Abstract
Progress in cancer treatment has improved the survival of patients with advanced-stage cancers. Consequently, the clinical courses of patients are prolonged and often accompanied by morbidity due to bone metastases. Skeletal-related events (SREs), such as pathological fractures and spinal paralysis, cause impairment in activities of daily life and quality of life (QOL). To avoid serious SREs causing impairment in QOL and survival, early diagnosis and a prophylactic approach are required. It is necessary to initiate a bone management program concurrently with the initiation of cancer treatment to prevent complications of bone metastasis. In addition, the requirement of a multidisciplinary approach through a cancer board focusing on the management of bone metastases and involving a team of specialists in oncology, palliative care, radiotherapy, orthopedics, nuclear medicine, radiology, and physiatrists has been emphasized. In the cancer board, a strong focus is placed on the prevention of complications due to bone metastases and on reductions in the high morbidity, hospitalization rate, and overall costs associated with advanced-stage cancers. Recent reports suggest the usefulness of such approaches. The multidisciplinary approach through a cancer board would improve QOL and prognosis of patients, leading to new or continued systemic therapy for primary cancers.
Keywords: bone metastasis, metastatic cancer, bone management, cancer board
1. Introduction
The bone is one of the most common metastatic sites of cancers and bone metastases are a major cause of morbidity in the patients with advanced-stage malignant diseases [1]. Recent progression in cancer treatments, such as the development of molecular-targeted agents and immune checkpoint inhibitors, has improved the survival of patients with advanced-stage cancers [2]. Consequently, the clinical courses of patients are prolonged and often accompanied by morbidity due to bone metastases. Morbidities, such as pathological fractures and spinal paralysis, cause impairment in activities of daily life (ADLs) and quality of life (QOL), and affect prognosis because of deterioration of the affected patient’s general condition and discontinuation of treatment for the primary disease [3,4,5,6,7,8].
Formerly, the management of bone metastasis did not hold much importance because it had been considered palliative and unassociated with prognosis. Thus, bone metastases were sometimes diagnosed after developing serious complications, such as pain, pathological fractures, and spinal cord compression. Recently, it has been necessary to initiate bone management programs concurrently with the initiation of cancer treatment to effectively prevent serious complications of bone metastasis. In addition, the requirement of a multidisciplinary approach involving a team of specialists in oncology, palliative care, radiotherapy, orthopedics, nuclear medicine, radiology, and physiatrics has been emphasized [1]. This review describes recent developments in the multidisciplinary team approach for bone management of patients with cancer with bone metastasis.
2. Epidemiology of Bone Metastasis
Bone is a common metastatic site in patients with breast, lung, and prostate cancer [9,10,11,12,13,14], whereas it can also occur in other cancers, such as liver cancer, kidney cancer, and multiple myeloma [15]. In prostate cancer, bone is the most frequent metastatic site and more than 90% of patients develop bone metastasis 2 years prior to death [14]. The bone is also the leading metastatic site in breast cancer, both at initial presentation of metastases and as the site of first recurrence [16]. The bone is the third most common site of metastasis after the lungs and the liver [13,17]. On the other hand, bone metastases are the most frequent malignancy of the bone [13]. Bone metastasis typically occurs via hematogenous dissemination [18]. The most frequent sites of metastases in the bone are the lumbar vertebrae, followed by the thoracic vertebrae, cervical vertebrae, and sacrum, whereas metastases in the appendicular skeleton are rare [19]. Pathological fractures and spinal paralysis caused by metastases in the vertebrae or femur have serious influences on the ADLs and QOL of affected patients.
3. Pathophysiology of Bone Metastasis
The reason why bone is a favorite site of metastasis from many types of cancer is not yet fully understood. One possible reason is that the microenvironment of the bone marrow is appropriate for the growth of cancer cells. In the bone marrow, metastatic cancer cells take advantage of the normal marrow physiology to survive distant from the primary site [20,21]. The bone marrow houses both cells of hematopoietic lineage and cells responsible for bone remodeling, such as osteoblasts and osteoclasts. Metastatic cancer cells in the bone interact with osteoclasts or the hematopoietic stem cell niche, which play important roles in the early colonization of bone [22]. Disseminated tumor cells (DTCs) positively influence bone remodeling to create a favorable environment for further recruitment and better survival of DTCs in the bone marrow [21,23]. Local production of osteolytic factors by the DTCs stimulates osteoclast-mediated bone resorption, which induces the production of growth factors and secretion of osteolytic cytokines [15]. Bone resorption may also play a role in the formation of osteoblastic lesions in patients with prostate cancers [15].
