Abstract
Study Design:
This study was a retrospective observational study.
Purpose:
The purpose of the study was to determine the radiological and clinical outcome of using locally sourced autologous bone graft in the surgical management of single-level lumbar lytic spondylolisthesis.
Background:
Many spinal surgeons supplement pedicle screw fixation of lumbar spondylolisthesis with cages. In developing countries, the high cost of interbody cages has precluded their use, with surgeons resorting to filling the interbody space with different types of bone graft instead. This study reports on the clinical and radiological outcome of posterior lumbar interbody fusions for low-grade lytic spondylolisthesis using locally sourced autologous bone graft.
Material and Methods:
Posterior interbody fusion was performed in 22 consecutive patients over 18-month period, using (BRAND) pedicle screw system and locally sourced bone graft, i.e., bone removed during neural decompression. There were no postoperative restrictions, and all patients underwent clinical outcome measurements using Oswestry Disability Index (ODI), visual analogue pain score (VAS) at a minimum follow-up of 12 months, and computed tomography (CT) assessment of fusion with intraobserver validation by radiology consultant blinded, at 6 and12 months. Nearly 50% of the population were smokers.
Results:
There was significant clinical improvement in ODI, VAS back pain, and VAS leg pain (P < 0.001). By contrast, the radiologic fusion rate measured by CT at 12 months was less satisfactory at 64%. There was no difference in clinical outcome between the fused group and nonfused population.
Conclusions:
These results indicate that the use of locally sourced bone graft in single-level lumbar lytic low-grade spondylolisthesis. Interbody fusion provides good clinical outcomes. The use of an interbody cage may not be clinically necessary. Our radiologic outcome, however, shows inferior fusion rates compared with published data. Future research will focus on long-term outcomes
Keywords: Back pain, interbody cage, lumbar fusion, lumbar spine, lytic spondylolisthesis
INTRODUCTION
Spondylolisthesis is the forward subluxation of one vertebra on another. Surgical fusion is an important method of stabilizing the spine in lumbar spondylolisthesis, which is used to reduce pain and decrease disability in patients with chronic low back pain.[1] There are several types of lumbar fusion have been described for surgical management of spondylolisthesis and among including posterior lumbar fusion (PLF), posterior lumbar interbody fusion (PLIF), anterior lumbar interbody fusion, circumferential 360 fusion (front and back), and more recently, the transforaminal lumbar interbody fusion (TLIF).[2] PLIF was introduced in 1940 by Cloward [3] where he used tricortical bone graft from iliac bone. Brantigan and Steffee developed a carbon-fiber-reinforced implant,[4] which was the first interbody cage and achieved excellent outcomes. In the following years, the interbody cages evoluted rapidly with many variant types including titanium cages and the use of cages combined with locally morcellized bone graft rather than tricortical iliac bone graft became the standard of current practice.[5] In developing countries, because of the cost, they practice a different technique with using locally morcellized autograft from the posterior elements removed during decompression in spondylolisthesis in instrumented interbody fusion without cages. This study aims to evaluate the radiological and clinical outcomes of this technique.
MATERIALS AND METHODS
Patient population
A total of 22 PLIFs using this technique were undertaken by one spinal consultant at one spine center in the Middle East between May 2014 and November 2015. The demographics are summarized in Table 1. The patients fulfilled the following criteria: persistent low back pain of more than 6 months and/or sciatica after routine conservative treatment (analgesia and physiotherapy), radiological evidence of low-grade lytic spondylolisthesis (Meyerding Grade 1 and 2) and the presence of spinal canal stenosis, and complete medical records. Patients who underwent more than one level of interbody fusion were excluded, also patients with severe comorbidities, for example, marked obesity, hepatitis C virus, HIV, and revision surgery were excluded from the study. Data were collected retrospectively, using the preoperative hospital admission sheets, operative notes, postoperative follow-up, and outpatient clinic documentations. Consent was obtained from patients and consultants to use their data in this study.
Table 1.
