Abstract
Selenium (Se) participates in several enzymatic reactions necessary for regulating the homeostasis of thyroid hormones. We aimed to analyze the association between dietary Se intake and subclinical hypothyroidism. Baseline data from the Longitudinal Study of Adult Health (Estudo Longitudinal de Saúde do Adulto—ELSA-Brasil) in Brazil were analyzed, with a final sample size of 14,283 employees of both sexes aged 35–74 years. Dietary data was collected using a previously validated food frequency questionnaire. Subclinical hypothyroidism was categorized as thyroid-stimulating hormone levels of >4.0 IU/mL and free prohormone thyroxine levels within normal limits, without administering drugs for thyroid disease. A multiple logistic regression model was used to assess the relationship between the presence of subclinical hypothyroidism and tertiles of Se consumption. The prevalence of subclinical hypothyroidism in the study sample was 5.4% (95% confidence interval [CI], 3.8–7.0%). Compared with the first tertile of Se intake, the second (odds ratio [OR], 0.79; 95% CI, 0.65–0.96%) and third (OR, 0.72; 95% CI, 0.58–0.90%) tertiles were inversely associated with subclinical hypothyroidism, however further research is needed to confirm the involvement of Se in subclinical hypothyroidism using more accurate methodologies of dietary assessment and nutritional status to evaluate this relationship.
Keywords: selenium, diet, subclinical hypothyroidism, adults, thyroid
1. Introduction
One of the diseases that affect the thyroid gland is subclinical hypothyroidism, which is characterized by elevated serum levels of thyroid-stimulating hormone (TSH) at a concentration recommended for prohormone thyroxine (T4) and active hormone triiodothyronine (T3). The decompensated levels of thyroid hormones may contribute to atherosclerotic events [1] and an increase in cardiovascular-related mortality [2]. Also, observational longitudinal studies have shown an inverse association between selenium exposure and risk of some cancer types but still to be confirmed [3]. It is estimated that subclinical hypothyroidism affects 3–8% of the general population and is more common in women than in men [4]. In Brazil, an epidemiological study in elderly reported that prevalence of subclinical hypothyroidism was 6.5% [5]. Olmos et al. [6], in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), reported that subclinical hypothyroidism prevalence was 5.4% overall.
Hypothyroidism is sometimes difficult to diagnosis, since most of the symptoms, such as fatigue, lack of concentration, dry skin, are nonspecific and frequently attributed to other causes or to the aging process itself [2]. Studies conducted in Brazil demonstrated the influence of race on the prevalence of hypothyroidism, which was lower in black and brown people [6,7]. Also, gender, race and socioeconomic status were reported to influence the diagnosis and treatment of hypothyroidism, with men, browns, blacks and subjects with low socioeconomic status having lower frequencies of treatment for hypothyroidism [6].
The thyroid gland contains high levels of selenium (Se) [8] and expresses a variety of selenoproteins that are involved in protection of oxidative stress and metabolism of thyroid hormones (TH) [9,10,11]. Se deficiency impairs regular synthesis of selenoproteins and adequate TH metabolism. However, on selenium deficient diets, endocrine organs and the brain are preferentially supplied [12], especially the thyroid gland, that retains the trace element very efficiently [11,13]. On the other hand, Parshukova et al. [14], studying the interrelationships between seasonal selenium levels and levels of thyroid gland hormones over a year, verified that low levels of plasma selenium affected thyroid hormone levels in humans living in North European Russia. Wu et al. [15] reported in a study on China that higher serum selenium was associated with lower chance to present subclinical hypothyroidism.
Se nutritional status varies worldwide because the Se content in food is related to the amount in the soil [16]. Thus, the plasma Se concentrations are variable in different populations around the world. For instance, plasma Se is higher in the USA compared to the South Islands of New Zealand [17]. A study in São Paulo, Brazil, using biomarkers of Se status, reported that plasma Se concentrations were very low compared with those observed in other healthy populations, such as the USA, New Zealand and UK [18]. They hypothesized that, as Se intake can be predicted by plasma Se concentrations, this lower concentration could be a consequence of low Se intake and the low Se content in foods in this southern region of Brazil. According to a study conducted by Favaro et al. [19], the food intake of selenium in Brazil can vary from 20 to 114 μg/day, that is, from low to adequate, depending on the region that was studied and the socioeconomic level of the population. Usually the main sources of Se are cereals, meats and fish [20]. Ferreira et al. [21], evaluated the selenium content in foods consumed in different states of Brazil and the ingredients that are considered staple food, such as beans, wheat flour, rice, cassava flour and maize, were poor sources of selenium, while animal sources, more expensive, were better sources.
Despite the expected relationship between Se and thyroid function, only one [22] of several studies [22,23,24,25,26], which evaluated thyroid metabolism in different populations, found a positive effect of Se supplementation on thyroid hormone levels.
The objective of this study was to analyze the association between the dietary intake of Se and subclinical hypothyroidism based on baseline data from the Longitudinal Study of Adult Health (Estudo Longitudinal de Saúde do Adulto—ELSA-Brasil).
2. Materials and Methods
This cross-sectional study analyzed baseline data from the ELSA study in Brazil, a multicenter cohort study focused on chronic diseases, particularly cardiovascular diseases and comprised 15,105 employees from six Brazilian institutions of higher education and research aged 35–74 years.
Baseline data were collected from 2008 to 2010 by conducting interviews to identify sociodemographic, lifestyle, anthropometric, dietary and clinical characteristics. Details of the study design were reported by Aquino et al. [27] and Bensenor et al. [28].
