Abstract
Epstein–Barr virus-associated gastric carcinoma (EBVaGC) is the most common malignancy caused by EBV infection. EBVaGC has definite histological characteristics similar to gastric carcinoma with lymphoid stroma. Clinically, EBVaGC has a significantly low frequency of lymph node metastasis compared with EBV-negative gastric cancer, resulting in a better prognosis. The Cancer Genome Atlas of gastric adenocarcinomas proposed a molecular classification divided into four molecular subtypes: (1) EBVaGC; (2) microsatellite instability; (3) chromosomal instability; and (4) genomically stable tumors. EBVaGC harbors a DNA methylation phenotype, PD-L1 and PD-L2 overexpression, and frequent alterations in the PIK3CA gene. We review clinical importance of EBVaGC and discuss novel therapeutic applications for EBVaGC.
Keywords: Epstein–Barr virus, gastric carcinoma, DNA methylation, programed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), immune checkpoint inhibitor, endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD)
1. Introduction
Epstein–Barr virus (EBV) is a ubiquitous human herpes virus that was originally discovered in Burkitt lymphoma. EBV can transform primary B lymphocytes in vitro and is associated with nasopharyngeal carcinoma, Hodgkin lymphoma, natural killer (NK)/T lymphoma, and post-transplant lymphoproliferative disorders [1,2]. Gastric carcinoma is the fourth most frequent cancer in the world and is the second leading cause of cancer deaths. More than 95,000 new patients are diagnosed each year, and there were 72,000 gastric cancer-related deaths worldwide in 2012 [3]. About 9% of gastric cancers have been identified as EBV-positive [4]. Thus, EBV-associated gastric carcinoma (EBVaGC) is the most common cancer among EBV-related malignancies. In the Cancer Genome Atlas (TCGA), gastric cancer is classified according to its molecular biology into EBVaGC, microsatellite unstable tumors, genomically stable tumors, and chromosomally unstable tumors [5]. We review the clinicopathologic and molecular features of EBVaGC and discuss novel therapeutic applications for EBVaGC.
2. Definition
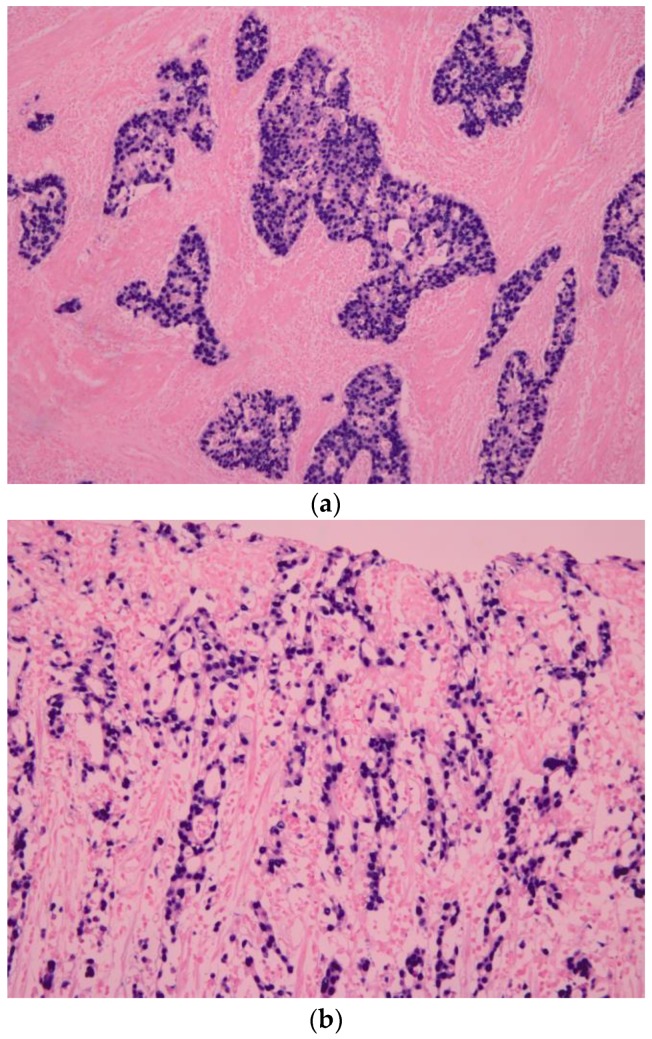
In 1990, Burke et al. reported EBV-positive gastric carcinoma by PCR [6]. In the early 1990s, various studies showed that EBVaGC comprises about 10% of all gastric cancers worldwide by using in situ hybridization (ISH) targeting EBV-encoded small RNA 1 (EBER1) [7,8]. EBER1 is abundant (10 million copies per cell) in latently EBV-infected cells. EBER1 ISH is commonly used to assess EBV infection in histopathologic samples. Typically, signals of EBER1 are detected in the nuclei of almost all carcinoma cells in EBVaGC (Figure 1). Imai et al. [9] and Fukayama et al. [10] reported that EBVaGC resulted from the monoclonal proliferation of EBV-infected cells.
Figure 1.
Histologic characteristic of Epstein–Barr virus-associated gastric carcinoma (EBVaGC). (a) EBV-encoded small ribonucleic acid (EBER1) in situ hybridization shows positive nuclei in the carcinoma cells, which are surrounded by infiltrating lymphocytes (×100); (b) Histologic characteristic of EBVaGC. The “lacy pattern” is composed of irregularly anastomosing tubules and moderate to dense lymphocytic infiltration (×100).
3. Pathologic Features
EBVaGC has definite histological relevance to carcinoma with lymphoid stroma (CLS) [11,12,13], which was originally reported by Watanabe et al. [14]. CLS is a poorly differentiated adenocarcinoma with diffuse and intense lymphocyte infiltration and resembles EBV-positive nasopharyngeal carcinoma (Figure 1a). The incidence of CLS was reported to be 3–4% of all gastric cancers. More than 80% of CLS is infected with EBV [11,12,13], whereas EBV was observed in 5–10% of ordinary-type gastric cancers that show features of moderately or poorly differentiated adenocarcinoma with various amount of lymphocytic infiltration.
