Abstract
Age-related effects were studied in 14 younger (M = 34 years) and 14 (M = 47 years) older healthy participants. Event-related potential (ERP) recording was done using a 256-channel EEG system. Results indicated that ERP is affected by advanced age. There was a significant difference in P200 mean latency between the younger participants and older participants for the target (low-probability) stimuli, but no such significance was evident for the P200 mean latency during the presentation of the standard (high-probability) stimuli. As for the P200 mean peak amplitude, the results for the target (low-probability) stimuli did show a significant difference between the two age groups, while the results for the standard (high-probability) stimuli did not show any significant difference between the two age groups. The results of this study are explained in light of aging effects on attentional recruitment and frontal lobe intactness.
Keywords: Electrophysiology, ERP, Neuroscience, Age, Aging, P200, P2, Oddball, EEG, Neurophysiology
Introduction
There exists a qualified general belief that aging and brain capacity are inversely linearly related (An increase in age results in a decrease in cognitive capacity). However, it remains unclear to what extent does aging affect electrophysiology and neurophysiology [1,2,3,4,5,6]. In the present paper, we examine how a neurophysiological signal associated with perceptual processing and attention changes during the course of normal aging. More specifically, we examine the P200 event-related potential (ERP) which is believed to signify attentional recruitment and modulates perceptual processing.
Aging may sometimes be characterized by a widespread decline in physiological, and psychological performance [9]. However, its effects on cognitive function are still not completely understood. When investigated across time, there are several capabilities that do differ in stability with increasing age, but it remains that age-induced changes in certain cognitive capacities, such as attention, are still not very well understood. In a study by Commodari et al. (2008), it was demonstrated that age affected attentive efficiency, but, notably, such a decline does not involve all attentional components [10]. More specifically, with regards to P200, age-related differences were found for novel stimuli (i.e. target/ low-probability stimuli) [11]. In this regard, enhancement of P200 has been shown to partly suggest the occurrence of an interaction between frontal brain areas used for evaluation and posterior brain areas used for perceptual representation while identifying a task-relevant stimuli [7], and it may be the case that P200 is an indication of stimulus evaluation rather than response production [8].
Bennett et al. (2004), with 13 younger participants and 12 older participants, found age-related differences in ERPs [12]. Also, age-related changes in the brain can affect inhibitory neurotransmission of cortical and subcortical neurons. Such an effect, in turn, may affect the response properties of neurons and temporal processing. Furthermore, inhibition is a very influential modulatory mechanism for different cortical and sub-cortical processes that influence neuronal network plasticity, oscillations, and control of response [13,14,15,16].
With regards to cognitive function decline and neurodegeneration, it has been shown that there exists a link between cognitive performance associated with pathological aging (i.e. Alzheimer’s disease) and deficit in the inhibition of redundant information and that specific ERP gating deficit in pathological aging correlates to frontal neuropsychological function [17,18]. Moreover, attentional mechanisms can be either bottom-up or top-down. The bottom-up system can result in automated reaction and unspecific alertness to sensory input. On the other hand, the top-down system allows for modulation of processing and stimulus recognition [14,19,20].
As detailed above, there is an inverse relationship between increasing age and brain capacity. Also, there is still no consensus on the effects of aging on neurophysiology and electrophysiology. Additionally, although studies have shown aging effects on attention, there is still no consensus on whether or not ERPs are affected by aging and, if so, how are such ERPs affected by aging. Such ERP studies and especially those investigating P200 remain limited. Therefore, the present study aims to investigate and assess any age-related differences in the P200 ERP waveform’s amplitude and latency for both standard (high-probability) stimuli, target (low-probability) stimuli.
Methods
Participants
Younger participants (YP; mean age = 34 years, SD = 3.0, n=14) and older participants (OP; mean age = 47 years, SD = 6.0, n = 14) were recruited from staff working at the Health Sciences Center, Kuwait University. Younger participants were in a young age adulthood category and older participants were in a middle age adulthood category. All participants were right-handed and did not complain from cognitive, neurological, or psychological problems. Ethical approval was granted from the Health Sciences Center Ethical Committee.