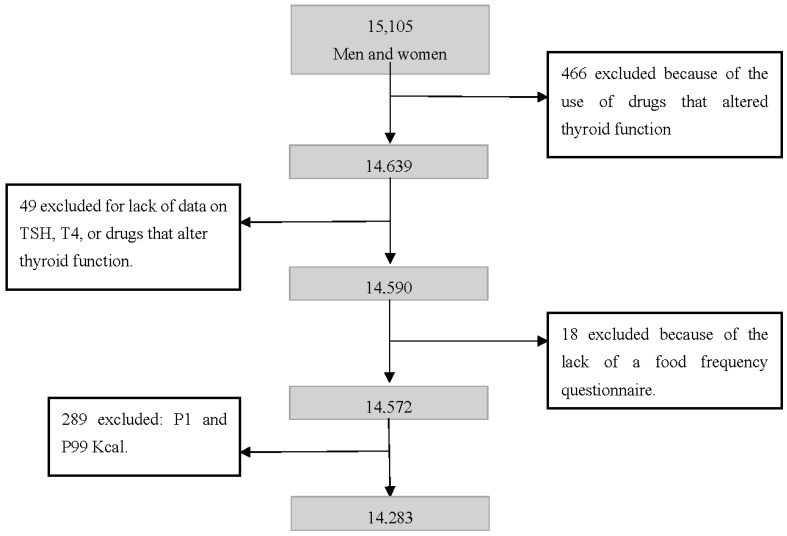
The study sample consisted of 14,283 participants. The exclusion criteria were the use of drugs that modified thyroid function, lack of information on TSH and T4, absence of a food frequency questionnaire (FFQ) and energy intake lower than the first percentile or higher than the 99th percentile of the distribution (Figure 1).
Figure 1.
Exclusion criteria and final sample. ELSA-Brasil, Brazil 2017.
2.1. Ethical Aspects
The ELSA-Brasil protocol was approved at all 6 centers: Oswaldo Cruz Foundation (Fiocruz), Federal University of Bahia (UFBA), Federal University of Espírito Santo (UFES), Federal University of Minas Gerais (UFMG), Federal University of Rio Grande do Sul (UFRGS) and University of São Paulo (USP), by the institutional review boards addressing research in human participants. All participants signed a written informed consent form.
2.2. Diet
Food intake was obtained using a validated FFQ with 114 food items to evaluate diet in the past 12 months [29], covering three sections: food products/food preparations, measures of consumed products and consumption frequencies with eight response options: “more than 3 times a day”, “2 to 3 times a day”, “once a day”, “5 to 6 times a week”, “2 to 4 times a week”, “once a week”, “1 to 3 times a month” and “never/rarely”. The measures of consumed foods were determined using a toolkit [30].
2.3. Subclinical Hypothyroidism
Venous blood was withdrawn from the ELSA participants after a 12-h fast and dosing of TSH. The levels of free T4 were analyzed in participants with low TSH (<0.4 IU/mL) or high TSH (>4.0 IU/mL). TSH and FT4 were measured using a third-generation immunoenzymatic assay (Siemens, Deerfield, IL, USA) in serum obtained from centrifuged venous blood samples after overnight fasting [31]. FT4 levels were measured in participants exhibiting altered TSH levels. In this study, reference range levels were 0.4–4.0 mIU/L for TSH and 10.3–24.45 pmol/L for FT4. We excluded participants using drugs that could interfere with thyroid function: amiodarone, carbamazepine, carbidopa, phenytoin, furosemide, haloperidol, heparin, interferon, levodopa, lithium, metoclopramide, propranolol, primidone, rifampicin and valproic acid.
ELSA-Brasil study participants were classified into five categories of thyroid function, according to TSH and FT4 levels and information related to the use of medication to treat thyroid disorders: clinical hyperthyroidism (low serum TSH and high FT4 levels or use of medication to treat hyperthyroidism), subclinical hyperthyroidism (low serum TSH, normal FT4 levels and no use of drugs to treat thyroid diseases), euthyroidism (normal TSH and no use of thyroid drugs), subclinical hypothyroidism (high TSH levels, normal FT4 levels and no use of drugs to treat thyroid diseases) and clinical hypothyroidism (high TSH and low FT4 levels, or use of levothyroxine to treat hypothyroidism). For the descriptive analysis all types were included, but, only participants with subclinical hypothyroidism or euthyroidism were included on the regression models.
The cutoff points used to determine subclinical hypothyroidism were TSH levels of >4.0 IU/mL with free T4 within the recommended doses, without the use of drugs that alter thyroid function.
2.4. Statistical Analysis
Multiple logistic regression models with nutrients adjusted for total energy, using the residuals method [32], were conducted in the sample that included only participants with subclinical hypothyroidism or euthyroidism. The models were adjusted for age (35–59 years, ≥60 years), sex (male and female), nutritional status (body mass index) in kg/m² (low weight, eutrophic, overweight and obese according to the cut-off points recommended by the World Health Organization) [33], smoking (no for ex-smokers and non-smokers and yes for smokers), hypertension (yes or no; obtained from systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, or use of drugs for treating hypertension), diabetes (yes or no, obtained from data on post-prandial glycaemia, glycated hemoglobin, use of medications for treating diabetes and previous diagnosis of diabetes), dyslipidemia (yes or no, obtained from previous diagnosis of the disease and use of medicines), per capita income (obtained from data on the net family income of the past month, by the average of extreme values of each category and number of family members who depended on this income to live), current alcohol use (yes or no), level of physical activity during leisure (low, moderate, or high) according to the International Physical Activity Questionnaire (IPAQ), change in diet (yes or no) and use of dietary supplements (regularly or not).
Micronutrients that correlated with the outcome of interest and thyroid function, including zinc, vitamin A, iodine and sodium, were also used as adjustment variables [34]. Urinary sodium (g/day) was used as a proxy for iodine consumption [35].
