Supplemental Digital Content is available in the text
Keywords: cardiovascular disease, clinical inertia, hypertension, quality improvement, risk factor management, therapeutic inertia
Abstract
Background:
Clinical Inertia is defined as “failure of health care providers to initiate or intensify therapy according to current guidelines”. This phenomenon is gaining increasing attention as a major cause of clinicians’ failure to adequately manage hypertension, thus leading to an increased incidence of cardiovascular events. We performed a systematic review and meta-analysis of randomized controlled trials to determine whether interventions aimed at reducing clinical inertia in the pharmacological treatment of hypertension improve blood pressure (BP) control.
Methods:
MEDLINE, Embase, and Cochrane Database of Systematic Reviews were searched from the start of their database until October 3, 2017 for the MESH terms “Hypertension” or “Blood Pressure”, their subheadings, and the keywords “Therapeutic Inertia” or “Clinical Inertia”. Studies were included if they addressed pharmacologic hypertension management, clinical inertia, were randomized controlled trials, reported an outcome describing prescriber behavior, and were available in English. Data for the included studies was extracted by two independent observers. Quality of studies was analyzed using the Cochrane Risk of Bias Assessment. Data was pooled for statistical analysis using both fixed- and random-effects models. The primary study outcome was the percentage of patients achieving blood pressure control as defined by the Joint National Committee guidelines or study authors.
Results:
Of 474 citations identified, ten met inclusion criteria comprising a total of 26,871 patients, and eight were selected for meta-analysis. Interventions included Physician Education, Physician Reminders, Patient Education, Patient Reminders, Ambulatory BP Monitoring, Digital Medication Offerings, Physician Peer Visits, and Pharmacist-led Counselling. Pooled event rates revealed more patients with controlled BP in the intervention group versus control (55%, 95% CI 46-63% versus 45%, 95% CI 37-53%) and interventions significantly improved the odds of BP control (OR = 1.19, 95% CI = 1.12−1.27, P < .001). Heterogeneity in the quantitative analysis was moderate.
Conclusions & Relevance:
Addressing clinical inertia through physician reminders, ambulatory BP monitoring, and educational interventions for primary care providers was associated with an improvement in blood pressure control. Our findings encourage further research to investigate strategies at reducing clinical inertia in the management of hypertension.
1. Introduction
Cardiovascular disease (CVD) remains the leading cause of death worldwide.[1] Hypertension (HTN), diabetes, dyslipidemia, and smoking remain the most important modifiable risk factors for CVD.[1] Well-established guidelines outline targets for pharmacological management of blood pressure (BP), glucose, and lipids,[2–4] and large randomized controlled trials (RCTs) and systematic reviews have shown that adherence to targets significantly lowers the risk of CVD and other adverse health outcomes.[5–9] However, risk factor modification remains suboptimal, and it is estimated that 47.5% of known hypertensive patients have inadequate BP control in the USA with similar findings observed worldwide.[10,11] This phenomenon is accentuated in low and middle-income nations where a recent study estimates less than a third of known hypertensive patients receive treatment and less than 10% have controlled BPs.[12]
Inadequate risk factor control may be due to a variety of patient and system-specific factors, such as missed appointments, patient adherence, resistance to polypharmacy, and cost of medication. Additionally, HTN is typically asymptomatic, creating challenges for early diagnosis and adherence.[13] Despite the many patient factors, physician prescribing behavior remains fundamental in the appropriate management of patient risk factors.[14,15]
Phillips introduced the concept of “clinical inertia,” also known as “therapeutic inertia,” as a failure of healthcare providers to initiate or intensify therapy when guidelines indicate doing so.[16,17] Numerous studies have attempted to identify reasons for clinical inertia in risk factor management, which may be summarized as patient characteristics, physician characteristics, and factors that impact the patient–provider interaction. Patient characteristics include older age, lower life expectancy, multiple comorbidities, particularly psychiatric conditions, and patients who are “near-target” or reaching physician-defined “acceptable” targets. Provider characteristics include lack of knowledge about appropriate goals, high patient volume, and time constraints. [11,13,16,18–25] In addition, differences in physician risk tolerance, ambiguity, and decision-making within the realm of uncertainty have been suspected to be facilitators of clinical inertia in such settings as multiple sclerosis care.[26]
A recent survey of primary care visits of diagnosed hypertensive patients found that treatment intensification occurred in only 16% of those visits. Importantly, medication initiation in diagnosed hypertensive patients occurred in only 26.4% of visits.[24] Thus, appropriate guideline-adherent treatment initiation and intensification of antihypertensive medication is a major opportunity to improve HTN control and reduce CVD risk.