Bone metastases can be lytic, blastic, or mixed depending on the type of cancer [1]. Osteoblastic metastases are typical in prostate cancer, and are sometimes detected in breast and undifferentiated type stomach cancer. Meanwhile, osteolytic metastases are detected in many types of cancers, such as breast, lung, thyroid, and stomach cancers. The frequency of serious complications depends on the site and type of lesion.
In addition, systemic treatment for patients with bone metastasis, such as hormone therapy, chemotherapy, and steroids, often influence the microenvironment of the bone and sometimes cause secondary osteoporosis. Aromatase inhibitors for breast cancer increase the risk of secondary osteoporosis and pathological fractures [24,25]. Androgen deprivation therapy (ADT) is a standard treatment for metastatic prostate cancer. Because the efficacy of ADT is very high and progression of prostate cancer is relatively slow, patients with prostate cancer are usually treated using ADT for long periods. Long-term administration of ADT yet increases the risk of secondary osteoporosis and pathological fractures, especially in elderly patients [26,27]. A prospective study evaluating bone mineral density (BMD) in a total of 84 patients with prostate cancer after administering ADT for 2 years revealed that BMD significantly decreased by 6.16% after 2 years compared to the baseline [27].
4. Skeletal-Related Events
Skeletal-related events (SREs) are skeletal complications associated with bone metastases, including cancer-induced bone pain, hypercalcemia, pathological bone fractures, and spinal cord compression [21]. Pain is one of the most frequent SREs in patients with cancer with metastatic disease; 68% of patients experience pain [28]. Meanwhile, other serious SREs, such as pathological fractures, spinal cord compression, and hypercalcemia, worsen patients’ QOL and reduce survival rates [3,4,5,29,30]. A retrospective cohort study including 832 patients with castration-resistant prostate cancer and bone metastasis revealed that 207 of them developed symptomatic skeletal events (SSEs) during follow-up period (median 2.1 years). Among them, 103 patients completed Functional Assessment of Cancer Therapy-Prostate (FACT-P) and Brief Pain Inventory-Short Form (BPI-SF) questionnaires. The SSE cohort had lower mean FACT-P functional well-being, higher mean pain severity and worst pain scores compared with the non-SSE cohort [29]. In addition, Howard et al. conducted a retrospective study including 233 men diagnosed with non-metastatic castration-resistant prostate cancer. During follow-up period (median 14.7 months), 88 (38%) patients had an SRE and 198 (85%) died. The SRE was significantly associated with increased mortality [30].
Information on the management of SREs is shown in Table 1. Importantly, randomized trials showed the effects of bisphosphonate and denosumab to prevent SREs in metastatic cancer. Zoledronic acid is the first bisphosphonate to demonstrate efficacy to reduce skeletal complications in patients with bone metastases from solid tumors including breast cancer, non-small cell lung carcinoma and multiple myeloma [31,32]. In addition, randomized trials showed the superiority of denosumab to zoledronic acid for prevention of SREs [33,34]. In the phase 3 randomized, double-blinded study, a total of 1904 patients with bone metastases from castration-resistant prostate cancer were assigned to either denosumab or zoledronic acid with a 1:1 allocation [34]. Median time to first SRE was significantly longer with denosumab than those with zoledronic acid (20.7 versus 17.1 months, respectively).
Table 1.
Management of skeletal related events.
| Skeletal Related Event | Management | Effects |
|---|---|---|
| Bone pain | NSAIDs, Opioids | Analgesic effects |
| Bisphosphonates | Inhibition of pathological bone resorption Analgesic effects | |
| Denosumab | Inhibition of pathological bone resorption Analgesic effects | |
| Radiation | Analgesic effects Tumor shrinkage | |
| Pathological bone fracture | Surgery | Stabilization of fracture |
| Radiation | Supportive therapy to prevent local recurrence | |
| Bisphosphonates | Prophylaxis | |
| Denosumab | Prophylaxis | |
| Spinal cord compression | Steroids | Stabilization of vascular membranes Reduction of inflammation and edema |
| Radiation | Tumor shrinkage effects | |
| Surgery | Relief for the compression | |
| Bisphosphonates | Prophylaxis | |
| Denosumab | Prophylaxis | |
| Hypercalcemia | Hydration | Promotion of renal calciuresis |
| Loop diuretics | Promotion of renal calciuresis | |
| Bisphosphonates | Inhibition of pathological bone resorption | |
| Denosumab | Inhibition of pathological bone resorption |
NSAID: nonsteroidal anti-inflammatory drug.