Study population demographics (n=22)
Surgical procedure
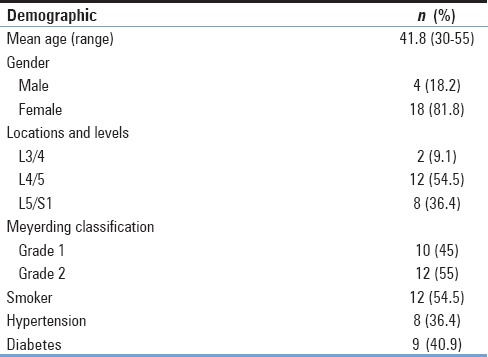
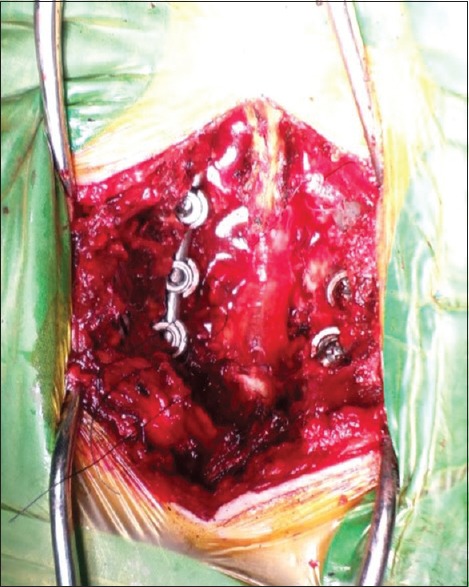
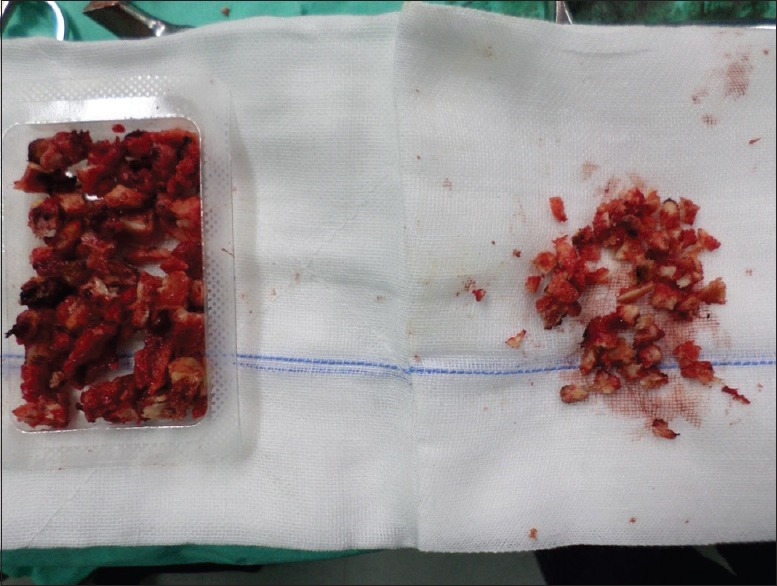
All patients were given antibiotics (cefazolin, 2.0 g) intravenously 1 h preoperatively. After exposing the spinous process and both laminae, the surrounding soft tissues such as ligamentum flavum and interspinous ligaments were removed. Instrumented fixation with interpedicular screws and rods was performed with support from an image intensifier. Piece meal resection of the spinous process and lytic laminae was performed using Rongeur and Kerrisons. The dura and nerve roots were protected using a nerve-root retractor [Figure 1]. All disc material and the articular cartilage from the superior and inferior apophyseal joints were removed. Locally morcellized bone graft was cut into small pieces [Figure 2]. Using a punch and rods, graft was inserted and impacted into the intervertebral disc space [Figure 3]. All patients were mobilized on the 2nd day after the surgery with a soft flexible lumbar support offered if back pain persisted beyond 3 weeks.
Figure 1.
Exposure of disc space with the protection of the dura and nerve root
Figure 2.
Preparation of bone graft
Figure 3.
Bone graft Impaction
Outcome measures
Functional outcome was assessed using the Oswestry Disability Index (ODI) and visual pain analog score (VAS).
Radiological assessment was performed blindly, independently by consultant neuroradiologist from a different center in a different country with intraobserver evaluation. The assessment included (1) fusion rate was determined using Brantigan and Steffe criteria for interbody fusion [4] [Table 2], (2) disc height calculated as the ratio between the disc height and the height of the superior vertebral body.
Table 2.