All analyses were performed using Stata Statistical Software (release 14, 2015, StataCorp LP, College Station, TX, USA) and the level of significance was set at 5%.
3. Results
The total sample had a higher proportion of participants who were Caucasian, female, aged 35–59 years, with per capita income in the first tertile, non-smokers, alcohol users, with low physical activity level during leisure, without significant changes in diet, overweight, non-hypertensive, non-diabetic, dyslipidemic and euthyroid (Table 1).
Table 1.
Description of the total population and selenium consumption per tertile in the ELSA-Brasil study, 2017.
| Total | Selenium Intake * | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Tertile (0–187 mg) |
Second Tertile (188–232 mg) |
Third Tertile (233–1087 mg) |
p Value ** | ||||||
| N | % | N | % | N | % | N | % | ||
| Sex | <0.001 | ||||||||
| Male | 6518 | 45.6 | 2519 | 53.0 | 1979 | 41.6 | 2020 | 42.4 | |
| Female | 7765 | 54.4 | 2242 | 47.0 | 2782 | 58.4 | 2741 | 57.6 | |
| Self-declared race | <0.001 | ||||||||
| Caucasian | 7418 | 52.5 | 2045 | 43.4 | 2540 | 53.9 | 2833 | 60.3 | |
| Black and Mixed | 6204 | 43.9 | 203 | 53.1 | 190 | 42.3 | 111 | 36.4 | |
| Others | 497 | 3.6 | 161 | 3.5 | 179 | 3.8 | 157 | 3.3 | |
| Age | <0.001 | ||||||||
| 35–59 years | 11,271 | 78.9 | 3892 | 80.5 | 3831 | 80.5 | 3548 | 74.5 | |
| ≥60 years | 3012 | 21.1 | 869 | 18.6 | 930 | 19.5 | 1213 | 25.5 | |
| Per capita income | <0.001 | ||||||||
| First tertile (USD 14.85–520.51) | 5175 | 36.1 | 2498 | 52.7 | 1665 | 35.1 | 1012 | 21.3 | |
| Second tertile (USD 529.87–1059.74) | 4992 | 34.9 | 1476 | 31.1 | 1739 | 36.6 | 1707 | 36.0 | |
| Third tertile (USD 1115.32–4238.97) | 4135 | 29.0 | 768 | 16.2 | 1342 | 28.3 | 2025 | 42.7 | |
| Current smoking | <0.001 | ||||||||
| No | 12,446 | 87.1 | 3979 | 83.6 | 4182 | 87.9 | 4285 | 90.0 | |
| Yes | 1836 | 12.9 | 782 | 16.4 | 578 | 12.1 | 476 | 10.0 | |
| Current alcohol use | <0.001 | ||||||||
| No | 4302 | 30.1 | 1675 | 35.2 | 1434 | 30.1 | 1193 | 25.1 | |
| Yes | 9978 | 69.9 | 3085 | 64.8 | 3325 | 69.9 | 3568 | 74.9 | |
| Physical activity during leisure | <0.001 | ||||||||
| Low | 10,796 | 76.7 | 3865 | 82.6 | 3621 | 77.3 | 3310 | 70.3 | |
| Moderate | 1986 | 14.1 | 499 | 10.7 | 683 | 14.6 | 804 | 17.2 | |
| Vigorous | 1287 | 9.2 | 315 | 6.7 | 379 | 8.1 | 593 | 12.5 | |
| Change in diet | <0.001 | ||||||||
| No | 9903 | 69.4 | 3517 | 73.9 | 3247 | 68.3 | 3139 | 66.0 | |
| Yes | 4366 | 30.6 | 1243 | 26.1 | 1507 | 31.7 | 1616 | 34.0 | |
| Use of dietary supplements | <0.001 | ||||||||
| No | 10,887 | 77.3 | 3940 | 84.2 | 3639 | 77.5 | 3308 | 70.5 | |
| Regularly | 1823 | 12.9 | 391 | 8.3 | 579 | 12.3 | 853 | 18.0 | |
| Not regularly | 1381 | 9.8 | 353 | 7.5 | 480 | 10.2 | 548 | 11.5 | |
| Nutritional status | <0.001 | ||||||||
| Low weight | 129 | 0.9 | 57 | 1.2 | 34 | 0.7 | 38 | 0.8 | |
| Eutrophic | 5175 | 36.2 | 1672 | 35.1 | 1662 | 34.9 | 1841 | 38.7 | |
| Overweight | 5740 | 40.2 | 1898 | 39.9 | 1940 | 40.8 | 1902 | 40.0 | |
| Obese | 3234 | 22.7 | 1,32 | 23.8 | 1124 | 23.6 | 978 | 20.5 | |
| Hypertension | <0.001 | ||||||||
| No | 9930 | 69.5 | 3047 | 64.0 | 3120 | 65.5 | 3163 | 66.4 | |
| Yes | 4951 | 34.7 | 1714 | 36.0 | 1640 | 34.5 | 1597 | 33.6 | |
| Diabetes | <0.001 | ||||||||
| No | 11,558 | 80.9 | 3797 | 79.8 | 3886 | 81.6 | 3875 | 81.4 | |
| Yes | 2724 | 19.1 | 963 | 20.2 | 875 | 18.4 | 886 | 18.6 | |
| Dyslipidemia | <0.001 | ||||||||
| No | 6007 | 42.4 | 2225 | 47.0 | 1926 | 40.8 | 1856 | 39.4 | |
| Yes | 8169 | 57.6 | 2510 | 53.0 | 2799 | 59.2 | 2860 | 60.6 | |
| Thyroid function | <0.001 | ||||||||
| Subclinical hypothyroidism | 770 | 5.4 | 276 | 5.8 | 252 | 5.3 | 242 | 5.1 | |
| Clinical hypothyroidism | 1061 | 7.4 | 256 | 5.4 | 383 | 8.0 | 422 | 8.9 | |
| Euthyroid | 12,171 | 85.3 | 4146 | 87.1 | 4022 | 84.5 | 4003 | 84.1 | |
| Subclinical hyperthyroidism | 186 | 1.3 | 57 | 1.2 | 70 | 1.5 | 59 | 1.2 | |
| Clinical hyperthyroidism | 95 | 0.6 | 26 | 0.5 | 34 | 0.7 | 35 | 0.7 | |
* Energy-adjusted nutrient; ** p values of the chi-square test.