Evoked Potential Procedure
ERP recording was done using a 256-channel dense array EEG system (GES 410 by Electrical Geodesics, Inc.), that uses saline for conduction, situated in an electrically shielded and sound-attenuated room. The 256 EEG electrodes were embedded in a HydroCel Geodesic Sensor Net (Electrical Geodesics, Inc.). The acquisition software used was Net Station 5.1 (Electrical Geodesics, Inc.). The sampling rate was set at 1000 Hz. Given that the EEG system is considered a high-impedance system, the electrode impedance was kept well below 50 kilo-ohms [21]. For each participant, the net was properly adjusted, and it was made sure, by scalp markings, that Cz was on the vertex and that the Fz-Cz-Pz were on the midline of the scalp. The remaining electrodes were placed in accordance with the geodesic structure of the net.
While being situated in a comfortable chair, in front of a computer screen, with their right index finger situated on the response keypad and the EEG net on their head, participants were respectively given verbal instructions on the task. The task used was an oddball task that was programmed in EPrime 2.0 (Psychology Software Tools, Inc.) stimuli presentation software. The oddball task consisted of standard (high-probability) stimuli and target (low-probability) stimuli. The ratio of standards to targets is 80:20. The standard was represented by an “O” and the target was represented by an “X”. The Xs and Os were displayed in random order for a 500 ms appearance and were interleaved by a fixation cross (ITI = 1000 – 2500 ms). Each participant was instructed to not respond to fixations and Os, and to only respond to Xs by pressing a response key. Each participant had two practice runs, followed by 3 experiment runs (each run was 6.7 minutes in duration).
Evoked Potential Analysis
All ERP data underwent pre-processing and post-processing using Net Station 5.1 (Electrical Geodesics, Inc.). All data were filtered using a 0.1-30 Hz Bandpass filter. Then, the recorded data was segmented into epochs. The epochs commence at 100 ms before the onset of the target stimulus and end 700 ms after the onset. An 18 ms offset for the segment was set, which was based on a timing test done prior to the experiment runs. Artifact detection and artifact removal algorithms of Net Station 5.1 (Electrical Geodesics, Inc.) were used to remove eye movements (max amplitude – min amplitude > 55 μV), eye blinks (max amplitude – min amplitude > 140 μV), bad channels (max amplitude – min amplitude > 200 μV).
In post-processing, first, bad-channel replacement was done where detected bad channels were replaced with interpolated data from the good channels that remained. Then all segments were averaged, and an average reference montage operation was done. Using a baseline of 100 ms before stimulus onset and lasting for 100 ms a baseline correction was performed.
Careful inspection of the post-processed data was carried out to find the P200 ERP peak across the scalp. Given our interest in the P200 and that it occurs primarily in frontal-central scalp electrode locations, at the 150-250 ms temporal domain, we have selected the following electrode sites for further analysis: Cz, Fp1, Fp2, Fz, F3, F4.
Using the statistic extraction method in Net Station 5.1 (Electrical Geodesics, Inc.), the P200 peaks and latencies were extracted for all participants. Then, a two-sample t test was performed for the P200 peak amplitude followed by another two-sample t test for the P200 latency. SPSS (IBM SPSS Statistics for Windows Version 25) was used to perform the t tests.
Results
YP’s P200 mean peak amplitude (4.3 μV) for the target (low-probability) stimuli was significantly greater than that of the OP group (3.3 μV) at p=0.032. As for the standard (high-probability) stimuli, there was no significant difference (p=0.646) between YP’s mean peak amplitude (2.4 μV) and OP’s mean peak amplitude (2.3 μV).
