrospective data on whether the patient was on or off of Ig replacement and whether the patient had been tested for response to tetanus vaccine at the time of blood sampling. Consistent with results published by us and by others,2,8-11,14,16 patients in the conditioned group were more likely to be off Ig replacement than those in the unconditioned group (76% vs 38%; P = .0004). In the group that had not received conditioning, 20/29 continued receiving Ig and had not been tested for vaccine response, whereas 9/29 had a documented detectable or protective response to tetanus vaccine. In the group who had received conditioning, 15/19 had a documented response to tetanus and only 4 had not been tested. As we and others have reported,2,11,16 median donor B-cell chimerism (99.65% vs 2%; P = .0008) was higher in patients who had received conditioning compared with those who had not (supplemental Figure 2).
Table 1.
Clinical data of participating patients divided by pre-HSCT treatment regime
| Without conditioning (n = 29) | With conditioning (n = 19) | P | |
|---|---|---|---|
| Age at time of sample, median (minimum-maximum), y | 12.65 (2.68-29.65) | 5.34 (2.17-16.73) | .0034 |
| Time posttransplant at time of sample, median (minimum-maximum), y | 12.63 (2.13-28.44) | 4.59 (1.97-16.73) | .0012 |
| Male sex, n (%) | 28 (97) | 16 (84) | |
| Genotype, n (%) | |||
| IL2RG | 27 (93) | 15 (79) | |
| JAK3 | 2 (7) | 4 (21) | |
| Positive family history, n (%) | 20 (69) | 13 (72)* | |
| Donor type, n (%) | |||
| Mismatched related | 27 (93) | 9 (47) | |
| Unrelated | 1 (3.5) | 10 (53) | |
| Matched other relative | 1 (3.5) | 0 (0) | |
| Age at transplant, mean (standard deviation), d | 122 (106) | 176 (136) | |
| Chimerism, median (minimum-maximum), % | |||
| T cell | 98 (70-100); n = 11 | 100 (64-100); n = 17 | |
| B cell | 2 (0-100); n = 28 | 99 (0-100); n = 16 | |
| Myeloid | 3 (1-100); n = 7 | 100 (4-100); n = 4 | |
| Off intravenous immunoglobulin, n (%) | 9 (31) | 16 (84) | |
| Vaccine status , n (%) | |||
| Not tested | 20 (69) | 4 (21) | |
| Detectable | 2 (7) | 1 (5) | |
| Protective | 7 (24) | 14 (74) |
Percentage of cases within conditioning category.
One unknown family history.
Naive CD4 T-cell percentages in patients undergoing HSCT are similar between those with and those without humoral immune function
We previously reported that naive CD4 T-cell numbers at 2 to 5 years post-HSCT were higher in patients with SCID who received conditioning than in those who did not,1 which may be a result of sustained seeding of the thymus with donor-derived HSC and improved thymic output in patients who have received conditioning. We analyzed naive CD4 T-cell percentage in this cohort according to time post-HSCT, given the known age dependence of naive CD4 T-cell percentage. We saw a trend toward lower naive CD4 T-cell percentage in the group that did not receive conditioning at later points post-HSCT; however, within groups of patients at the same time post-HSCT, naive CD4 T-cell percentage was not statistically different (supplemental Figure 3). Results of analysis of Ig replacement status were similar (supplemental Figure 3).