Description of fusion by Brantigan and Steffee
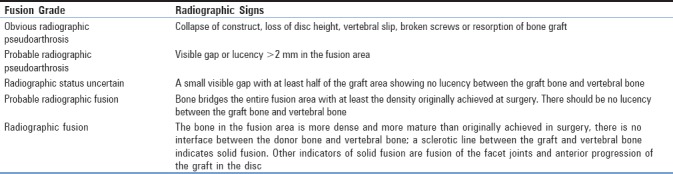
Radiographs were performed immediately postoperatively and at 6-month intervals after surgery. Computed tomography (CT) was performed routinely at 6“12 months intervals as routine current practice for the local operating team.
Statistical analysis
Paired samples t-tests were conducted to investigate whether there was a difference in patient-reported pain pre- and 12-month postsurgery, as identified using the ODI, VAS Back, and VAS leg assessment tools. An independent sample t-test was undertaken to identify whether there was a difference in mean score difference (pre- and 12-month postsurgery) between those who were clinically identified as having their surgery successfully fused and those who had not fused. Linear regression analyses were used to analyze the effect of patient factors on the difference between pre- and 12 months post-ODI, VAS back, and VAS leg scores. Covariates included in the models were age, gender, body mass index (≤/>30 kg/m2), diabetes status, hypertension, smoking status, level, fusion status, disc height (mm), occurrence of dural tear, occurrence of infection, and presurgery score.
RESULTS
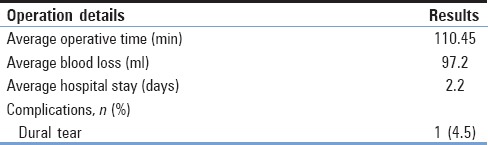
The data collected from 22 cases of surgically managed spondylolisthesis were analyzed [Table 3]. The average operative time was <2 h. The average blood loss was <100 ml. The average hospital stay was <3 days. Complications were dural tear in one patient 4.5%. No deep or superficial infection, nor graft subsidence, nor metal failure occurred. None of the patients needed revision surgery [Table 3].
Table 3.
Intraoperative and postoperative clinical data
Clinical outcome measures
All patients had improvement in back and leg pain symptoms by the final follow-up outpatient appointment.
ODI score improved significantly from mean (standard deviation [SD]) 61.1 (6.2) preoperatively to 14.2 (11.3) at 12 months postoperatively P < 0.001 [Figure 4].
Figure 4.
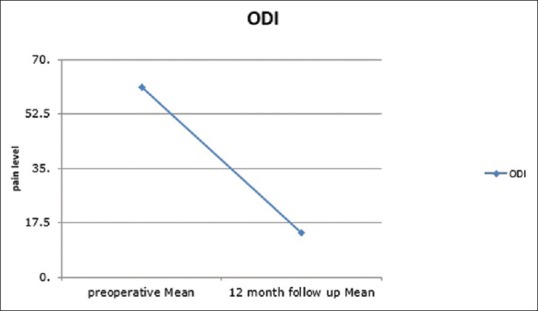
Oswestry Disability Index changes pre- and 12-month postoperative
Back pain VAS decreased from mean (SD) 6.4 (0.96) preoperatively to 1.5 (1.3) at 12 months postoperatively P < 0.001 [Figure 5].
Figure 5.
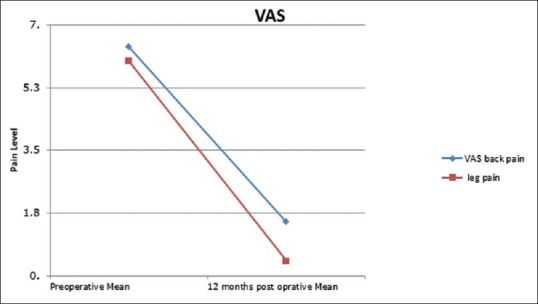
Visual analog pain score for back and leg changes pre- and 12 months postoperative
Leg pain VAS decreased from mean (SD) 6.0 (1.2) preoperatively to 0.41 (0.73) at 12 months postoperatively P < 0.001 [Figure 5].
Radiological outcomes
Fusion rate was 64% as assessed by CT (level 5 by Brantigan Steffe classification). Almost 18% did not fuse on CT (level 1), and 18% possibly fused on CT (level 3) by 12 months [Figure 6]. There was no significant difference in pain improvement between those whose surgery had resulted in CT-verified fusion and those whose surgery had not resulted in CT-verified fusion [Table 4]. For both ODI and VAS back, no significant covariates were identified, indicating that the level of pain improvement was due to the surgery and not significantly affected by any of the identified variables. For the difference in VAS leg pain score, the linear regression model showed that the difference in score was affected by the patient's gender (P = 0.042), diabetes status (P = 0.024), and disc height (P = 0.039) [Table 4]. This gave a regression equation of:
Figure 6.