Nine to 29% of patients with bone metastases develop pathological fractures [35,36]. Pathological fractures not only reduce QOL, but also impair the survival of patients [37,38]. Pathological fractures are mainly treated with surgery to stabilize the fractured bones to improve QOL via pain relief and restoration of function and mobility [39]. Radiation therapy is administered only for supportive therapy to prevent local recurrence by eliminating residual disease [40].
Spinal cord compression is an oncological emergency as it leads to reduced survival and QOL if accurate diagnosis is not made and treatment is not performed [41]. The spinal cord is damaged by compression or by vascular compromising due to tumor growth. The damage can be irreversible if the arterial flow to the spinal cord is disturbed [42,43,44]. The symptoms are pain; motor weakness; sensory deficits; gait disturbance; and urinary, bowel, and sexual dysfunction [41,45]. Immediate treatment is essential for spinal cord compression. Steroids are the first-line treatment to stabilize vascular membranes, and reduce inflammation and edema. Even though the efficacy of radiation is promising, surgery was shown to be more effective to relieve compression [46]. In a randomized, multi-institutional, non-blinded trial, patients with spinal cord compression caused by metastatic cancer were assigned to either surgery followed by radiotherapy (n = 50) or radiotherapy alone (n = 51). The primary endpoint was the ability to walk. Significantly more patients in the surgery group (42/50, 84%) than in the radiotherapy group (29/51, 57%) were able to walk after treatment. This trial show the significant role of surgery for the treatment of metastatic epidural spinal cord compression. Prompt decision-making is very important to reduce damage to the spinal cord. The optimum dose and treatment regimen of radiation for spinal cord compression is still controversial. The short course radiotherapy is preferable because the survival prognosis of most patients with metastatic epidural spinal cord compression is only a few months [47]. However, the high daily doses might be more toxic and less effective for the treatment of acute compression and prevention of recurrence. Only a randomized trial showed the non-inferiority of short course radiotherapy to the longer course [48]. In this trial, a total of 203 patients with metastatic epidural spinal cord compression and poor to intermediate expected survival were randomly assigned to either 4 Gy × 5 in 1 week (n = 101) or 3 Gy × 10 in 2 weeks (n = 102). The primary endpoint was overall response regarding motor deficits at 1 month after radiotherapy, defined as improvement or no further progression. The overall response rates regarding motor function were not significantly different, 87.2% after 4 Gy × 5 and 89.6% after 3 Gy × 10. However, both of the regimens was still non-standard short schedules. Further randomized trials is required to compare them with a standard, more protracted schedule.
Hypercalcemia is a paraneoplastic syndrome frequently observed in patients with breast cancer, lung cancer, and multiple myeloma [49]. Cancer-related hypercalcemia is mainly mediated by the activity of parathyroid hormone-related proteins produced by cancer cells, followed by bone resorption due to bone metastases. Common symptoms of hypercalcemia are nausea, vomiting, anorexia, and abdominal pain. It can also cause cognition disorder or arrhythmia [49]. As treatment for hypercalcemia, hydration and diuretics are administered to promote renal calciuresis, and bisphosphonates and denosumab are administered to inhibit pathological bone resorption [49,50,51].
5. Early Diagnosis and Prophylactic Approach for Bone Management
Early diagnosis and a prophylactic approach for serious SREs, such as pathological fractures and spinal cord compression are required because they will cause impairment of QOL and survival once they occur. Bone management in the former clinical system used to be fragmentary and unsatisfactory in that patients were referred to a series of specialists, often with long waiting lists, which created great psychological stress and led to poor continuity of healthcare [1]. In addition, physicians often referred to orthopedic surgeons after the patients had developed fractures or paralysis. Although the therapy for bone metastases is usually palliative, early diagnosis and treatment are imperative to prevent irreversible neurological damage [52,53]. Unfortunately, these procedures are subject to “doctor’s delay” when the urgent nature of the clinical state is not apprehended [54,55].
There have been reports indicating that surgical intervention for patients with cancer with impending pathologic fractures lead better outcomes than that for patients with cancer with completed pathologic fractures [56,57]. In a retrospective study with a total of 182 consecutive patients who underwent surgery for metastatic disease of the femur, treatment of 97 impending pathologic fracture yielded better results than treatment of 85 completed pathological fractures with less average blood loss, shorter hospital stay, greater likelihood of discharge to home as opposed to an extended care facility, and greater likelihood of resuming support-free ambulation [57]. A population-based study also revealed that patients who underwent prophylactic stabilizations of bone metastatic disease had better survival outcomes than those who underwent surgical interventions after fracture [58]. In the study, a total of 624 patients who had undergone femoral stabilization, either for pathological femoral fractures or for prophylactic fixation of femoral metastases before pathological fractures were identified. Patients who underwent prophylactic stabilization had significantly better overall survival after adjusting for age, sex, comorbidities and type of cancer [58].