Development of TFH post-HSCT recapitulates normal ontogeny and is unrelated to conditioning or humoral immune function
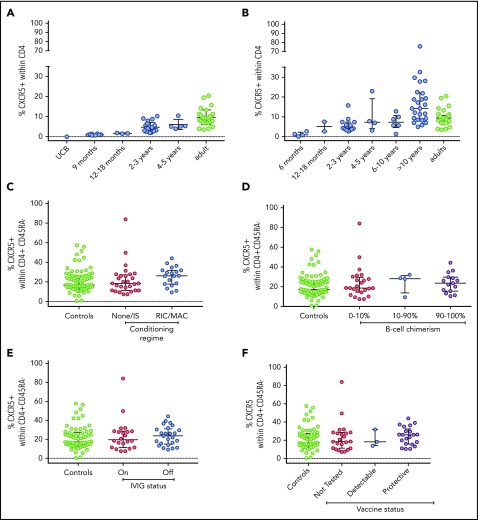
TFH produce IL-21 and are critical mediators of T-B-cell cooperation, Ig class switching, and terminal B-cell maturation into antibody-secreting cells.22-30 Although T-cell help is restored post-HSCT, how and when TFH reconstitute after HSCT is not well described. TFH are CD4+ CD45RA− and CXCR5+ T cells in spleen, lymph nodes, and tonsils; a phenotypically similar population circulates in peripheral blood and has some, although not all, of the same functional and transcriptional characteristics.27-30 We examined the percentage of TFH cells in umbilical cord blood and in healthy children aged 9 months to 5 years and found that the percentage of CD45RA− CXCR5+ cells within the CD4 compartment is less than 1% in umbilical cord blood, as reported,31 and increases with age (Figure 1A). Because patients with SCID enrolled in this study by definition lacked autologous T cells, development of donor-derived TFH after HSCT could be expected to recapitulate ontogeny. Indeed, in patients post-HSCT, TFH increased with kinetics similar to healthy pediatric control patients (Figure 1B).
Figure 1.
TFHdevelopment in healthy children and adults and in patients post-HSCT. Percentage of TFH (the percentage of CD4+ T cells that are CD45RA− and CXCR5+) was measured in (A) umbilical cord blood (UCB), healthy children of the ages shown, and healthy adults; (B) patients with IL2RG/JAK3 SCID at varying times post-HSCT; and patients with IL2RG/JAK3 SCID analyzed according to (C) conditioning, (D) donor B-cell chimerism, (E) Ig replacement status, and (F) response to tetanus vaccine. Medians and interquartile ranges are shown.
To investigate whether failure of TFH development explains a lack of B-cell function post-HSCT, we compared TFH in patients stratified by conditioning, B-cell chimerism, and humoral immune reconstitution. To account for age-associated differences in TFH percentage and CD45RA+ percentage, we analyzed the percentage of CXCR5+ cells within the CD4+ CD45RA− compartment, as this did not vary with age (supplemental Figure 4). Using this measurement, adults and children were similar and merged as controls. We found the proportion of TFH was similar in patients regardless of conditioning, B-cell chimerism, or Ig replacement status post-HSCT (Figure 1C-F). Within circulating TFH cells, those that secrete Th2 and Th17 cytokines make more IL-21 and have a far greater capacity to induce B-cell differentiation than those that secrete Th1 cytokines.27 We therefore analyzed the proportion of Th1-like (CXCR3+ CCR6−), Th2-like (CXCR3− CCR6−), and Th17-like (CXCR3− CCR6+) TFH according to B-cell function and conditioning, but found no differences between groups or between patients and adult control patients (supplemental Figure 5). Thus, lack of B-cell function cannot be attributed to a failure of TFH development as a whole, or to a skewing toward Th1-like TFH.
Switched memory B cells and in vitro B-cell response to IL-21 correlate with B-cell function after HSCT
We examined B-cell phenotype, including transitional B cells (CD24hi CD38hi), marginal zone-like (CD24hi CD38low), unswitched memory (IgD+ CD27+), and switched memory (IgD− CD27+) percentages within CD19+ B cells (supplemental Figure 1). The range and medians for adults and 7 pediatric control patients aged 3 to 11 years were similar (supplemental Table 1), and therefore were merged. Transitional, marginal zone-like, and unswitched memory B-cell percentage did not correlate with the use of conditioning, Ig replacement, vaccine response, or B-cell chimerism in the patient groups (supplemental Figure 5).