It is known that Helicobacter pylori (H. pylori) is causally associated with gastric cancer development and is a pathogenic factor of chronic gastritis and intestinal metaplasia [15]. Several studies reported the preventive effect of H. pylori eradication on gastric cancer development [16,17]. We have also reported that EBVaGC is derived from gastric mucosa with chronic inflammation induced by H. pylori infection [18]. Although EBVaGC predominantly localizes in the middle or upper stomach, the background mucosa of EBVaGC is accompanied by mucosal atrophy and intestinal metaplasia [18]. It is uncertain whether H. pylori eradication has a preventive effect on the development of EBVaGC.
When cases of CLS are observed, EBER ISH should be performed to aid in the diagnosis. Tokunaga et al. reported that early-stage EBVaGC shows a characteristic histology called a “lacy pattern” that is composed of irregularly anastomosing tubules and moderate to dense lymphocytic infiltration (Figure 1b) [19]. Other pathologic characteristics were also reported; however, EBER1 ISH is necessary to prove EBV infection in tumor cells.
4. Clinical Features
Almost all studies show male predominance of EBVaGC [4]. Camargo et al. reported that the association of smoking with gastric cancer is stronger for EBVaGCs than for control cases [20].
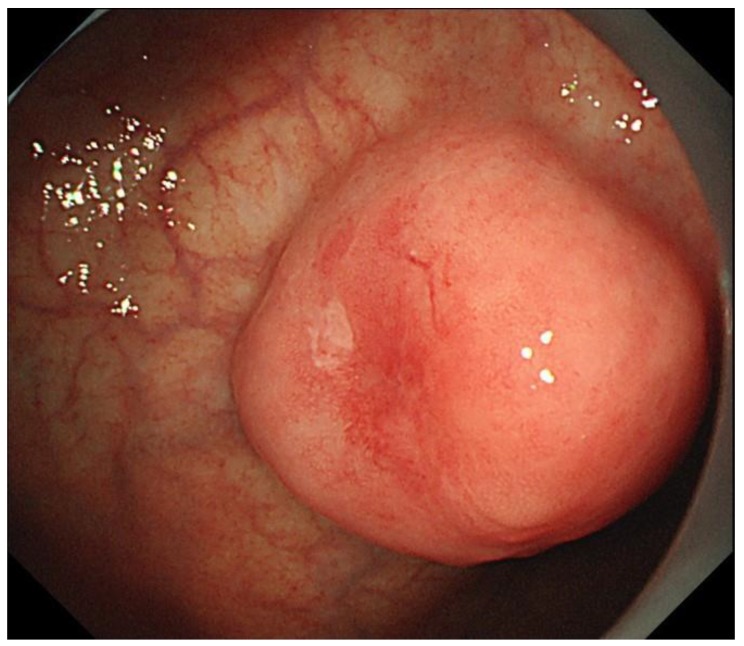
We reported endoscopic and endosonographic features of EBVaGC. EBVaGC predominantly localizes in the middle or upper stomach and appears as superficially depressed or ulcerated lesions [21]. It is considered that submucosal tumor-like morphology is one of the features of CLS. Although the majority of gastric CLS overlaps with EBVaGC, the submucosal tumor-like morphology may be helpful in discovering EBVaGC (Figure 2) [21]. Endosonography revealed a hypoechoic mass in the submucosal layer reflecting nodules, which is composed of tumor cells and infiltrating lymphocytes [22].
Figure 2.
Endoscopic images of an EBV-associated gastric carcinoma. Submucosal tumor-like morphology is one of the features of EBVaGC.
Gastric remnant cancer can arise after distal gastrectomy for gastroduodenal ulcers accompanied by bleeding or perforation. It is frequently (25% to 41.2%) associated with EBV infection [23,24]. A high prevalence of EBVaGC was reported in synchronous and metachronous gastric cancers [25,26]; however, this prevalence is not evident in meta-analyses of the characteristics of EBVaGC. Background mucosa in which EBVaGC had once been present might be at high risk for the redevelopment of EBVaGC.
A clinicopathological study showed that the frequency of lymph node metastasis (LNM) is significantly low in EBVaGCs compared with EBV-negative controls [27]. The rates of LNM in mucosa-confined and submucosal early gastric cancers are 2.2–4.6% and 14.0–23.6%, respectively [28,29,30,31,32,33]. The frequency of LNM is even lower in early EBVaGC. Tokunaga et al. reported that early-stage EBVaGC did not have LNM even in submucosally invading gastric cancers [34]. Park et al. showed that EBV negativity was an independent risk factor for LNM in submucosal early gastric cancers [35]. A recent meta-analysis revealed the infrequent tendency of EBVaGC toward LNM. After disease stage and prognostic factors were adjusted for, EBV positivity was associated with lower mortality [36].
5. Molecular Features of EBVaGC by TCGA Project
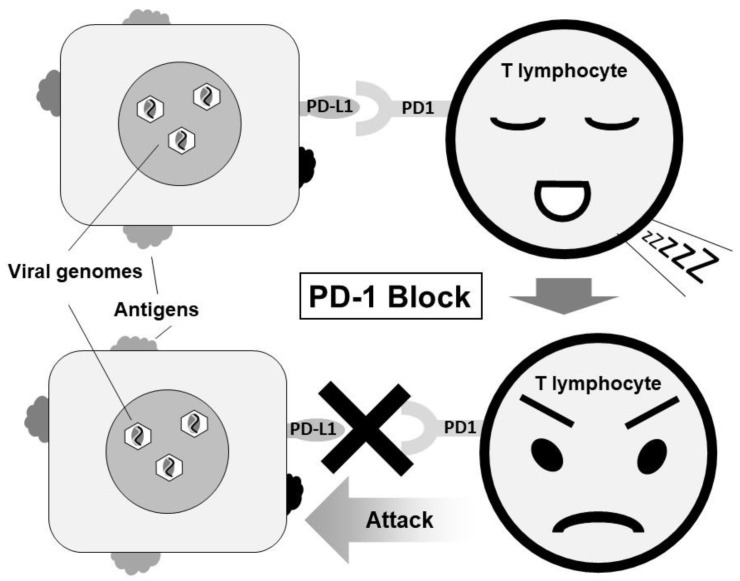
TCGA of gastric adenocarcinomas [5] divided gastric cancer into four molecular subtypes: (1) EBVaGC; (2) microsatellite instability; (3) chromosomal instability; and (4) genomically stable tumors. In TCGA project, 26 tumor samples of EBVaGCs were analyzed. The characteristics of EBVaGC are reported to include the harboring of recurrent PIK3CA mutations, DNA hypermethylation, amplification of JAK2, and overexpression of both PD-L1 and PD-L2 [5]. Recently, many investigators have reported PD-L1 overexpression in EBVaGC [37,38,39,40,41]. Crescenzi et al. reported that PD-L1 is expressed in gastric cancer cells, whereas PD-1 is expressed in infiltrating lymphocytes [42]. It seems that EBVaGC evades immunity from immunological recognition by T lymphocytes via the PD-1/PD-L1 pathway.