An understanding of the risk of pathological fractures in patients with bone metastases is an unmet need for prompt prevention, detection, and treatment. Mirels proposed a scoring system to quantify the risk of sustaining a pathologic fracture through a metastatic lesion in a long bone [59]. The scoring system was based on four characteristics: (1) site of the lesion; (2) nature of the lesion; (3) size of the lesion; and (4) pain. All the features were assigned progressive scores ranging from 1 to 3 (Table 2). This scoring system was extramurally validated and its reproducibility was demonstrated, in which the overall sensitivity was 91% and specificity was 35% [60,61]. A post-hoc analysis of prospective randomized trial of radiotherapy for patients with bone metastases from solid tumors also reported risk factors for pathological fractures with femoral metastases. Their study indicated that axial cortical involvement >30 mm and circumferential cortical involvement >50% were significant predictive factors of fractures, which were more reliable than Mirels’ scoring system [62].
Table 2.
Mirels’ scoring system for diagnosing impending pathologic fractures in a long bone [59].
| Score | Site of Lesion | Size of Lesion | Nature of Lesion | Pain |
|---|---|---|---|---|
| 1 | Upper limb | <1/3 of cortex | Blastic | Mild |
| 2 | Lower limb | 1/3–2/3 of cortex | Mixed | Moderate |
| 3 | Trochanteric region | >2/3 of cortex | Lytic | Functional |
In developing a care plan, the merits of prophylactic surgery should be considered for patients with bone metastases [15]. With early diagnosis, timely intervention is essential to prevent pathologic fractures.
6. Comprehensive Approach as a Cancer Board
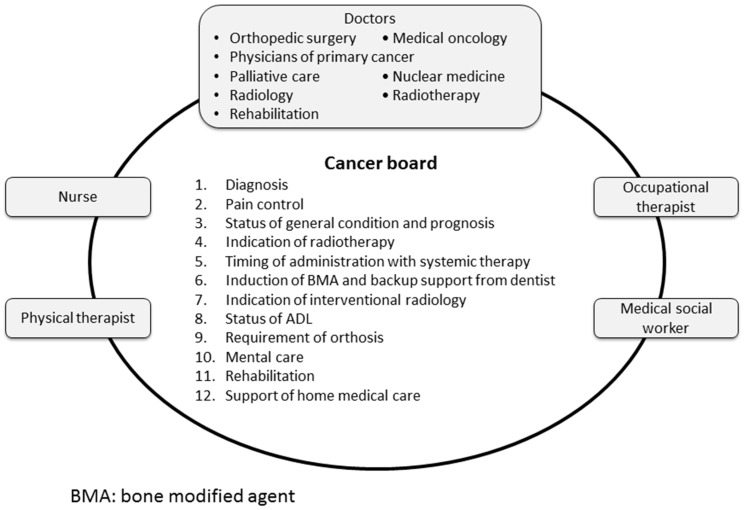
The treatment strategy for bone metastases from variable primary cancers should be planned comprehensively, taking into consideration the status of the primary cancer, prognosis, and social background of the patients. To achieve a multidisciplinary approach, a cancer board focusing on the management of bone metastases was organized involving a team of doctors in oncology, palliative care, radiotherapy, orthopedics, nuclear medicine, radiology, and rehabilitation; in addition, a nurse, physical therapist, occupational therapist, and medical social worker were included (Figure 1).
Figure 1.
Cancer board focusing the management of bone metastases.
A strong focus is placed on the prevention of complications due to bone metastases and on a reduction in the high morbidity, hospitalization rate, and overall costs associated with management of advanced-stage cancers [1].
Interdisciplinary meetings are held on a regular basis, every two to four weeks. An orthopedic surgeon or a medical oncologist usually coordinates members of the cancer board. In the cancer board of our institution, physicians of primary cancer or radiologists select cases and the radiologist presents an interpretation of the images. Issues that are focused on in the meetings are as follows: (1) confirmation of diagnosis of bone metastasis, (2) pain control by analgesic drugs or nerve-blocking agents, (3) status of general condition and prognosis, (4) indication of radiotherapy, (5) timing of administration with systemic therapy, (6) induction of bone-modifying agents and backup support from experts in oral surgery and preventive dentistry, (7) indication of interventional radiology, (8) status of ADLs, (9) requirement of orthosis, (10) mental care, (11) rehabilitation, and (12) support of home-based medical care [63]. In particular, clinical decisions to perform surgical interventions should be made in the context of the overall health status of the patients [15]. The major consideration is the functional status of the patient and what can be performed to preserve function from the point of view of bone metastases. Importantly, as surgical intervention requires a term of rehabilitation to restore function, patient prognosis is required to be predicted accurately.
