Abstract
Fever of unknown origin (FUO) is a frequently observed phenomenon in clinical practice. The present study was aimed to investigate potential causes of FUO, thereby improving clinical diagnosis of this disorder.
In this retrospective study, clinical data were collected from 215 patients who were diagnosed with FUO between January 2009 and December 2010, and an 18 to 36 months follow-up visit was also performed for these patients.
Among these FUO cases, the most common causes of the disease were infectious diseases (IDs) (42.3%), followed by connective tissue diseases (CTDs) (32.1%), miscellaneous (Mi) (10.7%) and neoplasm (N) (6.5%), while the causes for the other 18 cases (8.4%) were still unknown. The most common types of ID, CTD, and N were tuberculosis (16/91, 17.6%), adult onset Still disease (AOSD) (37/69, 53.6%) and non-Hodgkin lymphoma (6/14, 42.9%), respectively.
IDs still represent the most common causes of FUO. Regularly intermittent fever with urinary infections and irregularly intermittent fever with infective endocarditis may be regarded as some signs in clinical diagnosis of FUO.
Keywords: diagnosis, fever of unknown origin, retrospective analysis
1. Introduction
Fever of unknown origin (FUO) refers to a pathological condition with a fever higher than 38.3°C (101°F) lasting more than 3 weeks, and is characterized by easy relapse and uncertain diagnosis after 1 week of treatment in hospital.[1] Two major amendments to the initial definition of FUO were released in 1991: first, observation period was abridged to 3 days instead of 1 week, and the number of diagnosis operated only during in-hospital evaluation was no less 3; second, FUO was divided into 4 types: classical FUO and nosocomial-, neutropenic- and HIV-associated FUO.[2] Definite diagnosis of FUO is a great challenge in clinical practice due to its complex causes. Potential causes for FUO involve more than 200 diseases. Moreover, FUO diagnosis is also affected by various factors, such as countries, technical levels, and clinician experiences. Identifying etiological causes and characteristics of FUO is of great importance for clinical work all the time. In this study, we retrospectively analyzed the clinical characteristics of 215 patients with classical FUO, who were diagnosed in Chinese PLA General Hospital and received a mean 24-month follow-up investigation.
2. Patients and methods
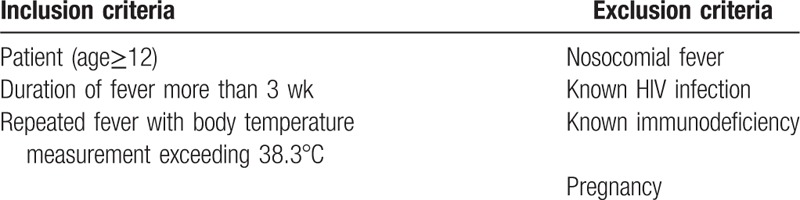
More than 600 patients presenting fever more than 3 weeks were collected from the department of fever related diseases of the Chinese PLA General Hospital between January 2009 and December 2010. The patients were more than 12 years old. After diagnosis according with the criteria of FUO,[2] 215 patients were finally recruited in this study, who had an 18 to 36 months follow-up visit. Patients with nosocomial infection, HIV infection, immunocompromise or in pregnancy were excluded from this study. None of the patients had received antibiotic treatments or hormone therapy within 3 months before the study. Immunocompromised patients, such as those with neutropenia (leukocyte count <1.0 × 109/L and/or granulocyte count <0.5 × 109/L) suffering fever at least 1 week within the past 3 months, those having known human immunodeficiency virus (HIV)-infection or known hypogammaglobulinemia (IgG<50% of the normal value) and those receiving the equivalent of more than 10 mg prednisone during at least 2 weeks in the past 3 months, were also excluded from this study.[3] Inclusion and exclusion criteria for study participants are listed in Table 1.
Table 1.
Inclusion and exclusion criteria.
After enrollment, detailed medical history, physical examination results, and laboratory data were obtained from each patient. Laboratory data included complete blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin (PCT), biochemical tests (urea, creatinine, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, bilirubins, albumin and globulin, lactate dehydrogenase, creatine kinase), antinuclear antibodies, rheumatoid factor, urinalysis, tuberculin skin test, blood and urine cultures, abdominal ultrasonography or computed tomography (CT), tuberculin skin test, and chest X-ray. Certain patients also had invasive examinations such as biopsies from bone marrow, liver, enlarged lymph node, and other relevant tissues.