As for target (low-probability) latency for P200, YP showed significantly shorter mean latency (180 ms) compared to OP (199 ms) at p=0.001. On the other hand, the P200 mean latency for the standard (high-probability) stimuli showed no significant difference (p=0.371) between YP (195 ms) and OP (199 ms).
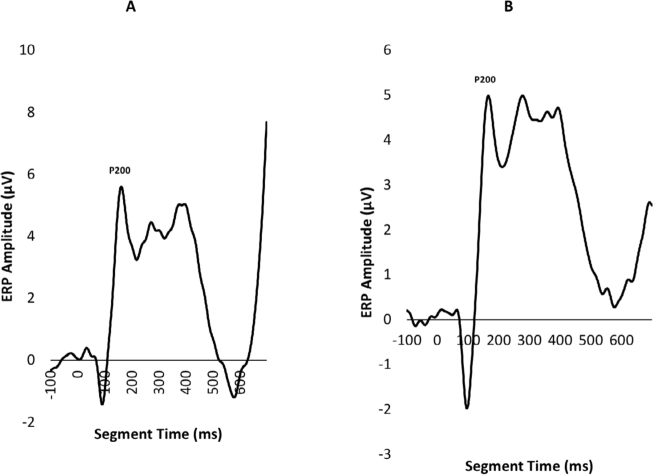
Both YP and OP age groups showed a clearly visual peak in electrophysiological signal intensity at frontal-central scalp locations between 150-250 ms after event onset. Figure 1 shows the averaged voltage scalp EEG map for the target (low-probability) P200 ERP for YP at 180 ms. Figure 2 shows the averaged voltage scalp EEG map for the standard (high-probability) P200 ERP for YP at 195 ms. Figure 3 shows the averaged voltage scalp EEG map for the standard (high-probability) P200 ERP for OP at 199 ms. Figure 4 shows the averaged voltage scalp EEG map for the target (low-probability) P200 ERP for OP at 199 ms. Figure 5 provides single subject ERP waveforms for the target stimuli (showed age-related significant difference in latency and amplitude) for both YP and OP.
Figure 1.
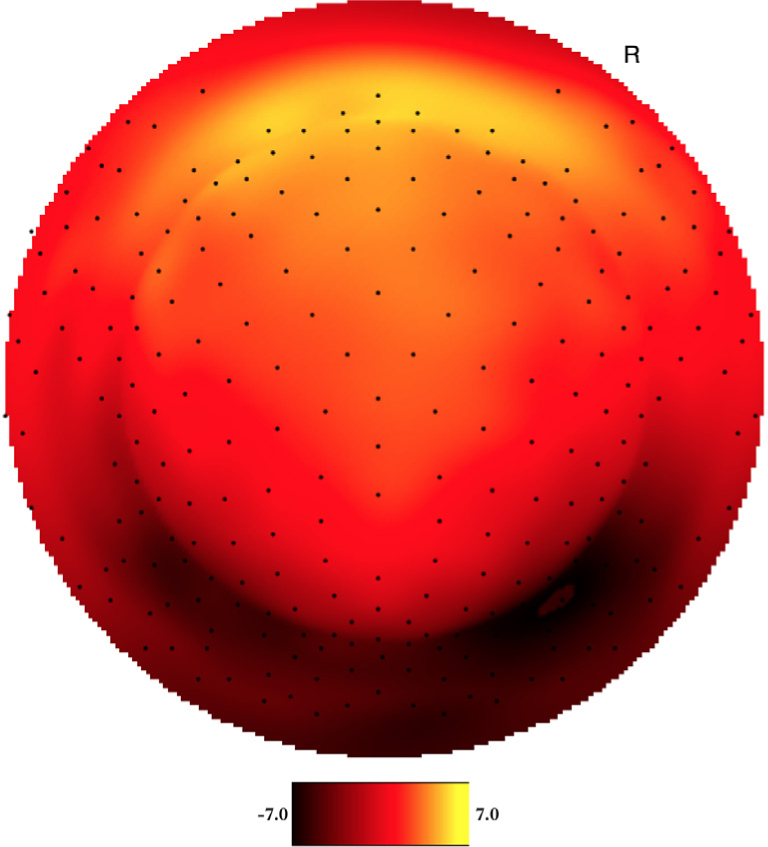
The two-dimensional averaged ERP voltage map for YP at 180 ms latency for the target (low-probability) stimuli. Dark red is negative and bright yellow is positive ( scale: -7.0μV - 7.0μV). The orientation of the figure is with the nose at the top of the figure and looking down at the top of the head.