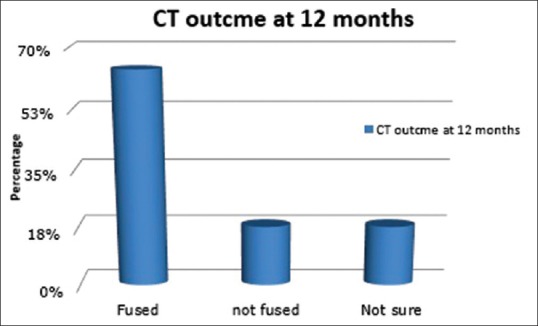
Computed tomography outcome at 12 months postoperative
Table 4.
Parameter estimates for linear regression model: Difference in pre- and 12 months postsurgery visual analog score leg score
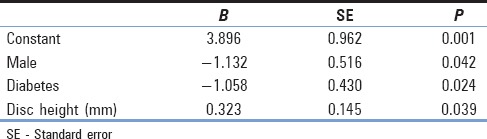
(y [VAS leg difference] = 3.896 − 1.132 male − 1.058 diabetes + 0.323 disc height), indicating that a female nondiabetic with a disc height of 8 mm would be expected to have an improvement in VAS leg score of 3.896 + (0.323 × 8) = 6.48, while a male diabetic with the same disc height would be expected to have an improvement of 3.896 − 1.132 − 1.058 + (0.323 × 8) = 4.29 [Table 4].
Case presentation 1
Male patient, aged 35 works as hard lifting worker, had low-grade lytic L4/5 spondylolisthesis operated on January 22, 2015. Successful fusion 100% in 12 months [Figure 7] with ODI improved from 52 preoperatively to 10 in 6-month follow-up and VAS back pain from 8 to 2 to 0 preoperatively, 6 months, 1 year respectively [Figure 7].
Figure 7.
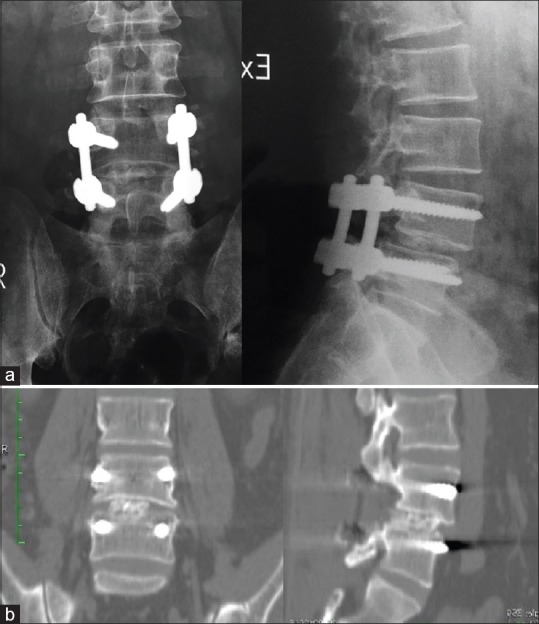
Case presentation: (a) immediate postoperative X-ray. (b) Computed tomography at 1 year follow up showing grade five fusion
Case presentation 2
Female patient aged 45, homemaker with Grade 2 lytic L4/5 spondylolisthesis, obese, and smoker. Had Operation Done in December 1, 2015 and had successful fusion in 6 months. [Figure 8]. ODI improved markedly from 60 preoperatively to 6, 2 in 6 and 12 months follow-ups respectively. VAS back pain also improved from 6 preoperatively to 0 at 6 months follow up.
Figure 8.