While numerous reviews have identified clinical inertia as a major factor in the undermanagement of HTN, we have yet to understand which interventions, if any, are effective at combating this phenomenon. We conducted a systematic review and meta-analysis to identify whether quality improvement initiatives are successful in overcoming clinical inertia and improving prescriber practices in the management of HTN.
2. Methods
2.1. Search strategy and study identification
Ovid MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews were searched from the earliest record until October 3, 2017. Search parameters included the MESH term “Hypertension” or “Blood Pressure,” and all subheadings, and were combined with the keywords “Clinical Inertia” or “Therapeutic Inertia” (see Document, Supplemental Digital Content 1, which presents the detailed search strategy). Appropriate synonyms and subheadings were included as defined by a medical librarian at the Li-Ka Shing Knowledge Institute, St. Michael's Hospital. It should be noted that “clinical inertia” and “therapeutic inertia” were searched for as a keyword as currently no MESH term exists. This methodology is in line with past studies.[27,28] Reference lists of relevant reviews and selected studies were screened to identify initial studies suitable for inclusion.
The primary author (TM) screened the preliminary search results to eliminate duplicates. Studies were screened by abstract and excluded if they were not RCTs, did not focus in part on HTN management, or were published in a language other than English. Method papers deemed potentially relevant were searched and replaced with updated citations if available. Two independent reviewers (TM and RJ) then screened the remaining studies. Disagreements among the independent reviewers regarding inclusion decisions were resolved by discussion and consensus, and the senior author (GS) was consulted if necessary.
2.2. Study selection
Criteria for inclusion in the review were studies that were RCTs, provided at least 1 measurable outcome that met the definition of clinical inertia, were completed studies, outlined an intervention that was a quality improvement initiative, and written in English. Inter-rater agreement was measured by Cohen kappa at multiple steps throughout the selection process to ensure agreement among reviewers. Bias and study quality were assessed by the Cochrane risk of bias method.[29]
2.3. Outcome measures
Studies selected for inclusion in the meta-analysis were analyzed and all relevant outcome measures were extracted that reflected changes in BP, and measures of clinical inertia. The primary outcome was the percentage of subjects with “controlled” BP as defined by the Joint National Committee (JNC) guidelines at time of study design or by study authors. Secondary BP outcomes included BP measurements at baseline and at study completion, and mean change in BP values. Inertia outcomes included number of treatment intensifications (dosage changes or medication additions), mean number of antihypertensive medications per patient, and adherence measures. When outcomes were reported at multiple time-points, values of the longest follow-up period or intervention length were used.
2.4. Statistical analysis
Of the studies included for systematic review, only those that reported percentage of controlled BPs were included in the meta-analysis. Pooled estimates and events rates with 95% confidence intervals (CIs) were combined using the random-effects (DerSimonian-Laird) model.[30] Statistical comparisons were performed using both fixed and random-effects model, and odds ratios (ORs) and 95% CIs were calculated. Funnel plot analysis was grossly symmetrical with the exception of a single outlier. A sensitivity analysis was conducted where the outlying study was removed from the aforementioned comparison models to determine whether significance was retained. The Z test was utilized to determine the significance of pooled ORs. We considered P values of less than 0.05 as significant. The I2 test was used to assess heterogeneity between studies. An I2 value of >75% were set to indicate significant heterogeneity in the analysis.[31] All statistical tests were performed using Comprehensive Meta-Analysis Software Suite (Biostat Inc., Englewood, NJ).
3. Results
Our search returned 474 citations (see Fig. 1 for flow diagram). In all, 296 citations remained after duplicates were deleted. One citation was found to be a protocol paper and the citation for its corresponding RCT was added to the review.[32,33] Among these, 9 studies met all inclusion criteria.[33–41] Selected studies were screened for relevant references, yielding 1 additional citation that was included in the data synthesis.[42]
Figure 1.