The major food sources of dietary selenium verified in this study were: rice (23%), meat (13%), bread (12%), beans (10%), milk (10%), fish (8%), pasta (5%) and nuts (4%).
The lower tertile of Se consumption had a higher proportion of participants who were males, of Black and mixed race, aged 35–59 years, in the lowest tertile of per capita income, non-smokers, alcohol consumers, with a low level of physical activity during leisure, without significant changes in diet, not using dietary supplements, overweight, non-hypertensive, non-diabetic, dyslipidemic and euthyroid. The highest tertile of Se intake had a predominance of participants who were Caucasian, female, aged 39–59 years, in the highest tertile of per capita income, non-smokers, alcohol users, with a low level of physical activity during leisure, without significant changes in the diet, not using food supplements, non-hypertensive, non-diabetic, dyslipidemic and euthyroid (Table 1).
The analysis of the other nutrients showed a correlation with thyroid function with respect to the consumption tertiles relative to Se intake tertiles (Table 2). All analyzed micronutrients were positively correlated with Se intake, particularly total fats, which presented a higher correlation coefficient (r = 0.33) (Table 2).
Table 2.
Intake of energy and micronutrients and urinary sodium per selenium intake tertile in the ELSA-Brasil study, 2017.
| Total | Selenium Intake * | R *** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| First Tertile (0–187 mg) |
Second Tertile (188–232 mg) |
Third Tertile (233–1087 mg) |
p Value ** | ||||||
| N | N | % | N | % | N | % | |||
| Energy (average) | <0.001 | _ | |||||||
| First tertile (1900 kcal) | 4761 | 1049 | 22.0 | 2202 | 46.2 | 1510 | 31.7 | ||
| Second tertile (2735 kcal) | 4761 | 1599 | 33.6 | 1529 | 32.1 | 1633 | 34.3 | ||
| Third tertile (4166 kcal) | 4761 | 2113 | 44.4 | 1030 | 21.6 | 1618 | 34.0 | ||
| Zinc * (average) | <0.001 | 0.16 | |||||||
| First tertile (13 mg) | 4761 | 2562 | 53.8 | 1226 | 25.7 | 973 | 20.4 | ||
| Second tertile (16 mg) | 4761 | 1278 | 26.8 | 1833 | 38.5 | 1650 | 34.7 | ||
| Third tertile (21 mg) | 4761 | 921 | 19.3 | 1702 | 35.8 | 2138 | 44.9 | ||
| Vitamin A * (average) | <0.001 | 0.11 | |||||||
| First tertile (71 mg) | 4761 | 1887 | 39.6 | 1614 | 33.9 | 1260 | 26.5 | ||
| Second tertile (125 mg) | 4761 | 1443 | 30.3 | 1714 | 36.0 | 1604 | 33.7 | ||
| Third tertile (220 mg) | 4761 | 1431 | 30.1 | 1433 | 30.7 | 1897 | 40.6 | ||
| Total fat * (average) | <0.001 | 0.33 | |||||||
| First tertile (74 g) | 4761 | 2335 | 49.0 | 1418 | 29.8 | 1008 | 21.2 | ||
| Second tertile (93 g) | 4761 | 1399 | 29.4 | 1815 | 38.1 | 1547 | 31.6 | ||
| Third tertile (112 g) | 4761 | 1027 | 21.6 | 1528 | 32.1 | 2206 | 47.2 | ||
| Saturated fat * (average) | <0.001 | 0.13 | |||||||
| First tertile (22 g) | 4761 | 2107 | 44.3 | 1354 | 28.4 | 1300 | 27.8 | ||
| Second tertile (30 g) | 4761 | 1315 | 28.1 | 1825 | 39.1 | 1621 | 34.7 | ||
| Third tertile (41 g) | 4761 | 1339 | 28.7 | 1582 | 33.9 | 1840 | 39.0 | ||
| Urinary sodium (average) | <0.001 | −0.08 | |||||||
| First tertile (6 g/day) | 4665 | 1345 | 28.8 | 1537 | 33.0 | 1773 | 38.1 | ||
| Second tertile (10 g/day) | 4667 | 1528 | 32.8 | 1563 | 33.6 | 1576 | 33.9 | ||
| Third tertile (19 g/day) | 4642 | 1782 | 38.4 | 1557 | 33.4 | 1303 | 28.0 | ||
* Energy-adjusted nutrient; ** p value of the chi-square test; *** Pearson correlation coefficient between selenium and other nutrients (p < 0.001).
Table 3 shows the inverse association between Se intake and subclinical hypothyroidism based on logistic regression models adjusted for gender, self-reported race, age, per capita income, current smoking, current alcohol use, physical activity, use of supplements, dietary change in the past 6 months, total energy intake, total and saturated fat consumption, zinc and vitamin A consumption, urinary sodium, nutritional status, diabetes, hypertension and dyslipidemia.
Table 3.