Figure 2.
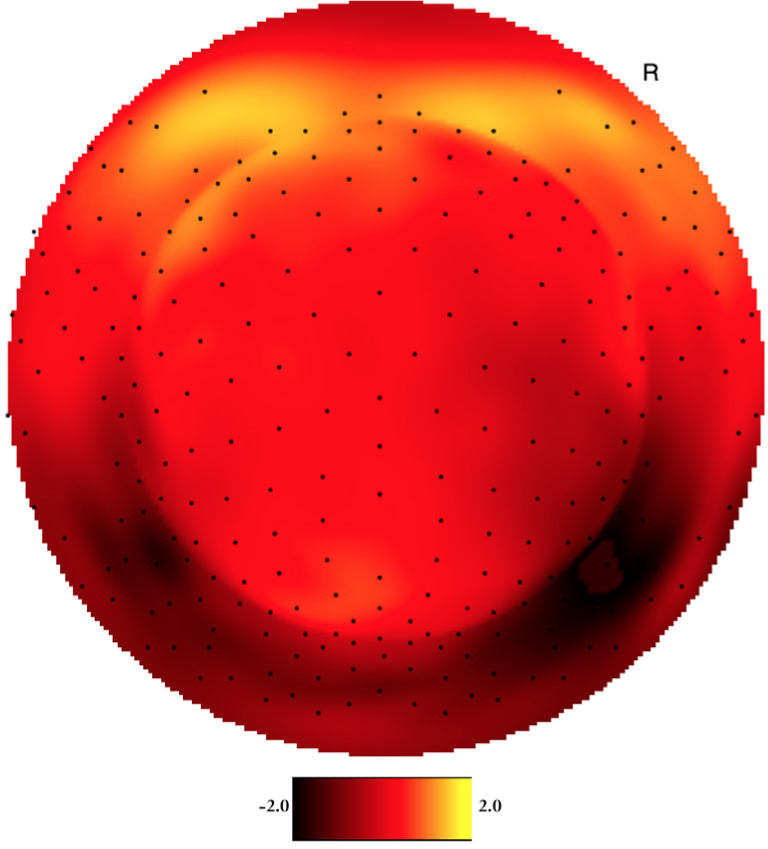
The two-dimensional averaged ERP voltage map for YP at 195 ms latency for the standard (high-probability) stimuli. Dark red is negative and bright yellow is positive (scale: -2.0μV - 2.0μV). The orientation of the figure is with the nose at the top of the figure and looking down at the top of the head.
Figure 3.