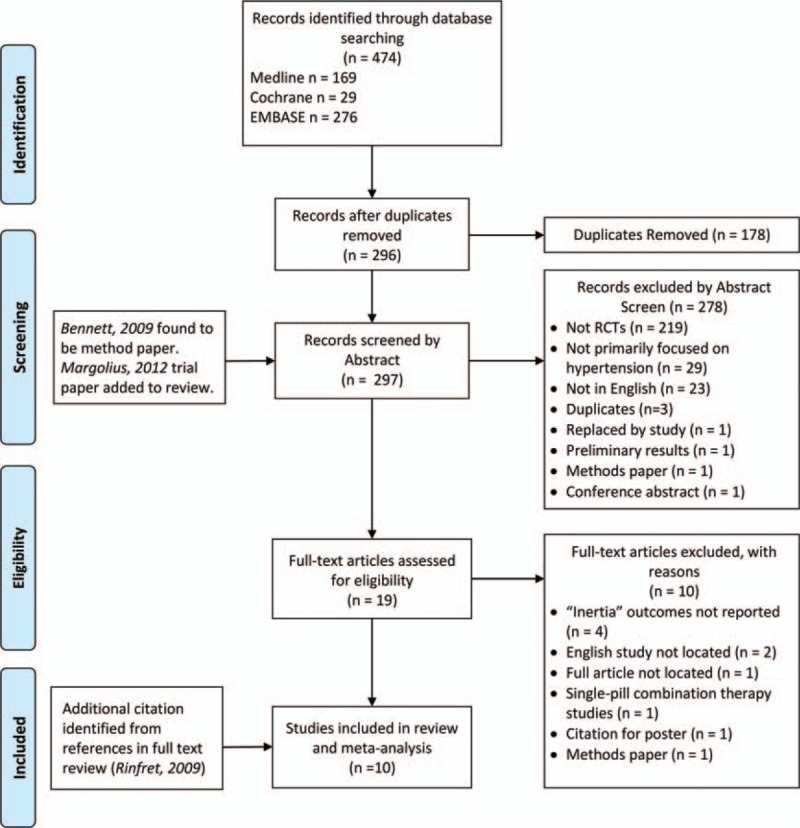
PRISMA schema of systematic review. RCT = randomized controlled trial.
Table 1 outlines the extracted information from the ten studies included in the systematic review (see Table, Supplemental Digital Content 2 for description of BP recording method, intervention details and additional study outcomes). Studies originated from the USA, Canada, Germany, and Argentina, and encompassed a total of 26,871 patients. Seven of the 10 study designs were clustered RCTs where randomization occurred at the provider level, and all patients treated by that provider belonged to the same randomized group. The remainder of the studies were randomized trials at the patient level. All studies reported BP outcome measures, although not all studies reported the percentage of patients with well-controlled BP. Other BP outcome measures included systolic BP change and diastolic BP change. Three of the studies additionally reported changes in other cardiovascular risk factors including low-density lipoprotein cholesterol and glycated hemoglobin.[35,36,41]
Table 1.
Characteristics, controlled blood pressure, inertia markers, and main findings of included studies.
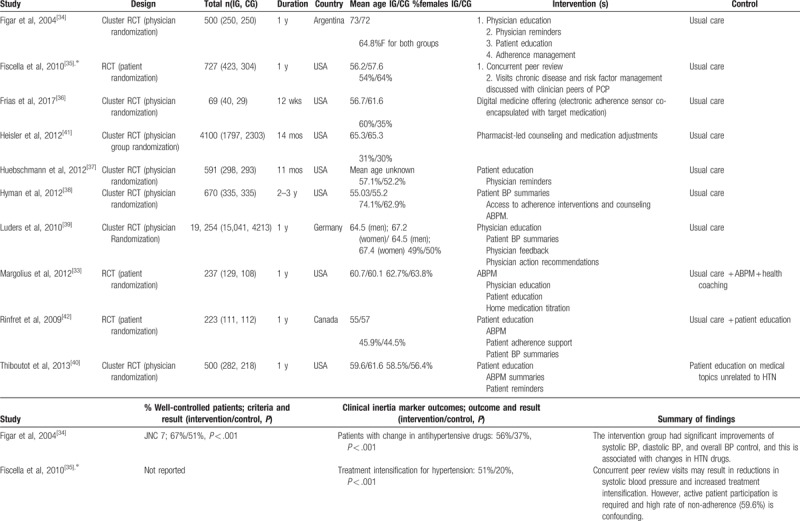
Table 1 (Continued).
Characteristics, controlled blood pressure, inertia markers, and main findings of included studies.