Logistic regression models between subclinical hypothyroidism (outcome)* and selenium intake adjusted for the consumption of zinc, vitamin A, total and saturated fats and urinary sodium. ELSA-Brasil study, 2017.
| Model 1 a | Model 2 b | Model 3 c | Model 4 d | Model 5 e | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||||
| Selenium ** | |||||||||||||||
| First tertile (0–187 mg) | 1 | _ | _ | 1 | _ | _ | 1 | _ | _ | 1 | _ | _ | 1 | _ | _ |
| Second tertile (188–232 mg) | 0.89 | 0.74 | 1.08 | 0.85 | 0.71 | 1.03 | 0.81 | 0.67 | 0.98 | 0.80 | 0.66 | 0.97 | 0.79 | 0.65 | 0.96 |
| Third tertile (233–1087 mg) | 0.90 | 0.75 | 1.08 | 0.86 | 0.70 | 1.05 | 0.74 | 0.60 | 0.92 | 0.73 | 0.59 | 0.91 | 0.72 | 0.58 | 0.90 |
* For this analysis the sample includes only participants with subclinical hypothyroidism or euthyroidism; ** Energy-adjusted nutrients using the residuals method; a Model used to assess the correlation between subclinical hypothyroidism and selenium intake tertiles adjusted for energy intake, use of dietary supplements and diet change in the past 6 months; b Model adjusted by model 1 variables plus, zinc, vitamin A, total and saturated fats and urinary sodium; c Model adjusted by model 2 variables plus gender, age, per capita income and self-declared race; d Model adjusted by model 3 variables plus current smoking, current alcohol use and practice of physical activity; e Model adjusted by model 4 variables plus presence of diabetes, arterial hypertension, dyslipidemia and nutritional status.
4. Discussion
Selenium intake showed an inverse association with subclinical hypothyroidism, independent of the intake of energy and other nutrients that were previously shown to be correlated with thyroid function.
Most studies that analyzed the effects of Se on thyroid function and hypothyroidism are experimental and three studies [36,37,38] assessed the effect of Se supplementation on the enzymatic and hormonal functions essential for thyroid maintenance in Se-deficient rats. These studies found a positive correlation between Se and the analyzed parameters. Understanding the health implications of Se in humans has been far more difficult, as Se intakes nor tissue levels of free-living people can seldom be ascertained with the levels of confidence typical of controlled animal experiments [17].
It is well known that the thyroid gland retains selenium and selenoprotein activity even under conditions of severe deficiency [39]. However, it remains unknown whether selenium modulates peripheral thyroid hormone action via less prioritized mechanisms [13]. A cross-sectional study [15] involving 6152 participants from two municipalities in China determined the prevalence of thyroid diseases in two similar areas, except in participants with extreme Se levels and showed that low selenium status was associated with increased risk of thyroid disease and concluded that increased selenium intake may reduce the risk in areas of low selenium intake. However, Thomson et al. [40] analyzed data from two cross-sectional and three interventional studies conducted in New Zealand on the effects of Se on thyroid metabolism and found no significant correlations, even after Se supplementation.
Despite data on large controlled trials, that would provide more reliable evidence, are scarce, randomized controlled trials, conducted in healthy and diseased participants and used different doses of supplemental Se, found no significant effects of this micronutrient on the outcome of interest [41,42,43,44]. Studies that reported detrimental [45,46] or worsening results also were found in literature [47]. Despite that, in general, an improvement on levels of Se were observed [45,48,49]. In a prospective randomized controlled study by Pirola et al. [50] involving 192 patients supplemented with Se for 4 months, euthyroidism was restored in 1/3 of subclinical hypothyroidism patients with autoimmune thyroiditis.
Despite the lack of studies and inconclusive evidence, Negro et al. [51] (2016) evaluated a sample of 778 Italian endocrinologists and observed that more than two-thirds of the study population used Se supplementation as a therapy for subclinical hypothyroidism and 60% of this sample suggested daily doses of 100–200 μg, 20–30% recommended doses of <100 μg and 10–20% suggested doses of >200 μg. These recommendations are higher than those proposed by the Dietary Reference Intakes [52].
The present study has limitations. Our estimates of selenium intake were obtained from a FFQ, a method that is widely used in large epidemiological studies to determine the frequency of consumption of specific food products in 1 year [29]. This method is appropriated to rank individual according to levels of intake, however, it is not considered the most appropriate for the quantitative analysis of micronutrients because it tends to overestimate dietary intake and. Moreover, as it lacks accuracy, does not allow the evaluation of the adequacy of the ingested micronutrients within this period, therefore only allowing a comparison between major and minor consumers. Nutrient intake can be better estimated using repeated 24-h recalls and dietary records because the mean values obtained from several days of dietary intake yield safer and more reliable results [53]. However, as these methods are based on the individual report, they still are susceptible to bias. For greater accuracy of the selenium levels in the participants, it would be necessary to use biomarkers capable of indicating more precisely the condition of these individuals [3,12,20]. Especially taking into account that there is enormous variability of Se levels in foods, as it is dependent exclusively on the soil properties from which they were harvested. So, the demonstrated selenium consumption tertiles may not correspond to the participants’ exact selenium levels. However, we used the obtained Se estimates to rank individual according to their intake and we used the distribution of this intake in our population to define cutoffs. We used the same composition table that was used in the last National Dietary Survey 2009–2009 in Brazil, carefully checked for the completeness of Se information. In this case, we expect a systematic bias and a hypothesized relationship is possible to identify, that need to be confirmed. Another important limitation lies in the design of the study, since it is a cross-sectional observational study and suffer from limitations inherent to the observational design, including exposure misclassification and unmeasured confounding. Although it has been adjusted by several dietary and non-dietary factors, these are data from ELSA-Brasil baseline and we did not longitudinally evaluate food consumption in this population, which would be more appropriate. Therefore, because it is an observational study, the results found on the relationship between selenium and the outcome cannot be interpreted as a causal relation, requiring that they would be confirmed in further studies.