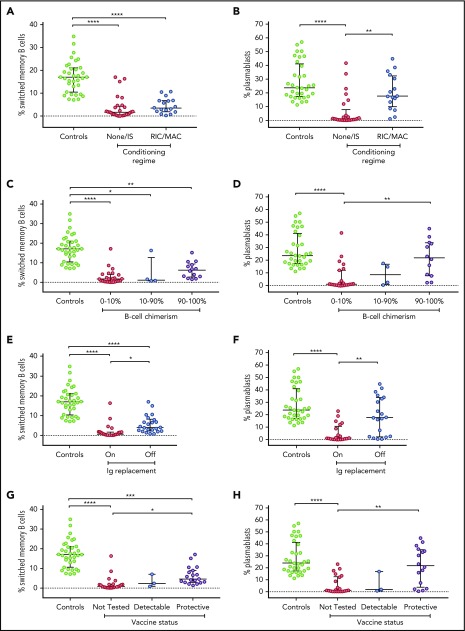
Given the important role of IL-21, a cytokine critically dependent on IL2RG/JAK3 function, for inducing germinal center responses and Ig class switching, we examined whether the level of switched memory B cells in our cohort correlated with variables including conditioning, donor B-cell chimerism, and/or humoral immune function. Of note, we found that patients who did not receive conditioning, had 0% to 10% donor B-cell chimerism, were receiving Ig replacement, or had not been tested for vaccine response had a lower percentage of switched memory B cells compared with healthy control patients. Switched memory B cells were similar between patients who had received or who had not received conditioning (Figure 2A; P = .8751). However, switched memory B-cell percentage did distinguish patients with vs without humoral immune function, as indicated by freedom from Ig replacement or vaccine response (Figure 2E,G; P = .0356 on vs off Ig, P = .0344 not tested vs detectable response).
Figure 2.
Switched memory B cells and in vitro plasmablast generation in patients post-HSCT. Percentage of switched memory B cells (the percentage of CD19+ B cells that are IgD− and CD27+) and percentage of plasmablasts (the percentage of CD19+ B cells that are CD27high and CD38high) after in vitro stimulation with CD40L and IL-21-Fc were measured in patients with IL2RG/JAK3 SCID analyzed according to conditioning (A-B), donor B-cell chimerism (C-D), Ig replacement status (E-F), and response to tetanus vaccine (G-H). Control patients were combined from healthy adults and children. Medians and interquartile ranges are shown. Brackets depict comparisons with significant P values: *P < .05; **P < .01; ***P < .001; ****P < .0001.
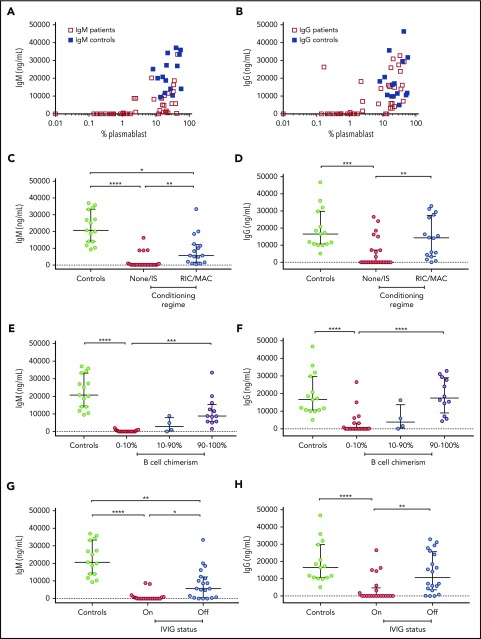
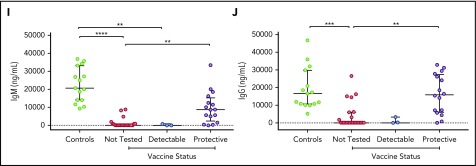
Our previous work in patients with IL2RG/JAK3 SCID who had host B cells post-HSCT showed that in vitro response to IL-21 of B cells was compromised.14 In this larger group of patients with IL2RG/JAK3 SCID post-HSCT, we investigated whether B-cell differentiation into plasmablasts and secretion of IgM and IgG in response to IL-21 in vitro correlated with the clinical parameters of conditioning, B-cell chimerism, and humoral immune function. We examined the percentage of CD27+ CD38hi plasmablasts produced after 5 to 7 days of culture with CD40L and IL-21-Fc, and measured the secretion of IgM and IgG in the supernatant of 41 patients, comparing results with measurements made for both adult and pediatric control patients. The range and means for adults and 7 pediatric control patients aged 3 to 11 years were similar (supplemental Table 1), and therefore the data were merged. Consistent with the analysis of switched memory B cells described here (Figure 2), the percentage of plasmablasts generated in vitro in