Abstract
Backgrounds and Aims
Because stomata in bryophytes occur on sporangia, they are subject to different developmental and evolutionary constraints from those on leaves of tracheophytes. No conclusive experimental evidence exists on the responses of hornwort stomata to exogenous stimulation.
Methods
Responses of hornwort stomata to abscisic acid (ABA), desiccation, darkness and plasmolysis were compared with those in tracheophyte leaves. Potassium ion concentrations in the guard cells and adjacent cells were analysed by X-ray microanalysis, and the ontogeny of the sporophytic intercellular spaces was compared with those of tracheophytes by cryo-scanning electron microscopy.
Key Results
The apertures in hornwort stomata open early in development and thereafter remain open. In hornworts, the experimental treatments, based on measurements of >9000 stomata, produced only a slight reduction in aperture dimensions after desiccation and plasmolysis, and no changes following ABA treatments and darkness. In tracheophytes, all these treatments resulted in complete stomatal closure. Potassium concentrations are similar in hornwort guard cells and epidermal cells under all treatments at all times. The small changes in hornwort stomatal dimensions in response to desiccation and plasmolysis are probably mechanical and/or stress responses of all the epidermal and spongy chlorophyllose cells, affecting the guard cells. In contrast to their nascent gas-filled counterparts across tracheophytes, sporophytic intercellular spaces in hornworts are initially liquid filled.
Conclusions
Our experiments demonstrate a lack of physiological regulation of opening and closing of stomata in hornworts compared with tracheophytes, and support accumulating developmental and structural evidence that stomata in hornworts are primarily involved in sporophyte desiccation and spore discharge rather than the regulation of photosynthesis-related gaseous exchange. Our results run counter to the notion of the early acquisition of active control of stomatal movements in bryophytes as proposed from previous experiments on mosses.
Keywords: Abscisic acid, dehiscence, evolution of land plants, potassium flux, plasmolysis, spore discharge, stomata, sporangia, X-ray microanalysis
INTRODUCTION
Stomata are a key innovation that enabled freshwater algae to colonize Earth’s land masses >500 million years ago (Morris et al., 2018). A long-held hypothesis posits that stomata evolved once in the common ancestor of land plants and that their role and regulation are conserved across all land plant lineages (Raven, 2002). However, this major assumption has been based on scarce or no structural and functional evidence for phylogenetically key groups at the base of the land plant tree: bryophytes (hornworts, mosses), the most basal clade, and pteridophytes (lycophytes, ferns), the sister group to the seed plants. Recent studies focusing on these groups have presented conflicting evidence either supporting the evolutionary theory of early acquisition of active stomatal control (Chater et al., 2011, 2013, 2017; Ruszala et al., 2011; Xu et al., 2016; Hõrak et al., 2017; Merilo et al., 2017) or rejecting it in favour of the alternative hypothesis of gradual acquisition of active control mechanisms (Brodribb et al., 2009; Brodribb and McAdam, 2011, 2013, 2017; Pressel et al., 2014; Field et al., 2015; Villarreal and Renzaglia, 2015; Sussmilch et al., 2017; Duckett and Pressel, 2017a; Merced and Renzaglia, 2017; Renzaglia et al., 2017). Following recent demonstrations of key stomatal developmental stages unique to bryophytes (Pressel et al., 2014; Villarreal and Renzaglia, 2015; Merced and Renzaglia, 2017), here we investigate stomatal behaviour in hornworts – the bryophyte clade currently considered sister to the vascular plants (Liu et al., 2014; Qiu et al., 2006, 2007).
Stomata occur in all embryophyte groups except liverworts. Unlike tracheophytes where stomata abound on vegetative organs, especially leaves, of the sporophyte, those in bryophytes are restricted to the sporangia. As monosporangiates, bryophytes are characterized by the occurrence of a single sporangium on a matrotrophic sporophyte (Ligrone et al., 1993). Undoubtedly, stomata on these sporangia have experienced a suite of evolutionary constraints independent from those in tracheophytes, potentially leading to fundamental differences in structure and function across these groups. The hornwort sporophyte is unparalleled in any living or extinct group in that it is an elongating sporangium with asynchronous meiosis. Such a system requires precise co-ordination of all portions of the sporophyte to ensure protection of all stages, from meristematic cells to dehiscence of mature spores. Much like the apical meristem of vegetative organs, it affords a dynamic experimental system in which to examine spatial and temporal changes in a static structure. It is perplexing therefore that, in spite of the multitude of physiological studies on tracheophyte stomata, no conclusive analysis has been conducted on the regulation of stomatal function in hornworts.
Conflicting reports exist on regulation of movement in hornwort stomata. Responses to environmental stimuli and exogenous abscisic acid (ABA) were reported by Bopp and Werner (1993), Hartung (2010) and Hartung et al. (1987, 1994). However, Lucas and Renzaglia (2002) found no evidence for stomatal closure in response to ABA or darkness, with some response to desiccation, in line with previous studies by Paton and Pierce (1957). Staining in the guard cells (GCs) with Fast Violet B was associated with pore opening, thus suggesting organic acid accumulation (e.g. malate), but cobaltous nitrate staining for potassium produced equivocal results between open and closed stomata. Lucas and Renzaglia (2002) concluded that hornwort stomata open only once, through osmotic changes, thereafter remaining open with a dual role in providing a passageway for photosynthesis-based gaseous exchange and facilitating sporophyte dehydration.
Consequent on a recent study of early stomatal ontogeny in hornworts, Pressel et al. (2014) came to the same conclusion about function (Lucas and Renzaglia, 2002), a view echoed most recently by Villarreal and Renzaglia (2015), Merced and Renzaglia (2017) and Renzaglia et al. (2017). An unexpected discovery by Pressel et al. (2014) was that in vascular plants, the schizogenous intercellular spaces associated with stomata are gas filled from their inception whereas those in hornworts are initially fluid filled. Opening of the stomatal pores is a prerequisite for drying the spaces and replacing the liquid with gas. The importance of this process and the ultimate drying of the sporophyte for spore release would seem to preclude any requirement for regulation of water loss through stomatal movements. Further additional pieces in the jigsaw of stomatal function and evolution are that asymmetric stomata along the sporophyte sutures in hornworts, originally considered abnormal (Pressel et al., 2014), are in fact an integral component in the dehiscence mechanism (Villarreal and Renzaglia, 2015) and that, in hornworts and mosses, apertures and numbers do not respond to different atmospheric CO2 concentrations (Field et al., 2015). In contrast, in vascular plants, species with passive stomatal control have reduced stomatal densities when grown in raised CO2 (Brodribb et al., 2009; Brodribb and McAdam, 2011, 2013, 2017), whereas those with active regulation respond by aperture reductions (Haworth et al., 2015; Lake et al., 2016). It should also be noted that, whilst some authors strongly promote early acquisition of active control on the basis of changes in stomatal number and density in response to both ABA and CO2 in ferns and angiosperms under different laboratory growth conditions (Xu et al., 2016; Hõrak et al., 2017; Merilo et al., 2017), more broadly based and critical evaluations by others make a strong case for gradual evolution (Sussmilch et al., 2017; Brodribb and McAdam, 2017).
