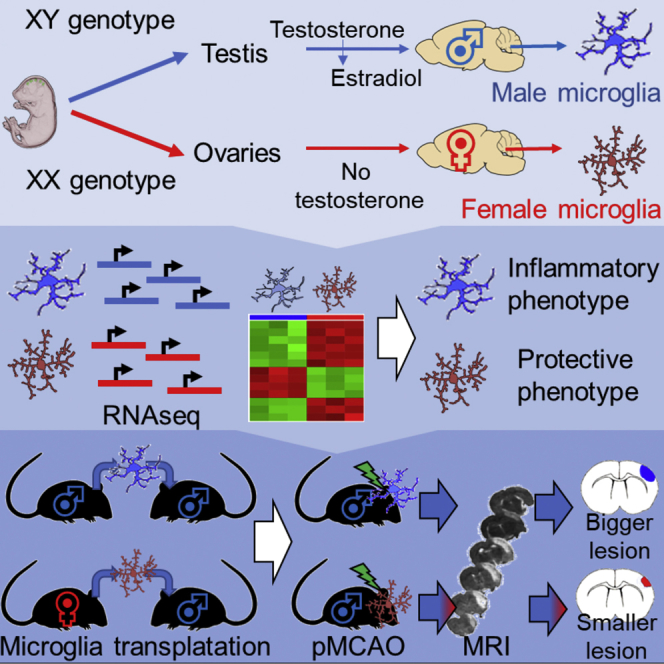
Summary
Sex has a role in the incidence and outcome of neurological illnesses, also influencing the response to treatments. Neuroinflammation is involved in the onset and progression of several neurological diseases, and the fact that estrogens have anti-inflammatory activity suggests that these hormones may be a determinant in the sex-dependent manifestation of brain pathologies. We describe significant differences in the transcriptome of adult male and female microglia, possibly originating from perinatal exposure to sex steroids. Microglia isolated from adult brains maintain the sex-specific features when put in culture or transplanted in the brain of the opposite sex. Female microglia are neuroprotective because they restrict the damage caused by acute focal cerebral ischemia. This study therefore provides insight into a distinct perspective on the mechanisms underscoring a sexual bias in the susceptibility to brain diseases.
Keywords: microglia, neuroinflammation, sexual differentiation, cell transfer, ischemic stroke, estrogens
Graphical Abstract
Highlights
-
•
Transcriptome sequencing indicates sexual differentiation in adult murine microglia
-
•
Female microglia show a neuroprotective phenotype, independent from hormonal cues
-
•
Female microglia phenotype is retained after transfer into male brains
-
•
The presence of female microglia protects male brains from ischemic stroke
Villa et al. find significant differences in the transcriptomes of microglia isolated from the brains of healthy adult male and female mice. They find that microglia from female mice are neuroprotective and that they retain this functional ability when transferred into the brains of male mice.
Introduction
Investigation into the role of microglia, the myeloid cells that reside in the CNS, has begun to receive intense interest, unravelling the complexity of the effects that these cells have on neural functions and establishing their major role in the development and life-long maintenance of brain homeostasis (Kierdorf and Prinz, 2017, Prinz and Priller, 2014, Salter and Stevens, 2017).
During development, microglia have been shown to influence neurodevelopmental processes such as axon guidance, neurite growth, and synaptic pruning (Kettenmann et al., 2013, Schafer et al., 2013, Wu et al., 2015), and to follow a precise and coordinated transcriptional program (Matcovitch-Natan et al., 2016). In addition, it has been demonstrated that microglia participate in the process of brain masculinization induced by the neonatal surge of male gonadal activity (Lenz et al., 2013); this process organizes brain architecture by structuring neuronal circuits to be activated by sexual functions after puberty (Arnold and Gorski, 1984, MacLusky and Naftolin, 1981). In adults, microglia are the first line of protection against noxious stimuli such as stress and pathogenic insults, and they maintain healthy brain function by pruning synapses (Kettenmann et al., 2013, Wu et al., 2015), clearing debris (Neumann et al., 2009), and synthesizing growth and repair factors (Hu et al., 2015). In spite of this, prolonged microglia stimulation may lead to neuronal damage. Indeed, several recent studies have highlighted the involvement of microglia and neuroinflammation in the manifestation of major neurological and neuropsychiatric diseases.
The finding that these cells are responsive to estrogens and that their immune functions and inflammatory response are significantly mitigated by estrogens (Vegeto et al., 2001) may point to a role of this hormone in determining the sex-specific prevalence, onset, and course of numerous brain diseases (Vegeto et al., 2002, Villa et al., 2016). Furthermore, increasing evidence suggests that neurological disorders have their roots in diversions from a normal developmental trajectory, and disturbances and a loss of microglial function have been associated with the onset of brain diseases (Prinz and Priller, 2014). Considering the role of microglia in brain sexual differentiation, it could be hypothesized that the effects of estrogens during brain differentiation may induce permanent effects in microglia, enabling these cells to contribute to sex-related manifestation of brain diseases (Villa et al., 2016).
The aim of the present study was to identify the differences in the transcriptome of male and female microglia isolated from adult healthy mice and to verify the extent to which microglia sex differences might affect the progress of pathologies where these immune cells play a major role.
Results
RNA-Sequencing Highlights Sex Differences in Adult Microglia
To study the expression profile of male and female microglia, we took advantage of a recently developed methodology to isolate these cells at high level of purity from adult brain (Hanamsagar et al., 2017, Pepe et al., 2014) (Figure S1A–S1D). To avoid the variability related to rearing, environment, or diets affecting the metabolism and the microbiome, both well-known regulators of the brain immune system (Hooper et al., 2012), care was taken to match the mice in terms of age and to take the same number of males and females from each of the litters utilized in the study. To limit any circadian influence and facilitate the analysis of the phase of the cycle, in all experiments, animals were euthanized at the same hours (between 2:00 and 4:00 p.m.); females were at metestrus, a phase with low circulating estrogens.
The transcriptome was investigated by RNA sequencing (RNA-seq; RNA-seq data are available at the NCBI Sequence Read Archive: SRP104620). The RNAs were prepared from pools of 6 brains each: 2 pools were from males and 2 pools from females; thus, we utilized a total of 12 mice/sex. Mice were 12 weeks old. The transcriptional data were initially analyzed by AltAnalyze (Olsson et al., 2016) and mapped over the Tissue Fate Map to demonstrate the absence of contaminations from other neural cells. Indeed, the reads per kilobase million (RPKM) relative to biomarkers of neutrophils (Ly6g), B cells (Cd19), T cells (Cd3–Cd8), astrocytes (Aldh1l1), neurons (Thy1), and oligodendrocytes (Olig1) were found to be negligible with respect to the predominant microglia markers.