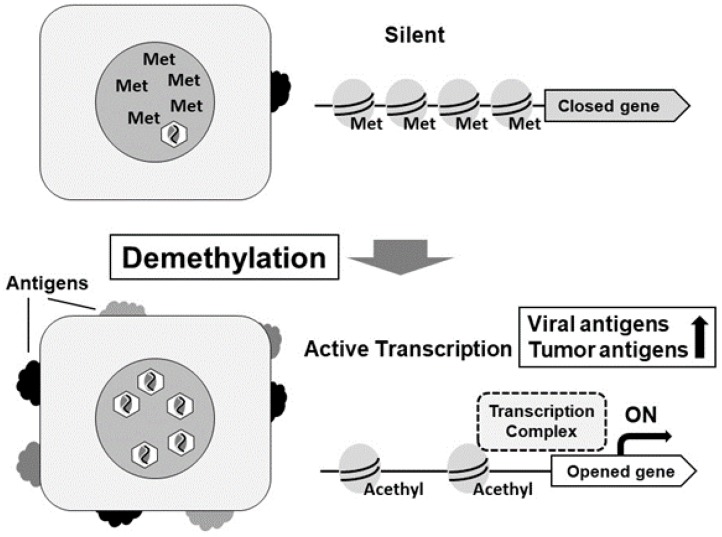
DNA methylation plays an important role in the development and progression of gastric cancer [43]. Methylation of both viral and host DNA is important for the development of EBVaGC. Viral DNA methylation controls the expression of EBV latent and lytic genes. Methylation of host cell DNA inactivated tumor suppressor genes and tumor-associated antigens [44]. Methylation frequencies of several tumor-related genes and DNA loci were reported to be significantly higher in EBVaGC [45,46,47,48,49,50]. The molecular mechanism that induces DNA methylation in EBVaGC has also been studied. Hino et al. showed that EBV latent membrane protein 2A (LMP2A) induces DNA methyltransferase 1 transcription via the phosphorylation of STAT3 [51]. Namba-Fukuyo et al. reported that TET2 downregulation was crucial in inducing DNA methylation in EBVaGC. Although the TET family genes encoded DNA demethylase, TET2 was downregulated by EBV transcripts such as BARF0 and LMP2A and TET2 targeting miRNAs [52]. Matsusaka et al. showed that when EBV was infected into MKN7 gastric cancer cells and the GES1 non-neoplastic gastric epithelial cell line, DNA methylation was induced in these cells [53,54].
The carcinogenic interaction between H. pylori and EBV was recently reported. Tyrosine-phosphorylated cytotoxin-associated gene A (Cag A) binds to the tyrosine phosphatase SHP2 and deregulates the phosphatase activity, which has been considered to play an important role in gastric carcinogenesis [55,56]. SHP2 homologue SHP1 also interacts with Cag A, but SHP1 dampens the oncogenic function of Cag A. Saju et al. reported that EBV infection can induce promoter methylation of SHP1 and downregulate the expression of SHP1, which might increase the oncogenic function caused by the interaction of Cag A and SHP2 [57].
6. Treatment of EBVaGC
Most EBVaGCs are surgically resected, and better prognosis in these patients has already been reported [36].
6.1. Treatment for Early-Stage EBVaGC
Early gastric cancer is defined as cancer confined to the gastric mucosa or submucosa, and the 5-year survival rate of early gastric cancer exceeds 90% in Japan. Because endoscopic examination is highly advanced in Japan, more than 50% of gastric cancer cases are early detections. Endoscopic mucosal resection (EMR) was designed as a local treatment for early gastric cancers appearing with no LNM [58]. The guideline criteria for EMR according to the Japanese Gastric Cancer Association is mucosal cancer ≤20 mm in size without ulcerative findings [59]. When lesions fit these criteria, EMR achieves results equal to those of surgical resection [60,61]. Endoscopic submucosal dissection (ESD) is a novel endoscopic technique that enables the resection of larger lesions en bloc. Gotoda et al. analyzed 5265 early gastric cancer patients who underwent gastrectomy with lymph node dissection. They provided important information on the risks of LNM and proposed expanded criteria for endoscopic resection: (1) mucosal cancer without ulcer findings, irrespective of tumor size; (2) mucosal cancer with an ulcer ≤3 cm in diameter; and (3) minimal (≤500 μm from the muscularis mucosa) submucosal invasive cancer ≤3 cm in size [28]. Use of the ESD method has achieved curative resections in both the guideline and the expanded-criteria lesions.
EBVaGC has a significantly low frequency of LNM compared with EBV-negative gastric cancer, especially in the early stage [34,35,36]. The criteria of endoscopic resection have been carefully expanded. In the future, EBV-associated early gastric cancers will become an indication for minimally invasive therapy such as EMR and ESD because LNM in submucosal invasive cancer with EBV infection is low.
6.2. Treatment for Advanced-Stage EBVaGC
6.2.1. DNA Methylation Inhibitors
The therapeutic application of DNA demethylating agents for EBVaGC is an attractive approach. Because demethylation agents induce lytic EBV infection in latently EBV-infected cells followed by apoptotic cell death, the demethylating agents may lead to the lysis of cancer cells. [47,48]. We investigated the anticancer effects of 5-aza-2′-deoxycytidine (decitabine) against EBVaGC cell lines. Decitabine induced G2/M arrest, apoptosis, and the expression of E-cadherin in the cells. The promoters of tumor suppressor genes were demethylated, and their expression was upregulated by decitabine treatment [62]. These facts strongly support the possible application of demethylating agents in the medical treatment of EBV-associated gastric cancer (Figure 3).
Figure 3.
Treatment by DNA demethylating agents for EBVaGC.
