Abstract
Objectives:
The purpose of this study was to extend the results of our previous study providing a minimum of 4-year follow-up results of a prospective study following implantation of a cervical cage with an integrated fixation system.
Summary of Background Data:
The use of cervical intersomatic cages with an integrated fixation system for anterior cervical discectomy and fusion (ACDF) has increased rapidly in this last decade. In addition to immediate stabilization, these implants allow avoidance of anterior plating and iliac crest bone-grafting.
Methods:
Patients were studied prospectively, and data were collected and analyzed. Intersomatic cages with an integrated fixation system were used in consecutive 100 patients operated on for ACDF. Intraoperative parameters, clinical, and outcome scores were recorded. Radiographs were taken to evaluate implant positioning and fusion rate, disc height (DH), and changes in adjacent disc spaces. All the patients had a minimum 4-year follow-up.
Results:
A total of 127 cages were implanted in the 100 patients. Compared to preoperatively, the visual analog scale, 36-item short-form health survey, the Japanese Orthopedic Association, and the Neck Disability Index scores were significantly improved at 1-year follow-up without change during subsequent follow-up. At 4 years, the fusion rate was 97%. Two patients complained about minor dysphagia-related symptoms, which resolved rapidly. DH index and cervical Cobb angle were significantly restored after surgery, and the results were maintained during the whole follow-up.
Conclusions:
This is a prospective, independently conducted study on cages with an integrated fixation system with 4-year long follow-up. Findings of this study seem to be interesting regarding outcomes and low complications rates compared to recent series using other implants with integrated fixation system. Larger, randomized controlled trials are warranted.
Keywords: Anterior cervical discectomy fusion, cervical disc herniation, cervical spondylosis functional scores
INTRODUCTION
Cervical discectomy is one of the most commonly and rapidly growing procedures done to rapidly restore radicular and medullar compression. Since fusion procedures are widely accepted, research and development have concentrated on improving and simplifying portions of this procedure in hopes that it would improve outcomes. Accordingly, many devices including the use of allograft bone and anterior plating, polyether ether ketone (PEEK) cages with anterior plating, and other interbody fusion devices have been used with different results.[1] Although a large number of technical and biomechanical advances have led to an array of available options for fusions, the use of cage and the supplementary fixation by an anterior cervical plate has enhanced the risk of complications such as dysphagia.[2,3,4,5] Technological advancements and new materials discovery have gained the evolution to zero-profile anchored cage systems tailored for stand-alone fusion [6] avoiding the disadvantages of plating. The ROI-C cage (Zimmer Biomet, Austin, TX, USA) is a cervical interbody cage with an integrated fixation that has been developed to increase cervical spine stability since the early postoperative period. In addition to immediate stabilization, use of these types of implants allows avoidance of anterior plating and iliac crest bone-grafting. Following the first study reporting a short-term outcome assessment,[7] the purpose of this study was to evaluate the efficacy and safety of anterior cervical discectomy and fusion (ACDF) with the ROI-C in a longer follow-up, spanning 9 years, following surgery
METHODS
Study design
This was a prospective multicenter study, of patients who underwent single- or multi-level ACDF with the ROI-C cages. Between January 2009 and January 2014, 100 consecutive patients affected by cervical disc herniation or spondylosis causing radiculopathy were enrolled. Patients had not experienced any cervical spine surgery before this operation. All patients attempted a trial of conservative therapy usually at least 6 weeks before surgery, including rest, physical, and/or pharmacotherapy. Patients were diagnosed based on the preoperative radiograph, computed tomography (CT) scan, and magnetic resonance imaging (MRI) findings. All patients enrolled in the study were suitable candidates for ACDF. Enrollment required a diagnosis of degenerative disc disease with symptomatic radiculopathy or myeloradiculopathy at one or two levels from C-3 to C-7 that correlated with appropriate level and side neural compression on MRI or CT. Patients who presented with other spinal degenerative conditions, such as stenosis or arthritis, were not excluded as long as the diagnosis indicated that the primary cause for complaints was clinically consistent with nerve root compression. Patients were also not excluded due to age, sex, compensation claims, diabetes, obesity, or other medical conditions that would not preclude surgery, in general. The exclusion criteria were prior spine surgery at the operative levels, ossification of the posterior longitudinal ligament, major degenerative or traumatic instability, cervical canal narrowing requiring posterior decompression associated with anterior fusion, fracture, infection, and tumor. Each patient was appropriately informed regarding medical data collection and the aim of the study.