The causes were classified into 5 groups: infectious diseases (IDs), connective tissue diseases (CTDs), neoplasm (N), miscellaneous (Mi), and undiagnosed (U).[4]
3. Ethical considerations
This study was reviewed and approved by the Research Ethics Committees of the Chinese PLA General Hospital. All participants signed written informed consents.
3.1. Statistical analysis
Comparisons between 2 groups were performed through Kruskal–Wallis test for variables which followed non-normal distribution, while t test was used to compare those in normal distribution. Chi-square test or Fischer exact test were adopted to analyze categorical data. All baseline variables were entered into a logistic regression analysis with a stepwise selection procedure, which was used to identify the association between factors and diagnosis of infectious diseases. The entry and removal criteria were P = .05 and P = .10, respectively. 95% confidence intervals (95% CIs) were calculated for each comparison. The area under the receiver-operating characteristic curve (AUC) was used to describe the diagnostic performance of the factors. P value less than .05 was considered statistical significance. Statistical analyses were performed using SAS software version 9.2.
4. Results
A total of 215 patients, containing 102 (47.4%) females and 113 (52.6%) males, were enrolled in our study, with a median age of 38.7 years (13–80). Within 15 days before hospitalization, the patients only received routine home therapy, including cold compress (33 cases), hot compress (35 cases), bath (27 cases), supplement liquid (39 cases), and dietary therapy (64 cases). The median fever duration of these patients was 166 days (21–7500). Moreover, the median follow-up time was 24.1 months, ranging from 18 to 36 months.