DNA methylation inhibitors 5-azacytidine and decitabine were approved by the US Food and Drug Administration in 2004 and 2006, respectively, for the treatment of patients with myelodysplastic syndromes. Decitabine was efficacious in solid tumors, including lung cancer, esophageal cancer, and pleural mesothelioma [63]. Epigenetic agents showed antitumor activity against solid tumors by the single or combinational use with standard anticancer treatment. A phase 1 study was conducted to identify a tolerable dose of 5-azacitidine prior to neoadjuvant chemotherapy in patients with locally advanced esophageal/gastric adenocarcinoma. Neoadjuvant administration together with 5-azacitidine with chemotherapy was tolerated with significant clinical and epigenetic responses. Several tumor suppressor genes were demethylated and expressed in the tumors resected surgically [64].
The efficacy of decitabine is limited because of its instability in vivo [65]. Nanoparticle-based drug delivery systems have rapidly developed in the field of cancer therapeutics. Many reports have confirmed the superiority of nanoparticles, such as decitabine-loaded nanogels [66]. Decitabine in nanogel sustains DNMT1 depletion and makes cancer cells stay in G2/M arrest. Nanoparticle-based drug delivery systems could potentially be explored to treat solid tumors as well [67].
6.2.2. Immune Checkpoint Inhibitor
Treatment by blocking PD-1/PD-L1, which is an immune checkpoint molecule for the immunoregulatory system, currently shows good prognosis in various cancers. The anti-PD-1 antibody nivolumab showed therapeutic effects in malignant melanoma, non-small cell lung cancer, renal cell carcinoma, and Hodgkin lymphoma [68,69,70,71,72,73]. Recently, patients with advanced or recurrent gastric cancer who could not undergo standard therapy due to their physical condition or treatment intolerance have been treated with nivolumab. These patients showed significantly prolonged overall survival in comparison with those receiving a placebo [74].
It is important to search for biomarkers that predict the effects of anti-PD-1/PD-L1 antibody therapy. Previous studies reported that tumors with high expression levels of PD-L1 and tumors with lymphocytic infiltration in the stroma are more likely to respond to anti-PD-1 antibody therapy [74,75]. The expression of PD-L1 is common in EBVaGC and EBV-associated post-transplant lymphoproliferative disorder, NK/T lymphoma, Hodgkin lymphoma, and nasopharyngeal carcinoma [76,77]. The constitutive expression of PD-L1 might be involved in the development of these EBV-related tumors. It is reported that carcinomas sensitive to immune checkpoint inhibitors have numerous genetic mutations by expressing diverse neoantigens. In particular, EBVaGC expresses EBV latent and lytic genes, which may also act as neoantigens (Figure 4).
Figure 4.
Treatment by blocking PD-1/PD-L1 for EBVaGC.
There is a strong urge to identify a biomarker predicting the response to checkpoint blockades because EBV-associated cancers, especially EBVaGC, could be therapeutic targets [78]. The new marker may also be applied to treat virus-related malignancy by developing novel immunotherapy.
6.3. Potential Therapies for EBVaGC
6.3.1. Proteasome Inhibitors
Proteasome is an enzyme complex localized in the cytoplasm and the nucleus that maintains the intracellular environment by degrading and removing ubiquitinated proteins. EBV infection protects infected cells from immunological attack and apoptosis by accelerating degradation of host proteins by proteasome. BDLF3 assists in evading immune recognition because this EBV protein induces ubiquitination of both major histocompatibility complex (MHC)-I and MHC-II molecules [79]. BZLF1 is an inducer of EBV lytic infection, which forms a complex with the apoptosis inducer p53, but is subject to ubiquitination [80]. Therefore, inhibition of proteasomal function in EBV infected cells induces viral lytic infection and apoptosis of infected cells. Single or combinational use of the proteasome inhibitor bortezomib with radiation therapy suppressed tumor formation in mice transplanted with primary EBV-associated gastric cancer cells [81]. However, therapeutic application of bortezomib is limited because this drug has serious side effects such as thrombocytopenia.
6.3.2. Infusion of EBV-Specific Cytotoxic T Cells
In immunologically competent persons who are infected with EBV, most of the EBV-antigen expressing cells have been removed from the body by EBV-specific cytotoxic T cells (EBV-CTL). Therefore, EBV-CTL therapy can be administered to the EBV-infected cancer. Such an attempt was applied to treat lymphoproliferative disorders caused after transplantation because EBV antigens are highly expressed on the proliferating B cells [82]. Moreover, a recent study extended EBV-CTL therapy to EBV-positive epithelial cell tumors. Straathof and colleagues reported that four of ten nasopharyngeal cancer patients who received EBV-CTL went into remission, and 2 of the 6 refractory patients exhibited immune responses [83]. However, the efficacy of EBV-CTL is influenced by the combination of MHC genes and antigen epitopes. It is hard to expect a therapeutic effect of EBV-CTL against EBV-associated gastric cancer because EBV-associated gastric cancer cells exhibit type I latency, which expresses EBNA1 with low antigenicity or LMP2A only in some of the cells.
6.3.3. Histone Deacetylase (HDAC) Inhibitors
Histone modification is known to control lytic infection of EBV [84]. Hui et al. reported that HDAC inhibitors induced lytic infection in latently EBV-infected gastric cancer cell lines [85]. EBV is not sensitive to ganciclovir because the viral thymidine kinase is not expressed during latent infection. However, Hui et al. succeeded in the targeted killing of infected cells by combinational use of ganciclovir and the HDAC inhibitor romidepsin by inducing lytic replicative infection [85].
6.3.4. Indoleamine 2,3-Dioxygenase (IDO) Inhibitors
Depletion of tryptophan inhibits the expression of NK cell receptor NKG2D, which results in the suppression of T-cell proliferation [86]. Expression of IDO, a tryptophan metabolizing enzyme, is elevated in EBV-infected cells. IDO induces immunological evasion of infected cells from NK cell recognition [87]. Thus, IDO inhibitors can be applied to remove EBV-infected cells.
6.3.5. Small-Molecule EBNA1 Inhibitors
EBNA1 plays an important role in establishing persistent infection of EBV because the molecule binds and cross-links viral genomes and human genomes. Thus, EBNA1 is expressed in any of EBV-associated cancer cells. Thompson and colleagues screened EBNA1 inhibitors and found 3 structural EBNA1 mimics from 14,000 substances [88]. Treatment of Raji cells with EBNA1 inhibitors reduced the EBV copy number of the cells in a dose-dependent manner. Similar inhibitors discovered by in silico virtual screening are regarded as attractive candidates for EBV eliminators [89].