Outcomes
Each patient was followed-up prospectively with preoperative and postoperative evaluations. Data including patient demographics (age and gender), intra-operative details (duration of operation, level of operation, types, and sizes of the implant, complications), postoperative details (length of hospitalization, and time to return to work), postoperative functional scores, radiological findings, and surgery related-complications were collected. During follow-up, clinical and radiographic data were collected on the last day of hospital stay, at 1 and 6 months, and every year. All the patients were included in the clinical and radiographic evaluations at each follow-up time point.
During follow-up, clinical and radiographic data were collected on the last day of hospital stay, at 6 weeks, at 3, 6, 12 months, and every year. Complications were recorded as implant-related, surgery-related, or general (not directly implant or surgery related). All the patients were included in the clinical and radiographic evaluations at each follow-up time. The minimum follow-up was 4 years (mean, 7.05 years; range, 4“9 years).
Outcome measures used were the Medical Outcomes Study 36-item short form health survey (SF-36), Neck Disability Index (NDI), and visual analog scale (VAS) scores for the neck and arm pain. All patients were asked to complete questionnaires, even in the form of an interview, before surgery and at each follow-up examination. The NDI and VAS scores ranged from 0 to 100. Odom's grading system (poor, fair, good, or excellent) was used to evaluate patient satisfaction with the surgery. Myelopathy was graded using the Japanese Orthopedic Association (JOA) score.
Dysphagia-related symptoms were graded by a physician, depending on the patient's state, as none (no episodes of swallowing problems), mild (rare episodes of dysphagia), moderate (occasional swallowing difficulty with specific food), and severe (frequent difficult swallowing with the majority of food).[8] In addition, the amount of pain (VAS 0“100) and the duration of dysphagia-related symptoms were recorded.
Anteroposterior, lateral, and flexion-extension radiographs were taken before surgery, within 1 week after surgery, and at 1 and 6 months after surgery. Subsequent follow-up examinations were performed every 12 months to detect implant failure, including segmental collapse, caused by implant subsidence. An implant penetration into the adjacent endplates of more than 2 mm was defined as segmental collapse.[9] MRI was routinely performed to evaluate preoperative spinal cord compression. Cervical alignment was calculated by the Cobb angle between the inferior margins of C2 and C7 vertebral bodies on the lateral radiograph [Figure 1a]. The disc height (DH) was measured on the lateral radiograph as the distance from the highest portion of the lower end-plate of the cephalad vertebra to the closest portion of the upper end-plate of the caudal vertebra [Figure 1b]. Degenerative changes in the adjacent segments were evaluated on MRI every year postoperatively. Disc degeneration was graded on T2-weighted sagittal and axial images as described by Miyazaki.[10] The evaluation of interbody fusion was according to Bridwell's classification.[11]
Figure 1.