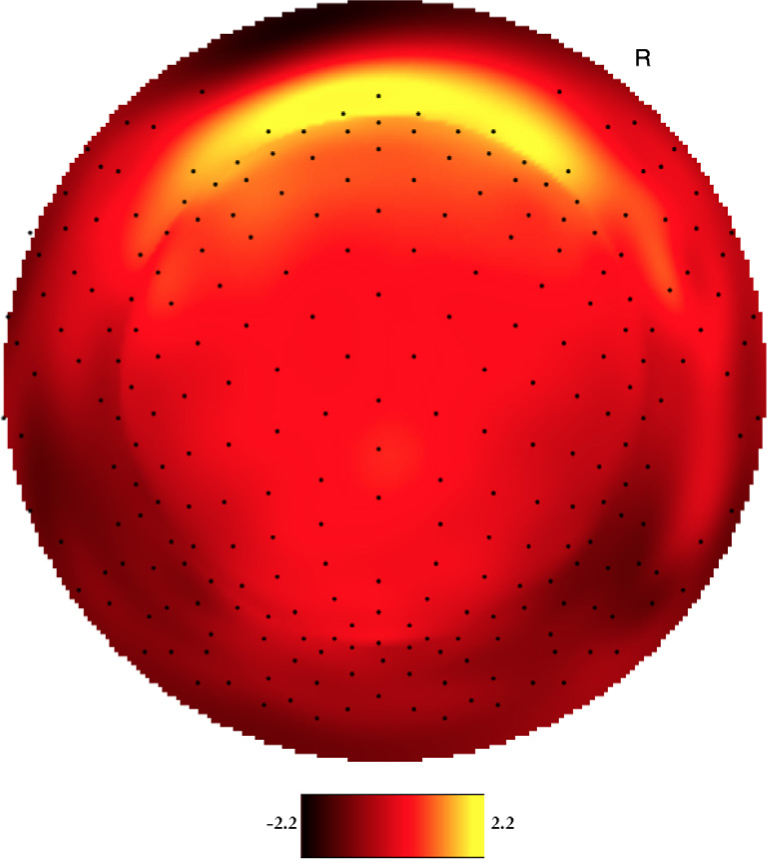
The two-dimensional averaged ERP voltage map for OP at 199 ms latency for the standard (high-probability) stimuli. Dark red is negative and bright yellow is positive (scale: -2.2μV - 2.2μV). The orientation of the figure is with the nose at the top of the figure and looking down at the top of the head.
Figure 4.
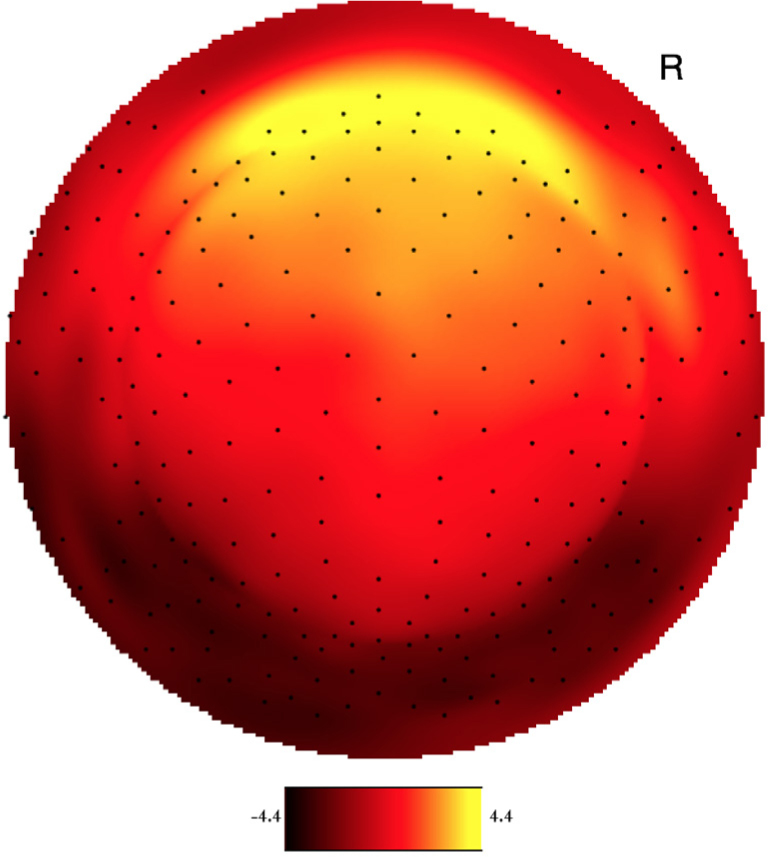
The two-dimensional averaged ERP voltage map for OP at 199 ms latency for the target (low-probability) stimuli. Dark red is negative and bright yellow is positive (scale: -4.4μV – 4.4μV). The orientation of the figure is with the nose at the top of the figure and looking down at the top of the head.