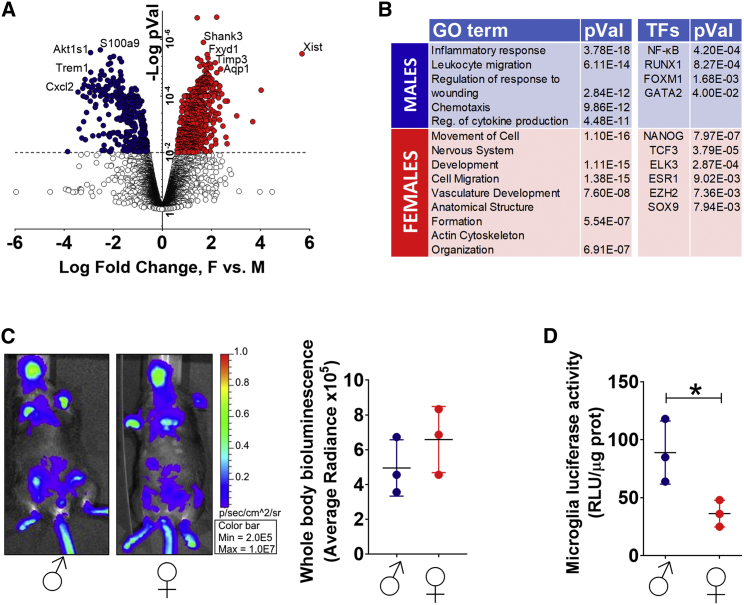
To determine the differently expressed genes (DEGs), we carried out the statistical analysis using the CuffDiff (Trapnell et al., 2012) software. By applying a threshold of 0.01 to the false discovery rate (FDR; Benjamini-Hochberg correction)-adjusted p values (pVal), we identified 546 DEGs (Figure 1A). The reliability of the RNA-seq results was tested on microglia isolated from 12 additional individuals (males, n = 6; females, n = 6); the results obtained with qPCR on a panel of 20 mRNAs randomly selected among those with sex-dependent and sex-independent expression demonstrated the reproducibility of the RNA-seq data (Figure S1E).
Figure 1.
Microglia Transcriptome in the Male and Female Mouse Brain
(A) Volcano plot of RNA-seq data obtained from microglia isolated from male and female adult C57BL/6 mice. Analyses were conducted on two pools of six brains each, a total of 12 males and 12 females. Blue dots represent genes for which RPKM values are significantly higher in males than in females (204 genes; p < 0.01 by Benjamini-Hochberg correction). Red dots represent genes for which RPKM values are significantly higher in females than in males (342 genes; p < 0.01).
(B) Gene Ontology and ChIP enrichment analysis (ChEA) transcription factor (TF) term enrichment analysis of transcripts with higher expression in male (blue) or in female (red) microglia.
(C) In vivo imaging of luciferase expression in male and female NFκB-luc2 mice. Bioluminescence is measured ventrally and whole body, and the pseudocolors represent radiance (p/s/cm2/sr). The image is representative of three independent measures on three mice per group per experiment. In the graph, each line corresponds to average radiance ± SEM.
(D) Luciferase activity measured in extracts of microglia isolated from the whole brains of male (n = 3) and female (n = 3) NFκB-luc2 mice and expressed as relative luciferase units (RLUs) per microgram of proteins. Each sample was measured in triplicates. Lines represent the mean ± SEM of n = 3. ∗p < 0.05 by unpaired, two-tailed t test.
Next, to identify whether the DEGs could be associated with specific functional categories, we carried out a clustering analysis by Enrichr (Kuleshov et al., 2016). The majority of the 204 genes more expressed in males belonged to Gene Ontology classes associated with inflammatory processes, including regulation of cell migration and cytokine production (Figure 1B). Whole-genome molecular signature analysis of transcription factors (TFs) pointed to nuclear factor κB (NF-κB) as the TF most involved in the regulation of the DEG preferentially expressed in males, together with other TFs associated with inflammatory processes (RUNX1) (Kierdorf and Prinz, 2013), migration (FOXM1) (Balli et al., 2012), and negative regulation of neurogenesis (GATA2) (El Wakil et al., 2006). In contrast, no association with inflammation was found in the DEGs distinctive of female microglia that were grouped in ontogenies associated with morphogenesis, development, or cytoskeleton organization under the control of several TFs such as NANOG (Duan et al., 2013) and TCF3 (Miao et al., 2014), linked to the inhibition of inflammatory response and promotion of repair mechanisms (Duan et al., 2013, Miao et al., 2014), aside from the estrogen receptor alpha (ESR1) itself. The lack of a sex-dependent pro-inflammatory profile in macrophages isolated from the peritoneum of the male mice studied above (G.P. and E.V., unpublished data) suggested that this could be a microglia-specific feature.
The Inflammatory Phenotype of Male Microglia
We continued our study in the NFκB-luc2 mouse model where the luciferase gene was under the control of an NF-κB-responsive synthetic promoter. This reporter mouse was specifically designed to study the inflammatory status in living cells and animals (Rizzi et al., 2017). Whole-body, in vivo imaging in unstimulated conditions showed that the bioluminescence was comparable in siblings of the two sexes (n = 3) (Figure 1C); this result suggested a similar, generalized state of NF-κB transcriptional activation in males and females. However, when we measured luciferase activity in microglia purified from adult siblings of both sexes (n = 3), the luciferase activity was 2.4-fold higher in males (Figure 1D). These data supported our previous bioinformatic analysis by demonstrating that in male microglia NF-κB was transcriptionally activated, suggesting that male microglia cells were more poised to inflammatory reactions than female microglia. To substantiate the hypothesis of male microglia more prone to inflammatory activation, we further analyzed the RNA-seq data, focusing on a total of 95 genes that had been experimentally demonstrated to be targets of NF-κB and involved in immune or inflammatory responses (http://www.bu.edu/nf-kb/gene-resources/target-genes/).
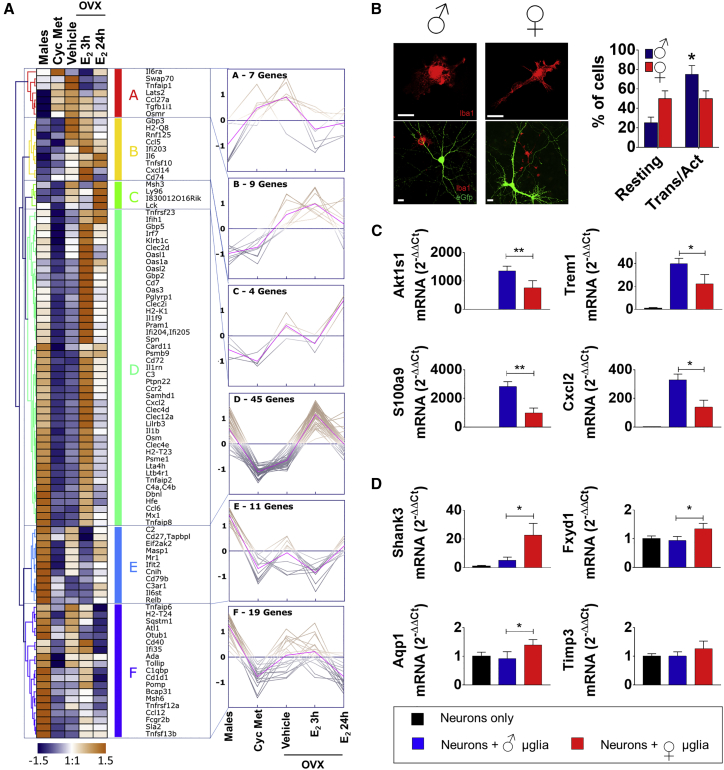
Consistent with previous results, unbiased male-female comparative analysis (Figure 2A) showed that 79% of the 95 inflammatory genes were more expressed in males, and 34 of these genes were differentially expressed with a threshold of 0.05 applied to the FDR (Benjamini-Hochberg correction)-adjusted p values (clusters D, E, and F); of the remaining genes, 14% were expressed similarly in the two sexes (clusters B and C), and 7% were grouped in cluster A, possibly for having a trend to be more expressed in females.