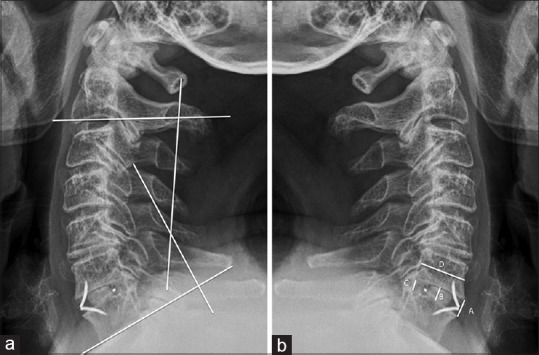
Lateral radiograph depicting a 4-year long follow-up in a C6“C7 case. (a), Image showing cervical alignment calculated by the Cobb angle between the inferior margins of C2 and C7 vertebral bodies; (b), lateral radiograph showing the disc height measurement. A, is anterior disc height, b is middle disc height, c is posterior disc height, and d is sagittal diameter of the up vertebral body. Disc height index = ([a + b + c]/3)
Statistical analysis
We computed means and standard deviations for continuous data. We determined differences in outcome measures scores between preoperative and postoperative time points and during further follow-up using the t-test for paired samples if a normality test was passed or a Wilcoxon signed-rank test if a normality test was failed. Intra-observer variability for radiographic evaluation was determined using kappa statistics. (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) software was used for all analyses.
RESULTS
Patient population
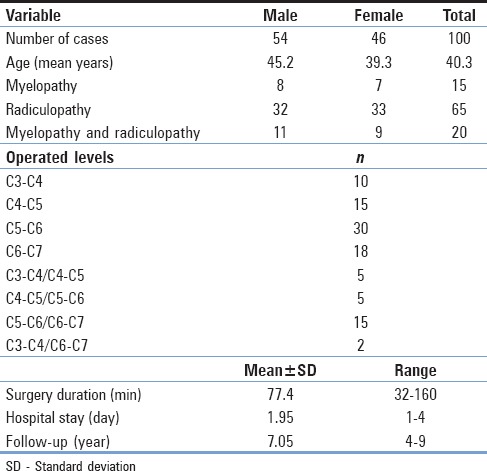
A total of 100 patients (54 men and 46 women; mean age 40.30 years, range 31“78 years) included in this study received 127 ROI-C devices. Sixty-five patients (65%) had radicular pain, 15 (15%) had myelopathy, and 20 (20%) had both radiculopathy and myelopathy. Most patients had a history of incapacitating neck and arm pain lasting longer than 6 weeks, which was unresponsive to physical therapy and anti-inflammatory medication or had new neurological deficits resulting from myelopathy. All the patients had a minimum of 4 years follow-up and a maximum of 9 years (mean, 7.05 years). The mean surgery duration was 77.4 min (range, 32“160). Seventy-three patients (73%) were treated at one level and 27 (27%) at two levels. Table 1 summarizes demographic, and surgery data.
Table 1.
Summary of demographic and surgery data
Outcomes
Postoperatively, a cervical collar was not used, except in some patients who asked the externals device as “comfort.” Usually, following 4 weeks postsurgery, patients were allowed to engage in normal activities of daily living including driving. After 5 weeks, patients were allowed to return to all normal activities except contact sports on a permanent basis. Postoperative oral pain medications were administered as needed. In the first 8 weeks, anti-inflammatory medication therapy was never administered. The average hospitalization time was 1.95 days (range, 1“4 days).
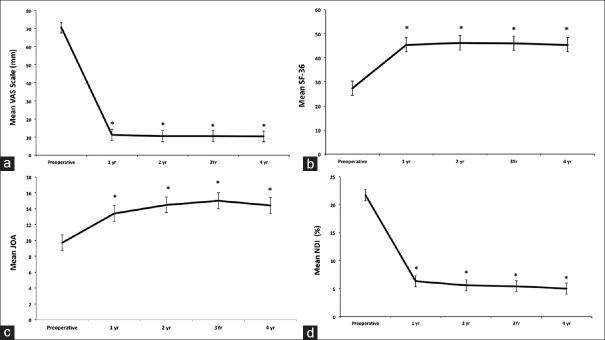
Overall, outcome measures improved immediately after surgery (P < 0.001) compared to baseline. At 4 years, all patients had a significant reduction in VAS, SF-36, JOA, and NDI scores (P < 0.05) [Figure 2]. Using Odom's criteria, 94% of patients rated their level of satisfaction with the surgery as excellent or good, without significant difference during the follow-up (P > 0.05). Only two patients complained about minor dysphagia (VAS score, 2 and 3), which resolved within 1 and 3 months, respectively.
