Abstract
This study aimed to determine if short-term exposure to particulate matter (PM2.5) and ozone (O3) is associated with increased symptoms or lung function decline in fibrotic sarcoidosis. Sixteen patients with fibrotic sarcoidosis complicated by frequent exacerbations completed pulmonary function testing and questionnaires every three months for one year. We compared 7-, 10-, and 14-day average levels of PM2.5 and O3 estimated at patient residences to spirometry (forced expiratory volume in 1 s (FEV1), to forced vital capacity (FVC), episodes of FEV1 decline > 10%) and questionnaire outcomes (Leicester cough questionnaire (LCQ), Saint George Respiratory Questionnaire (SGRQ), and King’s Sarcoidosis Questionnaire (KSQ)) using generalized linear mixed effect models. PM2.5 level averaged over 14 days was associated with lower KSQ general health status (score change −6.60 per interquartile range (IQR) PM2.5 increase). PM2.5 level averaged over 10 and 14 days was associated with lower KSQ lung specific health status (score change −6.93 and −6.91, respectively). PM2.5 levels were not associated with FEV1, FVC, episodes of FEV1 decline > 10%, or respiratory symptoms measured by SGRQ or LCQ. Ozone exposure was not associated with any health outcomes. In this small cohort of patients with fibrotic sarcoidosis, PM2.5 exposure was associated with increased severity of respiratory and quality of life symptoms.
Keywords: sarcoidosis, air pollution, particulate matter, ozone, signs and symptoms, respiratory, pulmonary function tests
1. Introduction
Chronic sarcoidosis progresses to fibrotic disease in approximately 10–20% of patients [1]. Fibrotic disease is characterized by a progression of chronic inflammation to fibrotic transformation, and may include upper and middle lung predominant findings of traction bronchiectasis, bronchial distortion, linear opacities, fibrotic masses, honeycombing, or cysts [1,2].
Patients with fibrotic sarcoidosis commonly experience acute episodes of clinical worsening and lung function decline [3,4]. These acute pulmonary exacerbations of sarcoidosis (APES) have been described by decline in pulmonary function, worsening pulmonary symptoms such as cough, sputum, and shortness of breath, increase in biomarkers of disease activity, need to start or restart corticosteroid therapy, and exclusion of alternative causes of pulmonary symptoms and dysfunction [3,4,5]. Risk factors for exacerbations include underlying bronchiectasis, longer disease duration, African American race, previous steroid treatment, and anti-tumor necrosis factor (TNF) therapy [4]. Acute pulmonary exacerbations are typically treated with antibiotics and/or corticosteroid therapy [3,4,5].
Short-term air pollution exposure is associated with exacerbations of other pulmonary diseases such as chronic obstructive pulmonary disease (COPD) [6], asthma [7], and cystic fibrosis [8], which share clinical characteristics with exacerbations of fibrotic sarcoidosis. The effect of elevated short-term air pollution exposure on sarcoidosis is unknown. We aimed to evaluate if short-term exposure to ozone and fine particulate matter (PM2.5) is associated with increased symptoms or lung function decline in an exploratory study of a small cohort of patients with fibrotic sarcoidosis.
2. Materials and Methods
2.1. Study Participants
We performed a secondary analysis of an electronic database of adult fibrotic sarcoidosis cases generated for a separate study [9]. All patients in this analysis had testing performed at the University of Cincinnati Medical Center. Patients with a history of two or more acute exacerbations in the previous year were enrolled in a randomized controlled trial evaluating roflumilast for reducing episodes of acute exacerbation in fibrotic sarcoidosis. All participants had experienced at least two exacerbations of their sarcoidosis in the prior year, with an exacerbation defined as an acute event requiring increase of prednisone, with or without use of antibiotics, and no other etiology identified [4]. For inclusion in the study, patients were between 18 and 70 years of age and had a diagnosis of sarcoidosis by American Thoracic Society (ATS) criteria [10], ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) of less than 80%, fibrosis on chest X-ray and/or high-resolution computed tomography (CT) of the chest, and were on a stable dose of corticosteroids and other agents for sarcoidosis for at least four weeks. Exclusion criteria included renal dysfunction with creatinine > 3 mg/dL, moderate or severe liver disease, unstable cardiac disease, non-cutaneous malignancy treated in the past two years, unable to complete questionnaires or lung function testing. Participants completed testing every three months for one year, including spirometry, 6-min walk test, blood draw, and questionnaires. Pulmonary function testing was performed and interpreted according to the 2005 ATS and European Respiratory Society (ERS) guidelines [11,12], and pre-bronchodilator spirometric measurements were used for analysis. For this analysis, we included all patients who completed three or more testing visits. We then excluded two individuals residing in areas lacking air quality data.