response to IL-21 was consistently lower in patients who had not received conditioning, who had 0% to 10% B-cell chimerism, who were receiving Ig replacement, or who had not been tested for vaccine response compared with healthy control patients (all P < .0001; Figure 2). Further, in vitro plasmablast generation was higher for patients who had received conditioning, had donor chimerism, or exhibited humoral immune function (all P < .01; Figure 2). Patient cells that had low plasmablast differentiation capacity generally produced negligible amounts of IgM and IgG in vitro (Figure 3A-B). The correlation between IgG and IgM secretion with humoral immune function was consistent: both IgM and IgG secretion were higher in patients who had received conditioning (P < .01), had more than 90% donor B-cell chimerism (IgM, P < .001; IgG, P < .0001), were not receiving Ig replacement (IgM, P < .05; IgG, P < .01), or had documented vaccine response (P < .01; Figure 3C-J). In vitro plasmablasts from patients who received conditioning (P < .05) or were not receiving Ig (P < .01) secreted less IgM than controls (Figure 3C,G).
Figure 3.
Secreted IgM and IgG in response to CD40L and IL-21-Fc. Percentage of plasmablasts after in vitro stimulation with CD40L and IL-21-Fc correlates with secretion of (A) IgM and (B) IgG in the supernatant. IgM and IgG secretion from patients post-HSCT were analyzed according to conditioning (C-D), donor B-cell chimerism (E-F), Ig replacement status (G-H), and response to tetanus vaccine (I-J). Control patients were combined from healthy adults and children. Medians and interquartile ranges are indicated. Brackets depict comparisons with significant P values: *P < .05; **P < .01; ***P < .001; and **** P < .0001.
Evaluation of sensitivity and specificity of serum IgM, switched memory B-cell percentage, and IL-21 response as correlates of humoral immune function
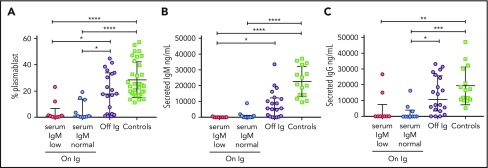
Clinicians may use serum IgM level as an indicator that discontinuing Ig replacement can be considered. Of 24 patients off Ig replacement, and with serum IgM levels available in clinical records, 23 had normal values for age (specificity, 0.96). In contrast, 9 of 21 patients receiving Ig replacement had low serum IgM, and 12 of 21 had normal serum IgM for age (sensitivity, 0.43). We investigated responsiveness of PBMC samples in vitro to IL-21, comparing patients remaining on Ig replacement who had low vs normal serum IgM for age. Cells from both groups of patients receiving Ig replacement were poorly responsive to IL-21 with low levels of plasmablasts generated in vitro and little IgM secretion into the supernatant compared with patients off Ig and healthy adult control patients. Patients who had normal levels of serum IgM were no different from patients who had low levels of serum IgM for age (Figure 4).
Figure 4.