4.1. Spectrum of diseases causing FUO
Diagnosis results of FUO cases are summarized in Table 2. IDs were observed in 91 (42.3%) of the FUO patients, representing the most common cause for the disease. The second common reason for FUO was CTDs, accounting for 32.1% (69/215) of the cases, followed by Mi (23/215, 10.7%) and N (14/215, 6.5%). However, there were still 18 (8.4%) undiagnosed FUO patients.
Table 2.
Etiological reasons of FUO cases.
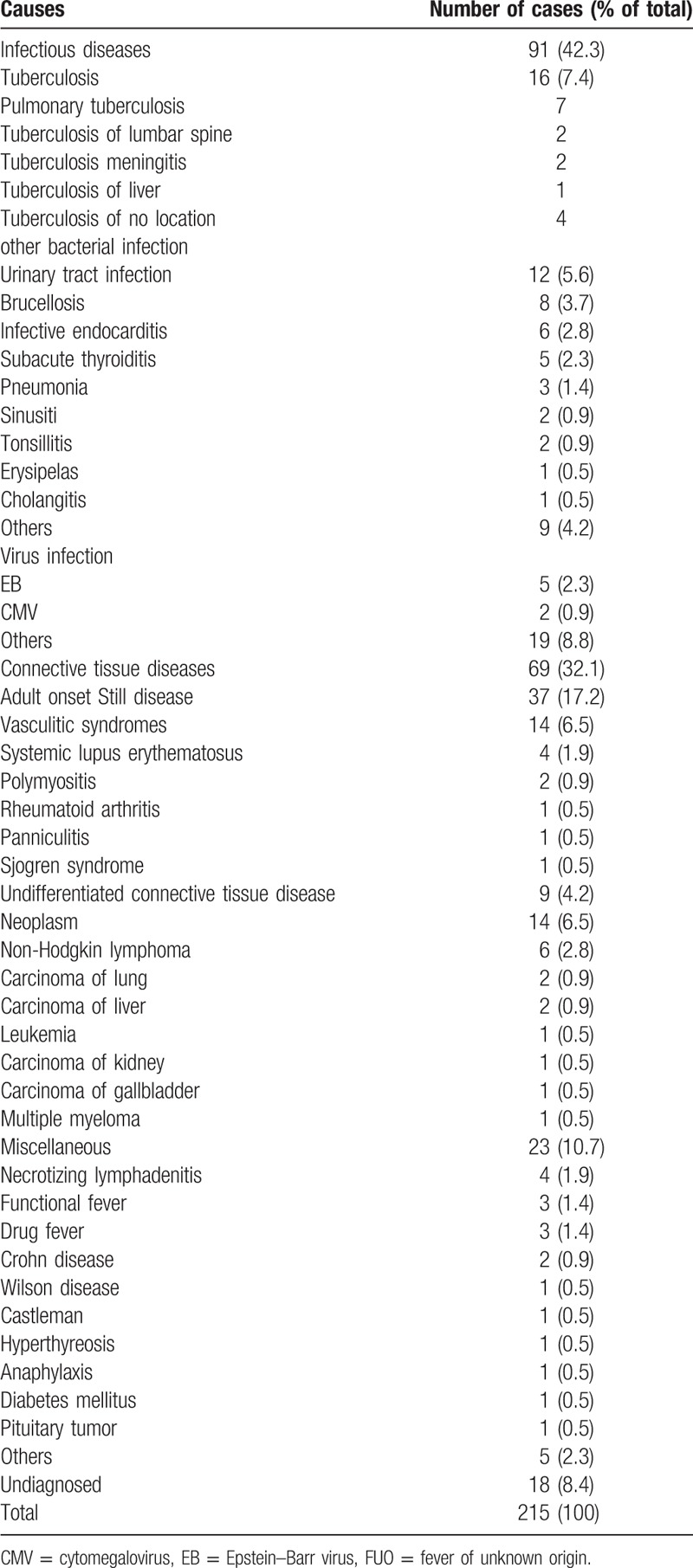
In our study, the most common diseases causing FUO were adult onset Still disease (AOSD) (37/215, 17.2%), followed by tuberculosis (16/215, 7.4%), vasculitic syndromes (14/215, 6.5%), and urinary tract infection (12/215, 5.6%). The most common diseases in ID, CTD, N, and Mi groups were tuberculosis (16/91, 17.6%), AOSD (37/69, 53.6%), non-Hodgkin lymphoma (6/14, 42.9%), and necrotizing lymphadenitis (4/23, 17.4%), respectively.
Fatality rate of FUO was 2.8% (6/215) during the follow-up. Among those deceased, 3 died of lymphoma and Leukemia; 1 died of lung cancer; 1 died of acute myocardial infarction, and 1 died of indefinite reason. The fever spontaneously subsided in 3 months among 3 patients, of whom FUO causes were not diagnosed.
4.2. Characteristics of FUO patients
4.2.1. IDs, CTDs, and N groups
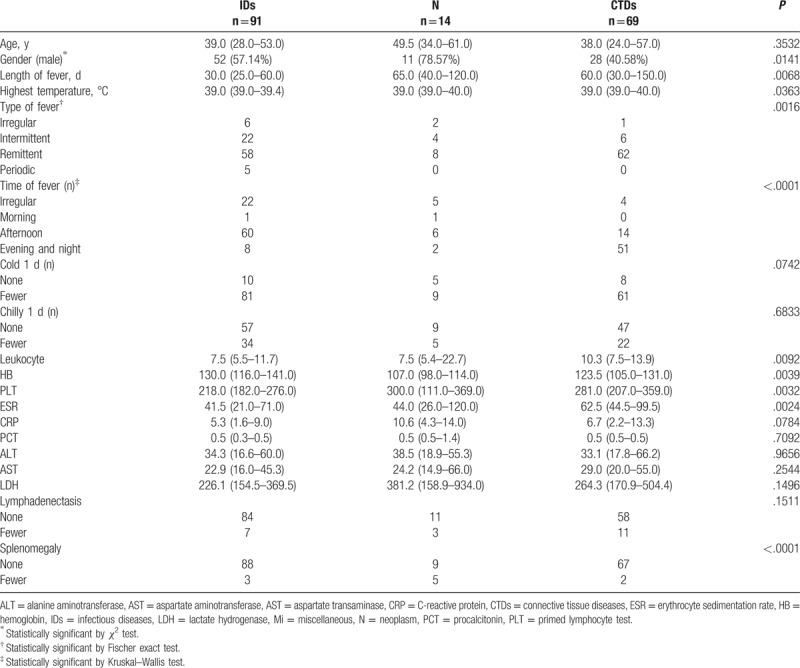
We compared the clinical characteristics of the patients between different groups classified according to the causes of FUO. CTDs were more common in women, while IDs and N were more frequently observed in men (P = .0141). We observed shorter duration of fever (P = .0068), lower temperature (P = .0363), and more frequent fever in the afternoon (P<.0001) in IDs. Remittent fever was more common in CTDs group, and typical periodic fever was more frequent in Brucellosis.