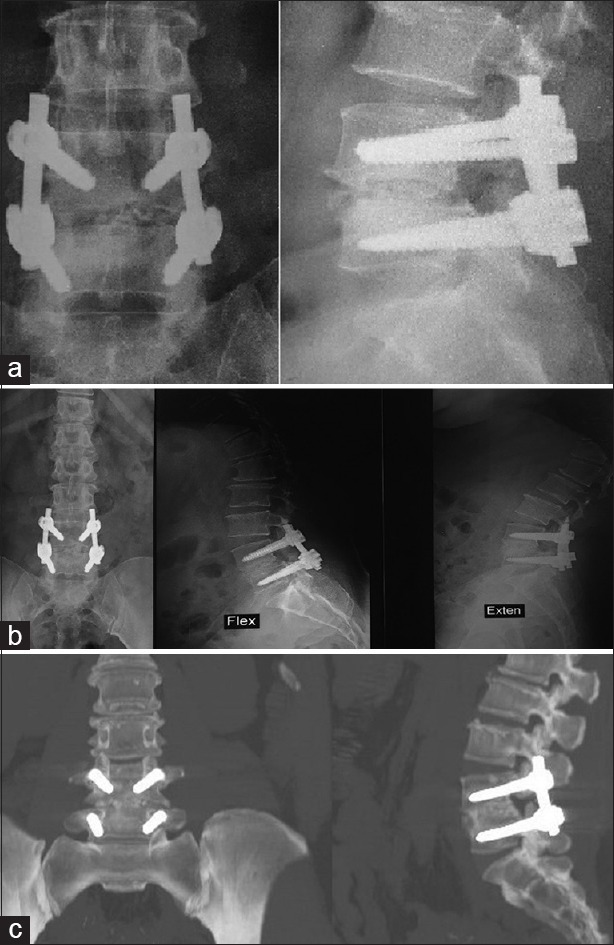
Case Presentation 2: (a) Immediate postoperative X-ray. (b) one year follow up X-ray showing full fusion. (c) One year Computed tomography showing grade 5 fusion
DISCUSSION
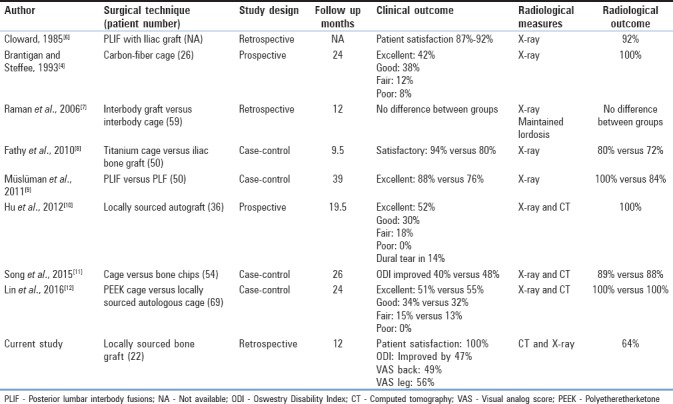
PLIF was pioneered by Cloward in 1940, using tricortical iliac graft.[3] In 1985, Cloward claimed excellent clinical and radiological outcomes in his 40-year experience.[6] In 1993, Brantigan developed the first interbody synthetic cage which was carbon-fiber-reinforced implant and achieved better fusion rate in 26 patients at 2 years postoperatively.[4] Table 5 summarizes the clinical outcomes and fusion rates of different fusion techniques employed in lumbar lytic spondylolisthesis as well as lumbar degenerative disease. Raman et al., in 2006, reported no difference in the outcome in using an interbody cage to treat single-level degenerative spondylolisthesis compared with interbody fusion without a cage.[7] In 2010, Fathy et al. compared titanium cage versus iliac bone graft in interbody fusion. Results were in favor of titanium cages.[8] In 2011, Müslüman et al. compared the results of PLIF and PLF in patients with Grade 1 and 2 isthmic spondylolisthesis. The clinical and radiological outcomes were better in the PLIF group.[9] In 2012, Hu et al., highlighted a new technique in China where they cut the posterior elements with an osteotome and reshape it as a locally sourced autograft cage, thus avoiding the hazards of iliac bone graft harvesting and the cost of a synthetic cage. The clinical outcome was excellent in 52%, fusion rate was 100% at 18 months, yet five patients (14%) out of 36 had dural tear with this technique.[10] In 2015, Song et al. compared PLIF with locally sourced bone chips to PLIF with a cage. Clinical and radiological outcomes showed no statistically significant difference.[11] In 2016, Lin et al. compared the polyetheretherketone cage versus autologous cage using the lumbar spinous process and laminae in lumbar interbody fusion. Again, results showed no significant difference between the groups clinically or radiologically.[12]
Table 5.