Figure 5.
ERP waveforms at Fz (A: Target stimuli for YP, B: Target stimuli for OP).
Discussion
Common behavioral indices are not specific enough to provide enough information about processes that are involved at the time of encoding. In fact, compared to ERP they are delayed relative to the process under investigation. ERPs provide an immediate record of brain activity that is associated with a given stimulus. Moreover, some ERP components may in fact reflect operations of cognitive processes caused by the stimulus within the context of the participant’s task [22; 23]. Among these components is the P200.
The present study examined the effects of age on P200 ERP latency and amplitude. ERP measures were obtained during the performance of an oddball task in both a younger age group (Younger participants; YP; mean age = 34 years, n=14) and an older age group (Older participants; OP; mean age = 47 years, n = 14). The results showed that advanced age resulted in slower ERP latency and larger ERP wave amplitude. More specifically, the P200 ERP mean latency of the younger age group for the target (low-probability) stimuli was significantly faster (180 ms) compared to the older age group (199 ms). However, the difference in P200 ERP mean latency between the age groups for the standard (high-probability) stimuli was not significantly different. As for the P200 ERP mean peak amplitude, the younger age group, for the target (low-probability) stimuli, showed significantly larger amplitude (4.3 μV) compared to the older age group (3.3 μV). On the other hand, the P200 ERP mean peak amplitude did not show any significant difference between the two age groups for the standard (high-probability) stimuli.
In a study of 12 younger and 12 older adults results showed that both ERP amplitude and ERP latency were affected by age, and that the older group had a smaller ERP amplitude and longer ERP latency compared to the younger group [22]. Also, using an oddball task, another study [24] found a strong relationship between age and ERP latency. On the other hand, Bahramali (1991) found no significant association between ERP amplitude and age [25]. Friedman et al. (1998), also using an oddball task, did find ERP age effects. In another study, Kok (2000) showed ERPs to be smaller and of longer duration in older than younger participants [26]. Given that, and due to relatively limited research efforts with regards to ERP and aging, there is still no consensus on how and if ERP is affected advanced age.
Moreover, in a 2001 study by McEvoy et al., in which ERPs were recorded from younger and older age groups (n=10 per group), it was found that the latency of the P200 was not affected by age, but the P200 amplitude was found to be larger for older participants compared to younger participants. Despite these findings, age-related changes in the amplitude of P200 is still not well understood [27]. In our present study, we found that the P200 target (low-probability) stimuli amplitude and latency to be affected by age, in which the younger participants (YP) showed faster latency and larger amplitude for P200 compared to older participants (OP).
One of the major neuropsychological hypotheses is that there is a change in frontal lobe function with increasing age [28; 29]. Also, it is more difficult for older adults to inhibit responses to task-relevant events [30,31,32] which is a function that depends on intact frontal lobe functioning [33; 34]. On a more functional level, the frontal lobe is involved in generating top-down control signals for attentive switching. These signals may then be fed to visual processing areas in the brain. Also, parts of the frontal lobe play a crucial role in the ability to switch attentional control on the basis of changing demands of a given task [35].
In the light of these evidence, and given that P200 is primarily frontally distributed, our findings indicate that the modification of the P200 characteristics seems to relate to age-related brain changes. These age-related changes seem to affect the inhibitory neurotransmission, which in-turn affects neuronal response properties and temporal processing. On the same note, it is worth noting that inhibition can influence neuronal plasticity and response control, which in return may have implications on higher level neuronal processing in the brain.