Figure 2.
The Role of Estrogens in Preventing Microglia Inflammatory Phenotype
(A) Heatmap and hierarchical clustering of expression profiles for a subset of NF-κB-regulated genes measured in microglia, isolated from adult C57BL/6 mice: males, intact females (Cyc Met), and ovariectomized females treated with vehicle (OVX) or 17β-estradiol for 3 hr (OVX + E2 3 hr) and 24 hr (OVX + E2 24 hr). The group size was n = 2 samples per condition, each sample consisting of a pool of six brains. The results were log-transformed, normalized, and centered, and populations and genes were clustered by Pearson correlation. Data were obtained from two pools of six brains each. The right panel shows general expression plots for each cluster. The purple line represents the mean expression of the clustered genes.
(B) Immunocytochemistry of microglia stained with Iba1 antibody (red signal) showing different phenotypes in males or females. Upper panel: details of microglia (×100 magnification); lower panel: lower magnification images (×20) including neurons expressing EGFP (green). In the graph, each column corresponds to the percentage of cells showing either the resting or the activated phenotype. We blind-counted the cells present in 18 ×20 images (for about 200 cells/sex in total). The blind count was repeated by three different operators. ∗p < 0.05 by unpaired, two-tailed t test. Scale bar: 10 μm.
(C and D) qPCR analyses of genes showing higher expression in male (C) or female (D) microglia. Data are expressed as 2−ΔΔCt using the 36B4 transcript as an internal reference standard. Each column represents the mean ± SEM of n = 6 plates measured in triplicate. ∗p < 0.05; ∗∗p < 0.01 by a one-way ANOVA and Tukey’s method for multiple comparisons versus expression in neurons + male microglia (blue column).
Is Circulating 17β-Estradiol Responsible for Microglial Sex Differences?
To investigate the extent to which estrogens contributed to the sex differences observed in the NF-κB-regulated genes, we extended the analysis to the RNA-seq data on microglia isolated from mice ovariectomized (OVX) with or without estrogen replacement for 3 or 24 hr; such data were generated using groups of animals reared and treated in parallel with those described above. After ovariectomy, we observed a tendency toward a reduction in differences between the two sexes, with a generalized increase in the expression of the 95 NF-κB-driven genes (Figure 2A). However, Pearson analysis did not show significant changes when we did correlation analyses between the NF-κB genes expressed in OVX females/males (R = 0.9327) and cycling females/males (R = 0.8589), leading us to conclude that the gene expression of microglia was not significantly influenced by the lack of circulating sex steroids. Moreover, in the OVX mice, 17 of the original 34 DEGs were still found to be less expressed (with a threshold of 0.05 applied to the adjusted p values) than in males despite the lack of circulating estrogens; this finding pointed to the contribution of factors other than estrogens to the low expression of NF-κB-regulated genes. Hormone replacement induced a trend in the expression of these genes; hierarchical clustering (Pearson correlation, average linkage) identified three clusters of genes for which estrogen administration induced a rapid, transient increase (78%, clusters B, D, and F; Figure 2A), and a smaller group of genes (22%, clusters A, C, and E) showed the opposite trend, with a decreased expression after 3 hr of 17β-estradiol (E2) treatment.
These findings suggested that the transcriptional differences of the NF-κB-regulated genes did not depend on estrogens in a predictable way, and that additional factors played a role in differentiating the male and female microglia transcriptomes.
To evaluate whether the sex differences observed were maintained in vitro, we prepared primary cultures of microglia isolated from adult male and female mice. To enable microglia to survive and to preserve their responsiveness to inflammatory stimuli, the adult microglia cells were co-cultured with neurons dissected from hippocampi of rat embryos differentiated to mature neurons with >98% homogeneity (Gardoni et al., 2002). Bias of the co-culture was avoided by using a mix of male and female neurons.
Morphological analysis of microglia in culture (Benedusi et al., 2017) showed significant sex differences: 75% of male microglia had the globular morphology associated with the pro-inflammatory phenotype, and the remaining had the ramified branches connected with the surveilling status; in females, the percentage of activated microglia cells was much lower (50%) (Figure 2B). Consistent with this observation, qPCR on a subset of DEGs showed that the microglia grown in culture preserved, by and large, the expression pattern of the sex of origin (Figures 2C and 2D). Thus, microglia in culture appeared to maintain a gene expression characteristic of the sex of origin.
Female Microglia Retain Their Sex after Transplantation in the Male Brain
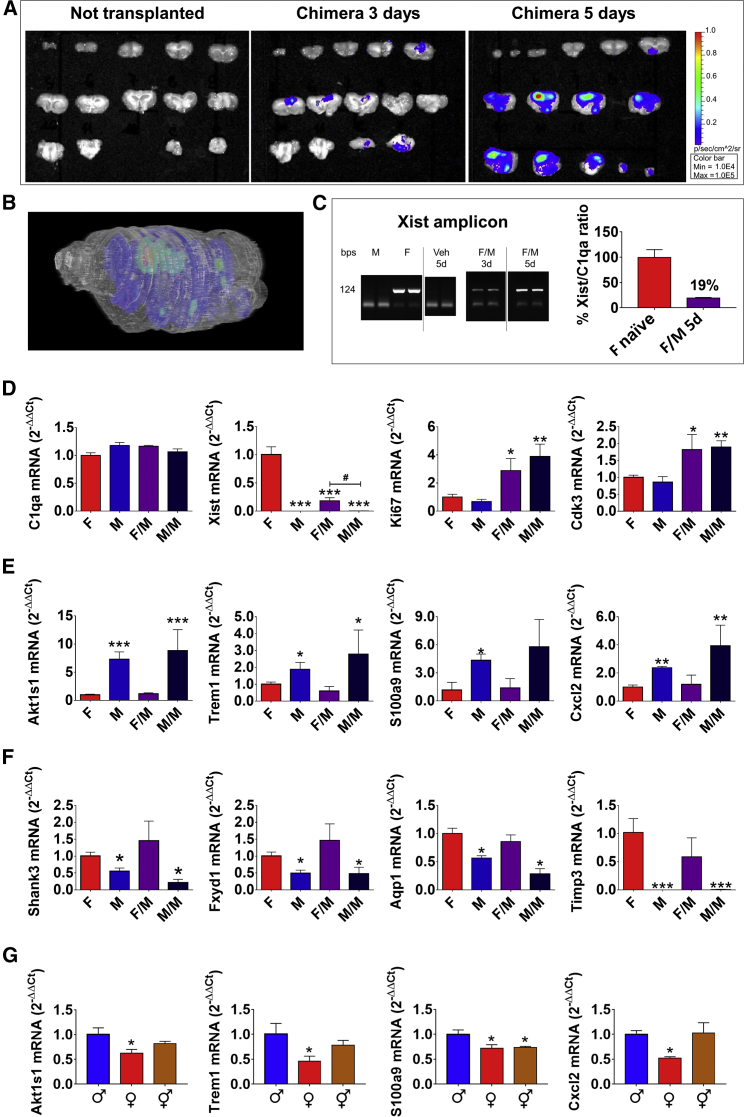
To better demonstrate that the transcriptome of adult microglia was sexually differentiated and independent of the in vivo hormonal environment, we investigated the gene expression profile of female microglia after transplantation into a male brain. In designing the transfer experiment, we anticipated several potential limitations, such as host versus graft reactions, microglia viability, and the ability to proliferate and migrate throughout the host brain. To facilitate the host brain repopulation by the transplanted microglia and limit adverse reactions, we first depleted the endogenous microglia. Previous reports had demonstrated that PLX3397, a small-molecule inhibitor of the CSF1 receptor (CSF1R) and related kinases, was able to promote selective microglia apoptosis (Elmore et al., 2014). This effect was transient, and microglia started to proliferate upon cessation of the treatment (Elmore et al., 2014). We administered PLX3397 via the transnasal (tsn) route to improve its permeation and diffusion throughout the brain parenchyma. Dose-response experiments allowed the identification of 100 μg/die as the minimum effective dose of PLX3397 necessary to deplete >90% of microglia after 7 days of treatment; no sign of toxicity was observed at higher dosages (data not shown). To be sure to maintain effective concentrations of PLX3397 in the brain, we treated the mice with 100 μg twice a day. The efficacy of the treatment was measured by the following: (1) counting the cells that were isolated from a single brain (Figure S2A); (2) measuring the whole brain content of the mRNA encoding the complement C1q subcomponent subunit A (C1qa), constitutively expressed in microglia (Fonseca et al., 2017) (Figure S2B); and (3) flow cytometry (Figure