Figure 2.
Line graphs showing the clinical outcome measures over follow-up. Results are expressed as mean ± standard error of the mean (a), visual analog scale (0“100 mm) for the neck and arm pain; (b) 36-item short form health survey score; (c), Japanese Orthopedic Association score; (d), Neck disability Index (0%“100%). Compared with preoperatively, the visual analog scale, 36-item short form health survey, Japanese Orthopedic Association, and Neck Disability Index scores were significantly improved at 1-year follow-up without change during subsequent follow-up (*P ≤ 0.05 compared to preoperative baseline)
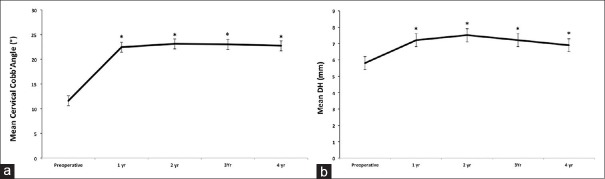
Intra-observer agreement for radiographic evaluation was satisfactory. Overall, we observed no implant-related complications. No segmental collapse during the follow-up was observed; there were no subsidence, no evidence of cage mobilization or implant dislodgment or mobilization of the anchoring system in both vertebral bodies. At 4 years, 97% of patients showed evidence of fusion in each operated segment according to the criteria suggested by Bridwell et al.[11] Three cases have been evaluated as doubtful fusion. Considering their good clinical outcome and the stability of the system as evaluated by dynamic radiographs, no further surgeries were undertaken. The cervical Cobb angle was also significantly improved from 11.6° ± 3.7°, measured before surgery, to 23.1° ± 2.2° at 1 week and 22.4° ± 3.4° at 24 months following the implant (P > 0.05). No statistical significant differences were observed among the subsequent evaluations (P > 0.05) [Figure 3a]. DH increased significantly after surgery. The mean DH at the treated level was significantly restored after surgery in all the cases. Briefly, the height of intervertebral space was significantly improved from 5.8 ± 0.5 mm measured before surgery to 7.2 ± 0.3 mm at 1 week and 7.5 ± 0.2 mm at 4 years following the surgical treatment (P < 0.05) [Figure 3b].
Figure 3.
Line graphs showing the mean cervical Cobb's angle (a) and disc height (b). Results are expressed as mean ± standard error of the mean (a) The cervical Cobb angle was significantly improved compared to baseline (*P ≤ 0.05). (b) The mean disc height, at the treated level, was significantly restored after surgery in all the cases (*P ≤ 0.05 compared to baseline)
DISCUSSION
In the present study, we prospectively evaluated the safety and efficacy the ROI-C, a cervical cage with an integrated fixation system, for ACDF. Results were presented through 4 years follow-up as an extension of a previous study in which we have shown the preliminary results.[7] The average hospitalization time was 1.95 days (range, 1-4 days). The mean surgery duration was 77.4 min (range, 32“160). Overall, outcome measures improved immediately after surgery (P < 0.001) compared to baseline. At 4 years, all patients had a significant reduction in VAS, SF-36, JOA, and NDI scores (P < 0.05). Using Odom's criteria, 94% of patients rated their level of satisfaction with the surgery as excellent or good, without significant difference during the follow-up (P > 0.05). Only two patients complained about minor dysphagia (VAS score, 1.6 and 2), which resolved rapidly. At 4 years, 97% of patients showed evidence of fusion in each operated segment. Overall, the results demonstrated that clinical scores improved significantly compared to baseline at all-time points after surgery until final follow-up. A high interbody fusion rate and a low complications rate were also observed. Similar observations have been reported in some studies evaluating the clinical efficacy of ACDF using cages with an integrated fixation system.[2,3,5,7,12,13,14]
The present study is the first objectively analyzing by a prospective study on the use of a cervical cage with an integrated fixation system for ACDF with a follow-up spanning 9 years. The results indicate that the use of this peek cage is safe in the standard ACDF procedure. Therefore our findings provide further information supporting the use of devices with an integrated fixation system. These systems appear to be acceptably safe, also in long-term follow-ups.