References
- [1].Hillyard SA, Anllo-Vento L.. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. 1998;95(3):781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Freunberger R, Klimesch W, Doppelmayr M, Höller Y.. Visual P2 component is related to theta phase-locking. Neurosci. Lett. 2007;426(3):181–186. doi: 10.1016/j.neulet.2007.08.062. [DOI] [PubMed] [Google Scholar]
- [3].Chen A, Xu P, Wang Q, Luo Y, Yuan J, Yao D. et al. The timing of cognitive control in partially incongruent categorization. Hum. Brain Mapp. 2008;29(9):1028–1039. doi: 10.1002/hbm.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Craik FIM, Byrd M. Aging and Cognitive Processes. Boston, MA: Springer US; 1982. Aging and Cognitive Deficits; pp. 191–211. [Google Scholar]
- [5].Bourisly AK, El-Beltagi A, Cherian J, Gejo G, Al-Jazzaf A, Ismail M.. A voxel-based morphometric magnetic resonance imaging study of the brain detects age-related gray matter volume changes in healthy subjects of 21-45 years old. Neuroradiol. J. 2015;28(5):450–9. doi: 10.1177/1971400915598078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bourisly AK.. Effects of aging on P300 between late young-age and early middle-age adulthood. Neuroreport. 2016;27(14):999–1003. doi: 10.1097/WNR.0000000000000644. [DOI] [PubMed] [Google Scholar]
- [7].Potts GF, Patel SH, Azzam PN.. Impact of instructed relevance on the visual ERP. Int. J. Psychophysiol. 2004;52(2):197–209. doi: 10.1016/j.ijpsycho.2003.10.005. [DOI] [PubMed] [Google Scholar]
- [8].Potts GF.. An ERP index of task relevance evaluation of visual stimuli. Brain Cogn. 2004;56(1):5–13. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- [9].Craik FIM, Salthouse TA. The handbook of aging and cognition. Psychology Press; 2008. [Google Scholar]
- [10].Commodari E, Guarnera M.. Attention and aging. Aging Clin. Exp. Res. 2008;20(6):578–84. doi: 10.1007/BF03324887. [DOI] [PubMed] [Google Scholar]
- [11].Riis JL, Chong H, McGinnnis S, Tarbi E, Sun X, Holcomb PJ. et al. Age-related changes in early novelty processing as measured by ERPs. Biol. Psychol. 2009;82(1):33–44. doi: 10.1016/j.biopsycho.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bennett IJ, Golob EJ, Starr A.. Age-related differences in auditory event-related potentials during a cued attention task. Clin. Neurophysiol. 2004;115(11):2602–15. doi: 10.1016/j.clinph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- [13].Lijffijt M, Moeller FG, Boutros NN, Burroughs S, Lane SD, Steinberg JL. et al. The Role of Age, Gender, Education, and Intelligence in P50, N100, and P200 Auditory Sensory Gating. J. Psychophysiol. 2009;23(2):52–62. doi: 10.1027/0269-8803.23.2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gmehlin D, Kreisel SH, Bachmann S, Weisbrod M, Thomas C.. Age Effects on Preattentive and Early Attentive Auditory Processing of Redundant Stimuli: Is Sensory Gating Affected by Physiological Aging? Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 2011;66A(10):1043–1053. doi: 10.1093/gerona/glr067. [DOI] [PubMed] [Google Scholar]
- [15].Caspary DM, Ling L, Turner JG, Hughes LF.. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J. Exp. Biol. 2008;211(11):1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Alitto HJ, Dan Y.. Function of inhibition in visual cortical processing. Curr. Opin. Neurobiol. 2010;20(3):340–346. doi: 10.1016/j.conb.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thomas C, vom Berg I, Rupp A, Seidl U, Schröder J, Roesch-Ely D. et al. P50 gating deficit in Alzheimer dementia correlates to frontal neuropsychological function. Neurobiol. Aging. 2010;31(3):416–424. doi: 10.1016/j.neurobiolaging.2008.05.002. [DOI] [PubMed] [Google Scholar]