Splenomegaly, high primed lymphocyte test (PLT) counts and low measures of hemoglobin (HB) were common in N group (P < .0001, P = .0032, P = .0039 respectively). High leukocyte counts and high measures of ESR were more frequently observed among patients in CTDs group (P = .0094, P = .0024 respectively) (Table 3).
Table 3.
Patients characteristics of infectious, neoplasm, and collagen vascular diseases.
4.2.2. Diagnosed and undiagnosed
In our study, there were still 18 patients (8.4%) with undiagnosed FUO, and 3 of them spontaneously recovered in 6 months. Chill number per day was fewer in these undiagnosed patients than the diagnosed patients (P = .0474). There were no significant differences in characteristics of history, physical examination findings or Laboratory data between the diagnosed and undiagnosed patients (data not shown).
5. Discussion
5.1. Etiological reasons of FUO
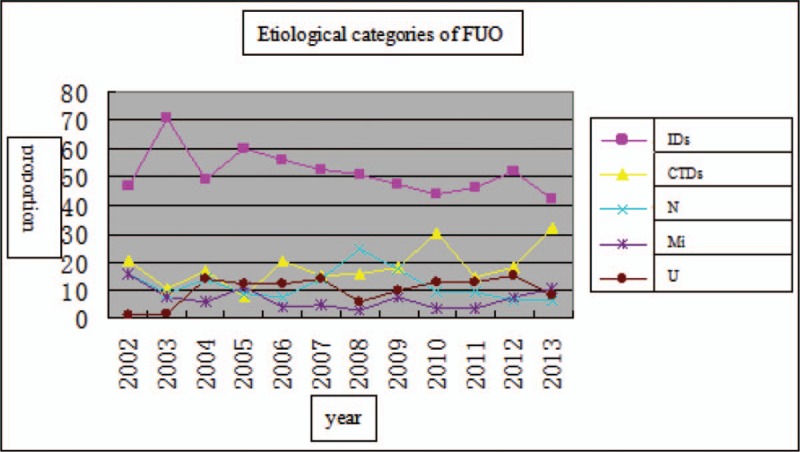
In this study, a total of 215 Chinese FUO patients were included. We analyzed the clinical characteristics and etiological causes for these patients. Identified etiological reasons of FUO for these patients included IDs (42.3%), CTDs (32.1%), N (6.5%),Mi (10.7%), and U (8.4%) (Table 4). IDs were the most common cause of FUO. Based on the series of retrospective reports on classical FUO among people living in northern China[5–15] which were published between 2002 and 2012, we found that the appearing frequencies of IDs, CTDs, N, and Mi were 51.5%, 18.4%, 11.9%, 7.1% respectively. Among 2502 FUO patients, 10.1% of them remained undiagnosed (Table 5). However, in Western counties, the etiology of FUO shows differences from that in China. Previously, 6 articles including a total of 837 FUO patients in Western counties were published between 2003 and 2016.[16–21] In these researches, the appearing frequencies of IDs, CTDs, N, Mi, and undiagnosed cases were 29.1%, 25.8%, 11.6%, 11.7%, and 26.1%, respectively. The differences in the frequencies between Chines and Western countries might be attributed to diverse geographical environments, climate changes, dietary habits, as well as the divergence in medical levels. In addition, after comparing the etiological reasons of FUO between the last decade of the last century and the first decade of this century, we found a downward tendency in the frequencies of IDs and Mi and an upward trend in N and U frequencies (P = .0002). Nonetheless, in recent years, the frequencies of IDs and N as FUO causes have declined, while CTDs exhibited an increasing trend in northern China (Fig. 1). And we hypothesized that these changes might be partly attributed to the advances in serological and immunological diagnostic tests.[15] The differences in undiagnosed rate (from 0.9% to 15.4%) might be partially explained by the changes in FUO definitions. There were 52 diseases described as FUO causes in the above 12 reports. Tuberculosis in IDs group was most common among FUO patients in north China, followed by AOSD in CTDs, lymphoma in N, and drug fever in Mi. Traditional Chinese drugs, used widely in China, have also received increasing attentions in FUO research field, being regarded as one of the most important reasons for drug fever.[22] Apart from the several aspects mentioned in our study, some other factors may be also involved in FUO, such as neurosarcoidosis, sarcoid granulomas.[23–26] In order to improve the diagnosis rate of FUO, more relevant studies will be required.