5. Conclusions
The results revealed an inverse correlation between Se intake and subclinical hypothyroidism. However, further research is needed to confirm the involvement of Se in subclinical hypothyroidism using more accurate methodologies of dietary assessment and nutritional status to evaluate this relationship.
Acknowledgments
Not applicable.
Author Contributions
Designed the study’s analytic strategy: G.R.G.A., B.G., I.M.B., D.M.M. Analyzed the data: G.R.G.A., B.G., D.M.M. Wrote the article: G.R.G.A. Discussion and final review: G.R.G.A., B.G., D.M.M., P.A.L., I.M.B. All authors participated in critically revising the manuscript and approved the final version.
Funding
The ELSA-Brasil baseline study was supported by the Brazilian Ministry of Health (Science and Technology Department) and the Brazilian Ministry of Science and Technology and National Research Council (grants 01 06 0010.00 RS, 01 06 0212.00 BA, 01 06 0300.00 ES, 01 06 0278.00 MG, 01 06 0115.00 SP and 01 06 0071.00 RJ). The research center of São Paulo was also supported by São Paulo Research Foundation (FAPESP; grant 2011/12256-4). G.R.G.A received a scholarship from the FAPESP (grant 2016/22077-3). No funding agencies had a role in the study design, data collection, analysis, decision to publish, or preparation of the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ichiki T. Thyroid hormone and vascular remodeling. J. Atheroscler. Thromb. 2016;23:266–275. doi: 10.5551/jat.32755. [DOI] [PubMed] [Google Scholar]
- 2.Bensenor I.M., Olmos R.D., Lotufo P.A. Hypothyroidism in the elderly: Diagnosis and management. Clin. Interv. Aging. 2012;7:97–111. doi: 10.2147/CIA.S23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinceti M., Filippini T., Del Giovane C., Dennert G., Zwahlen M., Brinkman M., Zeegers M.P., Horneber M., D’Amico R., Crespi C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018;1:CD005195. doi: 10.1002/14651858.CD005195.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatourechi V. Subclinical hypothyroidism: An update for primary care physicians. Mayo Clin. Proc. 2009;84:65–71. doi: 10.4065/84.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bensenor I.M., Goulart A.C., Lotufo P.A., Menezes P.R., Scazufca M. Prevalence of thyroid disorders among older people: Results from the São Paulo Ageing and Health Study. Cad. Saúde Pública. 2011;27:155–161. doi: 10.1590/S0102-311X2011000100016. [DOI] [PubMed] [Google Scholar]
- 6.Olmos R.D., Figueiredo R.C., Aquino E.M., Lotufo P.A., Bensenor I.M. Gender, race and socioeconomic influence on diagnosis and treatment of thyroid disorders in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Braz. J. Med. Biol. Res. 2015;48:751–758. doi: 10.1590/1414-431X20154445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sichieri R., Baima J., Marante T., de Vasconcellos M.T., Moura A.S., Vaisman M. Low prevalence of hypothyroidism among black and Mulatto people in a population-based study of Brazilian women. Clin. Endocrinol. 2007;66:803–807. doi: 10.1111/j.1365-2265.2007.02816.x. [DOI] [PubMed] [Google Scholar]
- 8.Ventura M., Melo M., Carrilho F. Selenium and thyroid disease: From pathophysiology to treatment. Int. J. Endocrinol. 2017;2017:1297658. doi: 10.1155/2017/1297658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schomburg L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat. Rev. Endocrinol. 2011;8:160–171. doi: 10.1038/nrendo.2011.174. [DOI] [PubMed] [Google Scholar]
- 10.O’Kane S.M., Mulhern M.S., Pourshahidi L.K., Strain J.J., Yeates A.J. Micronutrients, iodine status and concentrations of thyroid hormones: A systematic review. Nutr. Rev. 2018;76:418–431. doi: 10.1093/nutrit/nuy008. [DOI] [PubMed] [Google Scholar]
- 11.Behne D., Hilmert H., Scheid S., Gessner H., Elger W. Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim. Biophys. Acta. 1988;966:12–21. doi: 10.1016/0304-4165(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 12.Fairweather-Tait S.J., Bao Y., Broadley M.R., Collings R., Ford D., Hesketh J.E., Hurst R. Selenium in human health and disease. Antioxid. Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 13.Schomburg L., Riese C., Michaelis M., Griebert E., Klein M.O., Sapin R., Schweizer U., Köhrle J. Synthesis and metabolism of thyroid hormones is preferentially maintained in selenium-deficient transgenic mice. Endocrinology. 2006;147:1306–1313. doi: 10.1210/en.2005-1089. [DOI] [PubMed] [Google Scholar]
- 14.Parshukova O., Potolitsyna N., Shadrina V., Chernykh A., Bojko E. Features of selenium metabolism in humans living under the conditions of North European Russia. Int. Arch. Occup. Environ. Health. 2014;87:607–614. doi: 10.1007/s00420-013-0895-4. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q., Rayman M.P., Lv H., Schomburg L., Gao C., Chen P., Zhuang G., Zhang Z., Peng X., Li H., et al. Low population selenium status is associated with increased prevalence of thyroid disease. J. Clin. Endocrinol. Metab. 2015;100:4037–4047. doi: 10.1210/jc.2015-2222. [DOI] [PubMed] [Google Scholar]