- [18].Cancelli I, Cadore IP, Merlino G, Valentinis L, Moratti U, Bergonzi P. et al. Sensory Gating Deficit Assessed by P50/Pb Middle Latency Event Related Potential in Alzheimer???s Disease. J. Clin. Neurophysiol. 2006;23(5):421–425. doi: 10.1097/01.wnp.0000218991.99714.ee. [DOI] [PubMed] [Google Scholar]
- [19].Fritz JB, Elhilali M, David S V, Shamma SA.. Auditory attention— focusing the searchlight on sound. Curr. Opin. Neurobiol. 2007;17(4):437–455. doi: 10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- [20].Pinto Y, van der Leij AR, Sligte IG, Lamme VAF, Scholte HS.. Bottom-up and top-down attention are independent. J. Vis. 2013;13(3):16–16. doi: 10.1167/13.3.16. [DOI] [PubMed] [Google Scholar]
- [21].Ferree TC, Luu P, Russell GS, Tucker DM.. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001;112(3):536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- [22].Dujardin K, Derambure P, Bourriez JL, Jacquesson JM, Guieu JD.. P300 component of the event-related potentials (ERP) during an attention task: effects of age, stimulus modality and event probability. Int. J. Psychophysiol. 1993;14(3):255–267. doi: 10.1016/0167-8760(93)90040-v. [DOI] [PubMed] [Google Scholar]
- [23].Donchin E, Ritter W, McCallum C. Brain event-related potentials man. New York Acad. Press; 1978. Cognitive psychophysiology: The endogenous components of the ERP. [Google Scholar]
- [24].Coyle S, Gordon E, Howson A, Meares R.. The effects of age on auditory event-related potentials. Exp. Aging Res. 1991;17(2):103–111. doi: 10.1080/03610739108253889. [DOI] [PubMed] [Google Scholar]
- [25].Bahramali H.. The Effects of Age on Late Components of the ERP and Reaction Time. Exp. Aging Res. 1999;25(1):69–80. doi: 10.1080/036107399244147. [DOI] [PubMed] [Google Scholar]
- [26].Kok A.. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biol. Psychol. 2000;54(1–3):107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- [27].McEvoy LK, Pellouchoud E, Smith ME, Gevins A.. Neurophysiological signals of working memory in normal aging. Cogn. Brain Res. 2001;11(3):363–376. doi: 10.1016/s0926-6410(01)00009-x. [DOI] [PubMed] [Google Scholar]
- [28].Albert MS, Kaplan E. New Directions in Memory and Aging (PLE: Memory) Routledge: 2014. [Google Scholar]
- [29].Moscovitch M, Winocur G. The handbook of aging and cognition. Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc; 1992. The neuropsychology of memory and aging; pp. 315–372. [Google Scholar]
- [30].Hartman M, Hasher L.. Aging and suppression: memory for previously relevant information. Psychol. Aging. 1991;6(4):587–94. doi: 10.1037//0882-7974.6.4.587. [DOI] [PubMed] [Google Scholar]
- [31].Rabbitt P.. AN AGE-DECREMENT IN THE ABILITY TO IGNORE IRRELEVANT INFORMATION. J. Gerontol. 1965;20:233–8. doi: 10.1093/geronj/20.2.233. [DOI] [PubMed] [Google Scholar]
- [32].Tipper SP.. Less attentional selectivity as a result of declining inhibition in older adults. Bull. Psychon. Soc. 1991;29(1):45–47. [Google Scholar]
- [33].Lurii⊠a⊠ AR, Aleksandr R. The working brain; an introduction to neuropsychology. Basic Books; 1973. [Google Scholar]
- [34].Friedman D, Kazmerski VA, Cycowicz YM.. Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology. 1998;35(5):S0048577298970664. doi: 10.1017/s0048577298970664. [DOI] [PubMed] [Google Scholar]
- [35].Rossi AF, Pessoa L, Desimone R, Ungerleider LG.. The prefrontal cortex and the executive control of attention. Exp. brain Res. 2009;192(3):489–97. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]