There are few studies of moss stomatal structure and physiology (Sack and Paolillo, 1983a, b, c, 1985; Duckett et al., 2009; Merced and Renzaglia 2013, 2014; Merced, 2015). In Funaria hygrometrica and the closely related model moss Physcomitrella patens, the GCs are slightly sensitive to ABA, the fungal toxin fusicoccin that stimulates proton pumping across plant cell membranes, darkness and CO2 concentrations (Chater et al., 2011). Expanding green capsules of Funaria have only a short period of up to 5 d during which stomata are responsive to environmental stimuli (Garner and Paolillo, 1973), following which they remain open if hydrated. Merced and Renzaglia (2014) demonstrated that in Funaria, GC walls are thinner, contain higher pectin concentration and are flexible during this period when pores first form. With capsule maturation, GC walls thicken, contain reduced pectin and become less flexible. Stomatal function and homology have been called into question in Sphagnum, the sister group to other mosses (Newton et al., 2000; Cox et al., 2004; Shaw and Renzaglia, 2004). In Sphagnum, the paired GC-like structures adorning the capsule walls never open into intercellular spaces and there is no evidence of potassium fluxes between these and the adjacent epidermal cells (Beerling and Franks, 2009; Duckett et al., 2009). The conclusion from these and a more recent ultrastructural and immunocytochemical study of wall architecture (Merced, 2015) is that these structures in Sphagnum facilitate capsule desiccation leading to spore discharge, but their relationship to true stomata remains an open question. Echoing this notion, Chater et al. (2016) state that delayed dehiscence in stomata-less mutants of Physcomitrella implies that stomata might have promoted dehiscence in the first complex land-plant sporophytes, though this conclusion is difficult to reconcile with the fact that Physcomitrella sporophytes are cleistogamous (McDaniel et al., 2009).
Most recently it has been reported that hornwort stomata are short lived, with the GCs dying prior to drying of other sporophytic cells (Merced and Renzaglia, 2017; Renzaglia et al., 2017). These stomata subsequently collapse and remain locked with the apertures wide open over the sub-stomatal cavity. GC collapse precedes the final dehydration of the sporogenous tissue, which is surrounded by pectin-containing mucilage. Thus, as in mosses, hornwort stomata have a short window of potential physiological response to their environment in the green lower portions of the sporophyte.
Against the background of new information on stomatal and intercellular space development in hornworts, we report here an extensive experimental programme to determine whether or not: (1) aperture dimensions, immediately after initial opening, are subsequently capable of changing in response to the same external factors that affect mosses and vascular plants (i.e. ABA, desiccation, darkness and plasmolysis); and (2) opening is associated with a potassium flux into the GCs.
MATERIALS AND METHODS
Nomenclature of hornworts follows Duff et al. (2007) and Glenny (1998).
Electron microscopy observations were made on a range of freshly collected hornwort species: Anthoceros punctatus L. [UK]; Dendroceros javanicus (Nees) Nees [Peninsular Malaysia]; Folioceros fuciformis (Mont.) D.C. [Bharadwaj]; Notothylas levieri Schiffn. Ex Steph. [India]; Phaeoceros carolinianus (Michx.) Prosk. [USA]; P. laevis (L.) Prosk. [Wakehurst Place, Sussex, UK]; and Phaeomegaceros fimbriatus (Gottsche) Duff et al. [Panama]. Voucher specimens are in the herbarium at the Natural History Museum, London.
For all the experimental treatments to measure stomatal responses to exogenous and environmental cues (exogenous ABA, desiccation and darkness), we used wild-collected UK plants of A. punctatus and P. laevis. We also used the same plants to study the effects of loss of turgor pressure in hornwort GCs by plasmolysing these in 1.0 m sucrose. Since plasmolysis in seed plants results in closure of stomata due to loss of turgidity in GCs, open apertures following plasmolysis in hornwort stomata would be indicative of mechanical maintenance of open pores simply via cell wall properties.
The experiments on the wild plants were conducted over a period of 1–3 d after collection, i.e. as quickly as was feasible to record the large numbers of apertures needed. During this time, plants were maintained on their nutrient-poor native substrates in a growth cabinet with a constant irradiance of 100 μmol m–2 s–1 at 18 °C. We used constant illumination to compensate for the higher irradiances experienced by the plants in nature (typically up to 600–900 μmol m–2 s–1).
As well as rigorous hornwort controls and, as a further check on our procedures, during our hornwort experiments we also observed the effects of ABA and sucrose on angiosperms (arabidopsis, Lactuca and Hedera) kept in the same growth cabinets. Unlike the hornworts, their stomata closed completely.
Because hornwort stomata open at or just above the involucres (Pressel et al., 2014) and moss stomata are considered to be responsive to stimuli for only a few days after pore formation (Chater et al., 2011; Merced and Renzaglia, 2014), plus the recent demonstration that apertures become fixed open on older parts of sporophytes (Renzaglia et al., 2017), we restricted observations to stomata in the first 1 cm of the sporophytes above involucres. As a further precaution to minimize the risk of measuring possible dying or dead stomata, we selected sporophytes at least 3 cm long with the change in colour of the developing spores to yellow or black in Phaeoceros and Anthoceros, respectively, usually >2.5cm above the involucres. For the ABA treatments and desiccation, measurements were taken from ten sporophytes; for darkness and plasmolysis they were taken from five sporophytes.
Sporophytes were excised from the subjacent thalli level with the bases of the involucres and sliced longitudinally at right angles to the dehiscence grooves. The two halves were mounted in water, epidermis upwards, and digital images were taken of all the stomata using a Zeiss Axioscop 2 microscope equipped with an AxioCam MRc digital camera. Aperture dimensions (width, length and surface area – calculated with the equation for an ellipse) were measured using the associated Axiovision software. For the desiccation treatment, sporophytes were mounted in immersion oil to avoid rehydration. Control (hydrated) sporophytes were also mounted in immersion oil to discern any possible effect of the oil on aperture dimensions. There was no evidence that any of our manipulations led to the death of the GCs as the plasmolysed sporophytes in sucrose rapidly recovered when placed in water.
For the exogenous ABA treatment, we followed the protocol of Hartung (1983) and Hartung et al. (1987). Intact sporophytes and others sectioned longitudinally to optimize uptake were exposed to a range of ABA concentrations (100, 10 and 1 µm), dissolved either in water (unbuffered) or in a medium containing 10 mm KCl in 10 mm MES/NaOH (buffered) (Hartung et al., 1987) for 1, 2, 4 and 24 h in the controlled-environment cabinet. For the buffered treatment, sporophytes were placed in the medium for 1 h prior to addition of ABA as per Hartung et al. (1987). Control treatments were kept in water or in the same medium for corresponding lengths of time. The buffered and unbuffered ABA treatments, for intact or sectioned sporophytes, and irrespective of ABA concentration used and length of exposure, all gave similar results. Therefore, here we report only the results for the intact sporophytes in unbuffered 100 µm after 4 h exposure. The same ABA treatments were repeated using leaves of Lactuca sativa L, Arabidopsis thaliana (L.) Heyn. and Hedera helix L. as vascular plant controls.
For the desiccation treatment, sporophyte-bearing thalli were allowed to dry under natural conditions – after approx. 72 h, the dried thalli bearing flaccid sporophytes had lost >50 % of their original water (Fig. 1D) and were used for imaging and aperture measurements.
Fig. 1.