Correlation of patient serum IgM with in vitro response to IL-21. IL-21-induced in vitro plasmablast percentage (A), secreted IgM (B), and secreted IgG (C) are shown for healthy control patients, patients receiving Ig who had either normal or low serum IgM for age, or patients off Ig. Medians and interquartile ranges are indicated. Brackets depict comparisons with significant P values: *P < .05; **P < .01; ***P < .001; and ****P < .0001.
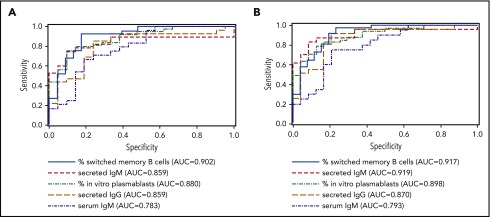
To evaluate the performance of switched memory B-cell percentage, in vitro response to IL-21, and serum IgM to classify patients’ humoral immune function, we generated receiver operating characteristic curves of these potential biomarkers with Ig replacement status and vaccine response (Figure 5). The percentage of switched memory B cells, percentage of in vitro plasmablasts, secretion of IgM and IgG by IL-21 stimulated cells, and serum IgM all correlated strongly with both Ig replacement status (AUC, 0.783-0.902) and vaccine response (AUC, 0.793-0.919). AUC of serum IgM was the lowest, although differences between the AUCs were not statistically different (P = .880 for Ig replacement status; P = .690 for vaccine response).
Figure 5.
Biomarkers of B-cell function correlate with clinical humoral immunity. Receiver operating curves measuring the sensitivity and specificity of the indicated parameters to predict Ig replacement status (A) and vaccine response (B) are shown.
Discussion
We sought to understand the determinants of B-cell function in patients with SCID after HSCT, focusing on patients with mutations in IL2RG or JAK3 who have intrinsic defects in B-cell function and lack of IL-21 signaling. Defects in a number of T-cell signaling molecules may lead to reduced numbers of TFH in murine models or in human immunodeficiency, such as deficiency of CD40L, ICOS, or STAT3 loss of function32-35; the lack of B-cell development in murine models or in BTK-deficient patients leads to a failure of TFH development.32,35,36 We thus explored whether and how TFH develop in patients with IL2RG/JAK3 SCID after HSCT, hypothesizing that the lack of B-cell function in some patients may compromise TFH development. We did not detect a difference in naive CD4 T-cell percentage between patients with and without B-cell function. We found that patients with poor B-cell function and residual host B cells nevertheless developed normal numbers of TFH. We went further to examine different functional subsets of TFH and found no difference between those patients who had or had not recovered humoral immunity and TFH in control patients. Our study is limited, in that we did not test the function of TFH in these patients. Although this circulating population of cells phenotypically resembles TFH in secondary lymphoid organs, circulating TFH may not be reflective of the cells present in tissues.
We showed that laboratory parameters critically dependent on IL-21 signaling, including the presence of class switched memory B cells, in vitro plasmablast differentiation, and antibody secretion in response to IL-21, correlated strongly with clinical humoral immune reconstitution, as indicated by freedom from Ig replacement and response to tetanus vaccination. We also confirmed the association of these B-cell parameters with conditioning and high-level engraftment of donor-derived B cells. Serum IgM is a widely available test that clinicians often use to consider whether Ig substitution may be stopped. We found that the specificity of normal serum IgM for age was excellent (0.96), but the sensitivity was poor (0.43). Cells from patients on Ig substitution with normal serum IgM for the most part still failed to respond to IL-21. Therefore, in practical terms, having normal serum IgM was not very predictive of humoral immune response. Evaluation of the capacity of functional tests of IL-21 response such as in vitro plasmablast generation to predict functional reconstitution of humoral immunity in individual patients would require a larger sample size and prospective analysis.