Table 4.
Distribution of diagnostic categories in patients with FUO during the last 2 decades.
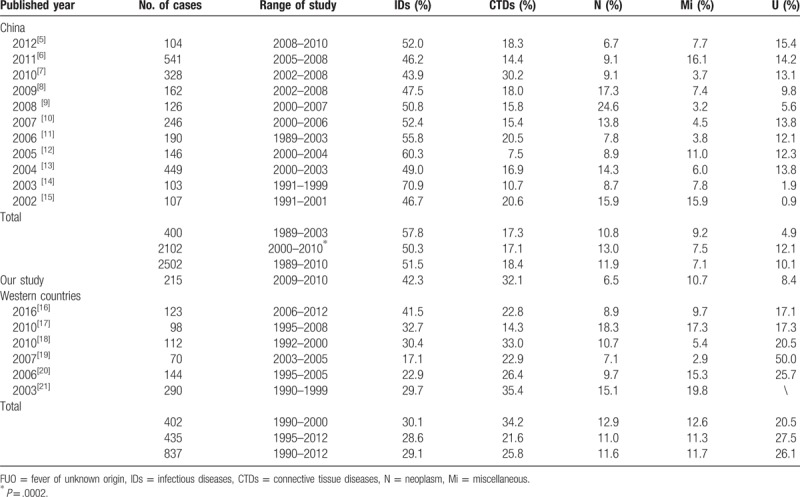
Table 5.
The common causes of FUO during the last two decade in north China.
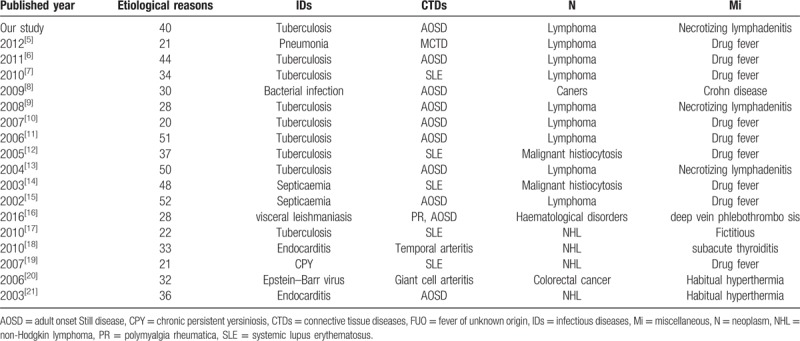
Figure 1.
Diagnostic categories of patients with FUO in north China during the last 2 decades. CTDs = connective tissue diseases, IDs = infectious diseases, Mi = miscellaneous, N = neoplasm, U = undiagnosed.
5.2. Comparing the characteristics of FUO patients
We compared the clinical characteristics of FUO patients between different groups divided according to the etiological reasons. Through the comparisons among IDs, CTDs, and N groups, we found that CTDs group had more females and higher ESR, while splenomegaly and lower HB level were frequently observed in N group. These results were similar with those from previous reports.[27,28] Because information about highly specific characteristics was insufficient,[29] we focused on the characteristics of fever in FUO cases, including duration, type, the highest body temperature, fever peak per day, with or without cold, and chill. Accordingly, we observed shorter fever duration, lower level of the highest body temperature and more fever in the afternoon among patients in IDs group; meanwhile, intermittent fever was more common in CTDs group in this study. Besides, we also noted 2 special types of fever in some cases, and FUO-triggering diseases affecting these patients might conduce to the diagnosis of FUO.
The first special type of fever was regularly intermittent, which was more common in FUO patients induced by urinary infections. Regularly intermittent fever was observed in 10 of 12 cases with urinary infections in this study. Regularly intermittent fever refers to regularly alternative appearance of fever and intermission, showing almost equal numerical value of fever peak and same time in every fever stage, and similar duration for both fever and intermission.