- 16.Oldfield J.E. Selenium World Atlas. Selenium-Tellurium Development Association; Grimbergen, Belgium: 2002. [Google Scholar]
- 17.Combs G.F. Selenium in global food systems. Br. J. Nutr. 2001;85:517–547. doi: 10.1079/BJN2000280. [DOI] [PubMed] [Google Scholar]
- 18.Donadio J.L., Guerra-Shinohara E.M., Rogero M.M., Cozzolino S.M. Influence of Gender and SNPs in GPX1 Gene on Biomarkers of Selenium Status in Healthy Brazilians. Nutrients. 2016;8:81. doi: 10.3390/nu8050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favaro D.I., Hui M.L., Cozzolino S.M., Maihara V.A., Armelin M.J., Vasconcellos M.B., Yuyama L.K., Boaventura G.T., Tramonte V.L. Determination of various nutrients and toxic elements in different brazilian regional diets by neutron activation analysis. J. Trace Elem. Med. Biol. 1997;11:129–136. doi: 10.1016/S0946-672X(97)80039-9. [DOI] [PubMed] [Google Scholar]
- 20.Combs G.F. Biomarkers of selenium status. Nutrients. 2015;7:2209–2236. doi: 10.3390/nu7042209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira K.S., Gomes J.C., Bellato C.R., Jordão C.P. Concentrações de selênio em alimentos consumidos no Brasil. Rev. Panam. Salud Publica. 2002;11:172–177. doi: 10.1590/S1020-49892002000300006. [DOI] [PubMed] [Google Scholar]
- 22.Kvicala J., Zamrazil V. Effect of iodine and selenium upon thyroid function. Cent. Eur. J. Public Health. 2003;11:107–113. [PubMed] [Google Scholar]
- 23.Combs G.F., Midthune D.N., Patterson K.Y., Canfield W.K., Hill A.D., Levander O.A., Taylor P.R., Moler J.E., Patterson B.H. Effects of selenomethionine supplementation on selenium status and thyroid hormone concentrations in healthy adults. Am. J. Clin. Nutr. 2009;89:1808–1814. doi: 10.3945/ajcn.2008.27356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson C.D., Campbell J.M., Miller J., Skeaff S.A., Livingstone V. Selenium and iodine supplementation: Effect on thyroid function of older New Zealanders. Am. J. Clin. Nutr. 2009;90:1038–1046. doi: 10.3945/ajcn.2009.28190. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Reyes R., Mathieu F., Boelaert M., Begaux F., Suetens C., Rivera M.T., Nève J., Perlmutter N., Vanderpas J. Selenium and iodine supplementation of rural Tibetan children affected by Kashin-Beck osteoarthropathy. Am. J. Clin. Nutr. 2003;78:137–144. doi: 10.1093/ajcn/78.1.137. [DOI] [PubMed] [Google Scholar]
- 26.Angstwurm M.W., Schopohl J., Gaertner R. Selenium substitution has no direct effect on thyroid hormone metabolism in critically ill patients. Eur. J. Endocrinol. 2004;151:47–54. doi: 10.1530/eje.0.1510047. [DOI] [PubMed] [Google Scholar]
- 27.Aquino E.M.L., Vasconcellos-Silva P.R., Coeli C.M., Araújo M.J., Santos S.M., De Figueiredo R.C., Duncan B.B. Aspectos éticos em estudos longitudinais: O caso do ELSA-Brasil. Rev. Saúde Pública. 2013;47:19–26. doi: 10.1590/S0034-8910.2013047003804. [DOI] [PubMed] [Google Scholar]
- 28.Bensenor I.M., Griep R.H., Pinto K.A., de Faria C.P., Felisbino-Mendes M., Caetano E.I., Albuquerque L.S., Schmidt M.I. Rotinas de organização de exames e entrevistas no centro de investigação ELSA-Brasil. Rev. Saúde Pública. 2013;47:37–47. doi: 10.1590/S0034-8910.2013047003780. [DOI] [PubMed] [Google Scholar]
- 29.Chor D., Alves M.G.M., Giatti L., Cade N.V., Nunes M.A., Molina M.C.B., Bensenor I.M., Aquino E.M.L., Passos V., Santos S.M., et al. Questionário do ELSA-Brasil: Desafios na elaboração de instrumento multidimensional. Rev. Saúde Pública. 2013;47:27–36. doi: 10.1590/S0034-8910.2013047003835. [DOI] [PubMed] [Google Scholar]
- 30.Molina M.C.B., Bensenor I.M., Cardoso L.O., Velazquez-Melendez G., Drehmer M., Pereira T.S.S., Faria C.P., Melere C., Manato L., Gomes A.L.C., et al. Reprodutibilidade e validade relativa do Questionário de Frequência Alimentar do ELSA-Brasil. Cad. Saúde Pública. 2013;29:379–389. doi: 10.1590/S0102-311X2013000600024. [DOI] [PubMed] [Google Scholar]
- 31.Fedeli L.G., Vidigal P.G., Leite C.M., Castilhos C.D., Pimentel R.A., Maniero V.C., Mill J.G., Lotufo P.A., Pereira A.C., Bensenor I.M. Logistics of collection and transportation of biological samples and the organization of the centrallaboratory in the ELSA-Brasil. Rev. Saude Publica. 2013;47:63–71. doi: 10.1590/S0034-8910.2013047003807. [DOI] [PubMed] [Google Scholar]
- 32.Willet W. Nutritional Epidemiology. Oxford University Press; New York, NY, USA: 2013. [Google Scholar]
- 33.World Health Organization . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. World Health Organization; Geneva, Switzerland: 2000. [(accessed on 10 April 2018)]. (WHO Technical Report Series 894). Available online: http://www.who.int/iris/handle/10665/42330. [PubMed] [Google Scholar]
- 34.Mezzomo T.R., Nadal J. Efeito dos nutrientes e substâncias alimentares na função tireoidiana e no hipotireoidismo. Demetra. 2016;11:427–443. doi: 10.12957/demetra.2016.18304. [DOI] [Google Scholar]