6.3.6. EBV Vaccine
Although the development of live vaccine is facing difficulties, development of component EBV vaccines for preventive and therapeutic use has been continuing. The gp350 glycoprotein is used for most vaccines as an antigen. Moreover, EBNA1 and LMP2A have also been used as antigens [90]. In addition to the development of component vaccines, another approach is also being investigated. AMMOM1 is a monoclonal antibody of EBV gH/gL, which inhibits viral infection to B cells and epithelial cells [91]. By utilizing the epitope recognized by the antibody, it might be possible to develop a novel type of EBV vaccine.
7. Summary
We reviewed the clinicopathologic and molecular features of EBVaGC. Due to the distinct characteristics of EBVaGC, minimally invasive surgery such as endoscopic resection could be indicated for EBVaGC in the early stage. DNA methylation inhibitor and immune checkpoint inhibitor treatment could be suitable targets for EBVaGC in the advanced stage.
Author Contributions
J.N. and H.Yo. conceived and designed the review. K.Sh., Y.K., S.S., M.N., H.I., K.Sa. and Y.S. collected and assembled the data. H.Ya., T.Y. and I.S. critically revised and approved the final article.
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Science and Technology of Japan (No. 16H05843 to H.Y. and No. 18K07974 to J.N.) and a Health Labor Sciences Research Grant from the Ministry of Health Labor and Welfare, Japan (No. 18fk0310105h0001 to H.Y.).
Conflicts of Interest
The authors declare no financial or commercial conflicts of interest.
References
- 1.Zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228:1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]
- 2.Rickinson A.B., Kieff E. Epstein-Barr virus. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology. 5th ed. Volume 2. Lippincott-Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 2655–2700. [Google Scholar]
- 3.Van Cutsem E., Sagaert X., Topal B., Haustermans K., Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 4.Murphy G., Pfeiffer R., Camargo M.C., Rabkin C.S. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke A.P., Yen T.S., Shekitka K.M., Sobin L.H. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod. Pathol. 1990;3:377–380. [PubMed] [Google Scholar]
- 7.Shibata D., Weiss L.M. Epstein-Barr virus-associated gastric adenocarcinoma. Am. J. Pathol. 1992;140:769–774. [PMC free article] [PubMed] [Google Scholar]
- 8.Tokunaga M., Land C.E., Uemura Y., Tokudome T., Tanaka S., Sato E. Epstein-Barr virus in gastric carcinoma. Am. J. Pathol. 1993;143:1250–1254. [PMC free article] [PubMed] [Google Scholar]
- 9.Imai S., Koizumi S., Sugiura M., Tokunaga M., Uemura Y., Yamamoto N., Tanaka S., Sato E., Osato T. Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA. 1994;91:9131–9135. doi: 10.1073/pnas.91.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukayama M., Hayashi Y., Iwasaki Y., Chong J., Ooba T., Takizawa T., Koike M., Mizutani S., Miyaki M., Hirai K. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab. Investig. 1994;71:73–81. [PubMed] [Google Scholar]
- 11.Oda K., Tamaru J., Takenouchi T., Mikata A., Nunomura M., Saitoh N., Sarashina H., Nakajima N. Association of Epstein-Barr virus with gastric carcinoma with lymphoid stroma. Am. J. Pathol. 1993;143:1063–1071. [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura S., Ueki T., Yao T., Ueyama T., Tsuneyoshi M. Epstein-Barr virus in gastric carcinoma with lymphoid stroma. Special reference to its detection by the polymerase chain reaction and in situ hybridization in 99 tumors, including a morphologic analysis. Cancer. 1994;73:2239–2249. doi: 10.1002/1097-0142(19940501)73:9<2239::AID-CNCR2820730902>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Matsunou H., Konishi F., Hori H., Ikeda T., Sasaki K., Hirose Y., Yamamichi N. Characteristics of Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma in Japan. Cancer. 1996;77:1998–2004. doi: 10.1002/(SICI)1097-0142(19960515)77:10<1998::AID-CNCR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe H., Enjoji M., Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976;38:232–243. doi: 10.1002/1097-0142(197607)38:1<232::AID-CNCR2820380135>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Warren J.R., Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;321:1273–1275. [PubMed] [Google Scholar]
- 16.Fukase K., Kato M., Kikuchi S., Inoue K., Uemura N., Okamoto S., Terao S., Amagai K., Hayashi S., Asaka M., et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 17.Ford A.C., Forman D., Hunt R.H., Yuan Y., Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanai H., Murakami T., Yoshiyama H., Takeuchi H., Nishikawa J., Nakamura H., Okita K., Miura O., Shimizu N., Takada K. Epstein-Barr virus-associated gastric carcinoma and atrophic gastritis. J. Clin. Gastroenterol. 1999;29:39–43. doi: 10.1097/00004836-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Uemura Y., Tokunaga M., Arikawa J., Yamamoto N., Hamasaki Y., Tanaka S., Sato E., Land C.E. A unique morphology of Epstein-Barr virus-related early gastric carcinoma. Cancer Epidemiol. Biomark. Prev. 1994;3:607–611. [PubMed] [Google Scholar]