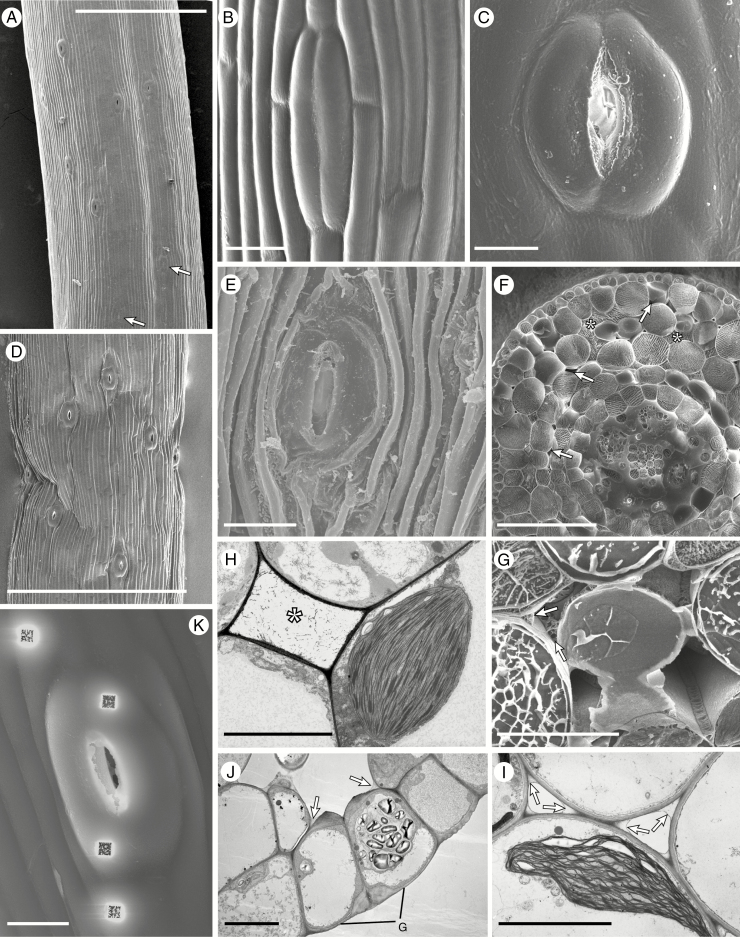
Cryo-scanning (A–G, K) and transmission (H–J) electron micrographs of Anthoceros punctatus (A–F), Phaeoceros laevis (G, J, K), Phaeomegaceros fimbriatus (H) and Folioceros fuciformis (I). (A) Portion of sporophyte just above the involucre with young stomata at the bottom (arrowed) and stomata with fully open apertures above. (B) Closed stoma with smooth walls. (C) Fully opened stoma. (D) Open stomata in a dehydrated sporophyte. (E) Stoma in the wall of a sporophyte well beyond the dehiscence point. (F) Cryo-fractured sporophyte with a mixture of liquid- (*) and gas-filled (arrowed) intercellular spaces. Note the liquid investing the developing spores and elaters. (G) Above the level where the stomata open the liquid is replaced by air, with liquid dried down to a thin layer lining the cell junctions (arrowed). (H, I) Liquid is also visible by TEM: (H) liquid-filled intercellular space (*) and (I) dried out liquid along the cell junctions in the assimilatory tissue (arrowed). (J) Section through stomatal guard cells; note the thickened inner and outer walls of the guard cells (G), the large starch-filled plastid and the dried down liquid in the corners of the intercellular spaces (arrowed). (K) An open stoma after ion milling. Scale bars: (A, D) 500 µm; (F) 100 µm; (B, C, E, J, K) 20 µm; (H, I) 10 µm.
Possible effects of darkness on aperture dimensions were studied on sporophytes kept in the dark for 15–24 h, and measurements were taken straight away, within 5 min of mounting these. While exposure to darkness was longer than that experienced by plants under natural conditions, the additional time reflects experimental time constraints and the fact that we had to record a large number of stomata. Sporophyte plasmolysis was brought about by submersion of intact sporophytes in 1.0 m sucrose for 15 min and compared with the leaves of a range of identically treated vascular plants (L. sativa, Osmunda regalis L. and Scolopendrium vulgare Sm.).
The protocol for transmission electron microscopy (TEM) follows Ligrone and Renzaglia (1990), and the cryo-scanning electron microscopy (cryo-SEM) protocol follows Duckett et al. (2009). For comparisons on intercellular space ontogeny in hornworts, we used representatives of all the major tracheophyte lineages: Huperzia selago (L.) Bernh. ex Schrank & Mart. (lycophyte), Scolopendrium vulgare (monilophyte), Ginkgo biloba L., Podocarpus nivalis Hook., Pinus mugo Turra (gymnosperms) and H. helix (angiosperm).
Element X-ray spectra were obtained for both GCs of at least five stomata plus adjacent epidermal cells from the following: (1) stomata inside involucres; (2) exposed stomata below and (3) above the dehiscence point; (4) exposed stomata in plants that had been allowed to dry out for 72 h; (5) exposed stomata on plants kept in the dark for 15–24 h (these were exposed to light for only 5 min, the time between mounting the specimens and freezing); and (6) plants treated with 1, 10 or 100 µm ABA for 1–24 h. Thallus epidermal cells provided controls for potassium concentrations in somatic cells. Spectra were obtained from pits in the cells produced by ion milling and directly from the lumina of fractured cells. Since both procedures produced closely similar results, these are not separated in Table 1.
Table 1.
Means from 8–9 readings of the percentage mass of potassium from X-ray microanalysis
| Species | Inside involucre | Exposed | Splitting | Desiccated | Dark | 4 h of 100 µm ABA |
|---|---|---|---|---|---|---|
| Phaeoceros laevis | ||||||
| Guard cells | 2.1 ± 0.18 | 0.9 ± 0.07 | 3.8 ± 0.42 | 1.6 ± 0.12 | 2.4 ± 0.16 | 2.4 ± 0.20 |
| Stomatal condition | Closed | Open | Open | Open | Open | Open |
| Epidermis | 1.7 ± 0.12 | 1.3 ± 0.10 | 3.9 ± 0.31 | 1.5 ± 0.09 | 2.1 ± 0.18 | 2.0 ± 0.21 |
| Ratio of K in guard cells to that in epidermal cells | 1.24 | 0.69 | 0.97 | 1.06 | 1.14 | 1.2 |
| Guard cell K relative to epidermis | Higher | Lower | Same | Same | Higher | Same |
| Thallus control | 0.98 ± 0.07 | |||||
| Anthoceros punctatus | ||||||
| Guard cells | 1.2 ± 0.06 | 0.9 ± 0.05 | 1.3 ± 0.05 | 1.1 ± 0.06 | 1.0 ± 0.04 | 6.0 ± 0.82 |
| Stomatal condition | Closed | Open | Open | Open | Open | Open |
| Epidermis | 1.0 ± 0.02 | 1.1 ± 0.02 | 1.3 ± 0.04 | 1.2 ± 0.04 | 0.6 ± 0.03 | 5.8 ± 0.74 |
| Ratio of K in guard cells to that in epidermal cells | 1.2 | 0.82 | 1.0 | 0.92 | 1.67 | 1.03 |
| Guard cell K relative to epidermis | Higher | Lower | Same | Same | Higher | Same |
| Arabidopsis* | Turgid | Wilted | ||||
| Guard cells | 3.36 ± 0.36 | 1.56 ± 0.12 | ||||
| Stomatal condition | Open | Closed | ||||
| Epidermis | 3.24 ± 0.45 | 3.91 ± 0.64 | ||||
| Ratio of K guard cells to epidermal cells | 1.04 | 0.40 | ||||
| Ratio of K in guard cells to that in epidermal cells | Same | Lower | ||||
*From Duckett et al. (2009).