- 35.McLean R.M. Measuring population sodium intake: A review of methods. Nutrients. 2014;6:4651–4662. doi: 10.3390/nu6114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chanoine J.P., Safran M., Farwell A.P., Tranter P., Ekenbarger D.M., Dubord S., Alex S., Arthur J.R., Beckett G.J., Braverman L.E., et al. Selenium deficiency and type II 5′-deiodinase regulation in the euthyroid and hypothyroid rat: Evidence of a direct effect of thyroxine. Endocrinology. 1992;131:479–484. doi: 10.1210/endo.131.1.1612029. [DOI] [PubMed] [Google Scholar]
- 37.Kralik A., Eder K., Kirchgessner M. Influence of zinc and selenium deficiency on parameters relating to thyroid hormone metabolism. Horm. Metab. Res. 1996;28:223–226. doi: 10.1055/s-2007-979169. [DOI] [PubMed] [Google Scholar]
- 38.Beckett G.J., MacDougall D.A., Nicol F., Arthur J.R. Inhibition of type I and type II iodothyronine deiodinase activity in rat liver, kidney and brain produced by selenium deficiency. Biochem. J. 1989;259:887–892. doi: 10.1042/bj2590887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köhrle J., Jakob F., Contempre B., Dumont J.E. Selenium, the thyroid and the endocrine system. Endocr. Rev. 2005;26:944–984. doi: 10.1210/er.2001-0034. [DOI] [PubMed] [Google Scholar]
- 40.Thomson C.D., McLachlan S.K., Grant A.M., Peterson E., Lillico A.J. The effect of selenium on thyroid status in a population with marginal selenium and iodine status. Br. J. Nutr. 2005;94:962–968. doi: 10.1079/BJN20051564. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs J., Kahana M.J. Neural representations of individual stimuli in humans revealed by gamma-band ECoG activity. J. Neurosci. 2009;29:10203–10214. doi: 10.1523/JNEUROSCI.2187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Zuuren E.J., Albusta A.Y., Fedorowicz Z., Carter B., Pijl H. Selenium supplementation for Hashimoto’s thyroiditis. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD010223.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eskes S.A., Endert E., Fliers E., Birnie E., Hollenbach B., Schomburg L., Köhrle J., Wiersinga W.M. Selenite supplementation in euthyroid subjects with thyroid peroxidase antibodies. Clin. Endocrinol. 2014;80:444–451. doi: 10.1111/cen.12284. [DOI] [PubMed] [Google Scholar]
- 44.Kahaly G.J., Riedl M., König J., Diana T., Schomburg L. Double-Blind, Placebo-Controlled, Randomized Trial of Selenium in Graves Hyperthyroidism. J. Clin. Endocrinol. Metab. 2017;102:4333–4341. doi: 10.1210/jc.2017-01736. [DOI] [PubMed] [Google Scholar]
- 45.Winther K.H., Bonnema S.J., Cold F., Debrabant B., Nybo M., Cold S., Hegedus L. Does selenium supplementation affect thyroid function? Results from a randomized, controlled, double-blinded trial in a Danish population. Eur. J. Endocrinol. 2015;172:657–667. doi: 10.1530/EJE-15-0069. [DOI] [PubMed] [Google Scholar]
- 46.Mao J., Pop V.J., Bath S.C., Vader H.L., Redman C.W., Rayman M.P. Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. Eur. J. Nutr. 2016;55:55–61. doi: 10.1007/s00394-014-0822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Contempre B., Dumont J.E., Ngo B., Thilly C.H., Diplock A.T., Vanderpas J. Effect of selenium supplementation in hypothyroid subjects of an iodine and selenium deficient area: The possible danger of indiscriminate supplementation of iodine-deficient subjects with selenium. J. Clin. Endocrinol. Metab. 1991;73:213–215. doi: 10.1210/jcem-73-1-213. [DOI] [PubMed] [Google Scholar]
- 48.Rayman M.P., Thompson A.J., Bekaert B., Catterick J., Galassino R., Hall E., Warren-Perry M., Beckett G.J. Randomized controlled trial of the effect of selenium supplementation on thyroid function in the elderly in the United Kingdom. Am. J. Clin. Nutr. 2008;87:370–378. doi: 10.1093/ajcn/87.2.370. [DOI] [PubMed] [Google Scholar]
- 49.Hawkes W.C., Keim N.L., Diane Richter B., Gustafson M.B., Gale B., Mackey B.R., Bonnel E.L. High-selenium yeast supplementation in free-living North American men: No effect on thyroid hormone metabolism or body composition. J. Trace Elem. Med. Biol. 2008;22:131–142. doi: 10.1016/j.jtemb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Pirola I., Gandossi E., Agosti B., Delbarba A., Cappelli C. Selenium supplementation could restore euthyroidism in subclinical hypothyroid patients with autoimmune thyroiditis. Endokrynol. Pol. 2016;67:567–571. doi: 10.5603/EP.2016.0064. [DOI] [PubMed] [Google Scholar]
- 51.Negro R., Attanasio R., Grimaldi F., Morcocci C., Guglielmi R., Papine E. A 2016 Italian survey about the clinical use of selenium in thyroid disease. Eur. Thyroid J. 2016;5:164–170. doi: 10.1159/000447667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Institute of Medicine . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. National Academy Press; Washington, DC, USA: 2000. [(accessed on 16 April 2018)]. Available online: http://books.nap.edu/catalog/9810.html. [Google Scholar]
- 53.Thompson F.E., Kirkpatrick S.I., Subar A.F., Reedy J., Schap T.E., Wilson M.M., Krebs-Smith S.M. The national cancer institute’s dietary assessment primer: A resource for diet research. J. Acad. Nutr. Diet. 2015;115:1986–1995. doi: 10.1016/j.jand.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]