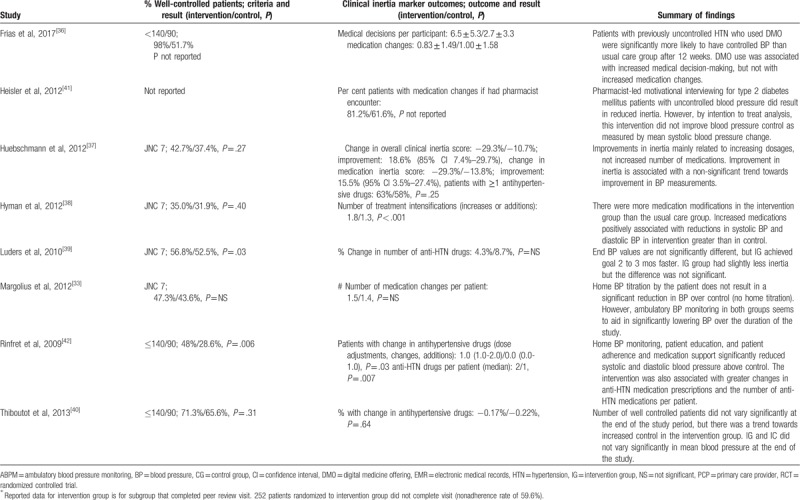
Seven of the 10 studies focused primarily on the physician–patient interaction and employed at least 1 of the following strategies: physician education, physician reminders and feedback, patient education, patient reminders and adherence counseling, and ambulatory BP monitoring. One study employed digital medication offerings (DMOs), edible sensors co-encapsulated with target medications that relay adherence data when swallowed and allow physicians and patients to track compliance.[36] Another study employed “physician peers,” who were physician colleagues of patient's primary care providers (PCPs), conducting visits solely to discuss chronic disease and risk factor management rather than a specific presenting complaint.[35]. One study employed a pharmacist-led counseling intervention that also allowed clinical pharmacists to increase antihypertensive medications. The latter 2 studies did not report the percentage of patients with well-controlled BP in the intervention and control groups (CGs), and therefore could not be included in the meta-analysis. Individually, all studies showed a greater percentage of patients with “controlled” BP in the intervention group (IG), indicating that all intervention types resulted in improved control (Table 1 ).
Studies varied in overall risk of bias (see Figure, Supplemental Digital Content 3, which presents the individual and summarized risk of bias analyses for all included studies). Specifically, allocation concealment and blinding of participants and personnel were found to be at highest risk of bias among studies. This risk was most prevalent in trials that were cluster-randomized at the provider level. The majority of studies reported results using intention-to-treat analyses, satisfying attrition bias. All studies reported both positive and negative results.
The pooled event rate for the percentage of participants with “controlled” BP was 55% (95% CI 46%–63%) in the IG versus 45% (95% CI 37%–53%) in the CG (Fig. 2). Both pooled event rates for groups were associated with high degree of heterogeneity (I2 > 90%). After calculating the weighted pooled ORs from each study, there was a significantly higher odds of achieving controlled BP in the IG versus control on both fixed-effect (OR 1.19, 95% CI 1.12–1.27, P < .001) and random-effect (OR 1.41, 95% CI 1.13–1.76, P = .003) models (Fig. 3). Heterogeneity level in the comparative analysis was moderate (69%).
Figure 2.

Forest plot of pooled events rates for the percentage of well-controlled blood pressure in control and intervention groups among included studies. Forest plot of pooled event rates for percentage of patients with controlled blood pressures in the control group (A) and intervention group (B) for each study. Square data markers indicate odds ratios (ORs) from primary studies, and size of squares indicates relative weight of the study using a random effects model. Horizontal lines indicate 95% confidence intervals. The blue diamond marker indicates overall OR and 95% confidence interval.
Figure 3.