2.2. Exposure Estimation
Eighteen air quality monitors in southwest Ohio were used for exposure estimation in this study; six recorded fine particulate matter (PM2.5) data only, eight recorded ozone data only, and four recorded both PM2.5 and ozone data. PM2.5 data was available at least every three days (one site provided daily data). Daily ozone data was available between the months of April and October (three monitors provided year-round data) during the study period (2013–2015). The data was gap-filled using linear interpolation to obtain daily values for each pollutant. The home street addresses for 16 patients (14 in the Cincinnati, OH, metro area, 1 in Dayton, OH, and 1 in Logan, OH, approximately 50 miles southeast of Columbus, OH) were geocoded to latitude/longitude coordinates. Using the home locations, daily pollutant exposure was estimated by kriging using the spherical semivariogram model [13]. Average pollutant exposure for 7, 10, and 14 days preceding each study visit was calculated for each patient. The interquartile range (IQR) was calculated for each pollutant and time period combination.
2.3. Outcomes
We evaluated three lung function outcomes and four questionnaire outcomes. Lung function outcomes included pre-bronchodilator FEV1 and FVC measured in liters, and episodes of FEV1 decline greater than 10% from each individual’s highest value. Lung function testing used Snowbird Criteria and Hankinson reference values and lung volumes were corrected for race [12,14].
Questionnaire outcomes included scores from Leicester cough questionnaire (LCQ), Saint George Respiratory Questionnaire (SGRQ), and King’s Sarcoidosis Questionnaire (KSQ) general health status and lung specific health status. Leicester cough questionnaire (LCQ) is a 19 item quality of life measure of cough over the prior two weeks [15]. Saint George Respiratory Questionnaire (SGRQ) measures the impact of respiratory symptoms on overall health, daily life, and perceived well-being, and queries symptoms over the past three months and “these days” [16]. King’s Sarcoidosis Questionnaire is a validated questionnaire for assessing health status in sarcoidosis and consists of five modules (General health status, Lung, Skin, Eye, Medications) [17]. The general health status collects information regarding ten general health and quality of life symptoms occurring over the past two weeks: frustration, trouble concentrating, lack of motivation, tiredness, anxiousness, muscle and joint pains, embarrassment, worry about weight, worry about sarcoidosis, and tiredness interfering with normal social activities. The lung specific health status collects information regarding six respiratory symptoms occurring over the past two weeks: cough causing pain/discomfort; breathlessness climbing stairs or walking up slight inclines; having to take deep breaths, also known as “air hunger”; chest feeling tight; episodes of breathlessness; and chest pains.
2.4. Statistical Analysis
We employed generalized linear mixed effect models to evaluate the adjusted association between average PM2.5 and ozone exposure and lung function and questionnaire outcomes. All models adjusted for age, gender, smoking status, and study drug assignment. We estimated the coefficients and adjusted odds ratio (aOR) for each outcome associated with an IQR increase in average PM2.5 or ozone level. Statistical analyses were conducted using R [18].
3. Results
Sixteen patients living in Ohio completed three or more testing visits between June 2013 and June 2015 and had complete pollutant exposure data available for their home address. There were a total of 69 testing visits, with an average of 3.6 per patient.
Patient characteristics and outcomes are shown in Table 1.
Table 1.