The other special type of fever was irregularly intermittent, which was more common in FUO patients caused by infective endocarditis. Irregularly intermittent fever was observed in all infective endocarditis cases in this study. Irregularly intermittent fever sees irregular appearance of fever and intermission, that is to say, numerical values and times of fever peak as well as the duration of fever and intermission are almost absolutely different between all fevers.
In conclusion, despite their decreased frequency, IDs were still the most common causes of FUO in north China in recent years. As another important etiological reason of FUO, CTDs exhibited increasing frequency, which could possibly be explained by the advances in serological and immunological diagnostic tests. The special types of fever might be regarded as potentially diagnostic clues which might improve clinical diagnosis of FUO.
Acknowledgments
The authors thank the study participants and their families.
Author contributions
Conceptualization: Yong-zhi Zhai.
Data curation: Yong-zhi Zhai.
Formal analysis: Yong-zhi Zhai.
Funding acquisition: Gang Liu.
Investigation: Xin Chen.
Methodology: Xin Chen.
Project administration: Gang Liu.
Resources: Xin Chen, Hong-ju Xiao.
Software: Xin Chen, Hong-ju Xiao.
Supervision: Xin Liu.
Validation: Xin Liu, Zhi-qiang Zhang.
Visualization: Xin Liu, Zhi-qiang Zhang.
Writing – original draft: Zhi-qiang Zhang, Hong-ju Xiao.
Writing – review & editing: Zhi-qiang Zhang, Hong-ju Xiao.
Footnotes
Abbreviations: 95% CIs = 95% confidence intervals, ALT = alanine aminotransferase, AOSD = adult onset Still disease, AST = aspartate transaminase, AUC = receiver-operating characteristic curve, CMV = cytomegalovirus, CRP = C-reactive protein, CT = computed tomography, CTDs = connective tissue diseases, EB = Epstein–Barr, ESR = erythrocyte sedimentation rate, FUO = fever of unknown origin, HB = hemoglobin, HIV = human immunodeficiency virus, IDs = infectious diseases, LDH = lactate hydrogenase, Mi = miscellaneous, N = neoplasm, PCT = procalcitonin, PLT = primed lymphocyte test, U = undiagnosed.
Y-zZ and XC equally contributed to this work and share the first authorship.
Financial support was provided by the following grants: 2012FC-TSYS-3033, 2013ZX1004805-003.
The authors have no conflicts of interest to disclose.
References
- [1].Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine 1961;40:1–30. [DOI] [PubMed] [Google Scholar]
- [2].Durack DT, Street AC. Fever of unknown origin—reexamined and redefined. Curr Clin Top Infect Dis 1991;11:35–51. [PubMed] [Google Scholar]
- [3].Bleeker-Rovers CP, Vos FJ, de Kleijn EM, et al. A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol. Medicine 2007;86:26–38. [DOI] [PubMed] [Google Scholar]
- [4].Kucukardali Y, Oncul O, Cavuslu S, et al. The spectrum of diseases causing fever of unknown origin in Turkey: a multicenter study. Int J Infect Dis 2008;12:71–9. [DOI] [PubMed] [Google Scholar]
- [5].Li JB, Zhang JP, Chen BY. Etiological factors for 541 patients with fever of unknown origin: a retrospective analysis. Chin J Nosocomiol 2011;21:1587–9. [Google Scholar]
- [6].Geng Q, Wang LP. A clinical review of 328 cases with fever of unknown origin. Guide China Med 2010;8:5–7. [Google Scholar]
- [7].Li TJ, Wang LQ. A analysis of 162 cases of fever of unknown origin. Shandong Med Drugs J 2009;49:89–90. [Google Scholar]
- [8].Gao QJ, Shi Z, Deng XF. Analysis of the fever of unknown origin in 126 patients. Chin J Crit Care Med 2008;28:928–30. [Google Scholar]
- [9].Shao ZC. Analysis of the diagnostic methods and etiological factors in patients with fever of unknown origin. J Chin Mod Med 2007;4:693–5. [Google Scholar]