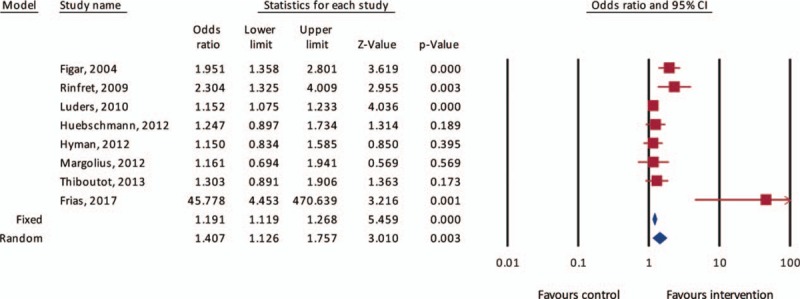
Forest plot of odds ratio for well-controlled blood pressure after intervention. Square data markers indicate odds ratios (ORs) of controlled blood pressures after intervention in each primary study. Horizontal lines indicate 95% confidence intervals. The blue diamond marker indicates overall OR and 95% confidence interval. Overall weighted ORs by fixed and random-effects models are represented by the blue diamonds, and width of diamonds represents 95% confidence interval.
Visualization of the funnel plot for each of the estimated effects revealed mild asymmetry and 1 study (Frias et al) to be a significant outlier (see Fig., Supplemental Digital Content 4, which reveals a cluster of effect estimates with a single outlier representing Frias et al). Accordingly, we conducted a random-effects model sensitivity analysis where the study by Frias et al was removed, revealing similar odds of achieving controlled BP on random-effects model (OR 1.3, 95% CI 1.1–1.6, P < .001) with a lower heterogeneity level (55%).
Table 1 also reveals that quantitatively, some additional measures of clinical inertia such as “number of medication changes per patient,” “percent with change in anti-hypertensive drugs,” “percent of patients with >1 antihypertensive drugs,” “number of antihypertensive drugs per patient,” and “number of treatment intensifications” were all greater in the IGs. However, due to the considerable heterogeneity in outcomes used to measure clinical inertia among the studies, a statistical analysis correlating prescriber behavior and BP outcomes could not be conducted.
4. Discussion
In this systematic review and meta-analysis, we found that interventions aimed at addressing clinical inertia in patient care, predominantly through physician or patient education, may be effective in improving HTN management as measured by percentage of patients with controlled BP. We found a 19% to 41% greater likelihood of achieving BP control among participants exposed to an intervention. These measures represent a number needed to treat ranging from 12 to 23. Moreover, measures of clinical inertia were also improved with intervention; however, there was substantial heterogeneity between studies.
Overall risk of bias among studies included for review is moderate. This is largely due to the difficulty of blinding patients who are given active roles in their care. Additionally, allocation concealment in cluster-randomized trials was not possible in trials that asked physicians to modify their behavior and office practices by offering education, adherence interventions, and counseling, or digital medication offerings. However, it is expected that part of the reason for overall improvement in HTN control among all included studies is greater provider awareness and focus on this health issue. Thus, physician blinding to these interventions in this case may be inappropriate for patient care.
The optimal management of vascular risk factors (eg, HTN, diabetes, dyslipidemia/hypercholesterolemia) is considered one of the most effective strategies to decrease the risk of CVDs.[43,44] Despite available guidelines and evidence from the literature, clinical inertia is ubiquitous to routine practice, and has been identified in many countries across different socioeconomic strata.[34,39,45,46]
Critics of the concept of clinical inertia suggest that there are numerous reasons for not intensifying treatment. These include time constraints due to complex patients, patients requiring time to discuss other illnesses, risk factors being managed by another physician, and patient factors such as noncompliance.[22,47] For BP management, suspected white coat HTN and delaying BP measurements until future visits are additional factors that physicians report as reasons to not intensify therapy.[22] Yet, regardless of the reasoning, nonintensification of therapy can have profound effects on patient health.[48–50] A study of clinical inertia in postischemic stroke patients found that therapy was not escalated in 30% of cases where office systolic BP measurements exceeded 160 mm Hg, potentially exposing patients to adverse outcomes.[51]
Few studies have attempted to address the issue of inertia at the level of the care provider. Interventions that educate physicians about BP targets and provide automated intensification suggestions may target some of the precipitating factors in clinical inertia .[22,34,37,39] Faria et al described a number of possible strategies to address clinical inertia in HTN treatment, including cognitive interventions, large educational programs, and “academic detailing” (one-on-one or small-group teaching sessions).[27] Educational and cognitive interventions have similarly proven to be effective at combating clinical inertia in multiple sclerosis care.[52] Various models of physician education, and also physician feedback were employed in the studies included in this review.[33,34,37,39,40,42]
Our study has multiple limitations. There was substantial heterogeneity among studies, and differences in intervention type, randomization level, and described inertia outcomes limited detailed quantitative analysis. Nevertheless, we were able to demonstrate a significant difference between groups in a summary measure of BP control. Additionally, all studies showed improvements in inertia markers (eg, increased treatment intensifications, number of prescribed antihypertensives per patient, dosage changes), although these changes could not be analysed due to the limited number of studies. These limitations highlight the need for more well-designed trials addressing interventions to improve physician practice, and greater consensus on appropriate HTN management outcomes.