Our study has a number of important limitations. First, clinical data were collected retrospectively. The decision to replace Ig or not in this cohort was based on the clinician’s judgment, and reasons for continuing or discontinuing Ig replacement were not always known. In addition, we cannot exclude the possibility that exogenous Ig could have impeded or delayed humoral immune recovery.37 Finally, a number of patients were never fully assessed for humoral response, and we were limited to tetanus vaccination data. It is therefore possible that some patients with responses to tetanus may lack response to other antigens, or that the responses observed were transient.
These data confirm the importance of stem cell ablative conditioning to promote donor-derived B-cell engraftment in patients with IL2RG/JAK3 SCID who receive transplants other than grafts from HLA matched siblings. Many of the patients in this study underwent high-dose busulfan-based conditioning regimens. These high-dose regimens have acute and long-term adverse effects that would ideally be avoided.38 It remains to be determined whether and to what degree lower-dose regimens could also reliably result in clinically relevant immune function. Determining the minimal amount of conditioning needed and targeting dosing to achieve the desired exposure is even more important in the era of universal newborn screening for SCID.39,40 Clinicians in the United States and elsewhere are now faced with the dilemma of how to treat very young infants quickly and safely.41 We plan to examine these same biomarkers and others in patients with IL2RG/JAK3 SCID enrolled in a prospective clinical HSCT trial developed by the PIDTC, using reduced intensity busulfan conditioning. In this and other future studies, we aim to determine whether these biomarkers can be used to identify which patients with SCID have sufficient B-cell function to be taken off Ig replacement post-HSCT.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The PIDTC (U54 AI082973-08) is part of Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, and the National Institute of Allergy and Infectious Diseases. The PIDTC is funded through collaboration between the National Center for Advancing Translational Sciences and the Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases.
This work was supported by cooperative agreements U54-AI082973 (principal investigator [PI]: M.J.C.), and U54-NS064808 and U01-TR001263 (PI: J. P. Krischer) and R13-AI094943 (M.J.C.), and the Laboratory of Host Defenses, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Collaborative work of the PIDTC with the Pediatric Blood and Marrow Transplant Consortium is supported by the U54 grants above along with support of the Pediatric Blood and Marrow Transplant Consortium Operations Center by the St. Baldrick’s Foundation and grant/cooperative agreement U10HL069254 (PI: M.A. Pulsipher) from the National Heart, Lung, and Blood Institute and the National Cancer Institute, National Institutes of Health. Collaborative work of the PIDTC with the Center for International Blood and Marrow Transplant Research is supported by grant/cooperative agreement U24-CA76518 (PI: M.M. Horowitz) from the National Cancer Institute, National Heart, Lung, and Blood Institute, and National Institute of Allergy and Infectious Diseases, and grant/cooperative agreement U01HL069294 from the National Heart, Lung, and Blood Institute and National Cancer Institute; contract HHSH250201200016C and HHSH234200637015C with the Health Resources and Services Administration, US Department of Health and Human Services; and grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research. This work was also supported by the Translational Investigator Service Award (S.-Y.P.), the Tania Treviño Fund (S.-Y.P.), and the American Society of Hematology Scholar Award (J.S.W.) Finally, funding for this study was also provided in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
The content and opinions expressed are solely the responsibility of the authors and do not represent the official policy or position of the National Institute of Allergy and Infectious Disease, the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, National Institutes of Health, Health Resources and Services Administration, or any other agency of the US government.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.M.M. and S.-Y.P. wrote the manuscript; A.M.M., G.H., and S.-Y.P. performed experiments; A.M.M. and B.R.L. performed statistical analysis; H.E., L.D.N., and S.-Y.P. conceived and designed the study; R.H.B., C.C.D., D.B.K., J.M.P., M.J.C., L.M.G., E.H., R.J.O., L.D.N., and S.-Y.P. developed the study as senior leadership for PIDTC and for Protocols 6901 and 6902; R.H.B., R.E.P., C.C.D., N.K., H.A.-A., S.E.P., D.S., H.D., I.C.H., A.G., B.J.D.S., L.D.N., and S.-Y.P. contributed patients to the study; H.E., J.E.W., and J.S.W. contributed critical reagents and contributed to experimental design; and all authors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for G.H. is bluebird bio, Cambridge, MA.