- [10].Liang ZF, Jiang HQ. Analysis of 190 cases of fever of unknown origin. Central Plains Med J 2006;33:8–10. [Google Scholar]
- [11].Zhang GH, Zhao HP. Clinical analysis of 146 cases of fever of unknown origin. Shanxi Med J 2005;34:816–8. [Google Scholar]
- [12].Ma XJ, Wang AX, Deng GH, et al. [A clinical review of 449 cases with fever of unknown origin]. Zhonghua Nei Ke Za Zhi 2004;43:682–5. [PubMed] [Google Scholar]
- [13].Yao QJ, Liao XH, Chen LP, et al. Analysis of 103 cases with fever of unknown origin. Chin J Infect Dis 2003;21:427–8. [Google Scholar]
- [14].Ni W, Miao XH, Zhang RQ, et al. Fever of unknown origin: a retrospective analysis of 107 clinical cases. Med J Chin PLA 2002;27:922–4. [Google Scholar]
- [15].Chen PD, Yu SL, Chen S, et al. Retrospective study of 61 patients with adult-onset Still's disease admitted with fever of unknown origin in China. Clin Rheumatol 2012;31:175–81. [DOI] [PubMed] [Google Scholar]
- [16].Bosilkovski M, Dimzova M, Stevanovic M, et al. Fever of unknown origin—diagnostic methods in a European developing country. Vojnosanit Pregl 2016;73:553–8. [DOI] [PubMed] [Google Scholar]
- [17].Moawad MA, Bassil H, Elsherif M, et al. Fever of unknown origin: 98 cases from Saudi Arabia. Ann Saudi Med 2010;30:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Efstathiou SP, Pefanis AV, Tsiakou AG, et al. Fever of unknown origin: discrimination between infectious and non-infectious causes. Eur J Intern Med 2010;21:137–43. [DOI] [PubMed] [Google Scholar]
- [19].Bleeker-Rovers CP, Vos FJ, Mudde AH, et al. A prospective multi-centre study of the value of FDG-PET as part of a structured diagnostic protocol in patients with fever of unknown origin. Eur J Nucl Med Mol Imaging 2007;34:694–703. [DOI] [PubMed] [Google Scholar]
- [20].Zenone T. Fever of unknown origin in adults: evaluation of 144 cases in a non-university hospital. Scand J Infect Dis 2006;38:632–8. [DOI] [PubMed] [Google Scholar]
- [21].Vanderschueren S, Knockaert D, Adriaenssens T, et al. From prolonged febrile illness to fever of unknown origin: the challenge continues. Arch Intern Med 2003;163:1033–41. [DOI] [PubMed] [Google Scholar]
- [22].Shi XC, Liu XQ, Zhou BT, et al. Major causes of fever of unknown origin at Peking Union Medical College Hospital in the past 26 years. Chin Med J (Engl) 2013;126:808–12. [PubMed] [Google Scholar]
- [23].Tana C, Wegener S, Borys E, et al. Challenges in the diagnosis and treatment of neurosarcoidosis. Ann Med 2015;47:576–91. [DOI] [PubMed] [Google Scholar]
- [24].Chokoeva AA, Tchernev G, Tana C, et al. Sarcoid-like pattern in a patient with tuberculosis. J Biol Regul Homeost Agents 2014;28:783–8. [PubMed] [Google Scholar]
- [25].Tana C, Giamberardino MA, Di Gioacchino M, et al. Immunopathogenesis of sarcoidosis and risk of malignancy: a lost truth? Int J Immunopathol Pharmacol 2013;26:305–13. [DOI] [PubMed] [Google Scholar]
- [26].Tchernev G, Chokoeva AA, Tana M, et al. Transcriptional blood signatures of sarcoidosis, sarcoid-like reactions and tubercolosis and their diagnostic implications. Sarcoidosis Vasc Diffuse Lung Dis 2016;33:5030. [PubMed] [Google Scholar]
- [27].Jin-ling M, Jian C, Yu-tang W, et al. Etiology and clinical features of fever of unknown origin. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2011;33:83–7. [DOI] [PubMed] [Google Scholar]
- [28].Salzberger B, Schneidewind A, Hanses F, et al. [Fever of unknown origin. Infectious causes]. Der Internist 2012;53:1445–53. [DOI] [PubMed] [Google Scholar]
- [29].Hayakawa K, Ramasamy B, Chandrasekar PH. Fever of unknown origin: an evidence-based review. Am J Med Sci 2012;344:307–16. [DOI] [PubMed] [Google Scholar]