Patient characteristics and outcomes.
| Characteristic | Statistic |
|---|---|
| Age, median years (IQR) | 59 (53.25, 62.5) |
| Female N (%) | 12 (75%) |
| African American N (%) | 9 (56%) |
| Current smoker N (%) | 1 (6%) |
| Former smoker N (%) | 10 (62%) |
| Assigned study drug | 8 (50%) |
| FEV1 (L) (mean (SD)) | 1.61 (0.68) |
| FEV1 % predicted (mean (SD)) | 62.38 (21.45) |
| FVC (L) (mean (SD)) | 2.32 (0.86) |
| FVC % predicted (mean (SD)) | 69.56 (19.81) |
| Had episode of FEV1 drop > 10% N (%) | 5 (31%) |
Patient characteristics and outcomes of study participants (N = 16). IQR: interquartile range; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
We observed low to moderate levels of PM2.5 and ozone during the study period, shown in Table 2. PM2.5 levels were similar between warm and cold seasons, with a mean 14-day average level of 11.9 μg/m3 and maximum of 23.5 μg/m3. Ozone levels were higher during the warm season, with mean 46 ppb and maximum 55 ppb. Interquartile range (IQR) for PM2.5 averaged over 7, 10, and 14 days was 4.92, 4.64, and 4.38 μg/m3, respectively. IQR for ozone averaged over 7, 10, and 14 days was 0.014, 0.014, and 0.014 ppm, respectively.
Table 2.
PM2.5 and O3 levels during the study period.
| Pollutant | Time Period | Statistic | 7-Day Average | 10-Day Average | 14-Day Average |
|---|---|---|---|---|---|
| PM2.5 (μg/m3) | All days (N = 69) | Mean (SD) | 11.6 (4.5) | 11.9 (4.3) | 11.9 (3.8) |
| Median (IQR) | 10.6 (4.9) | 10.9 (4.6) | 11.2 (4.4) | ||
| Range | (5.8, 25.3) | (6, 24.7) | (6, 23.5) | ||
| May–October (N = 33) | Mean (SD) | 10.7 (3.1) | 11.3 (2.8) | 11.6 (2.7) | |
| Median (IQR) | 10.7 (3.0) | 11.2 (3.4) | 11.4 (4.5) | ||
| Range | (6.1, 19.1) | (6.1, 17.8) | (6, 17) | ||
| November–April (N = 36) | Mean (SD) | 12.5 (5.5) | 12.5 (5.4) | 12.1 (4.6) | |
| Median (IQR) | 10.6 (6.7) | 10.2 (6.1) | 10.3 (5.4) | ||
| Range | (5.8, 25.3) | (6, 24.7) | (6.1, 23.5) | ||
| O3 (ppm) | All days (N = 69) | Mean (SD) | 0.04 (0.009) | 0.041 (0.008) | 0.041 (0.009) |
| Median (IQR) | 0.041 (0.014) | 0.042 (0.014) | 0.043 (0.014) | ||
| Range | (0.023, 0.058) | (0.023, 0.056) | (0.023, 0.055) | ||
| May–October (N = 33) | Mean (SD) | 0.044 (0.007) | 0.045 (0.007) | 0.046 (0.006) | |
| Median (IQR) | 0.044 (0.008) | 0.047 (0.008) | 0.048 (0.007) | ||
| Range | (0.026, 0.058) | (0.027, 0.056) | (0.032, 0.055) | ||
| November–April (N = 36) | Mean (SD) | 0.037 (0.008) | 0.037 (0.008) | 0.037 (0.008) | |
| Median (IQR) | 0.037 (0.012) | 0.036 (0.012) | 0.035 (0.013) | ||
| Range | (0.023, 0.052) | (0.023, 0.052) | (0.023, 0.052) |
7-, 10-, and 14-day average levels of PM2.5 and O3 occurring during the study period.