The current affiliations for J.E.W. are University of South Florida, Tampa, FL; Johns Hopkins All Children’s Hospital, St. Petersburg, FL; and Massachusetts General Hospital for Children, Boston, MA.
Correspondence: Sung-Yun Pai, Boston Children’s Hospital, Karp Family Research Laboratories, Room 08214, 300 Longwood Ave, Boston, MA 02115; e-mail: sung-yun.pai@childrens.harvard.edu.
References
- 1.Buckley RH, Schiff SE, Schiff RI, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340(7):508-516. [DOI] [PubMed] [Google Scholar]
- 2.Pai S-Y, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med. 2014;371(5):434-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Reilly RJ, Dupont B, Pahwa S, et al. Reconstitution in severe combined immunodeficiency by transplantation of marrow from an unrelated donor. N Engl J Med. 1977;297(24):1311-1318. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak CC, Hassan A, Slatter MA, et al. Comparison of outcomes of hematopoietic stem cell transplantation without chemotherapy conditioning by using matched sibling and unrelated donors for treatment of severe combined immunodeficiency. J Allergy Clin Immunol. 2014;134(4):935-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haddad E, Leroy S, Buckley RH. B-cell reconstitution for SCID: should a conditioning regimen be used in SCID treatment? J Allergy Clin Immunol. 2013;131(4):994-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley RH, Win CM, Moser BK, Parrott RE, Sajaroff E, Sarzotti-Kelsoe M. Post-transplantation B cell function in different molecular types of SCID. J Clin Immunol. 2013;33(1):96-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith LM, Cowan MJ, Notarangelo LD, et al. ; workshop participants. Primary Immune Deficiency Treatment Consortium (PIDTC) update. J Allergy Clin Immunol. 2016;138(2):375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorak CC, Cowan MJ, Logan BR, et al. The natural history of children with severe combined immunodeficiency: baseline features of the first fifty patients of the primary immune deficiency treatment consortium prospective study 6901. J Clin Immunol. 2013;33(7):1156-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gennery AR, Slatter MA, Grandin L, et al. ; European Society for Immunodeficiency. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126(3):602-610. [DOI] [PubMed] [Google Scholar]
- 10.Haddad E, Le Deist F, Aucouturier P, et al. Long-term chimerism and B-cell function after bone marrow transplantation in patients with severe combined immunodeficiency with B cells: A single-center study of 22 patients. Blood. 1999;94(8):2923-2930. [PubMed] [Google Scholar]
- 11.Cavazzana-Calvo M, Carlier F, Le Deist F, et al. Long-term T-cell reconstitution after hematopoietic stem-cell transplantation in primary T-cell-immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood. 2007;109(10):4575-4581. [DOI] [PubMed] [Google Scholar]
- 12.White H, Thrasher A, Veys P, Kinnon C, Gaspar HB. Intrinsic defects of B cell function in X-linked severe combined immunodeficiency. Eur J Immunol. 2000;30(3):732-737. [DOI] [PubMed] [Google Scholar]
- 13.Cattaneo F, Recher M, Masneri S, et al. Hypomorphic Janus kinase 3 mutations result in a spectrum of immune defects, including partial maternal T-cell engraftment. J Allergy Clin Immunol. 2013;131(4):1136-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recher M, Berglund LJ, Avery DT, et al. IL-21 is the primary common γ chain-binding cytokine required for human B-cell differentiation in vivo. Blood. 2011;118(26):6824-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298(5598):1630-1634. [DOI] [PubMed] [Google Scholar]
- 16.Abd Hamid IJ, Slatter MA, McKendrick F, Pearce MS, Gennery AR. Long-term outcome of hematopoietic stem cell transplantation for IL2RG/JAK3 SCID: a cohort report. Blood. 2017;129(15):2198-2201. [DOI] [PubMed] [Google Scholar]
- 17.Kienzler A-K, Rizzi M, Reith M, Nutt SL, Eibel H. Inhibition of human B-cell development into plasmablasts by histone deacetylase inhibitor valproic acid. J Allergy Clin Immunol. 2013;131(6):1695-1699. [DOI] [PubMed] [Google Scholar]