S2C). As reported previously in the literature (Elmore et al., 2014), at day 7, only a very small percentage (<8%) of the endogenous microglia was spared by the treatment; interruption of drug administration resulted in a rapid microglia proliferation starting 24 hr after the end of treatment, and 5 days after ending PLX3397 administration, the brain content of microglia cells was back to normal (Figure S2A). Having established the procedure to eliminate the majority of the host microglia, we proceeded with the transplantation using microglia purified from female UBC-luc2 (G4063), a transgenic mouse model where the expression of the luciferase reporter is constitutive and not affected by sex and age (Rizzi et al., 2017). Prior to cell transplantation, we showed that in the isolated microglia, the light emission was directly proportional to the cell number (Figure S2D). For the cell transfer, we avoided brain injections and opted for the tsn route to preserve brain inflammatory homeostasis and blood-brain barrier integrity. A total number of 350,000–400,000 female bioluminescent microglia cells were tsn-transferred to the microglia-depleted male brains 24 hr after the interruption of PLX3397 treatment. The tsn administration enabled the distribution of cells throughout the forebrain and the caudal brain, including the brainstem and cerebellum (see Figure S2E and its legend). Ex vivo imaging (Stell et al., 2008) established that 3 days after the tsn administration, the bioluminescent exogenous microglia cells were located mainly in the cortex (Figure 3A). This was expected on the basis of the route of administration selected (Figure S2E). At day 5, the significant increase of bioluminescence indicated that the transplanted female microglia were actively proliferating; furthermore, these cells had spread in most brain areas including the cortex, hippocampus, caudate and putamen, thalamus, hypothalamus, mid-brain, and pons (Figure S2F). The presence in the recipient male brain of an mRNA that is exclusively expressed in females, the X-inactive specific transcript (Xist), demonstrated that the transfer experiment had been successful (Figure 3C). In addition, the exogenous microglia were alive and transcriptionally active because the amount of Xist mRNA detectable 3 days after the transfer was increased at day 5 (Figure 3C). At day 5, the ratio of Xist to C1qa mRNAs indicated that the transplanted female cells were approximately 19% of the total host brain microglia (Figure 3C); this amount was confirmed by flow cytometry of CX3CR1-GFP male microglia transplanted in wild-type (males or females) (Figure S2G); the same experiment allowed to demonstrate that the transplanted cells maintained the expression of microglia-specific biomarkers (Tmem119) (Bennett et al., 2016) (Figure S2H). Further experiments with female or male microglia transplanted into male recipients (F/M or M/M, respectively) established that the sex of origin did not influence the ability of these cells to proliferate; indeed, Figure 3D shows that the expressions of selected genes (C1qa marker of total microglia content, Ki67 associated with cell proliferation, and Cdk3 expressed during G0–G1 and G1–S cell-cycle transitions) was the same in male or female transplanted cells. To investigate the extent to which the transplanted microglia preserved their sex-specific identity, we transplanted fluorescent microglia (from male or female CX3CR1-GFP mice) in WT males and analyzed the expression of selected genes in the fluorescent cells isolated by FACS. Male fluorescent cells transplanted in males (M/M) maintained the male-specific (M) profile of expression (relatively high Akt1s1, Trem1, S100a9, and Cxcl2 and low Shank3, Fxyd1, Aqp1, and Timp3); female cells transplanted in males (F/M) were able to keep a profile of expression superimposable with that of the control females (F) (Figures 3E and 3F).
Figure 3.
Female Microglia Maintain Their Characteristic Gene Expression When Transplanted in Male Brain
(A) Bioluminescence-based optical imaging of brain slices of WT male mice before (left panel) and after (3 days, central panel; 5 days, right panel) transnasal administration of 400,000 bioluminescent microglial cells isolated from female G4063 mice; pseudocolors represent the intensity of light emission (p/s/cm2/sr). The images are representative of three independent measures on n = 3 individual animals/group.
(B) Three-dimensional representation of the distribution of bioluminescent signals in WT mouse brain, 5 days after transnasal administration of bioluminescent microglial cells isolated from G4063 mice. Pseudocolors represent the intensity of light emission (p/s/cm2/sr), according to the color bar reported in (A).
(C) RT-PCR analysis was performed using primers for Xist mRNA (amplicon: 124 bp) on microglia RNA isolated from naive male (M) or female (F) mice, vehicle-treated male mice (Veh, 5 days [5d]) or after transnasal administration of 400,000 microglial cells isolated from female C57BL/6 mice (F/M 3d: microglia isolated 3 days after transnasal administration; F/M 5d: microglia isolated 5 days after transnasal administration). Bars represent the percentage ratio of Xist and C1qa mRNA accumulation in naive female microglia (two independent measures, n = 3) or in microglia derived from males, 5 days after transnasal administration of 400,000 microglial cells isolated from female C57BL/6 mice (5d: microglia isolated 5 days after transnasal administration, two independent measures, n = 3). Data are normalized to naive female mice.
(D) qPCR analyses of C1qa, Xist, Ki67, and Cdk3 mRNA accumulation in microglia isolated from naive female (F) or male (M) mice or 5 days after transnasal administration of 400,000 microglial cells isolated from female (F/M) or male (M/M) C57BL/6 mice. Data are expressed as 2−ΔΔCt using the 36B4 transcript as an internal reference standard. Columns represent the mean ± SEM of n = 6 animals measured in triplicates. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 by a one-way ANOVA and Tukey’s method for multiple comparisons versus (F). #p < 0.05 by a two-way ANOVA and Tukey’s method for multiple comparisons.
(E) qPCR analyses of Shank3, Fxyd1, Aqp1, and Timp3 mRNA accumulation in microglia isolated from naive female (F) or male (M) mice or fluorescence-sorted 5 days after transnasal administration of 400,000 microglial cells isolated from female (F/M) or male (M/M) CX3CR1-GFP mice. Data are expressed as 2−ΔΔCt using the 36B4 transcript as an internal reference standard. Columns represent the mean ± SEM of n = 6 animals measured in triplicates. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 by a one-way ANOVA and Tukey’s method for multiple comparisons versus (F). #p < 0.05 by a two-way ANOVA and Tukey’s method for multiple comparisons.