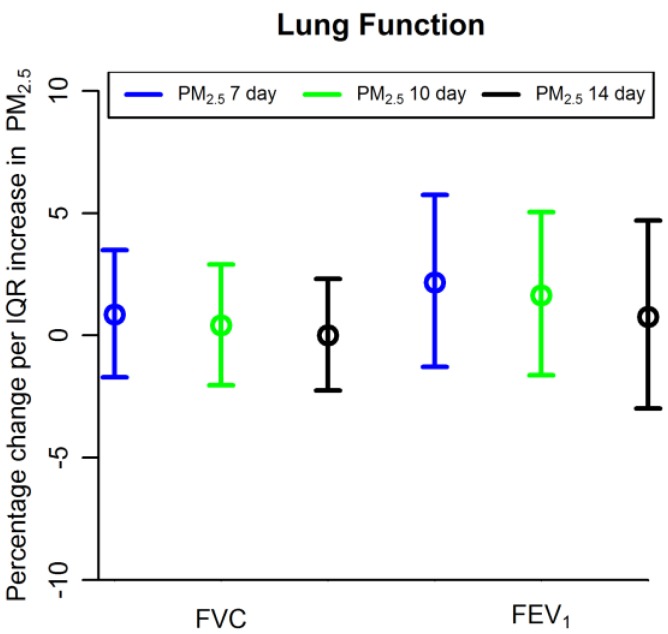
Table 3 and Figure 1 show results for lung function outcomes. Average PM2.5 was not associated with FVC (percentage change <0.01 per IQR increase in 14-day average PM2.5, 95% confidence interval (CI) −2.25 to 2.31), FEV1 (percentage change 0.76 per IQR increase in 14-day average PM2.5, CI −2.99 to 4.70), or episodes of FEV1 decline > 10% (Odds ratio (OR) 0.85 per IQR increase in 14-day average PM2.5, CI 0.32 to 1.38). Average ozone was not associated with FVC (percentage change 0.80 per IQR increase in 14-day average ozone, CI −3.13 to 4.88), FEV1 (percentage change −0.03, CI −5.18 to 5.40), or episodes of FEV1 decline > 10% (OR 0.98, CI 0.04 to 1.93).
Table 3.
Association between air pollution exposure and lung function outcomes.
| Lung Function Outcome | PM2.5 | Ozone | ||||
|---|---|---|---|---|---|---|
| 7-Day Average | 10-Day Average | 14-Day Average | 7-Day Average | 10-Day Average | 14-Day Average | |
| FVC (L) (% change) 1 |
0.86 (−1.71, 3.50) | 0.41 (−2.03, 2.91) | 0.0 (−2.25, 2.31) | 0.59 (−3.51, 4.86) | 0.46 (−3.58, 4.66) | 0.80 (−3.13, 4.88) |
| FEV1 (L) (% change) 1 |
2.17 (−1.28, 5.75) | 1.65 (−1.63, 5.04) | 0.76 (−2.99, 4.70) | 0.57 (−4.83, 6.29) | 1.11 (−4.25, 6.77) | −0.03 (−5.18, 5.40) |
| Episodes of FEV1 > 10% decline 2 | 0.74 (0.18, 1.19) | 0.76 (0.30, 1.22) | 0.85 (0.32, 1.38) | 0.9 (0.02, 1.79) | 0.93 (0.01, 1.85) | 0.98 (0.04, 1.93) |
Adjusted association between short-term air pollution exposure (PM2.5 and ozone) and lung function outcomes in fibrotic sarcoidosis. Shown are 1 percentage change (95% confidence interval) of FEV1 and FVC for each IQR change in air pollution exposure, and 2 odds ratios (95% confidence interval) for episodes of FEV1 decline > 10% for each IQR change in air pollution exposure. Interquartile range (IQR) for PM2.5 averaged over 7, 10, and 14 days = 4.92, 4.64, and 4.38 μg/m3, respectively. IQR for ozone averaged over 7, 10, and 14 days = 0.014, 0.014, and 0.014 ppm, respectively. All models adjusted for age, sex, smoking status, and study drug assignment. PM2.5 = fine particulate matter with diameter less than 2.5 μm; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Figure 1.
Association between particulate matter (PM2.5) and lung function outcomes. Shown are percentage change of FEV1 and FVC per interquartile range (IQR) increase in PM2.5 averaged over 7, 10, and 14 days. FEV1: forced expiratory volume in 1 s. FVC: forced vital capacity.