- 20.Camargo M.C., Koriyama C., Matsuo K., Kim W.H., Herrera-Goepfert R., Liao L.M., Eurgast-EPIC Group. Yu J., Carrasquilla G., Sung J.J., et al. Case-case comparison of smoking and alcohol risk associations with Epstein-Barr virus-positive gastric cancer. Int. J. Cancer. 2014;134:948–953. doi: 10.1002/ijc.28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanai H., Nishikawa J., Mizugaki Y., Shimizu N., Takada K., Matsusaki K., Toda T., Matsumoto Y., Tada M., Okita K. Endoscopic and pathologic features of Epstein-Barr virus-associated gastric carcinoma. Gastrointest. Endosc. 1997;45:236–242. doi: 10.1016/S0016-5107(97)70265-7. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa J., Yanai H., Mizugaki Y., Takada K., Tada M., Okita K. Case report: Hypoechoic submucosal nodules: A sign of Epstein-Barr virus-associated early gastric cancer. J. Gastroenterol. Hepatol. 1998;13:585–590. doi: 10.1111/j.1440-1746.1998.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto N., Tokunaga M., Uemura Y., Tanaka S., Shirahama H., Nakamura T., Land C.E., Sato E. Epstein-Barr virus and gastric remnant cancer. Cancer. 1994;74:805–809. doi: 10.1002/1097-0142(19940801)74:3<805::AID-CNCR2820740304>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa J., Yanai H., Hirano A., Okamoto T., Nakamura H., Matsusaki K., Kawano T., Miura O., Okita K. High prevalence of Epstein-Barr virus in gastric remnant carcinoma after Billroth-II reconstruction. Scand. J. Gastroenterol. 2002;37:825–829. doi: 10.1080/gas.37.7.825.829. [DOI] [PubMed] [Google Scholar]
- 25.Kaizaki Y., Hosokawa O., Sakurai S., Fukayama M. Epstein-Barr virus-associated gastric carcinoma in the remnant stomach: De novo and metachronous gastric remnant carcinoma. J. Gastroenterol. 2005;40:570–577. doi: 10.1007/s00535-005-1590-3. [DOI] [PubMed] [Google Scholar]
- 26.Liu S., Zhao Z., Han L., Liu S., Luo B. Epstein-Barr Virus Infection in Gastric Remnant Carcinoma and Recurrent Gastric Carcinoma in Qingdao of Northern China. PLoS ONE. 2016;11:e0148342. doi: 10.1371/journal.pone.0148342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Beek J., zur Hausen A., Klein Kranenbarg E., van de Velde C.J., Middeldorp J.M., van den Brule A.J., Meijer C.J., Bloemena E. EBV-positive gastric adenocarcinomas: A distinct clinicopathologic entity with a low frequency of lymph node involvement. J. Clin. Oncol. 2004;22:664–670. doi: 10.1200/JCO.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 28.Gotoda T., Yanagisawa A., Sasako M., Ono H., Nakanishi Y., Shimoda T., Kato Y. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/PL00011720. [DOI] [PubMed] [Google Scholar]
- 29.Yamao T., Shirao K., Ono H., Kondo H., Saito D., Yamaguchi H., Sasako M., Sano T., Ochiai A., Yoshida S. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602–606. doi: 10.1002/(SICI)1097-0142(19960215)77:4<602::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Seto Y., Shimoyama S., Kitayama J., Mafune K., Kaminishi M., Aikou T., Arai K., Ohta K., Nashimoto A., Honda I., et al. Lymph node metastasis and preoperative diagnosis of depth of invasion in early gastric cancer. Gastric Cancer. 2001;4:34–38. doi: 10.1007/s101200100014. [DOI] [PubMed] [Google Scholar]
- 31.An J.Y., Baik Y.H., Choi M.G., Noh J.H., Sohn T.S., Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: Analysis of a single institutional experience. Ann. Surg. 2007;246:749–753. doi: 10.1097/SLA.0b013e31811f3fb7. [DOI] [PubMed] [Google Scholar]
- 32.Holscher A.H., Drebber U., Monig S.P., Schulte C., Vallbohmer D., Bollschweiler E. Early gastric cancer: Lymph node metastasis starts with deep mucosal infiltration. Ann. Surg. 2009;250:791–797. doi: 10.1097/SLA.0b013e3181bdd3e4. [DOI] [PubMed] [Google Scholar]
- 33.Nam M.J., Oh S.J., Oh C.A., Kim D.H., Bae Y.S., Choi M.G., Noh J.H., Sohn T.S., Bae J.M., Kim S. Frequency and predictive factors of lymph node metastasis in mucosal cancer. J. Gastric Cancer. 2010;10:162–167. doi: 10.5230/jgc.2010.10.4.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokunaga M., Land C.E. Epstein-Barr virus involvement in gastric cancer: Biomarker for lymph node metastasis. Cancer Epidemiol. Biomark. Prev. 1998;7:449–450. [PubMed] [Google Scholar]
- 35.Park J.H., Kim E.K., Kim Y.H., Kim J.H., Bae Y.S., Lee Y.C., Cheong J.H., Noh S.H., Kim H. Epstein-Barr virus positivity, not mismatch repair-deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer. Gastric Cancer. 2016;19:1041–1051. doi: 10.1007/s10120-015-0565-1. [DOI] [PubMed] [Google Scholar]
- 36.Camargo M.C., Kim W.H., Chiaravalli A.M., Kim K.M., Corvalan A.H., Matsuo K., Yu J., Sung J.J., Herrera-Goepfert R., Meneses-Gonzalez F., et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: An international pooled analysis. Gut. 2014;63:236–243. doi: 10.1136/gutjnl-2013-304531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawazoe A., Kuwata T., Kuboki Y., Shitara K., Nagatsuma A.K., Aizawa M., Yoshino T., Doi T., Ohtsu A., Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20:407–415. doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
- 38.Böger C., Behrens H.M., Mathiak M., Krüger S., Kalthoff H., Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269–24283. doi: 10.18632/oncotarget.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derks S., Liao X., Chiaravalli A.M., Xu X., Camargo M.C., Solcia E., Sessa F., Fleitas T., Freeman G.J., Rodig S.J., et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7:32925–32932. doi: 10.18632/oncotarget.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma C., Patel K., Singhi A.D., Ren B., Zhu B., Shaikh F., Sun W. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am. J. Surg. Pathol. 2016;40:1496–1506. doi: 10.1097/PAS.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 41.Saito R., Abe H., Kunita A., Yamashita H., Seto Y., Fukayama M. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1+ immune cells in Epstein-Barr virus-associated gastric cancer: The prognostic implications. Mod. Pathol. 2017;30:427–439. doi: 10.1038/modpathol.2016.202. [DOI] [PubMed] [Google Scholar]