The reason for the development of alternative stabilization prosthetic devices in ACDF is avoidance of complications arising from the use of autologous or allergenic bone-grafting. These complications include persistent donor site pain, infection, hematoma formation, iliac crest fracture, and meralgia paresthetica. To prevent these complications, cages have been studied and applied in humans as potential bone substitutes for autograft in interbody fusion. Titanium or carbon fiber cages were widely used for cervical interbody fusion, but subsidence, migration, and structure failure have occurred.[15] PEEK is a nonabsorbable biopolymer that has been used in a variety of industries including medical devices. The PEEK cages are biocompatible, radiolucent, and have a modulus of elasticity similar to the bone. Satisfactory results were obtained with bone substitute regarding the fusion rate; although, fusion was delayed as compared with that in a cage-containing autograft.[16] The ROI-C cage is a cervical intersomatic cage made by PEEK optima (radiolucent) using a double anchoring system to obtain an intervertebral fusion. This cage is designed specifically for implantation into the cervical disc space after discectomy. It enables the filling of a graft either autologous fusion or bone substitute. The self-guided, curved plating is delivered in the plane of the disc through a direct anterior approach. In such a way, surgery can be achieved with less exposure than may be required to implant a traditional cervical plate or even contemporary stand-alone systems with screws that should be inserted at oblique angles. The cages are simple and intuitive, dedicated instruments are very few, and the learning curve is almost brief. Stabilization appears to be immediately afforded following implants, and the double anchoring system avoids effectively the use of a postoperative collar. Reasonably, the two wings firmly introduced in the vertebral bodies can provide initial stability until the fusion time. We found that the intervertebral height was significantly increased from 5.8 ± 0.5 mm measured before surgery to 7.2 ± 0.3 mm at 1 week and 7.5 ± 0.2 mm at 4 years following the surgical treatment (P > 0.05), and the cervical Cobb angle was significantly improved from 11.6° ± 3.7°, measured before surgery, to 22.4° ± 3.4° at 24 months following the implant without significant difference among the subsequent evaluations (P > 0.05).
Although ACDF has been shown to increase fusion rates, maintain or improve cervical sagittal alignment and primary and secondary stability, ACDF with plate and screws has been associated with complications including screw or plate dislodgment, soft-tissue injury, dysphagia, and adjacent segment disease.[17] We have shown that the use of cervical cage with an integrated fixation system, such as ROI-C, can reduce the rate of these complications, including the rate of pseudarthrosis. Pseudarthrosis following ACDF has been has been associated with poor clinical outcomes.[18] In a study analyzing 2682 patients, fusion rates have been found in 92.1%, 79.9%, and 65% for one-level, two-level, and three-level ACDF, respectively. In our series, the fusion rate at 4 years was 97%.[19] Previous studies investigating the same device, but with a shorter follow-up, reported similarly high rates of bony fusion ranging between 95.2% and 100%.[7,20,21] In 127 operated levels, among the patients examined 4 years after ACDF, there were three defined as doubtful fusion. Considering their good clinical outcome and the stability of the system as evaluated by dynamic radiographs, no further surgeries were undertaken.
One of the most reported complications following ACDF is chronic dysphagia that can reach 21% in some studies.[2,3,6,22,23] For the early postoperative period, the rate of dysphagia in our study was lower than that reported in the current literature.[8] The exact pathophysiological mechanisms underlying postoperative dysphagia remain unknown. It has been postulated that postoperative soft-tissue edema, esophageal injury, postoperative hematoma, and adhesive formations around implanted cervical plates might be possible explanations for dysphagia.[24] Some authors have advocated a strict correlation between the prosthesis thickness and dysphagia rate with decreased dysphagia incidence when thinner cages are used.[25] Cage with very low profile avoids an implant contact to the soft tissue in front of the cervical spine. This might avoid any mechanical irritation of the esophagus and may explain the low dysphagia rate in our patients. Only two patient (2%) experienced dysphagia, which resolved 1 and 3 months, after the surgery. A dysphagia rate of 2% is low compared to plate-augmented ACDF in the literature.[12] The results obtained are by the results of a recent meta-analysis showing that zero-profile anchored cages had a lower risk of postoperative dysphagia than cages with anterior plate fixation after ACDF.[6] Furthermore, although subsidence is one of the major concerns when using cages without plate fixation, the present study reported no case of subsidence in the follow-up. The same results have been reported in previous studies employing the same device.[7,12]
Overall, the present study confirms our previous findings [7] and expands the results on safety and effectiveness of cervical ROI-C system device in patients with cervical radiculopathy and myelopathy, also in a long follow-up.