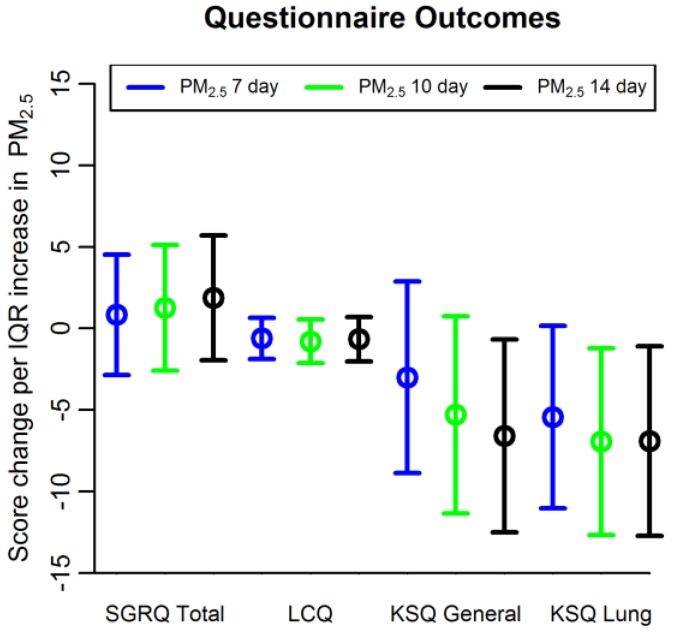
Table 4 and Figure 2 show results for questionnaire outcomes. PM2.5 level averaged over 14 days was associated with lower KSQ general health status (score change −6.60 per IQR increase in 14-day average PM2.5, 95% confidence interval (CI) −12.51 to −0.68). PM2.5 level averaged over 10 and 14 days was associated with lower KSQ lung specific health status (score change −6.93 per IQR increase in 10-day average PM2.5, 95% confidence interval (CI) −12.67 to −1.21, and score change −6.91 per IQR increase in 14-day average PM2.5, CI −12.73 to −1.09). PM2.5 levels were not associated with respiratory symptoms measured by SGRQ (score change 1.87 per IQR increase in 14-day average PM2.5, CI −1.96 to 5.70) or LCQ (score change −0.66, CI −2.03 to 0.70). Short-term ozone exposure was not associated with respiratory symptoms measured by SGRQ, LCG, or KSQ.
Table 4.
Association between air pollution exposure and questionnaire outcomes.
| Questionnaire Outcome | PM2.5 | Ozone | ||||
|---|---|---|---|---|---|---|
| 7-Day Average | 10-Day Average | 14-Day Average | 7-Day Average | 10-Day Average | 14-Day Average | |
| SGRQ Total Score | 0.84 (−2.85, 4.53) | 1.26 (−2.59, 5.11) | 1.87 (−1.96, 5.70) | −0.33 (−4.73, 4.07) | −1.08 (−5.5, 3.3) | −0.87 (−5.41, 3.66) |
| LCQ score | −0.61 (−1.87, 0.65) | −0.80 (−2.13, 0.54) | −0.66 (−2.03, 0.70) | −1.00 (−2.57, 0.57) | −0.97 (−2.55, 0.61) | −0.84 (−2.45, 0.77) |
| KSQ General Health Status | −3.00 (−8.87, 2.88) | −5.3 (−11.35, 0.75) | −6.60 (−12.51, −0.68) * | −1.22 (−8.88, 6.44) | −0.69 (−8.5, 7.1) | −0.48 (−8.37, 7.42) |
| KSQ Lung Health Status | −5.44 (−11.03, 0.15) | −6.93 (−12.67, −1.21) * | −6.91 (−12.73, −1.09) * | −3.57 (−10.9, 3.75) | −2.85 (−10.28, 4.58) | −2.34 (−9.98, 5.19) |
Adjusted association between short-term air pollution exposure (PM2.5 and ozone) and questionnaire outcomes in fibrotic sarcoidosis. Shown are regression coefficients (95% confidence interval) indicating questionnaire score change per IQR increase in air pollution exposure, * = p value < 0.05 for association. Interquartile range (IQR) for PM2.5 averaged over 7, 10, and 14 days = 4.92, 4.64, and 4.38 μg/m3, respectively. IQR for ozone averaged over 7, 10, and 14 days = 0.014, 0.014, and 0.014 ppm, respectively. All models adjusted for age, sex, smoking status, and study drug assignment. SGRQ: St. George’s Respiratory Questionnaire; LCQ: Leicester Cough Questionnaire; KSQ: King’s Sarcoidosis Questionnaire.
Figure 2.
Association between PM2.5 and questionnaire outcomes. Shown are questionnaire score changes per interquartile range (IQR) increase in PM2.5 averaged over 7, 10, and 14 days. SGRQ = St. George’s Respiratory Questionnaire; LCQ = Leicester Cough Questionnaire; KSQ = King’s Sarcoidosis Questionnaire.