(F) qPCR analyses of Akt1s1, Trem1, S100a9, and Cxcl2 mRNA accumulation in microglia isolated from naive female (F) or male (M) mice or fluorescence-sorted 5 days after transnasal administration of 400,000 microglial cells isolated from female (F/M) or male (M/M) CX3CR1-GFP mice. Data are expressed as 2−ΔΔCt using the 36B4 transcript as an internal reference standard. Columns represent the mean ± SEM of n = 6 animals measured in triplicates. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 by a one-way ANOVA and Tukey’s method for multiple comparisons versus microglia isolated from naive female mice. #p < 0.05; ###p < 0.001 by a two-way ANOVA and Tukey’s method for multiple comparisons.
(G) qPCR analyses of Akt1s1, Trem1, S100a9, and Cxcl2 mRNA accumulation in microglia isolated from naive male (♂), female (♀), or masculinized female (⚥) C57BL/6 mice. Data are expressed as 2−ΔΔCt using the 36B4 transcript as an internal reference standard. Columns represent the mean ± SEM of n = 6 animals measured in triplicates. ∗p < 0.05 by a one-way ANOVA and Tukey’s method for multiple comparisons versus ♂.
The combination of all the experiments conducted highlighted the possibility of a genomic and/or epigenetic component that sexually differentiates microglia gene expression. It is known that the perinatal androgen surge from male testis and the subsequent local aromatization of testosterone to estradiol is crucial for the permanent modification of neuronal functions characteristic of brain masculinization (Arnold and Gorski, 1984, McCarthy and Nugent, 2015, Villa et al., 2016). In line with these reports, imaging experiments on ERE-Luc mice showed that perinatal activation of ERα is restricted to males (Figures S3A and S3B). To directly show that in addition to neurons the perinatal exposure to estrogens could affect microglia gene expression permanently, we followed a classical protocol of brain masculinization by treating male and female pups with E2 benzoate at days P2, P5, and P8 (McCarthy, 2008, Wu et al., 2009). The masculinized females were unable to cycle at 6–8 weeks of age, demonstrating the efficacy of the protocol. The extent to which the treatment had affected microglia gene expression was assessed by qPCR of the four DEGs previously identified as more expressed in males. Figure 3G shows that in the microglia extracted from the masculinized brains, the expression of Akts, Trem1, and CxCl2 was not significantly different than in males; S100a9 was not affected by the brain masculinization.
Protective Action of Female Microglia in Ischemia
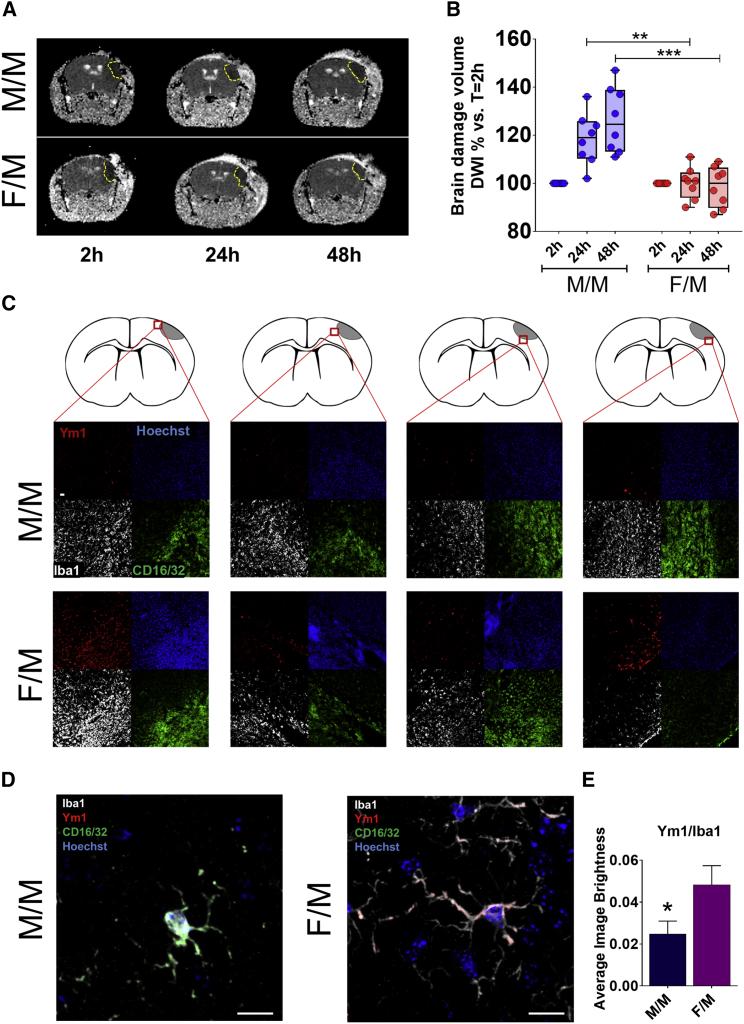
We next asked whether microglia sex-specific gene expression had any functional relevance. Several authors demonstrated that in the case of forebrain or focal ischemia, young adult female rodents sustain smaller injury than males, and females have lower mortality than males (Murphy et al., 2004, Spychala et al., 2017). The course of microglia activation after cerebral ischemia has been largely described, and it is well known that microglia proliferation at the infarct site limits the ischemic damage possibly because microglia generate neurotrophic factors that are beneficial for neuronal plasticity (Lalancette-Hébert et al., 2007). Considering this fact, and based on the results obtained with our RNA-seq analyses that unstimulated female microglia have a higher propensity than male microglia to express genes involved in cell plasticity, the control of the inflammatory response, and repair mechanisms, we questioned whether transferring female microglia into male brains would have restricted the brain damage consequent to ischemia. First, we subjected adult WT male (n = 6) and female (n = 6) mice to permanent focal cerebral ischemia (permanent middle cerebral artery occlusion [pMCAO]) (Gelosa et al., 2014), and we carried out diffusion-weighted imaging (DWI) at 2, 24, and 48 hr after the ischemic insult. In DWI, the ischemic lesion appears hypointense, consistent with a decrease in the apparent diffusion coefficient of water (ADC), which is a weighted average between the intracellular (assumed to be lower) and extracellular diffusion coefficients (assumed to be higher). The decrease in ADC reflects the changes in the ratio of intracellular and extracellular volume, because ischemia-induced energy impairment and membrane pump failure allow the osmotic drainage of water from extracellular to intracellular spaces that leads to cytotoxic edema (van Gelderen et al., 1994). The results obtained in control animals (Figure S4A) showed that in males, the damaged area at 24 and 48 hr was larger than in females, which is in line with previous reports (Spychala et al., 2017). Next, we transplanted 16 adult male mice with microglia purified either from male (n = 8 M/M) or female mice (n = 8 F/M). Figure 4A shows the DWI of the transplanted mice at 2, 24, and 48 hr after ischemia. At 2 hr after the infarct, the sex of origin of the transplanted microglia did not influence the volume of the damage (Figure S4B). This was expected because in our experience, changes in the damage size are not detectable at early phase after ischemia (Gelosa et al., 2014). In the M/M group, the progression of the damage was significantly higher (+26% after 48 hr) than in the F/M group (Figure 4B), thus pointing to a protective action of the female microglia. Male microglia into female brain (n = 8) did not provide as clear and significant results (Figure S4C).