- 42.Crescenzi A., Taffon C., Donati M., Guarino M.P., Valeri S., Coppola R. PD-L1/PD-1 check-point in gastric carcinoma with lymphoid stroma case report with immunochemical study. Medicine. 2017;96:e5730. doi: 10.1097/MD.0000000000005730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feinberg A.P., Tycko B. The history of cancer epigenetics. Nat. Rev. Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 44.Kang G.H., Lee S., Kim W.H., Lee H.W., Kim J.C., Rhyu M.G., Ro J.Y. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am. J. Pathol. 2002;160:787–794. doi: 10.1016/S0002-9440(10)64901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vo Q.N., Geradts J., Gulley M.L., Boudreau D.A., Bravo J.C., Schneider B.G. Epstein-Barr virus in gastric adenocarcinomas: Association with ethnicity and CDKN2A promoter methylation. J. Clin. Pathol. 2002;55:669–675. doi: 10.1136/jcp.55.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong J.M., Sakuma K., Sudo M., Ushiku T., Uozaki H., Shibahara J., Nagai H., Funata N., Taniguchi H., Aburatani H., et al. Global and non-random CpG-island methylation in gastric carcinoma associated with Epstein-Barr virus. Cancer Sci. 2003;94:76–80. doi: 10.1111/j.1349-7006.2003.tb01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang M.S., Uozaki H., Chong J.M., Ushiku T., Sakuma K., Ishikawa S., Hino R., Barua R.R., Iwasaki Y., Arai K., et al. CpG island methylation status in gastric carcinoma with and without infection of Epstein-Barr virus. Clin. Cancer Res. 2006;12:2995–3002. doi: 10.1158/1078-0432.CCR-05-1601. [DOI] [PubMed] [Google Scholar]
- 48.Kusano M., Toyota M., Suzuki H., Akino K., Aoki F., Fujita M., Hosokawa M., Shinomura Y., Imai K., Tokino T. Genetic, epigenetic, and clinicopathologic features of gastric carcinomas with the CpG island methylator phenotype and an association with Epstein-Barr virus. Cancer. 2006;106:1467–1479. doi: 10.1002/cncr.21789. [DOI] [PubMed] [Google Scholar]
- 49.Saito M., Nishikawa J., Okada T., Morishige A., Sakai K., Nakamura M., Kiyotoki S., Hamabe K., Okamoto T., Oga A., et al. Role of DNA methylation in the development of Epstein-Barr virus-associated gastric carcinoma. J. Med. Virol. 2013;85:121–127. doi: 10.1002/jmv.23405. [DOI] [PubMed] [Google Scholar]
- 50.Okada T., Nakamura M., Nishikawa J., Sakai K., Zhang Y., Saito M., Morishige A., Oga A., Sasaki K., Suehiro Y., et al. Identification of genes specifically methylated in Epstein-Barr virus-associated gastric carcinomas. Cancer Sci. 2013;104:1309–1314. doi: 10.1111/cas.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hino R., Uozaki H., Murakami N., Ushiku T., Shinozaki A., Ishikawa S., Morikawa T., Nakaya T., Sakatani T., Takada K., et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 2009;69:2766–2774. doi: 10.1158/0008-5472.CAN-08-3070. [DOI] [PubMed] [Google Scholar]
- 52.Namba-Fukuyo H., Funata S., Matsusaka K., Fukuyo M., Rahmutulla B., Mano Y., Fukayama M., Aburatani H., Kaneda A. TET2 functions as a resistance factor against DNA methylation acquisition during Epstein-Barr virus infection. Oncotarget. 2016;7:81512–81526. doi: 10.18632/oncotarget.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsusaka K., Kaneda A., Nagae G., Ushiku T., Kikuchi Y., Hino R., Uozaki H., Seto Y., Takada K., Aburatani H., et al. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res. 2011;71:7187–7197. doi: 10.1158/0008-5472.CAN-11-1349. [DOI] [PubMed] [Google Scholar]
- 54.Matsusaka K., Funata S., Fukuyo M., Seto Y., Aburatani H., Fukayama M., Kaneda A. Epstein-Barr virus infection induces genome-wide de novo DNA methylation in non-neoplastic gastric epithelial cells. J. Pathol. 2017;242:391–399. doi: 10.1002/path.4909. [DOI] [PubMed] [Google Scholar]
- 55.Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J. Gastroenterol. 2009;44:239–248. doi: 10.1007/s00535-009-0014-1. [DOI] [PubMed] [Google Scholar]
- 56.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 57.Saju P., Murata-Kamiya N., Hayashi T., Senda Y., Nagase L., Noda S., Matsusaka K., Funata S., Kunita A., Urabe M., et al. Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein-Barr virus. Nat. Microbiol. 2016;1:16026. doi: 10.1038/nmicrobiol.2016.26. [DOI] [PubMed] [Google Scholar]
- 58.Tada M., Murakami A., Karita M., Yanai H., Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445–450. doi: 10.1055/s-2007-1010365. [DOI] [PubMed] [Google Scholar]
- 59.Japanese Gastric Cancer Association Japanese Classification of Gastric Carcinoma, 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 60.Ono H., Kondo H., Gotoda T., Shirao K., Yamaguchi H., Saito D., Hosokawa K., Shimoda T., Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uedo N., Iishi H., Tatsuta M., Ishihara R., Higashino K., Takeuchi Y., Imanaka K., Yamada T., Yamamoto S., Yamamoto S., et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88–92. doi: 10.1007/s10120-005-0357-0. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura M., Nishikawa J., Saito M., Sakai K., Sasaki S., Hashimoto S., Okamoto T., Suehiro Y., Yamasaki T., Sakaida I. Decitabine inhibits tumor cell proliferation and up-regulates e-cadherin expression in Epstein-Barr virus-associated gastric cancer. J. Med. Virol. 2017;89:508–517. doi: 10.1002/jmv.24634. [DOI] [PubMed] [Google Scholar]
- 63.Schrump D.S., Fischette M.R., Nguyen D.M., Zhao M., Li X., Kunst T.F., Hancox A., Hong J.A., Chen G.A., Pishchik V., et al. Phase I study of decitabine-mediated gene expression in patients with cancers involving the lungs, esophagus, or pleura. Clin. Cancer Res. 2006;12:5777–5785. doi: 10.1158/1078-0432.CCR-06-0669. [DOI] [PubMed] [Google Scholar]
- 64.Schneider B.J., Shah M.A., Klute K., Ocean A., Popa E., Altorki N., Lieberman M., Schreiner A., Yantiss R., Christos P.J., et al. Phase I Study of Epigenetic Priming with Azacitidine Prior to Standard Neoadjuvant Chemotherapy for Patients with Resectable Gastric and Esophageal Adenocarcinoma: Evidence of Tumor Hypomethylation as an Indicator of Major Histopathologic Response. Clin. Cancer Res. 2017;23:2673–2680. doi: 10.1158/1078-0432.CCR-16-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vijayaraghavalu S., Labhasetwar V. Efficacy of decitabine-loaded nanogels in overcoming cancer drug resistance is mediated via sustained DNA methyltransferase 1 (DNMT1) depletion. Cancer Lett. 2013;331:122–129. doi: 10.1016/j.canlet.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu F.L., Li R.T., Yang M., Yue G.F., Wang H.Y., Liu Q., Cui F.B., Wu P.Y., Ding H., Yu L.X., et al. Gelatinases-stimuli nanoparticles encapsulating 5-fluorouridine and 5-aza-2′-deoxycytidine enhance the sensitivity of gastric cancer cells to chemical therapeutics. Cancer Lett. 2015;363:7–16. doi: 10.1016/j.canlet.