4. Discussion
In this small cohort of patients with fibrotic sarcoidosis, increased PM2.5 exposure was associated with increased severity of respiratory and quality of life symptoms indicated by a decrease in lung specific health status and general health status using the validated King’s Sarcoidosis Questionnaire. These findings suggest that short-term exposure to PM2.5 may adversely affect patients with fibrotic sarcoidosis.
To our knowledge, this is the first study to evaluate effects of short-term air pollution exposure on health outcomes in fibrotic sarcoidosis. Prior epidemiologic studies have demonstrated associations between environmental exposures and development of sarcoidosis. These have included microbially-rich environments such as agricultural exposures and mold and mildew, insecticides, industrial organic and inorganic dust, particulate matter and debris from the World Trade Center disaster, and metal industries [19,20,21,22,23,24,25]. These studies suggest that exposure to many different antigens in the environment and workplace may play a role in triggering a granulomatous immune response leading to sarcoidosis. Our study suggests that environmental exposures may also contribute to episodes of clinical worsening in this disease.
We did not detect an association of air pollution exposure with lung function outcomes. Even in the absence of objective changes in pulmonary function, increased respiratory symptoms carry significance for patients’ overall health status. In idiopathic pulmonary fibrosis, dyspnea is a stronger prognostic parameter than other physiologic markers in predicting survival [26]. Patients with pulmonary fibrosis due to sarcoidosis have increased risk for respiratory failure and death and this phase of the disease does not respond as well to the usual treatments in sarcoidosis [27,28]. Identification of environmental exposures that contribute to clinical worsening may lead to opportunities for clinical improvement by reducing exposure.
We did not find any association of ozone exposure with lung function or symptom outcomes. Ozone levels were very low during this study and therefore we cannot exclude the possibility of health effects at higher levels. Additionally, there was less ozone data available during the winter months of the year due to some monitors only recording during warm months.
There are several important features of our study. We evaluated a well-defined cohort of patients with uncommon and severe manifestations of sarcoidosis. All participants had experienced two or more exacerbations of sarcoidosis requiring pharmacologic treatment in the year prior to the study period. This is the first time air pollution exposure effects have been evaluated in this specific disease state. Multiple evaluations of lung function and respiratory symptoms allowed for an evaluation of exposures over multiple time points. We were able to estimate air pollutant exposure at individual place of residence based on data from 18 monitors.
We recognize several limitations to our study. Exposure measures were imperfect, as they were based on observations from multiple monitoring stations recording pollutant data only every three days, and some ozone monitors were without year-round measurements. Similarly, the pollutant concentration estimates were made only for the place of residence, ignoring variability in exposure due to time spent indoors and at locations other than the primary residence. We were only able to evaluate effects of PM2.5 and ozone due to a limited number of monitoring stations in the region measuring other air pollutants. Air pollutants other than PM2.5 and ozone may have also contributed to respiratory symptoms. PM2.5 and ozone exposure levels in this region of Southwest Ohio, USA, were low to moderate, and thus our findings may not accurately reflect exposure effects for patients residing in regions with more extreme exposure patterns. Our sample size was very small, thus we were unable to accurately estimate the effect size, and can only identify that there is an association between PM2.5 exposure and increased respiratory symptoms. We consider this study to be exploratory, and our findings suggest further study with larger sample sizes is warranted to better explore the association of short-term air pollution exposure with health outcomes in fibrotic sarcoidosis.
5. Conclusions
In this exploratory study evaluating a small cohort of patients with fibrotic sarcoidosis, increased PM2.5 exposure was associated with increased severity of respiratory and quality of life symptoms indicated by a decrease in lung-specific and general health status using the validated King’s Sarcoidosis Questionnaire. A larger sample size is needed to evaluate the association of short-term air pollution exposure with other health outcomes in fibrotic sarcoidosis.