Figure 4.
Sex of Microglia Influence the Progression of Ischemic Stroke
(A) Time-dependent evolution of infarct volume in permanent middle cerebral artery occlusion (pMCAO) mice. Diffusion-weighted imaging (DWI) representative of male mice transnasally administered with male (M/M) or female (F/M) microglia, taken at 2, 24, and 48 hr after pMCAO. The ischemic lesion is detectable as a hypointense area in the right cerebral hemisphere (delineated by the yellow dotted line). The image is representative of n = 8 animals.
(B) Scatterplot showing the quantitative analysis of the brain damage volume determined by DWI measurements and expressed as percent change relative to the initial 2 hr value set to 100%. Solid lines represent mean ± SEM for M/M (n = 8) and F/M mice (n = 8). ∗∗p < 0.01; ∗∗∗p < 0.001 by two-way ANOVA and Sidak’s method for multiple comparisons.
(C) Representative, low-magnification (×20) immunofluorescence analysis of coronal sections of brains excised from adult mice that had undergone pMCAO, stained for Iba1 (white), CD16/32 (green), Ym1 (red), and Hoechst 33258 (blue). M/M: male recipient transplanted with male microglia; F/M: male recipient transplanted with female microglia. Images are representative of n = 3 mice per experimental group and show the fluorescence images acquired in the areas indicated by red squares in the upper schematics. Scale bar: 10 μm.
(D) Representative high-magnification (×100) confocal z stack projections of cells stained for Iba1 (white), CD16/32 (green), Ym1 (red), and Hoechst 33258 (blue) in mice that had undergone pMCAO. The pictures show the colocalization of pro- (CD16/32) and anti-inflammatory markers with Iba1-stained microglia. M/M: male recipient transplanted with male microglia; F/M: male recipient transplanted with female microglia. Images are representative of n = 3 mice per experimental group. Scale bar: 10 μm.
(E) Graph columns show the mean ± SEM of fluorescence brightness for Ym1 (red channel) in Iba1-positive cells, measured in a double-blind manner. ∗p < 0.05 by unpaired, two-tailed t test.
To determine the ability of exogenous microglia to migrate to the infarct site, we subjected mice transplanted with bioluminescent G4063 microglia to pMCAO. Bioluminescence imaging revealed that after pMCAO, a large amount of the bioluminescent, exogenous microglia accumulated in the vicinity of the infarct site (Figure S4D). The phenotype of microglia at the lesion site was investigated by immunostaining of the pMCAO lesioned brain. We used Ab against Ym1 as a marker for microglia anti-inflammatory activation and against CD16/32 as a marker for pro-inflammatory activation (Figure 4C). Low-magnification confocal imaging of the fields of view surrounding the ischemic lesion revealed that Ym1 immunoreactivity was higher in brain samples obtained from the F/M animals. At higher magnification (×100), the immunofluorescence showed that both CD16/32 and Ym1 co-localized with Iba1 immunoreactivity (Figure 4D). Semiquantitative, double-blind analyses of the average fluorescence brightness in Iba1-positive cells surrounding the ischemic lesion showed Ym1 immunoreactivity significantly higher (+92%) in F/M versus M/M (Figure 4E). CD16/32 staining was comparable in both experimental groups (Figure S4E).
Discussion
The present study shows that microglia cells are sexually differentiated, as indicated by the sex-specific expression of a significant number of genes. Microglia cells maintain sex-specific expression independently by the circulating sex steroids, which was demonstrated by putting adult microglia in culture or by their transplant in the opposite sex. In addition, ovariectomy did not show very significant changes in microglia gene expression. The mechanism involved in microglia sexual differentiation remains to be determined. The fact that neonatal treatment of female brain with estrogens (using a protocol known to induce brain defemination) altered the expression of selected genes may suggest that similar to what was reported for neurons, the sex of microglia is determined at birth. Microglia are an autonomous self-proliferating population of cells that migrate from the yolk sac prior to hematopoiesis. Following brain colonization, these cells differentiate into microglia, and the formation of the blood-brain barrier prevents the infiltration of peripheral cells, at least under healthy conditions. Therefore, the masculinizing surge of testosterone might induce the differentiation of this cell population, because neonatal microglia express the estrogen receptor alpha (Crain et al., 2013, Sierra et al., 2008). Other authors studying microglia in embryos, neonates, and adult mice showed that there are precise temporal phases that characterize microglia development (Matcovitch-Natan et al., 2016), and that the immune reactivity of these cells is sexually differentiated very early in development (Hanamsagar et al., 2017). In addition, during the first week postpartum in mice, there are sexual differences in the morphology and the number of these cells (Lenz et al., 2013), and microglia have an inflammatory morphology that converts to a largely ramified state occurring by the third week after birth when male defemination is completed. These observations have suggested that microglia in the neonatal brain are involved in more than a response to brain injury or inflammation; that is, microglia actively participate in the formation of specific brain circuitries by regulating a variety of developmental processes and physiological functions including synapse elimination, spinogenesis, spine elimination, and synaptic physiology (Tremblay et al., 2011).
Our study suggests that the local neonatal synthesis of estrogen associated with the androgen surge from the male testis may not be a cause of transient activation of microglia but might have permanent (organizational) effects on these cells by inducing a sexual phenotype that is maintained in the adult animals. This would explain why male or female microglia have the tendency to maintain the same gene expression when transplanted in the opposite sex. Indeed, analogous to what occurs in neurons where their sensitivity and ability to respond to specific hormonal and environmental stimuli (activational effects) is permanently affected by the neonatal estrogen priming, our data suggest that in male microglia, the estrogen priming changes their immune capacity by enhancing their ability to react to inflammatory stimuli. These data are in agreement with a very recent study aimed at comparing the male and female microglia transcriptome in relation with the stage of development (Hanamsagar et al., 2017).
We do not know what the teleological meaning of this sexual differentiation is; certainly, our studies show that this biological event has functional repercussions on the damage caused from acute cerebral ischemia (MCAO) (Figure 4). It is well known that the damage induced by MCAO is significantly higher in males than in females, and several studies have debated whether this is due to a lower susceptibility of female neurons to ischemia or to differences in the evolution of the neuroinflammatory response (Murphy et al., 2004). The findings reported here that female microglia are better apt than male microglia to reduce the ischemic damage also when transplanted in males highlight an intrinsic sex-specific microglia phenotype that might be independent from the hormonal environment. This is in line with the observation that in humans, males have a higher incidence of stroke and poorer outcomes afterward (Golomb et al., 2009). However, we cannot definitively conclude that estrogens do not influence microglia activity, because aromatase is expressed in the male brain and may convert locally the circulating testosterone into estradiol (McCullough et al., 2003).