In our institution, the cancer board is held monthly. Radiologists review all imaging studies of patients with advanced cancer and pick up cases those possibly require a prophylactic approach to prevent serious SREs in advance. This process is very important and effective in our experience. Physicians sometimes overlook signs of serious SREs such as pain, motor dysfunction and sensory disturbance if they are not sever or the patients have more serious symptom. The physicians review the medical records of the patient and the prophylactic approach is discussed multidisciplinarily. Physicians of primary cancer also pick up and present the patients those may require intervention.
Even though it is difficult to evaluate the efficacy of the multidisciplinary approach of cancer boards accurately, there have been reports suggesting the usefulness of such approaches. Ibrahim et al. reported preliminary results of a new organized Osteo-Oncology Center involving 19 specialists in their institution [1]. A total of 425 patients were assessed by the team in its first three years. Anonymous questionnaires about the quality of the service revealed that 98% of patients were very satisfied or satisfied, suggesting the high efficacy of the cancer board, especially in terms of decreasing psychological suffering. The management of bone metastases by such a multidisciplinary approach would improve the total health situation of patients, leading to new or continued systemic therapy for primary cancers. Further studies are required to evaluate the influence of cancer board on the oncological outcomes of patients with bone metastasis.
7. Current Pitfalls and Future Directions
Although the multidisciplinary approach of cancer boards is effective method for management of bone metastases, there are some pitfalls. It is sometimes difficult to manage patients impending to develop serious SREs timely by the meeting held biweekly or monthly. More instantaneous meeting using web conference systems might resolve this problem. Picking up patients those require such an approach is sometimes difficult. Therefore, the role of radiologists to pick up patients those have serious bone metastases is important. In addition, physicians should learn the method to diagnose signs of impending SREs by orthopedists. Accurate prediction of prognosis of the patient is also important. The prognosis of the patients influences on the approach to the SREs. However, physicians tend to predict the prognosis more optimistically. Such a multidisciplinary approach can establish common understanding among the member of the cancer board.
8. Conclusions
Bone metastases cause serious morbidities, such as pathological fractures and spinal paralysis, which cause decreases in ADLs and QOL. Treatment strategies for bone metastases should be planned comprehensively from several points of view of the health status, prognosis, and social background of the patients. Multidisciplinary approaches through a cancer board focusing on the management of bone metastases consisting of specialists will support comprehensive healthcare and treatment of patients.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Ibrahim T., Flamini E., Fabbri L., Serra P., Mercatali L., Ricci R., Sacanna E., Falasconi M.C., Casadei R., Galassi R., et al. Multidisciplinary approach to the treatment of bone metastases: Osteo-oncology center, a new organizational model. Tumori. 2009;95:291–297. doi: 10.1177/030089160909500304. [DOI] [PubMed] [Google Scholar]
- 2.Bandini M., Pompe R.S., Marchioni M., Zaffuto E., Gandaglia G., Fossati N., Cindolo L., Montorsi F., Briganti A., Saad F., et al. Improved cancer-specific free survival and overall free survival in contemporary metastatic prostate cancer patients: A population-based study. Int. Urol. Nephrol. 2018;50:71–78. doi: 10.1007/s11255-017-1744-2. [DOI] [PubMed] [Google Scholar]
- 3.Coleman R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 4.Coleman R.E. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Plunkett T.A., Rubens R.D. The biology and management of bone metastases. Crit. Rev. Oncol. Hematol. 1999;31:89–96. doi: 10.1016/S1040-8428(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 6.Norgaard M., Jensen A.O., Jacobsen J.B., Cetin K., Fryzek J.P., Sorensen H.T. Skeletal related events, bone metastasis and survival of prostate cancer: A population based cohort study in Denmark (1999 to 2007) J. Urol. 2010;184:162–167. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Yong M., Jensen A.O., Jacobsen J.B., Norgaard M., Fryzek J.P., Sorensen H.T. Survival in breast cancer patients with bone metastases and skeletal-related events: A population-based cohort study in Denmark (1999–2007) Breast Cancer Res. Treat. 2011;129:495–503. doi: 10.1007/s10549-011-1475-5. [DOI] [PubMed] [Google Scholar]