A further limitation is the challenge in distinguishing between the impact of interventions on clinical inertia from patients’ compliance with medication regimens. Previous literature has identified that poor adherence and clinical inertia are independent risk factors for poor cardiovascular risk factor control.[53] However, poor adherence has also been described and self-reported by clinicians as a major contributing factor to inertia.[16,17]Additionally, Grant et al[54] reported an inverse correlation between medication adherence and clinical inertia, with greater adherence to regimens being associated with more appropriate treatment intensification. However, in a large meta-analysis, there was insufficient evidence to confirm an association between improved adherence to antihypertensives and greater BP control.[55] Furthermore, in patients with resistant HTN, treatment intensification, but not medication adherence, was associated with improved BP control.[56] In our systematic review, the study by Rinfret et al. was the only study to report adherence data for both the control and IGs. There was a significant difference in clinical inertia, but not in adherence, favoring the IG, in addition to significantly greater odds of BP control. Further studies are needed to clarify the relationship between clinical inertia, adherence, and patient outcomes.
Despite the aforementioned limitations, our study highlights the following: the consistency of improved outcomes among participants randomized to interventions addressing clinical inertia, the need for more studies targeting specific factors implicated in clinical inertia, and the value of educational interventions for physicians to assist with risk factor management and provide decision-making reminders.
The present systematic review and meta-analysis should be seen as a call to action for PCPs, and also researchers in the fields of health policy, quality improvement, cardiovascular, and cerebrovascular disease. The limited number of included studies and the heterogeneity among outcomes stress the need for further research in the field. A future direction of inquiry would be to examine administrative and population-level datasets where practices to reduce clinical inertia in HTN management have been employed. Importantly, the studies identified for inclusion in this review are from multiple nations, indicating the worldwide relevance of this phenomenon and the in international interest in finding strategies to combat inertia in risk factor management.
5. Conclusions
This review and meta-analysis shows that interventional strategies are effective in reducing clinical inertia and improving HTN management. The approach of targeting clinical inertia to optimize patient care is likely broadly applicable, and should be studied in other areas of cardiovascular health. The burden of global CVD is high, and innovative strategies are essential to address physician and patient factors, and ensure that risk factors are optimally managed.
Acknowledgments
The authors would like to thank David Lightfoot, Information specialist, and Librarian at the Li Ka Shing Knowledge Institute, St. Michael's Hospital, Toronto, ON, Canada, for assistance with the literature search.
Author contributions
Concept and design: Milman, Saposnik.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Milman, Joundi, Alotaibi.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis and figure design: Alotaibi.
Study supervision: Saposnik.
Conceptualization: Tal Milman, Gustavo Saposnik.
Data curation: Tal Milman.
Formal analysis: Tal Milman, Raed A. Joundi.
Investigation: Tal Milman, Raed A. Joundi, Naif M. Alotaibi.
Methodology: Tal Milman, Raed A. Joundi, Naif M. Alotaibi.
Supervision: Gustavo Saposnik.
Writing – original draft: Tal Milman, Raed A. Joundi, Naif M. Alotaibi.
Writing – review & editing: Tal Milman, Raed A. Joundi, Naif M. Alotaibi, Gustavo Saposnik.
Supplementary Material
Footnotes
Abbreviations: BP = blood pressure, CG = control group, CI = confidence interval, HTN = hypertension, IG = intervention group, OR = odds ratio, PCP = primary care provider, RCT = randomized controlled trial.
Supplemental Digital Content is available for this article.