For comparison with hornworts, element spectra sets were obtained from five open stomata and adjacent epidermal cells immediately following removal of leaves from well-watered arabidopsis plants. Other detached leaves were allowed to dry out in the laboratory and five further sets of spectra were collected as soon as the stomata closed (after approx. 1 h and 10 % water loss).
Statistical analysis
Because in our experiments we start from the proposition that experimental treatments have no effect when compared with the controls (reference value) and since ‘absence of evidence is not evidence of absence’, here we take a different approach from the usual statistical analyses, e.g. Student’s t-tests, to avoid using the null hypothesis significance test (NHST) and its well-known defects, including giving only a binary result.
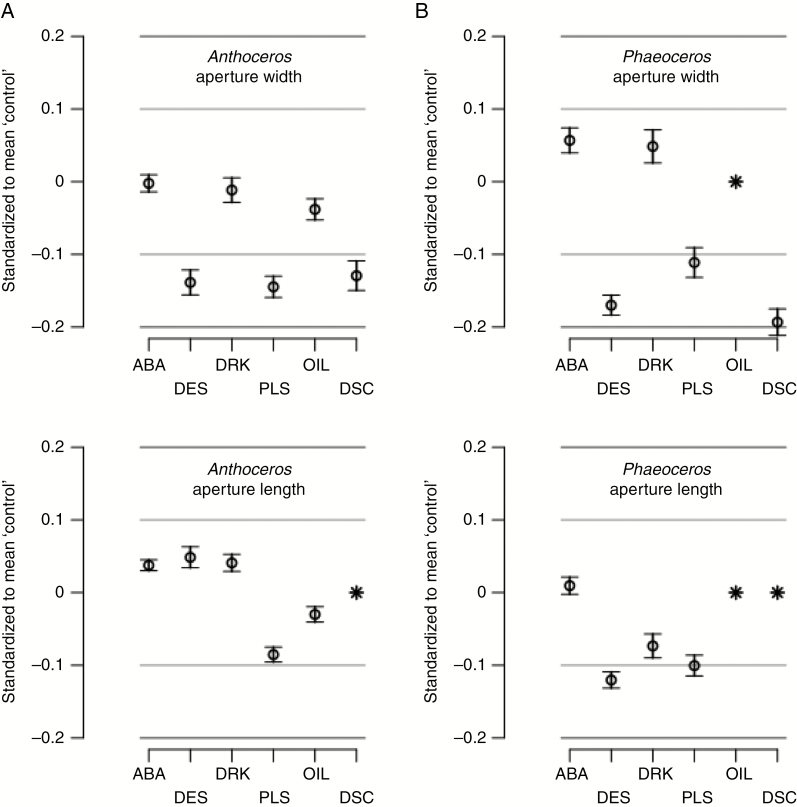
Instead we define a zone of indifference (ZoI) on either side of the reference value, and plot (T – R)/R, and its 95 % confidence interval (CI95); where R is the reference mean, and T the treatment mean. This plot (see Fig. 5) has a central y-axis value of 0, with the symmetrical ZoI lines above and below set at 0.1 and 0.2, because we judge that a difference of up to ±10 % of the control indicates a small treatment effect that we suggest is, in the circumstances, of little biological importance since it would correspond to a change in aperture width of less than about 1 and 2 µm against a mean of about 8–16 µm for Anthoceros and Phaeoceros, respectively (see Figs 4 and 5). We suggest that a 20 % difference (ZoI ± 0.2) is, again in the circumstances, of some but small biological importance.
Fig. 5.
Anthoceros punctatus (A) and Phaeoceros laevis (B). The 95 % confidence interval (CI95) of differences in means of ‘experimental’ treatment and ‘control’, expressed as a proportion of the ‘control’ mean, i.e. (x – c)/c. This standardized measure allows comparison of different measures. The ‘controls’ were kept moist and in light. Treatments: ABA, 100 μm for 4 h; DES, desiccated; DRK, in the dark for 15–24 h; PLA, plasmolysed; OIL, measurements made under oil (Anthoceros only); DCS, non-stomatal epidermal cells desiccated (Anthoceros only; cell widths only). Missing items are shown by an asterisk.
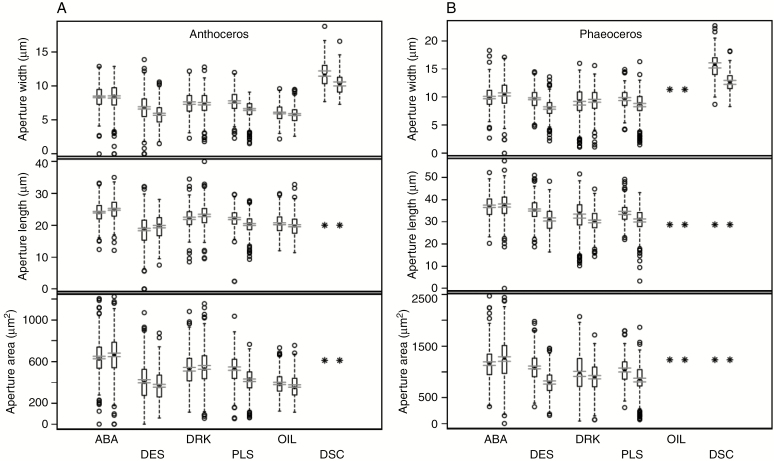
Fig. 4.
Box plots of measurements (width, length and surface area) on Anthoceros punctatus (A) and Phaeoceros laevis (B) in the experimental treatments and corresponding controls. The central bar is the median; the box defines the interquartile range (IQR); dashed ‘whiskers’ extend 1.5 IQR beyond the box; beyond that individual measurements are circles. The two parallel horizontal lines near the median define the 95 % confidence limits on the mean. To the left are controls which were kept moist and in light; to the right are experiment treatments: ABA, 100 μm for 4 h; DES, desiccated; DRK, in the dark for 15–24 h; PLA, plasmolysed; OIL, measurements made under oil (Anthoceros only); DCS, non-stomatal epidermal cells desiccated (Anthoceros only; cell widths only). Missing items are shown by an asterisk.
RESULTS
Stomatal architecture and ontogeny of intercellular spaces
In sporophytes of this length and stage of maturation, the first few (1–5) stomata immediately above the involucre are recently formed and unopened (Fig. 1A, B; Supplementary Data Fig. S1a). Further up the sporophyte, stomata open (Supplementary Data Fig. S1b, c) with full aperture dimensions in stomata 1 mm above the involucre (Fig. 1A, C; Supplementary data Fig. S1d, e); see also Pressel et al. (2014).
Whereas the outer walls in GCs of unopened stomata (Fig. 1B) are smooth, additional wall materials are progressively deposited around pores and over GCs with maturation (Supplementary Data Fig. S1f). Above the point of sporophyte dehiscence, the desiccated epidermal cells are deeply ridged and GCs remain broad in surface view with apertures remaining wide open (Fig. 1E) (see also Villarreal and Renzaglia, 2015; Renzaglia et al., 2017).