- 8.Sathiakumar N., Delzell E., Morrisey M.A., Falkson C., Yong M., Chia V., Blackburn J., Arora T., Kilgore M.L. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: A population-based analysis of us medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14:177–183. doi: 10.1038/pcan.2011.7. [DOI] [PubMed] [Google Scholar]
- 9.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Z., Deng G., Huang X., Li X., Xie X., Wang J., Shuang Z., Wang X. Bone metastasis pattern in initial metastatic breast cancer: A population-based study. Cancer Manag. Res. 2018;10:287–295. doi: 10.2147/CMAR.S155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawood S., Broglio K., Ensor J., Hortobagyi G.N., Giordano S.H. Survival differences among women with de novo stage iv and relapsed breast cancer. Ann. Oncol. 2010;21:2169–2174. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y., Ma Y., Sheng J., Huang Y., Zhao Y., Fang W., Hong S., Tian Y., Xue C., Zhang L. A multicenter, retrospective epidemiologic survey of the clinical features and management of bone metastatic disease in China. Chin. J. Cancer. 2016;35:40. doi: 10.1186/s40880-016-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hage W.D., Aboulafia A.J., Aboulafia D.M. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop. Clin. N. Am. 2000;31:515–528. doi: 10.1016/S0030-5898(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 14.Pezaro C., Omlin A., Lorente D., Rodrigues D.N., Ferraldeschi R., Bianchini D., Mukherji D., Riisnaes R., Altavilla A., Crespo M., et al. Visceral disease in castration-resistant prostate cancer. Eur. Urol. 2014;65:270–273. doi: 10.1016/j.eururo.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum R.H., Novetsky D., Shasha D., Fleishman S. The multidisciplinary approach to bone metastases. Oncology. 2003;17:845–857; discussion 862–863, 867. [PubMed] [Google Scholar]
- 16.Solomayer E.F., Diel I.J., Meyberg G.C., Gollan C., Bastert G. Metastatic breast cancer: Clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res. Treat. 2000;59:271–278. doi: 10.1023/A:1006308619659. [DOI] [PubMed] [Google Scholar]
- 17.Hosono N., Yonenobu K., Fuji T., Ebara S., Yamashita K., Ono K. Orthopaedic management of spinal metastases. Clin. Orthop. Relat. Res. 1995;312:148–159. [PubMed] [Google Scholar]
- 18.Karr J.P., Yamanaka H. Prostate Cancer and Bone Metastasis. 1st ed. Springer; New York, NY, USA: 1992. [Google Scholar]
- 19.Moriwaki S. Histopathology and statistical analysis of spinal metastases of carcinomas in autopsy cases. Orthop. Surg. Traumatol. 1993;36:233–241. [Google Scholar]
- 20.Olechnowicz S.W., Edwards C.M. Contributions of the host microenvironment to cancer-induced bone disease. Cancer Res. 2014;74:1625–1631. doi: 10.1158/0008-5472.CAN-13-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuzuki S., Park S.H., Eber M.R., Peters C.M., Shiozawa Y. Skeletal complications in cancer patients with bone metastases. Int. J. Urol. 2016;23:825–832. doi: 10.1111/iju.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiozawa Y., Pedersen E.A., Havens A.M., Jung Y., Mishra A., Joseph J., Kim J.K., Patel L.R., Ying C., Ziegler A.M., et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clines G.A., Guise T.A. Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocr. Relat. Cancer. 2005;12:549–583. doi: 10.1677/erc.1.00543. [DOI] [PubMed] [Google Scholar]
- 24.Chien A.J., Goss P.E. Aromatase inhibitors and bone health in women with breast cancer. J. Clin. Oncol. 2006;24:5305–5312. doi: 10.1200/JCO.2006.07.5382. [DOI] [PubMed] [Google Scholar]
- 25.Henry N.L., Giles J.T., Ang D., Mohan M., Dadabhoy D., Robarge J., Hayden J., Lemler S., Shahverdi K., Powers P., et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res. Treat. 2008;111:365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazawa Y., Sekine Y., Syuto T., Nomura M., Koike H., Matsui H., Shibata Y., Ito K., Suzuki K. Evaluation of bone turnover/quality markers and bone mineral density in prostate cancer patients receiving androgen deprivation therapy with or without denosumab. Anticancer Res. 2017;37:3667–3671. doi: 10.21873/anticanres.11737. [DOI] [PubMed] [Google Scholar]