References
- [1].Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics: 2017 update: a report from the American Heart Association. Circulation 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- [3].Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines 2014;129(25 suppl 2):S1–45. [DOI] [PubMed] [Google Scholar]
- [4].American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes – 2018. Diabetes Care 2018;41(suppl 1):S55–64. [DOI] [PubMed] [Google Scholar]
- [5].Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev 2013;Cd008277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang Q, Chang A, Ritchey MD, et al. Antihypertensive medication adherence and risk of cardiovascular disease among older adults: a population-based cohort study. J Am Heart Assoc 2017;6: doi: 10.1161/JAHA.117.006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78. [DOI] [PubMed] [Google Scholar]
- [8].Nathan DM, Cleary PA, Backlund JY. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- [10].Kearney PM, Whelton M, Reynolds K, et al. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004;22:11–9. [DOI] [PubMed] [Google Scholar]
- [11].Zafar A, Stone MA, Davies MJ, et al. Acknowledging and allocating responsibility for clinical inertia in the management of type 2 diabetes in primary care: a qualitative study. Diabet Med 2015;32:407–13. [DOI] [PubMed] [Google Scholar]
- [12].Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016;134:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Basile J. Clinical inertia and blood pressure goal attainment. J Clin Hypertens 2009;11(suppl 1):S5–12. [Google Scholar]
- [14].Phillips P. Diabetes care: therapeutic inertia in doctors and patients. Med Today 2008;9:50–6. [Google Scholar]
- [15].Reach G, Pechtner V, Gentilella R, et al. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab 2017;43:501–11. [DOI] [PubMed] [Google Scholar]
- [16].Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med 2001;135:825–34. [DOI] [PubMed] [Google Scholar]
- [17].Allen JD, Curtiss FR, Fairman KA. Nonadherent, clinical inertia, or therapeutic inertia? J Manag Care Pharm 2009;15:690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gil-Guillen V, Orozco-Beltran D, Mraquez-Contreras E, et al. Is there a predictive profile for clinical inertia in hypertensive patients? An observational, cross-sectional, multicentre study. Drugs Aging 2011;28:981–92. [DOI] [PubMed] [Google Scholar]
- [19].Strain WD, Cos X, Hirst M, et al. Time to do more: addressing clinical inertia in the management of type 2 diabetes mellitus. Diabetes Res Clin Pract 2014;105:302–12. [DOI] [PubMed] [Google Scholar]
- [20].Balkau B, Halimi S, Blickle J, et al. Reasons for non-intensification of therapy in type 2 diabetes patients uncontrolled by oral monotherapy in general practice in France: the DIAttitude study. Diabetologia 2013;56:S117. [Google Scholar]
- [21].Moise N, Davison K, Chaplin W, et al. Depression is associated with clinical inertia in management of hypertension in the primary care setting. J Gen Intern Med 2014;29:S65. [Google Scholar]
- [22].Salanitro A, Agee B, Burczyk-Brown J, et al. Appropriate inaction and clinical inertia. J Gen Intern Med 2010;25:S223–4. [Google Scholar]
- [23].Ferrari P. Reasons for therapeutic inertia when managing hypertension in clinical practice in non-Western countries. J Hum Hypertens 2009;23:151–9. [DOI] [PubMed] [Google Scholar]
- [24].Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc 2016;5: e004188. doi:10.1161/JAHA.116.004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harle CA, Harman JS, Yang S. Physician and patient characteristics associated with clinical inertia in blood pressure control. J Clin Hypertens (Greenwich) 2013;15:820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saposnik G, Sempere AP, Raptis R, et al. Decision making under uncertainty, therapeutic inertia, and physicians’ risk preferences in the management of multiple sclerosis (DIScUTIR MS). BMC Neurol 2016;16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens 2009;3:267–76. [DOI] [PubMed] [Google Scholar]
- [28].Lebeau JP, Cadwallader JS, Aubin-Auger I, et al. The concept and definition of therapeutic inertia in hypertension in primary care: a qualitative systematic review. BMC Fam Pract 2014;15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [31].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bennett H, Laird K, Margolius D, et al. The effectiveness of health coaching, home blood pressure monitoring, and home-titration in controlling hypertension among low-income patients: protocol for a randomized controlled trial. BMC Public Health 2009;9:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Margolius D, Bodenheimer T, Bennett H, et al. Health coaching to improve hypertension treatment in a low-income, minority population. Ann Fam Med 2012;10:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Figar S, Waisman G, De Quiros FGB, et al. Narrowing the gap in hypertension: effectiveness of a complex antihypertensive program in the elderly. Dis Manag 2004;7:235–43. [DOI] [PubMed] [Google Scholar]