Cryo-sections of sporophytes within involucres of the genera with stomata we examined (Anthoceros, Folioceros, Phaeoceros and Phaeomegaceros) invariably show liquid-filled intercellular spaces (data not shown, but see Pressel et al., 2014, Fig. 7A). In TEM micrographs, liquid is also visible as a finely granular network (Fig. 1H). Above the involucres, the liquid is gradually replaced by gas (Fig. 1F) and the assimilatory cell chloroplasts become positioned next to the gas-filled spaces (Fig. 1I). This gradual drying process is illustrated by the presence higher up the sporophytes of liquid that has dried down to a thin layer lining the cell junctions (Fig. 1G, I).
In sporophytes of hornworts that lack stomata such as Nothoceros (Fig. 2A, B), Dendroceros and Notothylas (data not shown), intercellular spaces are absent in the assimilatory tissues (Fig. 2A), which dry down and collapse post-dehiscence (Fig. 2B).
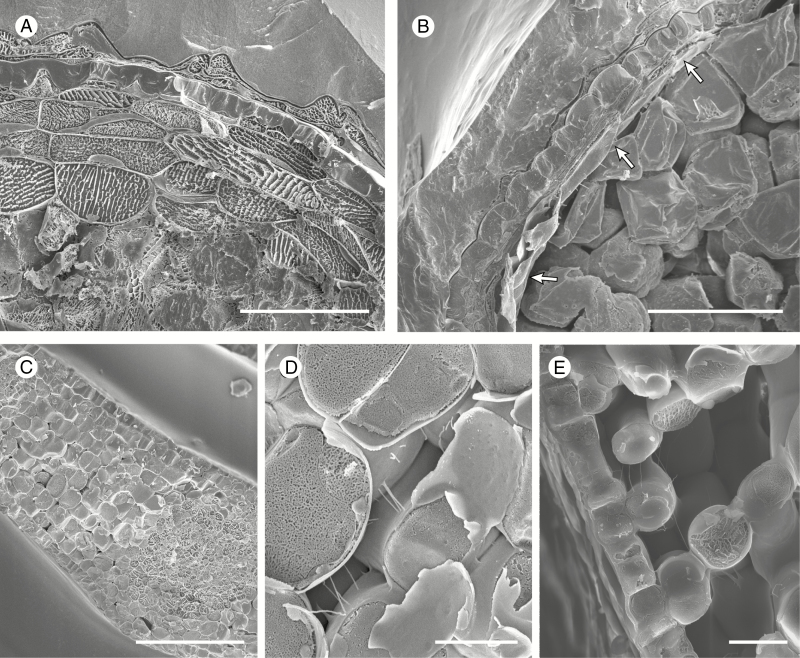
Fig. 2.
Cryo-scanning electron micrographs of Notothylas levieri (A, B) and Hedera helix (C–E). (A, B) Cryo-fractured sporophytes of the estomate hornwort species Notothylas levieri, young (A) and mature (B); note the complete absence of intercellular spaces in the assimilatory layers (A) which collapse and dry out at maturity (B, arrowed). (C–E) Cryo-fractured young leaves. (C) General aspect of a leaf approximately one-tenth its final size with nascent intercellular spaces. (D) Detail of an intercellular space from (C). (E) Spongy mesophyll and lower epidermis in a leaf approximately a quarter of its final size. Scale bars: (C) 100 µm; (A, B) 50 µm; (E) 20 µm; (D) 10 µm.
In contrast to hornworts, the intercellular spaces in the leaves from representatives of all major tracheophyte groups (lycophytes, ferns, gymnosperms and angiosperms) are gas filled from the outset, as illustrated here in H. helix (Fig. 2C–E). In all stages of development in the array of species we examined, thin pectic strands (Carr et al., 1980) extend across intercellular spaces with no remnants of liquid lining the cell junctions around spaces (Fig. 2D).
X-ray microanalysis
Fig. 1K shows a cryo-SEM image of ion beam milling prior to X-ray microanalysis in an open stoma and adjacent epidermal cells. The milling holes are precisely positioned to penetrate into the vacuoles of the guard and epidermal cells (Fig. 1J).
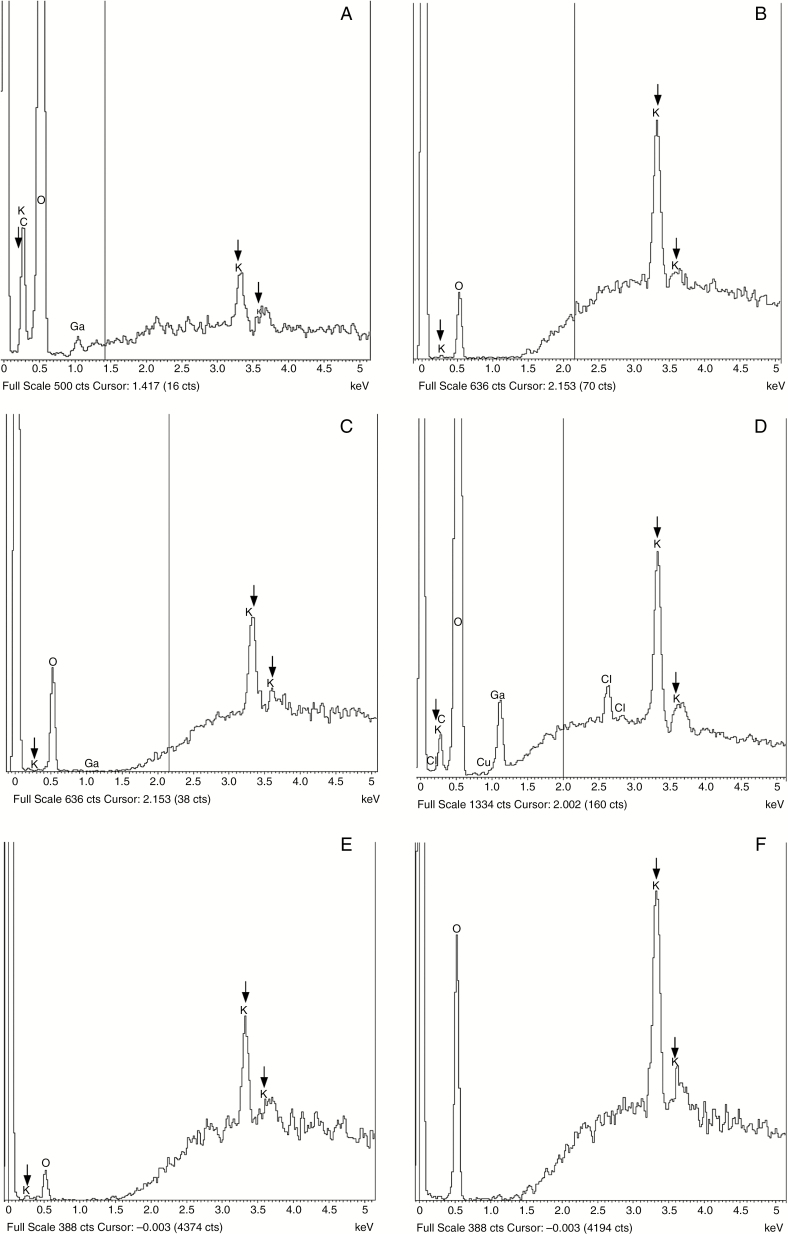
The mass-proportion of potassium, expressed as a percentage, detected by the probe in GCs of P. laevis and A. punctatus at different developmental stages and in adjacent epidermal cells, is summarized in Table 1. Thallus epidermal cells serve as controls. Typical spectra from which the numerical data were obtained are illustrated in Fig. 3. Potassium was not detected in the sporophytic intercellular liquid or on the surface of the thalli. Peaks (Fig. 3A) and mass-proportions from gametophytic cells were lower than in the sporophytes (Fig. 3B, C).