Our results point to microglia as major actors in the evolution of the ischemic insult; this is in accordance with prior literature, as our observations were conducted at 24 and 48 hr after ischemia. Morphological, immunohistochemical, MRI-based, and pharmacological studies have shown that in the first 72 hr after ischemia, the CNS resident microglia control the course of inflammation, generating anti-inflammatory or repair molecules in the insult area (Gelderblom et al., 2009, Rupalla et al., 1998). Additionally, microglia participate in attracting peripheral immune cells that populate the damaged area at later times (Gelderblom et al., 2009). At this point, it is possible that in females, the presence of circulating estrogens limits the damage caused by an excessive inflammatory response, because female monocytic cells were shown to better transit toward the anti-inflammatory phenotype (Villa et al., 2015) and were likely able to confine the tissue damage induced by the hypoxic stimulus, possibly by virtue of the limited expression of genes encoding inflammatory proteins. Interestingly, when we transplanted male microglia into female brains, we did not observe an increase in the ischemic area with time. This might be because the female resident microglia buffered the effects of the grafted cells.
Neuropsychiatric or neurological diseases may manifest in a large percentage of the population (approximately 25% of individuals suffer from brain disorders in their lifespan) with a pattern of susceptibility that is associated with sex. Females are more affected by diseases that occur during adulthood, whereas males are particularly vulnerable to life-long illnesses of neurodevelopmental origin. Considering the number of studies underscoring the relevance of inflammation in brain disturbances, the finding of a sex difference in male and female susceptibility to neuroinflammation provides a basis for a sex-related approach to therapy that must be based on a better understanding of the relationships among the endocrine, immune, and nervous systems in young and adult individuals.
Experimental Procedures
Animals
Animal studies were carried out at the Department of Pharmacological and Biomolecular Sciences. All animal experimentation was carried out in accordance with the ARRIVE and European Guidelines for Animal Care. All animal experiments were approved by the Italian Ministry of Research and University (permission numbers: 12-12-30012012, 547/2015, 479/2015) and controlled by a departmental panel of experts.
C57BL/6 and CX3CR1-GFP mice at 3 months of age were supplied by Charles River (Charles River Laboratories, Calco, Italy). Both male and female mice were used throughout experiments as described earlier. Animals were allowed access to food and water ad libitum and kept in temperature-controlled facilities on a 12-hr light and dark cycle. More details are available in the Supplemental Experimental Procedures.
Pharmacological Manipulations
E2 (Sigma-Aldrich, Italy) was administered by a 100 μL s.c. injection of 5 μg/kg E2 (which results in circulating E2 levels comparable with those at proestrus [Ciana et al., 2003]) dissolved in corn oil by overnight (o/n) stirring in the dark at room temperature; control animals received corn oil injection alone. E2 benzoate was administered by a s.c. injection of 5 μg in 50 μL of corn oil; control animals received corn oil alone.
Microglia Sorting
Isolation of microglia from the whole brains of adult mice was performed as described in Pepe et al. (2014). More details are available in the Supplemental Experimental Procedures.
PLX3397 Administration
8.3 mg of PLX3397 was dissolved in 1 mL of 5% DMSO + 45% polyethylene glycol 300 (PEG300) + ddH2O (double-distilled water) solution. Prior to tsn administration, animals were anesthetized with s.c. injection of 50 μL solution of ketamine (93.6 mg/kg, Ketavet 100; Intervet, Milan, Italy) and xylazine (7.2 mg/kg, Rompun; Bayer, Milan, Italy). More details are available in the Supplemental Experimental Procedures.
Cell Cultures
Primary neuronal cultures were obtained from the hippocampi of 18-day-old fetal Sprague Dawley rats of both sexes (Charles River Italia). Neurons were transfected at 7 days in vitro (DIV7) using the calcium-phosphate method with 2 μg of EGFP plasmid. At DIV10 microglia were isolated from the brain of adult mice and seeded over rat neurons with a microglia:neuron ratio of 1:10. At DIV14, cells were either processed for immunocytochemistry or resuspended in TRIzol reagent (Invitrogen, Milan, Italy) and processed for RNA preparation.
Morphological Analysis of Iba1-Stained Microglia
Three images of six slides per experimental condition were taken. The size of the fields analyzed was 173 μm × 218 μm. Morphological analyses were performed in a double-blind manner as previously described (Benedusi et al., 2017). ImageJ software was used to measure immunoreactivity through a threshold method, and the number of positive pixels and the extension of area of interest were used to determine the fractional area covered by the specific signal.
Brain Masculinization
Pregnant C57BL/6 mice were individually housed after pups’ birth. All pups within a litter were subcutaneously injected with vehicle (50 μL corn oil) or treatment (5 μg E2 benzoate in 50 μL corn oil) (Wu et al., 2009) at post-natal day 2 (P2), P5, and P8. At P21, females and males were housed in separate cages. At 10–14 weeks of age, vaginal smears were carried out to study the ability of the females to cycle.
MRI Analysis
Brain infarct size was visualized by DWI at 2, 24, and 48 hr after MCAO using a 4.7 T, vertical super-wide bore magnet of a Bruker Avance II spectrometer with microimaging accessory. More details are available in the Supplemental Experimental Procedures.
Statistical Analyses
Statistical analysis of RNA-seq data were carried out using the CuffDiff (Trapnell et al., 2012) software. A t test was used to calculate the p value for differential expression. A threshold of 0.01 was applied to FDR-adjusted p values (q values) in order to select the DEGs to use in downstream analyses. Cluster analyses were performed with the Genesis software tool (https://genome.tugraz.at/genesisclient/genesisclient_description.shtml) to log-transform, normalize, and center gene expressions, and populations and genes were clustered by Pearson correlation. Overrepresentation analysis (ORA) on DEG lists was performed using Enrichr for enrichment analysis. The mouse genome was used as background list. Biological processes, molecular functions, and KEGG pathways were investigated focusing on enriched terms with a Benjamini-adjusted p value less than 0.05.
Statistical significance of the other reported data were calculated using Prism 7 software (GraphPad); unless otherwise indicated in the figure legend, one-way ANOVA for single-treatment comparisons or two-way ANOVA for multiple treatment comparisons was applied. A p value less than 0.05 was considered as statistically significant.
Acknowledgments
We thank Monica Rebecchi and Clara Meda for their continuous assistance throughout the study, Nicolò Panini for technical assistance, and Tiziana Borsello for the critical reading of the manuscript. The authors are supported by European Union grant ERC-2012-ADG322977-Ways and the Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 278850 (INMiND).
Author Contributions
Conceptualization, A.M. and A.V.; Methodology, A.M., A.V., and N.R.; Investigation, A.V., P.G., M.C., N.R., E.M., F.L., G.P., and L.C.; Writing – Original Draft, A.M. and A.V.; Writing – Review & Editing, A.V., A.M., L.S., E.M., and P.G.; Funding Acquisition, A.M.; Resources, A.M. and L.S.; Supervision, A.M., L.S., and E.V.
Declaration of Interests
The authors declare no competing interests.
Published June 19, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.05.048.
Data and Software Availability
The accession number for the RNA-seq data reported in this paper is NCBI Sequence Read Archive: SRP104620.