CONCLUSIONS
Cervical arthrodesis by using a cervical interbody cage with integrated fixation showed that this system is safe and effective for the treatment of cervical degenerative disc disease. It incorporates a different philosophy of insertion besides the guarantee of fusion and low rate of postoperative complications. Large randomized controlled trials are warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Anderson DG, Albert TJ. Bone grafting, implants, and plating options for anterior cervical fusions. Orthop Clin North Am. 2002;33:317–28. doi: 10.1016/s0030-5898(01)00011-6. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Wang H, Li X, Chen J, Sun H, Wang G, et al. Comparison of a zero-profile anchored spacer (ROI-C) and the polyetheretherketone (PEEK) cages with an anterior plate in anterior cervical discectomy and fusion for multilevel cervical spondylotic myelopathy. Eur Spine J. 2016;25:1881–90. doi: 10.1007/s00586-016-4500-x. [DOI] [PubMed] [Google Scholar]
- 3.Dong J, Lu M, Lu T, Liang B, Xu J, Zhou J, et al. Meta-analysis comparing zero-profile spacer and anterior plate in anterior cervical fusion. PLoS One. 2015;10:e0130223. doi: 10.1371/journal.pone.0130223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Chen D, Wang X, Yang L, He H, Yuan W, et al. Zero-profile integrated plate and spacer device reduces rate of adjacent-level ossification development and dysphagia compared to ACDF with plating and cage system. Arch Orthop Trauma Surg. 2015;135:781–7. doi: 10.1007/s00402-015-2212-z. [DOI] [PubMed] [Google Scholar]
- 5.Tong MJ, Xiang GH, He ZL, Chen DH, Tang Q, Xu HZ, et al. Zero-profile spacer versus cage-plate construct in anterior cervical diskectomy and fusion for multilevel cervical spondylotic myelopathy: Systematic review and meta-analysis. World Neurosurg. 2017;104:545–53. doi: 10.1016/j.wneu.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 6.Xiao S, Liang Z, Wei W, Ning J. Zero-profile anchored cage reduces risk of postoperative dysphagia compared with cage with plate fixation after anterior cervical discectomy and fusion. Eur Spine J. 2017;26:975–84. doi: 10.1007/s00586-016-4914-5. [DOI] [PubMed] [Google Scholar]
- 7.Grasso G, Giambartino F, Tomasello G, Iacopino G. Anterior cervical discectomy and fusion with ROI-C peek cage: Cervical alignment and patient outcomes. Eur Spine J. 2014;23(Suppl 6):650–7. doi: 10.1007/s00586-014-3553-y. [DOI] [PubMed] [Google Scholar]
- 8.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: A prospective study. Spine (Phila Pa 1976) 2002;27:2453–8. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 9.Zdeblick TA, Ducker TB. The use of freeze-dried allograft bone for anterior cervical fusions. Spine (Phila Pa 1976) 1991;16:726–9. doi: 10.1097/00007632-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki M, Hong SW, Yoon SH, Morishita Y, Wang JC. Reliability of a magnetic resonance imaging-based grading system for cervical intervertebral disc degeneration. J Spinal Disord Tech. 2008;21:288–92. doi: 10.1097/BSD.0b013e31813c0e59. [DOI] [PubMed] [Google Scholar]
- 11.Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976) 1995;20:1410–8. [PubMed] [Google Scholar]