- 27.Miyazawa Y., Sekine Y., Syuto T., Nomura M., Koike H., Matsui H., Shibata Y., Ito K., Suzuki K. Effect of androgen-deprivation therapy on bone mineral density in Japanese patients with prostate cancer. In Vivo. 2018;32:409–412. doi: 10.21873/invivo.11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Beuken-van Everdingen M.H., de Rijke J.M., Kessels A.G., Schouten H.C., van Kleef M., Patijn J. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann. Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 29.McKay R., Haider B., Duh M.S., Valderrama A., Nakabayashi M., Fiorillo M., Ristovska L., Wen L., Kantoff P. Impact of symptomatic skeletal events on health-care resource utilization and quality of life among patients with castration-resistant prostate cancer and bone metastases. Prostate Cancer Prostatic Dis. 2017;20:276–282. doi: 10.1038/pcan.2017.4. [DOI] [PubMed] [Google Scholar]
- 30.Howard L.E., De Hoedt A.M., Aronson W.J., Kane C.J., Amling C.L., Cooperberg M.R., Terris M.K., Divers C.H., Valderrama A., Freedland S.J. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis. 2016;19:380–384. doi: 10.1038/pcan.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen L.S., Gordon D., Kaminski M., Howell A., Belch A., Mackey J., Apffelstaedt J., Hussein M.A., Coleman R.E., Reitsma D.J., et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 32.Rosen L.S., Gordon D., Tchekmedyian S., Yanagihara R., Hirsh V., Krzakowski M., Pawlicki M., de Souza P., Zheng M., Urbanowitz G., et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: A phase iii, double-blind, randomized trial—The zoledronic acid lung cancer and other solid tumors study group. J. Clin. Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 33.Lipton A., Fizazi K., Stopeck A.T., Henry D.H., Brown J.E., Yardley D.A., Richardson G.E., Siena S., Maroto P., Clemens M., et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: A combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer. 2012;48:3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Fizazi K., Carducci M., Smith M., Damiao R., Brown J., Karsh L., Milecki P., Shore N., Rader M., Wang H., et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aaron A.D. Treatment of metastatic adenocarcinoma of the pelvis and the extremities. J. Bone Jt. Surg. Am. 1997;79:917–932. doi: 10.2106/00004623-199706000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Bohm P., Huber J. The surgical treatment of bony metastases of the spine and limbs. J. Bone Jt. Surg. Br. 2002;84:521–529. doi: 10.1302/0301-620X.84B4.12495. [DOI] [PubMed] [Google Scholar]
- 37.Hansen B.H., Keller J., Laitinen M., Berg P., Skjeldal S., Trovik C., Nilsson J., Walloe A., Kalen A., Wedin R. The scandinavian sarcoma group skeletal metastasis register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop. Scand. Suppl. 2004;75:11–15. doi: 10.1080/00016470410001708270. [DOI] [PubMed] [Google Scholar]
- 38.Mavrogenis A.F., Pala E., Romagnoli C., Romantini M., Calabro T., Ruggieri P. Survival analysis of patients with femoral metastases. J. Surg. Oncol. 2012;105:135–141. doi: 10.1002/jso.22061. [DOI] [PubMed] [Google Scholar]
- 39.Bryson D.J., Wicks L., Ashford R.U. The investigation and management of suspected malignant pathological fractures: A review for the general orthopaedic surgeon. Injury. 2015;46:1891–1899. doi: 10.1016/j.injury.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Frassica D.A. General principles of external beam radiation therapy for skeletal metastases. Clin. Orthop. Relat. Res. 2003;415:S158–S164. doi: 10.1097/01.blo.0000093057.96273.fb. [DOI] [PubMed] [Google Scholar]
- 41.Prasad D., Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6:15–24. doi: 10.1016/S1470-2045(05)70022-X. [DOI] [PubMed] [Google Scholar]
- 42.Ushio Y., Posner R., Posner J.B., Shapiro W.R. Experimental spinal cord compression by epidural neoplasm. Neurology. 1977;27:422–429. doi: 10.1212/WNL.27.5.422. [DOI] [PubMed] [Google Scholar]
- 43.Ramsey R., Doppman J.L. The effects of epidural masses on spinal cord blood flow. An experimental study in monkeys. Radiology. 1973;107:99–103. doi: 10.1148/107.1.99. [DOI] [PubMed] [Google Scholar]