Fig. 3.
X-ray spectra from Phaeoceros laevis (A–D) and Anthoceros punctatus (E, F). Three vertical arrows indicate peaks attributable to potassium. (The peaks at 0.5 keV are gallium contamination from the ion milling.) (A) Low potassium peaks in a thallus epidermal cell (control). (B, C) The guard cell from an unopened stoma inside an involucre (B) has more pronounced potassium peaks than those in a newly opened stoma (C). (D) High potassium peaks in a guard cell of an open stoma after 4 h exposure to 100 µm ABA. (E) Guard cell in an unopened stoma. (F) High potassium peaks in a guard cell of an open stoma after 4 h exposure to 100 µm ABA.
In newly opened stomata of both species, the potassium mass-proportion in GCs is lower than that in epidermal cells. Thereafter it remains the same in both cell types even when sporophytes are desiccated. Also, potassium mass-proportions of unopened stomatal GCs are higher (Fig. 3B) than those of newly opened stomata (Fig. 3B). The mass-proportions of potassium were higher in Anthoceros after treatment with ABA (Fig. 3D–F) and were the same whether or not the pores were open or closed. After periods in darkness, potassium mass- proportions in GCs of Anthoceros appeared slightly higher than in the epidermal cells. In striking contrast, when stomata of arabidopsis close, the GC potassium mass-proportion falls significantly from parity between open GCs and epidermis in the turgid open state (see Duckett et al., 2009).
Stomatal responses to environmental stimuli
Aperture dimensions (widths and lengths) for the first centimetre of sporophytes above the involucre in control plants of A. punctatus and P. laevis are shown in Supplementary Data Fig. S2.
Results from the experimental treatments are summarized in Fig. 4, and indicate that stomatal aperture dimensions in both Anthoceros and Phaeoceros do not decrease in response to exposure to exogenous ABA or darkness, while desiccation and plasmolysis appear to elicit a slight reduction, but never the complete closure observed in ABA-treated (data not shown) and plasmolysed vascular plant stomata (Supplementary Data Fig. S3). A similar decrease in size (width only) was also elicited by desiccation in hornwort sporophyte non-stomatal epidermal cells. After 1–2 h in water, both stomata and epidermal cells regained their pre-desiccation dimensions (data not shown).
Plotting mean values of aperture sizes (width and length) of treated hornwort stomata and non-stomatal epidermal cells (width only) (CI95) against the reference (control) value (Fig. 5) confirms that both ABA and darkness treatments have no or only a trivial effect on aperture sizes, as values fall well within a ZoI of ±10 %. Desiccation and plasmolysis, on the other hand, have an effect at ZoI ±10 %, especially on aperture width sizes and, for desiccation only, on the lumen width of non-stomatal epidermal cells. However, these values do fall within a ZoI set at ±20 %. The results for the oil treatment on Anthoceros indicate that mounting sporophytes in immersion oil does not affect stomatal aperture sizes when compared with the same mounted in water.
DISCUSSION
The present study reveals major physiological differences between hornwort stomata and those in both mosses and vascular plants. Hornwort stomata do not respond to external stimuli and there are no potassium fluxes between epidermal cells and GCs. Identical to hornworts, there is no evidence of potassium fluxes associated with moss stomata (Duckett and Pressel, 2017a).
Developmental data (see also Pressel et al., 2014; Renzaglia et al., 2017) and responses to desiccation and exogenous ABA show that in hornworts, stomatal apertures open in early development and are not subsequently actively regulated. Hornwort stomata thus appear to be different from those in the mosses Physcomitrella and Funaria where ABA, darkness and reduced CO2 all decrease aperture dimensions (Chater et al., 2011). These authors also demonstrate, through cross-species complementation and knockout experiments, that stomata in the moss Physcomitrella behave like those in seed plants. However, Merced and Renzaglia (2017) and Renzaglia et al. (2017), drawing attention to the frequent incidence of pore occlusion by waxes and additional wall materials in bryophytes, illustrate nearly mature capsules of Physcomitrella with blocked open pores and/or liquid-filled intercellular spaces.
The present physiological data, coupled with liquid-filled sporophytic intercellular spaces, point to the primary role of hornwort stomata as facilitation of sporophyte drying leading to spore discharge, a conclusion reinforced by the early death of the GCs and locking of the apertures in an open position (Renzaglia et al., 2017). The same might well be true in mosses since recent observations over the entire life cycles of the sporophytes of five common mosses revealed that the stomata are invariably open regardless of the prevailing environmental conditions (Duckett and Pressel, 2017b).
Functional considerations
X-ray microanalysis in hornworts demonstrates that potassium mass-proportion is lower in GCs of newly opened stomata than in the adjacent epidermal cells, and that similar potassium mass-proportions occur in GCs and epidermal cells in stomata on older regions, desiccated sporophytes and those treated with ABA. These findings eliminate the possibility that potassium fluxes are involved in stoma formation and any putative opening or closing mechanism. We view the slightly higher GC potassium mass-proportion in dark-grown specimens as simply reflecting the ability of sporophytes to grow in the dark (Ligrone and Fioretto, 1987). In the absence of potassium fluxes, it seems plausible that stoma formation is the result of uneven and specialized wall deposition in GCs coupled with mobilization of abundant starch reserves in the plastids of young GCs (Pressel et al., 2014). The same arguments are equally applicable to moss stomata (Duckett and Pressel, 2017a).
Another major difference that separates hornwort stomata from those in other plant groups (Chater et al., 2011) is their lack of responsiveness to the stress hormone ABA, to darkness and to exogenous/environmental cues widely known to close apertures in seed plants and to result in decreased aperture size in mosses (Chater et al., 2011). Even though we followed the same protocol with far more samples, the results from our ABA treatment contrast with those reported previously by Hartung et al. (1987) who showed a dose-dependent ABA response in the stomata of A. punctatus. In trying to find an explanation for this disparity, we noticed that the two images of control and ABA-treated stomata in Hartung et al. (1987; see figs 5 and 6) seem to represent very different developmental stages unsuitable for comparison (compare with our Supplementary Data Fig. S1a, b and e), especially given the conspicuous difference in aperture wall thickness between the two stomata. Hartung et al. (1987) had no knowledge of the major ontogenetic changes subsequently described in hornwort stomata (Pressel et al., 2014; Villarreal and Renzaglia, 2015; Renzaglia et al., 2017) and which formed the basis for choices in the present work. There are no indications in Hartung et al. (1987) that they chose particular regions or ages of sporophytes to examine.