- 12.Bucci MN, Oh D, Cowan RS, Davis RJ, Jackson RJ, Tyndall DS, et al. The ROI-C zero-profile anchored spacer for anterior cervical discectomy and fusion: Biomechanical profile and clinical outcomes. Med Devices (Auckl) 2017;10:61–9. doi: 10.2147/MDER.S127133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song KJ, Taghavi CE, Lee KB, Song JH, Eun JP. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine (Phila Pa 1976) 2009;34:2886–92. doi: 10.1097/BRS.0b013e3181b64f2c. [DOI] [PubMed] [Google Scholar]
- 14.Grasso G. Clinical and radiological features of hybrid surgery in multilevel cervical degenerative disc disease. Eur Spine J. 2015;24(Suppl 7):842–8. doi: 10.1007/s00586-015-4281-7. [DOI] [PubMed] [Google Scholar]
- 15.Niu CC, Chen LH, Lai PL, Fu TS, Chen WJ. Trapezoidal titanium cage in anterior cervical interbody fusion: A clinical experience. Chang Gung Med J. 2005;28:212–21. [PubMed] [Google Scholar]
- 16.Cho DY, Lee WY, Sheu PC, Chen CC. Cage containing a biphasic calcium phosphate ceramic (Triosite) for the treatment of cervical spondylosis. Surg Neurol. 2005;63:497–503. doi: 10.1016/j.surneu.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Song KJ, Choi BW, Jeon TS, Lee KB, Chang H. Adjacent segment degenerative disease: Is it due to disease progression or a fusion-associated phenomenon. Comparison between segments adjacent to the fused and non-fused segments? Eur Spine J. 2011;20:1940–5. doi: 10.1007/s00586-011-1864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser MG, Haid RW, Jr, Subach BR, Barnes B, Rodts GE., Jr Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229–36. doi: 10.1097/00006123-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: A metaanalysis of fusion rates. J Neurosurg Spine. 2007;6:298–303. doi: 10.3171/spi.2007.6.4.2. [DOI] [PubMed] [Google Scholar]
- 20.Hofstetter CP, Kesavabhotla K, Boockvar JA. Zero-profile anchored spacer reduces rate of dysphagia compared with ACDF with anterior plating. J Spinal Disord Tech. 2015;28:E284–90. doi: 10.1097/BSD.0b013e31828873ed. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Jiang W, Li X, Wang H, Shi J, Chen J, et al. The application of zero-profile anchored spacer in anterior cervical discectomy and fusion. Eur Spine J. 2015;24:148–54. doi: 10.1007/s00586-014-3628-9. [DOI] [PubMed] [Google Scholar]
- 22.Shao H, Chen J, Ru B, Yan F, Zhang J, Xu S, et al. Zero-profile implant versus conventional cage-plate implant in anterior cervical discectomy and fusion for the treatment of degenerative cervical spondylosis: A meta-analysis. J Orthop Surg Res. 2015;10:148. doi: 10.1186/s13018-015-0290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Zhao Y, Tang J, Ren D, Guo J, Wang H, et al. Acomparison of a new zero-profile, stand-alone fidji cervical cage and anterior cervical plate for single and multilevel ACDF: A minimum 2-year follow-up study. Eur Spine J. 2017;26:1129–39. doi: 10.1007/s00586-016-4739-2. [DOI] [PubMed] [Google Scholar]
- 24.Fountas KN. Anterior cervical fusion using dense cancellous allografts and dynamic plating. Neurosurgery. 2005;56:E1166. [PubMed] [Google Scholar]
- 25.Lee MJ, Bazaz R, Furey CG, Yoo J. Influence of anterior cervical plate design on dysphagia: A 2-year prospective longitudinal follow-up study. J Spinal Disord Tech. 2005;18:406–9. doi: 10.1097/01.bsd.0000177211.44960.71. [DOI] [PubMed] [Google Scholar]