List of Abbreviations
| O3 | ozone |
| PM2.5 | fine particulate matter with diameter less than 2.5 μm |
| FEV1 | forced expiratory volume in 1 s |
| FVC | forced vital capacity |
| SGRQ | St. George’s Respiratory Questionnaire |
| LCQ | Leicester Cough Questionnaire |
| KSQ | King’s Sarcoidosis Questionnaire |
| IQR | interquartile range |
| APES | acute pulmonary exacerbations of sarcoidosis |
Author Contributions
C.S.P., M.B.S., D.L.M., Y.Z., and R.P.B. contributed to study concept and design. D.L.M., Y.Z., and Y.X. contributed to collection and analysis of data. C.S.P. drafted the manuscript. All authors provided important intellectual content and participated in interpretation of data, manuscript writing, and critical revision.
Funding
This work was supported by Astra Zeneca as well as the Center for Clinical and Translational Science and Training (CCTST) at the University of Cincinnati, which is funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program (grant number 1UL1TR001425-01). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of Interest
Dr. Baughman has received research support from Astra Zeneca, Genentech, Mallinckrodt, Gilead, and Bayer in the area of sarcoidosis treatment. Dr. Baughman has consulted for Mallinckrodt and Genentech in the area of sarcoidosis treatment. This study was approved by the Institutional Review Board of the University of Cincinnati. Individual patient consent was not required.
References
- 1.Bonham C.A., Strek M.E., Patterson K.C. From granuloma to fibrosis: Sarcoidosis associated pulmonary fibrosis. Curr. Opin. Pulm. Med. 2016;22:484–491. doi: 10.1097/MCP.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennebicque A.-S., Nunes H., Brillet P.-Y., Moulahi H., Valeyre D., Brauner M.W. CT findings in severe thoracic sarcoidosis. Eur. Radiol. 2005;15:23–30. doi: 10.1007/s00330-004-2480-4. [DOI] [PubMed] [Google Scholar]
- 3.Panselinas E., Judson M.A. Acute Pulmonary Exacerbations of Sarcoidosis. CHEST. 2012;142:827–836. doi: 10.1378/chest.12-1060. [DOI] [PubMed] [Google Scholar]
- 4.Baughman R.P., Lower E.E. Frequency of acute worsening events in fibrotic pulmonary sarcoidosis patients. Respir. Med. 2013;107:2009–2013. doi: 10.1016/j.rmed.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Panselinas E., Judson M.A. Pulmonary Sarcoidosis. Springer; New York, NY, USA: 2014. Acute Pulmonary Exacerbation of Sarcoidosis; pp. 65–78. [Google Scholar]
- 6.Li J., Sun S., Tang R., Qiu H., Huang Q., Mason T.G., Tian L. Major air pollutants and risk of COPD exacerbations: A systematic review and meta-analysis. COPD. 2016;11:3079–3091. doi: 10.2147/COPD.S122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng X.-Y., Ding H., Jiang L.-N., Chen S.-W., Zheng J.-P., Qiu M., Zhou Y.-X., Chen Q., Guan W.-J. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0138146. doi: 10.1371/journal.pone.0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goeminne P.C., Kiciński M., Vermeulen F., Fierens F., De Boeck K., Nemery B., Nawrot T.S., Dupont L.J. Impact of air pollution on cystic fibrosis pulmonary exacerbations: A case-crossover analysis. CHEST. 2013;143:946–954. doi: 10.1378/chest.12-1005. [DOI] [PubMed] [Google Scholar]
- 9.Baughman R.P., Birring S., Judson M.A., Culver D.A., Parambil J.G., Cordova F., Lower E.E. A Double Blind, Placebo Controlled Study of Roflumilast to Prevent Acute Events in Fibrotic Sarcoidosis. Am. J. Respir. Crit. Care Med. 2017;195:A4752. [Google Scholar]
- 10.Hunninghake G.W., Costabel U., Ando M., Baughman R., Cordier J.F., du Bois R., Eklund A., Kitaichi M., Lynch J., Rizzato G., et al. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG) Adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Volume 160. American Thoracic Society; New York, NY, USA: 1999. Statement on sarcoidosis; pp. 736–755. [DOI] [PubMed] [Google Scholar]