- 44.Kato A., Ushio Y., Hayakawa T., Yamada K., Ikeda H., Mogami H. Circulatory disturbance of the spinal cord with epidural neoplasm in rats. J. Neurosurg. 1985;63:260–265. doi: 10.3171/jns.1985.63.2.0260. [DOI] [PubMed] [Google Scholar]
- 45.Cole J.S., Patchell R.A. Metastatic epidural spinal cord compression. Lancet Neurol. 2008;7:459–466. doi: 10.1016/S1474-4422(08)70089-9. [DOI] [PubMed] [Google Scholar]
- 46.Patchell R.A., Tibbs P.A., Regine W.F., Payne R., Saris S., Kryscio R.J., Mohiuddin M., Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 47.Rades D., Abrahm J.L. The role of radiotherapy for metastatic epidural spinal cord compression. Nat. Rev. Clin. Oncol. 2010;7:590–598. doi: 10.1038/nrclinonc.2010.137. [DOI] [PubMed] [Google Scholar]
- 48.Rades D., Segedin B., Conde-Moreno A.J., Garcia R., Perpar A., Metz M., Badakhshi H., Schreiber A., Nitsche M., Hipp P., et al. Radiotherapy with 4 gy x 5 versus 3 gy x 10 for metastatic epidural spinal cord compression: Final results of the score-2 trial (aro 2009/01) J. Clin. Oncol. 2016;34:597–602. doi: 10.1200/JCO.2015.64.0862. [DOI] [PubMed] [Google Scholar]
- 49.Stewart A.F. Clinical practice. Hypercalcemia associated with cancer. N. Engl. J. Med. 2005;352:373–379. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 50.Hosking D.J., Cowley A., Bucknall C.A. Rehydration in the treatment of severe hypercalcaemia. QJM Int. J. Med. 1981;50:473–481. [PubMed] [Google Scholar]
- 51.Thosani S., Hu M.I. Denosumab: A new agent in the management of hypercalcemia of malignancy. Future Oncol. 2015;11:2865–2871. doi: 10.2217/fon.15.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maranzano E., Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: Final results from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-G. [DOI] [PubMed] [Google Scholar]
- 53.Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol. Scand. 1996;94:269–275. doi: 10.1111/j.1600-0404.1996.tb07064.x. [DOI] [PubMed] [Google Scholar]
- 54.Loven D., Gornish M., Fenig G.E., Sulkes A., Rappaport Z., Klir I., Rotenberg Z., Gadoth N. malignant epidural cord compression. Harefuah. 1996;131:457–462, 536. [PubMed] [Google Scholar]
- 55.Solberg A., Bremnes R.M. Metastatic spinal cord compression: Diagnostic delay, treatment, and outcome. Anticancer Res. 1999;19:677–684. [PubMed] [Google Scholar]
- 56.Johnson S.K., Knobf M.T. Surgical interventions for cancer patients with impending or actual pathologic fractures. Orthop. Nurs. 2008;27:160–171. doi: 10.1097/01.NOR.0000320543.90115.d5. [DOI] [PubMed] [Google Scholar]
- 57.Ward W.G., Holsenbeck S., Dorey F.J., Spang J., Howe D. Metastatic disease of the femur: Surgical treatment. Clin. Orthop. Relat. Res. 2003;415:S230–S244. doi: 10.1097/01.blo.0000093849.72468.82. [DOI] [PubMed] [Google Scholar]
- 58.Ristevski B., Jenkinson R.J., Stephen D.J., Finkelstein J., Schemitsch E.H., McKee M.D., Kreder H.J. Mortality and complications following stabilization of femoral metastatic lesions: A population-based study of regional variation and outcome. Can. J. Surg. 2009;52:302–308. [PMC free article] [PubMed] [Google Scholar]
- 59.Mirels H. Metastatic disease in long bones: A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256–264. doi: 10.1097/00003086-198912000-00027. [DOI] [PubMed] [Google Scholar]
- 60.Damron T.A., Morgan H., Prakash D., Grant W., Aronowitz J., Heiner J. Critical evaluation of mirels’ rating system for impending pathologic fractures. Clin. Orthop. Relat. Res. 2003;415:S201–S207. doi: 10.1097/01.blo.0000093842.72468.73. [DOI] [PubMed] [Google Scholar]
- 61.Jawad M.U., Scully S.P. In brief: Classifications in brief: Mirels’ classification: Metastatic disease in long bones and impending pathologic fracture. Clin. Orthop. Relat. Res. 2010;468:2825–2827. doi: 10.1007/s11999-010-1326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van der Linden Y.M., Dijkstra P.D., Kroon H.M., Lok J.J., Noordijk E.M., Leer J.W., Marijnen C.A. Comparative analysis of risk factors for pathological fracture with femoral metastases. J. Bone Jt. Surg. Br. 2004;86:566–573. doi: 10.1302/0301-620X.86B4.14703. [DOI] [PubMed] [Google Scholar]
- 63.Takagi T. Comprehensive approach for spinal metastases: Transdisciplinary managementa takagi. J. Jt. Surg. 2016;35:368–373. [Google Scholar]