Supplemental Information
References
- Arnold A.P., Gorski R.A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Balli D., Ren X., Chou F.S., Cross E., Zhang Y., Kalinichenko V.V., Kalin T.V. Foxm1 transcription factor is required for macrophage migration during lung inflammation and tumor formation. Oncogene. 2012;31:3875–3888. doi: 10.1038/onc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedusi V., Della Torre S., Mitro N., Caruso D., Oberto A., Tronel C., Meda C., Maggi A. Liver ERα regulates AgRP neuronal activity in the arcuate nucleus of female mice. Sci. Rep. 2017;7:1194. doi: 10.1038/s41598-017-01393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.L., Bennett F.C., Liddelow S.A., Ajami B., Zamanian J.L., Fernhoff N.B., Mulinyawe S.B., Bohlen C.J., Adil A., Tucker A. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P., Raviscioni M., Mussi P., Vegeto E., Que I., Parker M.G., Lowik C., Maggi A. In vivo imaging of transcriptionally active estrogen receptors. Nat. Med. 2003;9:82–86. doi: 10.1038/nm809. [DOI] [PubMed] [Google Scholar]
- Crain J.M., Nikodemova M., Watters J.J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z., Ma C., Han Y., Li Y., Zhou H. Nanog attenuates lipopolysaccharide-induced inflammatory responses by blocking nuclear factor-κB transcriptional activity in BV-2 cells. Neuroreport. 2013;24:718–723. doi: 10.1097/WNR.0b013e328363fd67. [DOI] [PubMed] [Google Scholar]
- El Wakil A., Francius C., Wolff A., Pleau-Varet J., Nardelli J. The GATA2 transcription factor negatively regulates the proliferation of neuronal progenitors. Development. 2006;133:2155–2165. doi: 10.1242/dev.02377. [DOI] [PubMed] [Google Scholar]
- Elmore M.R., Najafi A.R., Koike M.A., Dagher N.N., Spangenberg E.E., Rice R.A., Kitazawa M., Matusow B., Nguyen H., West B.L., Green K.N. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca M.I., Chu S.H., Hernandez M.X., Fang M.J., Modarresi L., Selvan P., MacGregor G.R., Tenner A.J. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J. Neuroinflammation. 2017;14:48. doi: 10.1186/s12974-017-0814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F., Bellone C., Viviani B., Marinovich M., Meli E., Pellegrini-Giampietro D.E., Cattabeni F., Di Luca M. Lack of PSD-95 drives hippocampal neuronal cell death through activation of an alpha CaMKII transduction pathway. Eur. J. Neurosci. 2002;16:777–786. doi: 10.1046/j.1460-9568.2002.02141.x. [DOI] [PubMed] [Google Scholar]
- Gelderblom M., Leypoldt F., Steinbach K., Behrens D., Choe C.U., Siler D.A., Arumugam T.V., Orthey E., Gerloff C., Tolosa E., Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Gelosa P., Lecca D., Fumagalli M., Wypych D., Pignieri A., Cimino M., Verderio C., Enerbäck M., Nikookhesal E., Tremoli E. Microglia is a key player in the reduction of stroke damage promoted by the new antithrombotic agent ticagrelor. J. Cereb. Blood Flow Metab. 2014;34:979–988. doi: 10.1038/jcbfm.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M.R., Fullerton H.J., Nowak-Gottl U., Deveber G., International Pediatric Stroke Study Group Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke. 2009;40:52–57. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R., Alter M.D., Block C.S., Sullivan H., Bolton J.L., Bilbo S.D. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia. 2017;65:1504–1520. doi: 10.1002/glia.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Leak R.K., Shi Y., Suenaga J., Gao Y., Zheng P., Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H., Kirchhoff F., Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Kierdorf K., Prinz M. Factors regulating microglia activation. Front. Cell. Neurosci. 2013;7:44. doi: 10.3389/fncel.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K., Prinz M. Microglia in steady state. J. Clin. Invest. 2017;127:3201–3209. doi: 10.1172/JCI90602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q., Wang Z., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hébert M., Gowing G., Simard A., Weng Y.C., Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz K.M., Nugent B.M., Haliyur R., McCarthy M.M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky N.J., Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan O., Winter D.R., Giladi A., Vargas Aguilar S., Spinrad A., Sarrazin S., Ben-Yehuda H., David E., Zelada González F., Perrin P. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353:aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- McCarthy M.M. Estradiol and the developing brain. Physiol. Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.M., Nugent B.M. At the frontier of epigenetics of brain sex differences. Front. Behav. Neurosci. 2015;9:221. doi: 10.3389/fnbeh.2015.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough L.D., Blizzard K., Simpson E.R., Oz O.K., Hurn P.D. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J. Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Q., Ku A.T., Nishino Y., Howard J.M., Rao A.S., Shaver T.M., Garcia G.E., Le D.N., Karlin K.L., Westbrook T.F. Tcf3 promotes cell migration and wound repair through regulation of lipocalin 2. Nat. Commun. 2014;5:4088. doi: 10.1038/ncomms5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.J., McCullough L.D., Smith J.M. Stroke in the female: role of biological sex and estrogen. ILAR J. 2004;45:147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- Neumann H., Kotter M.R., Franklin R.J. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A., Venkatasubramanian M., Chaudhri V.K., Aronow B.J., Salomonis N., Singh H., Grimes H.L. Single-cell analysis of mixed-lineage states leading to a binary cell fate choice. Nature. 2016;537:698–702. doi: 10.1038/nature19348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe G., Calderazzi G., De Maglie M., Villa A.M., Vegeto E. Heterogeneous induction of microglia M2a phenotype by central administration of interleukin-4. J. Neuroinflammation. 2014;11:211. doi: 10.1186/s12974-014-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M., Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Rizzi N., Rebecchi M., Levandis G., Ciana P., Maggi A. Identification of novel loci for the generation of reporter mice. Nucleic Acids Res. 2017;45:e37. doi: 10.1093/nar/gkw1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupalla K., Allegrini P.R., Sauer D., Wiessner C. Time course of microglia activation and apoptosis in various brain regions after permanent focal cerebral ischemia in mice. Acta Neuropathol. 1998;96:172–178. doi: 10.1007/s004010050878. [DOI] [PubMed] [Google Scholar]
- Salter M.W., Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- Schafer D.P., Lehrman E.K., Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61:24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Gottfried-Blackmore A., Milner T.A., McEwen B.S., Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Spychala M.S., Honarpisheh P., McCullough L.D. Sex differences in neuroinflammation and neuroprotection in ischemic stroke. J. Neurosci. Res. 2017;95:462–471. doi: 10.1002/jnr.23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell A., Belcredito S., Ciana P., Maggi A. Molecular imaging provides novel insights on estrogen receptor activity in mouse brain. Mol. Imaging. 2008;7:283–292. [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M.E., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. The role of microglia in the healthy brain. J. Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen P., de Vleeschouwer M.H., DesPres D., Pekar J., van Zijl P.C., Moonen C.T. Water diffusion and acute stroke. Magn. Reson. Med. 1994;31:154–163. doi: 10.1002/mrm.1910310209. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Bonincontro C., Pollio G., Sala A., Viappiani S., Nardi F., Brusadelli A., Viviani B., Ciana P., Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J. Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E., Ciana P., Maggi A. Estrogen and inflammation: hormone generous action spreads to the brain. Mol. Psychiatry. 2002;7:236–238. doi: 10.1038/sj.mp.4001007. [DOI] [PubMed] [Google Scholar]
- Villa A., Rizzi N., Vegeto E., Ciana P., Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci. Rep. 2015;5:15224. doi: 10.1038/srep15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Vegeto E., Poletti A., Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr. Rev. 2016;37:372–402. doi: 10.1210/er.2016-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.V., Manoli D.S., Fraser E.J., Coats J.K., Tollkuhn J., Honda S., Harada N., Shah N.M. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Dissing-Olesen L., MacVicar B.A., Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36:605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.