- 11.Miller M.R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., Crapo R., Enright P., van der Grinten C.P.M., Gustafsson P., et al. ATS/ERS Task Force Standardisation of spirometry. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 12.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 13.Lee P.-C., Roberts J.M., Catov J.M., Talbott E.O., Ritz B. First Trimester Exposure to Ambient Air Pollution, Pregnancy Complications and Adverse Birth Outcomes in Allegheny County, PA. Matern. Child Health J. 2012;17:545–555. doi: 10.1007/s10995-012-1028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Thoracic Society ATS statement–Snowbird workshop on standardization of spirometry. Am. Rev. Respir. Dis. 1979;119:831–838. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 15.Birring S.S., Prudon B., Carr A.J., Singh S.J., Morgan M.D.L., Pavord I.D. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58:339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones P.W., Quirk F.H., Baveystock C.M., Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am. Rev. Respir. Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 17.Patel A.S., Siegert R.J., Creamer D., Larkin G., Maher T.M., Renzoni E.A., Wells A.U., Higginson I.J., Birring S.S. The development and validation of the King’s Sarcoidosis Questionnaire for the assessment of health status. Thorax. 2013;68:57–65. doi: 10.1136/thoraxjnl-2012-201962. [DOI] [PubMed] [Google Scholar]
- 18.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 19.Newman L.S., Rose C.S., Bresnitz E.A., Rossman M.D., Barnard J., Frederick M., Terrin M.L., Weinberger S.E., Moller D.R., McLennan G., et al. ACCESS Research Group A case control etiologic study of sarcoidosis: Environmental and occupational risk factors. Am. J. Respir. Crit. Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 20.Izbicki G., Chavko R., Banauch G.I., Weiden M.D., Berger K.I., Aldrich T.K., Hall C., Kelly K.J., Prezant D.J. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. CHEST. 2007;131:1414–1423. doi: 10.1378/chest.06-2114. [DOI] [PubMed] [Google Scholar]
- 21.Jordan H.T., Stellman S.D., Prezant D., Teirstein A., Osahan S.S., Cone J.E. Sarcoidosis diagnosed after September 11, 2001, among adults exposed to the World Trade Center disaster. J. Occup. Environ. Med. 2011;53:966–974. doi: 10.1097/JOM.0b013e31822a3596. [DOI] [PubMed] [Google Scholar]
- 22.Newman K.L., Newman L.S. Occupational causes of sarcoidosis. Curr. Opin. Allergy Clin. Immunol. 2012;12:145–150. doi: 10.1097/ACI.0b013e3283515173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terčelj M., Salobir B., Harlander M., Rylander R. Fungal exposure in homes of patients with sarcoidosis-an environmental exposure study. Environ. Health. 2011;10:8. doi: 10.1186/1476-069X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnard J., Rose C., Newman L., Canner M., Martyny J., McCammon C., Bresnitz E., Rossman M., Thompson B., Rybicki B., et al. ACCESS Research Group Job and industry classifications associated with sarcoidosis in A Case-Control Etiologic Study of Sarcoidosis (ACCESS) J. Occup. Environ. Med. 2005;47:226–234. doi: 10.1097/01.jom.0000155711.88781.91. [DOI] [PubMed] [Google Scholar]
- 25.Deubelbeiss U., Gemperli A., Schindler C., Baty F., Brutsche M.H. Prevalence of sarcoidosis in Switzerland is associated with environmental factors. Eur. Respir. J. 2010;35:1088–1097. doi: 10.1183/09031936.00197808. [DOI] [PubMed] [Google Scholar]
- 26.Nishiyama O., Taniguchi H., Kondoh Y., Kimura T., Kato K., Kataoka K., Ogawa T., Watanabe F., Arizono S. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010;36:1067–1072. doi: 10.1183/09031936.00152609. [DOI] [PubMed] [Google Scholar]
- 27.Baughman R.P., Winget D.B., Bowen E.H., Lower E.E. Predicting respiratory failure in sarcoidosis patients. Sarcoidosis Vasc. Diffuse Lung Dis. 1997;14:154–158. [PubMed] [Google Scholar]
- 28.Nardi A., Brillet P.-Y., Letoumelin P., Girard F., Brauner M., Uzunhan Y., Naccache J.-M., Valeyre D., Nunes H. Stage IV sarcoidosis: Comparison of survival with the general population and causes of death. Eur. Respir. J. 2011;38:1368–1373. doi: 10.1183/09031936.00187410. [DOI] [PubMed] [Google Scholar]