The slight reductions in aperture dimensions after desiccation and plasmolysis in hornwort stomata also contrast with the much more pronounced responses typical of vascular plant stomata to the same cues (Supplementary Data Fig. S3). The absence of closure following plasmolysis is completely at odds with the notion that stomatal movement is modulated by turgor pressure. This finding is consistent with the early development of GC walls that mechanically restrain the apertures of dead stomata in an open configuration as sporophytes dry out (Renzaglia et al., 2017). Similarly, the slight reduction in stoma dimensions after desiccation is most likely to be related to physical, not physiological events, resulting from a general lateral shrinkage of all the sporophyte epidermal cells rather than a stoma-specific response (Merced and Renzaglia, 2017; Renzaglia et al., 2017). The width reductions associated with plasmolysis and desiccation also indicate that the vast majority of our measurements were on living stomata. Dead stomata would not respond. It should be noted that the duration of our darkness treatment (15–24 h), a necessity of our experimental protocol, exceeds what plants experience in the wild and therefore might have caused carbon starvation in hornwort sporophytes potentially leading to stress-induced stomatal opening. However, it is extremely unlikely that 15–24 h, rather than 12 h, could have had such dramatic effects on our experimental plants; further, our results are in line with previous observations of a lack of response to darkness in hornwort stomata (Lucas and Renzaglia, 2002). Although, the dimension reductions following desiccation and plasmolysis were small and not per se significant statistically, the remote possibility that these might be significant functionally still requires close scrutiny in the context of relationships between conductance of water vapour and CO2 which are determined by aperture sizes. The smaller the apertures the greater are the effects of changes in their sizes (Parlange and Waggoner, 1970; Brown and Escombe, 1900; Kaiser, 2009). For example, a width reduction of just 1 µm in a small 4 µm aperture width produces a 25 % reduction in conductance, whereas the corresponding figures for hornworts with larger mean aperture widths of between 6 and 10 µm (Fig. 4) are only 15 and 5 % reductions, respectively. The possibility that these last two small differences might have any real functional significance is even less likely because the stomatal densities in hornworts are an order of magnitude or more lower than those in most angiosperms (Willmer and Fricker, 1996; Field et al., 2015). This observation also appears to rule out in hornworts possible interstomatal diffusion interference, an important parameter in vascular plant leaves particularly in the context of density changes related to atmospheric CO2 concentrations (Woodward, 1987; Beerling et al., 2001; Beerling and Royer, 2002) to which bryophyte stomata are unresponsive (Field et al., 2015). Interstomatal diffusion interference becomes negligible when spacing is over three times stomatal length (Parlange and Waggoner, 1970). Less than 20 % of the stomata of hornworts, even with their very large GC sizes, lie outside this range, in contrast to the much more closely spaced stomata in many mosses (see Field et al., 2015; Table 1; Fig. 3).
Similar mechanical constraints in guard and subsidiary cell walls that restrict stomatal movements have also been noted in tracheophytes (Franks and Farquhar, 2007; Shtein et al., 2017a). The interpretation that hornwort stomata are structures facilitating sporophyte desiccation and spore dispersal finds parallel in anthers. Stomata on young anthers may open and close, but permanent maturational opening is thought to facilitate drying out and dehiscence leading to faster pollen release (Schmid, 1976; Heslop-Harrison et al., 1987; Lersten, 2004). Permanently open stomata are also a feature of nectaries, where they act as secretory pores rather than in gas exchange (Davis et al., 1986), of hydathodes, where the open pores may become occluded with granular, crystalline and waxy materials (Stevens, 1956; Takeda et al., 1991), and in aquatic angiosperms where loss of function is associated with altered patterns of cellulose crystallinity (Shtein et al., 2017b).
Evolution of stomata
The lack of stomata in four hornwort genera represents two independent losses and may be readily understood in terms of this postulated role in sporophyte desiccation (Pressel et al., 2014; Renzaglia et al., 2017). All four taxa fill ecological niches where drying via stomata is not adaptive. In the ephemeral Notothylas, the spores are usually water dispersed from highly reduced sporophytes that are, in many species, immersed in involucres throughout development (Hasegawa, 1980; Schuster, 1984; Singh, 2002). In the Megaceros/Nothoceros/Dendroceros lineage, the loss of stomata is consistent with the habitats they occupy. Megaceros and Nothoceros occur in highly humid regions with long growing seasons where they do not experience seasonal cycles of desiccation as do most temperate taxa. In the epiphytic and epiphyllous Dendroceros, the only desiccation-tolerant hornwort genus, cycles of rapid drying and re-wetting are the norm. Long involucres in these three genera provide additional protection of vulnerable tissue in environments where spores are dispersed over long growing seasons.
The absence of any experimental evidence for regulation of aperture movements and liquid-filled subjacent intercellular spaces supports the conclusion that the primary role of hornwort stomata is sporophyte desiccation. This conclusion points to a rethink of the possible course of stomatal evolution in land plants. Although the positions of mosses sister to hornworts and hornworts sister to vascular plants (Qiu et al., 2006, 2007; Liu et al., 2014) are consistent with a single origin for stomata (Raven, 2002), this hypothesis infers that the absence of stomatal regulation, as shown in the present study, was lost in hornworts following an earlier acquisition in mosses (Chater et al., 2011). On the other hand, phylogenetic analyses placing hornworts sister to other land plants (Cox et al., 2014; Wickett et al., 2014) are in line with the evolution of the stomatal regulatory molecular toolkit described by Chater et al. (2011, 2013, 2016, 2017) after the split of hornworts from the remaining land plants. A recent analysis of possible stomatal gains and losses (Rensing, 2018) shows the most parsimonious configuration to be one in which stomata were independently gained during land plant evolution, with a mosses-liverworts clade as sister to all other land plants and hornworts sister to the vascular plants (Puttick et al., 2018). All other configurations involve more losses and/or gains. When the hornwort genome becomes available, thus enabling the rapid identification of stomatal genes, we predict that a host of both stomatal patterning and identity genes will be shared across stomatophytes. However, the function of those genes will require further genetic and experimental analyses.
Pertinent to these evolutionary considerations are contrasting views on stomatal physiology between seed plants and early divergent tracheophytes (Brodribb and McAdam, 2011, 2012, 2017; Chater et al., 2011; McAdam and Brodribb, 2011; Ruszala et al., 2011). In seed plants, control of water balance is by active metabolic regulation of stomatal aperture via ion-pumping processes and closure induced by ABA. On the basis of molecular data and physiological experiments showing small changes in aperture and stomatal conductance, Ruszala et al. (2011) conclude that stomatal responses in Selaginella to both CO2 and the physiologically active enantiomer of ABA are similar to those in arabidopsis. They also report differences in potassium mass-proportion between GCs of light- and dark-grown Selaginella, although the cobaltinitrite staining method they used produced largely qualitative data. From their experiments, they argue that physiologically active stomatal control originated as least as far back as the origin of the lycophytes approximately 420 million years ago. From Physcomitrella studies, Chater et al. (2011, 2013) posit the same for mosses; they contend that mosses possess the same core regulatory components as flowering plants in GC ABA signalling, and that these originated in a common ancestor of bryophytes and tracheophytes. Phylogenomic studies of stomatal genes that include Physcomitrella support the single origin of GCs across land plants as Physcomitrella possesses GC patterning and development genes found in arabidopsis (Bergmann and Sack, 2007; Peterson et al., 2010; Rychel and Peterson, 2010; Caine et al., 2016; Chater et al., 2017).