Literature review for clinical and radiological outcomes of different fusion techniques
In our study, the technique is a simplified technique as it does not contain any more steps for the posterior decompression fixation procedure in lytic spondylolisthesis. Our clinical outcomes compared favorably with historical case series. Our CT-established fusion rate was 64%, which is a more sensitive method of assessing fusion compared with plain X-ray. Our complication rate was low. We did not encounter any screw cavitation postoperatively due to any subsidence within the disc space which contained the morcellized bone graft. We believe that the main advantages of this technique are low morbidity compared with iliac grafting and low cost compared with synthetic cage use in the interbody space.
CONCLUSIONS
On the basis of these results, we suggest that the use of an interbody cage may not be clinically necessary. Our radiologic outcome, however, shows inferior fusion rates compared with published data and future research will focus on long-term outcomes.
Limitation to this study
This is a retrospective study with no control group to compare the results. Our reported sample size is also relatively small with short-term follow up and as such, caution should be exercised when interpreting the results of the regression analysis, since the incorporation of multiple variables from a small number of patients may hide significant relationships between variables and pain improvement, inadequate X-ray material to comment on lordosis globally and segmentally.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ha KY, Na KH, Shin JH, Kim KW. Comparison of posterolateral fusion with and without additional posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Spinal Disord Tech. 2008;21:229–34. doi: 10.1097/BSD.0b013e3180eaa202. [DOI] [PubMed] [Google Scholar]
- 2.Lee CK, Kopacz KJ. In: Rothman “ Simeone the Spine. Herkowitz HN, Garfin SR, Eismont FJ, Bell GR, Balderston RA, editors. Philadelphia: Elsevier; 2006. pp. 350–60. [Google Scholar]
- 3.Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154–68. doi: 10.3171/jns.1953.10.2.0154. [DOI] [PubMed] [Google Scholar]
- 4.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine (Phila Pa 1976) 1993;18:2106–7. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 5.Okuyama K, Kido T, Unoki E, Chiba M. PLIF with a titanium cage and excised facet joint bone for degenerative spondylolisthesis “ In augmentation with a pedicle screw. J Spinal Disord Tech. 2007;20:53–9. doi: 10.1097/01.bsd.0000211243.44706.2b. [DOI] [PubMed] [Google Scholar]
- 6.Cloward RB. Posterior lumbar interbody fusion updated. Clin Orthop Relat Res. 1985;193:16–9. [PubMed] [Google Scholar]
- 7.Raman AS, Crawford R, Kakkar R, Rai AS, Crawford RJ. Posterior lumbar interbody fusion for degenerative spondylolisthesis: A comparison of results: Using non-structural autograft alone versus interbody cage with autograft. J Bone Joint Surg. 2006;90(B Suppl III):456–57. [Google Scholar]
- 8.Fathy M, Fahmy M, Fakhri M, Aref K, Abdin K, Zidan I, et al. Outcome of instrumented lumbar fusion for low grade spondylolisthesis; evaluation of interbody fusion with & without cages. Asian J Neurosurg. 2010;5:41–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Müslüman AM, Yılmaz A, Cansever T, Cavuşoğlu H, Colak I, Genç HA, et al. Posterior lumbar interbody fusion versus posterolateral fusion with instrumentation in the treatment of low-grade isthmic spondylolisthesis: Midterm clinical outcomes. J Neurosurg Spine. 2011;14:488–96. doi: 10.3171/2010.11.SPINE10281. [DOI] [PubMed] [Google Scholar]
- 10.Hu MW, Liu ZL, Zhou Y, Shu Y, Chen CL, Yuan X, et al. Posterior lumbar interbody fusion using spinous process and laminae. J Bone Joint Surg Br. 2012;94:373–7. doi: 10.1302/0301-620X.94B3.27629. [DOI] [PubMed] [Google Scholar]
- 11.Song D, Chen Z, Song D, Li Z. Comparison of posterior lumbar interbody fusion (PLIF) with autogenous bone chips and PLIF with cage for treatment of double-level isthmic spondylolisthesis. Clin Neurol Neurosurg. 2015;138:111–6. doi: 10.1016/j.clineuro.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Lin B, Yu H, Chen Z, Huang Z, Zhang W. Comparison of the PEEK cage and an autologous cage made from the lumbar spinous process and laminae in posterior lumbar interbody fusion. BMC Musculoskelet Disord. 2016;17:374. doi: 10.1186/s12891-016-1237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