- [35].Fiscella K, Volpe E, Winters P, et al. A novel approach to quality improvement in a safety-net practice: concurrent peer review visits. J Natl Med Assoc 2010;102:1231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Frias J, Virdi N, Raja P, et al. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. J Med Internet Res 2017;19:e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Huebschmann AG, Mizrahi T, Soenksen A, et al. Reducing clinical inertia in hypertension treatment: a pragmatic randomized controlled trial. J Clin Hypertens (Greenwich) 2012;14:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hyman DJ, Pavlik VN, Greisinger AJ, et al. Effect of a physician uncertainty reduction intervention on blood pressure in uncontrolled hypertensives: a cluster randomized trial. J Gen Intern Med 2012;27:413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Luders S, Schrader J, Schmieder RE, et al. Improvement of hypertension management by structured physician education and feedback system: cluster randomized trial. Eur J Cardiovasc Prev Rehabil 2010;17:271–9. [DOI] [PubMed] [Google Scholar]
- [40].Thiboutot J, Sciamanna CN, Falkner B, et al. Effects of a web-based patient activation intervention to overcome clinical inertia on blood pressure control: cluster randomized controlled trial. J Med Internet Res 2013;15:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Heisler M, Hofer TP, Schmittdiel JA, et al. Improving blood pressure control through a clinical pharmacist outreach program in patients with diabetes mellitus in 2 high-performing health systems: the adherence and intensification of medications cluster randomized, controlled pragmatic trial. Circulation 2012;125:2863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rinfret S, Lussier M-T, Peirce A, et al. The impact of a multidisciplinary information technology: supported program on blood pressure control in primary care. Circ Cardiovasc Qual Outcomes 2009;2:170–7. [DOI] [PubMed] [Google Scholar]
- [43].Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke. A quantitative modeling study. Stroke 2007;38:1881–5. [DOI] [PubMed] [Google Scholar]
- [44].Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747–57. [DOI] [PubMed] [Google Scholar]
- [45].Huang LY, Shau WY, Yeh HL, et al. A model measuring therapeutic inertia and the associated factors among diabetes patients: a nationwide population-based study in Taiwan. J Clin Pharmacol 2015;55:17–24. [DOI] [PubMed] [Google Scholar]
- [46].Kaplan SH, Billimek J, Sorkin DH, et al. Reducing racial/ethnic disparities in diabetes: the Coached Care (R2D2C2) project. J Gen Intern Med 2013;28:1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Stange KC. Is ’clinical inertia’ blaming without understanding? Are competing demands excuses? Ann Fam Med 2007;5:371–4. [Google Scholar]
- [48].Paul S, Shaw J, Klein K. Therapeutic inertia for glycaemic and blood pressure control in patients with type 2 diabetes mellitus and the cardiovascular consequences. Diabetologia 2015;58(Suppl 1): S63. doi: 10.1007/s00125-015-3687-4. [Google Scholar]
- [49].Boan AD, Bachman DL, Adams RJ, et al. High blood pressure treatment among black and white stroke patients. J Clin Hypertens 2011;13: A138. doi:10.1111/j.1751-7176.2011.00459.x. [Google Scholar]
- [50].Laiteerapong N, John PM, Meltzer DO, et al. Estimating the health effects of different delays in achieving systolic blood pressure control in adults with diabetes. J Gen Intern Med 2011;26:S223–4. [Google Scholar]
- [51].Cheng EM, Jaynes HA, Myers LJ, et al. Clinical inertia in the management of blood pressure after a stroke. Stroke 2014;45:AW303. [Google Scholar]
- [52].Saposnik G, Maurino J, Sempere AP, et al. Overcoming therapeutic inertia in multiple sclerosis care: a pilot randomized trial applying the traffic light system in medical education. Front Neurol 2017;8:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Turchin A. Poor outcomes in diabetes care: Is medication nonadherence or lack of treatment intensification to blame? Natl Clin Pract Endocrinol Metab 2008;4:536–7. [DOI] [PubMed] [Google Scholar]
- [54].Grant R, Adams AS, Trinacty CM, et al. Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care 2007;30:807–12. [DOI] [PubMed] [Google Scholar]
- [55].Gwadry-Sridhar FH, Manias E, Lal L, et al. Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: a systematic review by the ISPOR Medication Adherence and Persistence Special Interest Group. Value Health 2013;16:863–71. [DOI] [PubMed] [Google Scholar]
- [56].Daugherty SL, Powers JD, Magid DJ, et al. The association between medication adherence and treatment intensification with blood pressure control in resistant hypertension. Hypertension 2012;60:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