- 18.Sic H, Kraus H, Madl J, et al. Sphingosine-1-phosphate receptors control B-cell migration through signaling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. J Allergy Clin Immunol. 2014;134(2):420-428. [DOI] [PubMed] [Google Scholar]
- 19.Pieper K, Rizzi M, Speletas M, et al. A common single nucleotide polymorphism impairs B-cell activating factor receptor's multimerization, contributing to common variable immunodeficiency. J Allergy Clin Immunol. 2014;133(4):1222-1225. [DOI] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. [PubMed] [Google Scholar]
- 21.Roberts JL, Lengi A, Brown SM, et al. Janus kinase 3 (JAK3) deficiency: clinical, immunologic, and molecular analyses of 10 patients and outcomes of stem cell transplantation. Blood. 2004;103(6):2009-2018. [DOI] [PubMed] [Google Scholar]
- 22.Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29(1):138-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suto A, Kashiwakuma D, Kagami S, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205(6):1369-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29(1):127-137. [DOI] [PubMed] [Google Scholar]
- 25.Ma CS, Suryani S, Avery DT, et al. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87(8):590-600. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt N, Morita R, Bourdery L, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31(1):158-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita R, Schmitt N, Bentebibel S-E, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasheed A-U, Rahn H-P, Sallusto F, Lipp M, Müller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36(7):1892-1903. [DOI] [PubMed] [Google Scholar]
- 29.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62(1):234-244. [DOI] [PubMed] [Google Scholar]
- 30.Chevalier N, Jarrossay D, Ho E, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186(10):5556-5568. [DOI] [PubMed] [Google Scholar]
- 31.Förster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84(3):830-840. [PubMed] [Google Scholar]
- 32.Tangye SG, Deenick EK, Palendira U, Ma CS. T cell-B cell interactions in primary immunodeficiencies. Ann N Y Acad Sci. 2012;1250(1):1-13. [DOI] [PubMed] [Google Scholar]
- 33.Bossaller L, Burger J, Draeger R, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177(7):4927-4932. [DOI] [PubMed] [Google Scholar]
- 34.Ma CS, Hare NJ, Nichols KE, et al. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115(4):1049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma CS, Wong N, Rao G, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136(4):993-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deenick EK, Chan A, Ma CS, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33(2):241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan KM, Storek J, Kopecky KJ, et al. A controlled trial of long-term administration of intravenous immunoglobulin to prevent late infection and chronic graft-vs.-host disease after marrow transplantation: clinical outcome and effect on subsequent immune recovery. Biol Blood Marrow Transplant. 1996;2(1):44-53. [PubMed] [Google Scholar]
- 38.Allewelt H, El-Khorazaty J, Mendizabal A, et al. Late effects after umbilical cord blood transplantation in very young children after busulfan-based, myeloablative conditioning. Biol Blood Marrow Transplant. 2016;22(9):1627-1635. [DOI] [PubMed] [Google Scholar]
- 39.Puck JM; SCID Newborn Screening Working Group. Population-based newborn screening for severe combined immunodeficiency: steps toward implementation. J Allergy Clin Immunol. 2007;120(4):760-768. [DOI] [PubMed] [Google Scholar]
- 40.Kwan A, Abraham RS, Currier R, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312(7):729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heimall J, Logan BR, Cowan MJ, et al. Immune reconstitution and survival of 100 SCID patients post-hematopoietic cell transplant: a PIDTC natural history study. Blood. 2017;130(25):2718-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.