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Hong Y.D., Zhang J., Zhuang M., Li W., Wu P.U., Li R.T., Hu N., Bian B.X., Song Z.Y., Wu F.L. Efficacy of decitabine-loaded gelatinases-stimuli nanoparticles in overcoming cancer drug resistance is mediated via its enhanced demethylating activity to transcription factor AP-2 epsilon. Oncotarget. 2017;8:114495–114505. doi: 10.18632/oncotarget.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamid O., Robert C., Daud A., Hodi F.S., Hwu W.J., Kefford R., Wolchok J.D., Hersey P., Joseph R.W., Weber J.S., et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolchok J.D., Kluger H., Callahan M.K., Postow M.A., Rizvi N.A., Lesokhin A.M., Segal N.H., Ariyan C.E., Gordon R.A., Reed K., et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 71.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ansell S.M., Lesokhin A.M., Borrello I., Halwani A., Scott E.C., Gutierrez M., Schuster S.J., Millenson M.M., Cattry D., Freeman G.J., et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang Y.K., Boku N., Satoh T., Ryu M.H., Chao Y., Kato K., Chung H.C., Chen J.S., Muro K., Kang W.K., et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 75.Lawrence M.S., Stojanov P., Polak P., Kryukov G.V., Cibulskis K., Sivachenko A., Carter S.L., Stewart C., Mermel C.H., Roberts S.A., et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Green M.R., Rodig S., Juszczynski P., Ouyang J., Sinha P., O’Donnell E., Neuberg D., Shipp M.A. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: Implications for targeted therapy. Clin. Cancer Res. 2012;18:1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang W., Zhang J., Hong S., Zhan J., Chen N., Qin T., Tang Y., Zhang Y., Kang S., Zhou T., et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget. 2014;5:12189–12202. doi: 10.18632/oncotarget.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Panda A., Mehnert J.M., Hirshfield K.M., Riedlinger G., Damare S., Saunders T., Kane M., Sokol L., Stein M.N., Poplin E., et al. Immune Activation and Benefit From Avelumab in EBV-Positive Gastric Cancer. J. Natl. Cancer Inst. 2018;110:316–320. doi: 10.1093/jnci/djx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quinn L.L., Williams L.R., White C., Forrest C., Zuo J., Rowe M. The Missing Link in Epstein-Barr Virus Immune Evasion: The BDLF3 Gene Induces Ubiquitination and Downregulation of Major Histocompatibility Complex Class I (MHC-I) and MHC-II. J. Virol. 2015;90:356–367. doi: 10.1128/JVI.02183-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato Y., Kamura T., Shirata N., Murata T., Kudoh A., Iwahori S., Nakayama S., Isomura H., Nishiyama Y., Tsurumi T. Degradation of phosphorylated p53 by viral protein-ECS E3 ligase complex. PLoS Pathog. 2009;5:e1000530. doi: 10.1371/journal.ppat.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu D.X., Tanhehco Y., Chen J., Foss C.A., Fox J.J., Chong J.M., Hobbs R.F., Fukayama M., Sgouros G., Kowalski J., et al. Bortezomib-induced enzyme-targeted radiation therapy in herpesvirus-associated tumors. Nat. Med. 2008;14:1118–1122. doi: 10.1038/nm.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haque T., Taylor C., Wilkie G.M., Murad P., Amlot P.L., Beath S., McKiernan P.J., Crawford D.H. Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells. Transplantation. 2001;72:1399–1402. doi: 10.1097/00007890-200110270-00012. [DOI] [PubMed] [Google Scholar]
- 83.Straathof K.C., Bollard C.M., Popat U., Huls M.H., Lopez T., Morriss M.C., Gresik M.V., Gee A.P., Russell H.V., Brenner M.K., et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus–specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 84.Chang L.K., Liu S.T. Activation of the BRLF1 promoter and lytic cycle of Epstein-Barr virus by histoneacetylation. Nucleic Acids Res. 2000;28:3918–3925. doi: 10.1093/nar/28.20.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hui K.F., Cheung A.K., Choi C.K., Yeung P.L., Middeldorp J.M., Lung M.L., Tsao S.W., Chiang A.K. Inhibition of class I histone deacetylases by romidepsin potently induces Epstein-Barr virus lytic cycle and mediates enhanced cell death with ganciclovir. Int. J. Cancer. 2016;138:125–136. doi: 10.1002/ijc.29698. [DOI] [PubMed] [Google Scholar]
- 86.Munn D.H., Shafizadeh E., Attwood J.T., Bondarev I., Pashine A., Mellor A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song H., Park H., Kim J., Park G., Kim Y.S., Kim S.M., Kim D., Seo S.K., Lee H.K., Cho D., et al. IDO metabolite produced by EBV-transformed B cells inhibits surface expression of NKG2D in NK cells via the c-Jun N-terminal kinase (JNK) pathway. Immunol. Lett. 2011;136:187–193. doi: 10.1016/j.imlet.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 88.Thompson S., Messick T., Schultz D.C., Reichman M., Lieberman P.M. Development of a high-throughput screen for inhibitors of Epstein-Barr virus EBNA1. J. Biomol. Screen. 2010;15:1107–1115. doi: 10.1177/1087057110379154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li N., Thompson S., Schultz D.C., Zhu W., Jiang H., Luo C., Lieberman P.M. Discovery of selective inhibitors against EBNA1 via high throughput in silico virtual screening. PLoS ONE. 2010;5:e10126. doi: 10.1371/journal.pone.0010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen J.I. Epstein–Barr virus vaccines. Clin. Transl. Immunol. 2015;4:e32. doi: 10.1038/cti.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Snijder J., Ortego M.S., Weidle C., Stuart A.B., Gray M.D., McElrath M.J., Pancera M., Veesler D., McGuire A.T. An Antibody Targeting the Fusion Machinery Neutralizes Dual-Tropic Infection and Defines a Site of Vulnerability on Epstein-Barr Virus. Immunity. 2018;48:799–811. doi: 10.1016/j.immuni.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]