In contrast, Brodribb and McAdam’s (2011) finding of only a 15 % reduction in stomatal apertures in the presence of ABA in a lycophyte and further extensive studies on pteridophytes (reviewed by Brodribb and McAdam, 2017; Sussmilch et al., 2017) points to a lack of or very limited active control of water loss. These authors suggested that early land plants possessed passive stomatal control with the absence of an active ion-pumping mechanism, much like that demonstrated here in hornworts, and the gradual acquisition of active control mechanisms occurred as plants diversified. However, differently from hornworts, desiccation and plasmolysis did cause rapid stomatal closure in some ferns and lycophytes. The general lack of stomatal response in hornworts clearly conforms to the notion of gradual acquisition of stomatal regulation (Brodribb and McAdam, 2017; Sussmilch et al., 2017). Added to the present results is the demonstration that stomatal density and aperture in both hornworts and mosses do not respond to atmospheric CO2 concentrations (Field et al., 2015). The single study (Chater et al., 2011) based on actual measurements of stomata rather than analysis of stoma-related genes provides the only direct evidence for the early acquisition of active regulation. That study showed small responses rather than complete closure in two mosses with unusual pores in single GCs (Funaria and Physcomitrella). Merced and Renzaglia (2017) highlight that studies on Physcomitrella stomata are challenging; ‘they might not reflect the reality of the great majority of mosses because of its reduced cleistocarpic capsule with few stomata, small apertures and poorly developed intercellular spaces.’ It is difficult to envisage how absence of stomata might delay the dehiscence of cleistocarpous capsules with this suite of characters (Chater et al., 2016). Physiological and genetic studies including aperture measurements now need to be replicated in additional mosses with structurally elaborate capsules that contain well-developed apophyses and a system of intercellular spaces leading to regularly spaced stomata with two guard cells. No matter how many more stomata-related genes are discovered to be common across land plants, future considerations of structural and functional continuity must now take into account the recent demonstration of the multiple evolution of intercellular spaces in bryophytes (Duckett and Pressel, 2017a). Whereas this integral component of the stomatal apparatus is present at the foot of the hornwort tree (Leiosporoceros), it is strikingly absent from the basal moss clades, Sphagnum, Takakia and Andreaea.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: light micrographs of living sporophytes illustrating the ontogenetic changes in stomatal apertures in Anthoceros punctatus. Figure S2: Anthoceros punctatus and Phaeoceros laevis scatter plots of stomatal width and length for the four experimental treatents and corresponding controls. Figure S3: effects of plasmolysis on Phaeoceros laevis, Lactuca sativa, Osmunda regalis and Scolopendrium vulgare stomata.
ACKNOWLEDGEMENTS
We are grateful for skilled assistance in operating the cryo-SEM provided by Ken M. Y. P’ng, NanoVision Centre, School of Engineering and Materials Science, Queen Mary University of London E1 4NS, UK, and by Integrated Microscopy and Graphics Expertise staff for use of electron microscope facilities at Southern Illinois University. Support from DEFRA (Darwin Initiative), The New Phytologist Trust, the Royal Society (UK), a Leverhulme Emeritus Fellowship (J.G.D.) and a Leverhulme Early Career Fellowship (S.P.) enabled J.G.D. and S.P. to collect specimens used in this study from Chile and New Zealand. We thank Brian Butterfield (University of Canterbury) and David Glenny (Landcare, New Zealand) for help with collecting, and the New Zealand Department of Conservation for collecting permits. This project was supported by two NSF Tree of Life initiatives (DEB-0228679, DEB-0531751).
LITERATURE CITED
- Beerling DJ, Franks PJ. 2009. Evolution of stomata in ‘lower’ land plants. New Phytologist 183: 921–925. [DOI] [PubMed] [Google Scholar]
- Beerling DJ, Royer DL. 2002. Reading a CO2 signal from fossil stomata. New Phytologist 153: 387–397. [DOI] [PubMed] [Google Scholar]
- Beerling DJ, Osborne CP, Chaloner WG. 2001. Evolution of leaf-form in land plants linked to atmospheric CO2 decline in the Late Palaeozoic era. Nature 410: 352–354. [DOI] [PubMed] [Google Scholar]
- Bergmann FC, Sack FD. 2007. Stomatal development. Annual Review of Plant Biology 58: 163–181. [DOI] [PubMed] [Google Scholar]
- Bopp M, Werner O. 1993. Abscisic acid and desiccation tolerance in mosses. Botanica Acta 106: 103–106. [Google Scholar]
- Brodribb TJ, McAdam SAM. 2011. Passive origins of stomatal control in vascular plants. Science 331: 582–585. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM. 2012. Fern and lycophyte guard cells do not respond to endogenous ABA. The Plant Cell 24: 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM. 2013. Unique responsiveness of angiosperm stomata to elevated CO2 explained by calcium signalling. PLoS One 8: e82057. doi: 10.1371/journal.pone.0082057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SA. 2017. Evolution of the stomatal regulation of plant water content. Plant Physiology 174: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Feild TS. 2009. Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants. New Phytologist 183: 839–847 [DOI] [PubMed] [Google Scholar]
- Brown H, Escombe F. 1900. Static diffusion of gases and liquids in relation to the assimilation of carbon and translocation in plants. Philosophical Transactions of the Royal Society B: Biological Sciences 193: 223–291. [Google Scholar]
- Caine R, Chater CC, Kamisugi Y, et al. 2016. An ancestral stomatal patterning module revealed in the non-vascular plant Physcomitrella patens. Development 143: 3306–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Oates K, Carr SGM. 1980. Studies on intercellular pectic strands of leaf palisade parenchyma. Annals of Botany 45: 403–413. [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, et al. 2011. Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Current Biology 21: 1025–1029. [DOI] [PubMed] [Google Scholar]
- Chater C, Gray JE, Beerling DJ. 2013. Early evolutionary acquisition of stomatal control and development gene signalling networks. Current Opinion in Cell Biology 16: 638–646. [DOI] [PubMed] [Google Scholar]
- Chater C, Caine RS, Tomek M, et al. 2016. Origin and function of stomata in the moss Physcomitrella patens. Nature Plants 2: 16179. doi:10.1038/nplants.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Caine RS, Fleming AJ, Gray JE. 2017. Origins and evolution of stomatal development. Plant Physiology 174: 624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CJ, Goffinet B, Shaw AJ, Boles SB. 2004. Phylogenetic relationships among the mosses based on heterogeneous Bayesian analysis of multiple genes from multiple genomic compartments. Systematic Botany 29: 234–250. [Google Scholar]
- Cox CJ, Li B, Foster PG, Embley TM, Civáň P. 2014. Conflicting phylogenies for early land plants are caused by composition biases among synonymous substitutions. Systematic Biology 63: 862–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AR, Peterson RL, Shuel RW. 1986. Anatomy and floral nectaries of Brassica napus (Brassicaceae). Canadian Journal of Botany 64: 2508–2516. [Google Scholar]
- Duckett JG, Pressel S. 2017aThe evolution of the stomatal apparatus: intercellular spaces and sporophyte water relations—two ignored dimensions. Philosophical Transactions of the Royal Society B: Biological Sciences 373: doi: 10.1098/rstb.2016.0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett JG, Pressel S. 2017b. The colorful phenology of five common terricolous mosses in London, England. Bryophyte Diversity and Evolution 39: 44–56. [Google Scholar]
- Duckett JG, Pressel S, P’ng KMY, Renzaglia KS. 2009. Exploding a myth: the capsule dehiscence mechanism and the function of pseudostomata in Sphagnum.New Phytologist 183: 1053–1063. [DOI] [PubMed] [Google Scholar]
- Duff JE, Villarreal JC, Cargill DC, Renzaglia KS. 2007. Progress and challenges toward developing a phylogeny and classification of the hornworts. Bryologist 110: 214–243. [Google Scholar]
- Field KJ, Duckett JG, Cameron DD, Pressel S. 2015. Stomatal density and aperture in non-vascular land plants are non-responsive to atmospheric CO2 concentrations. Annals of Botany 115: 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD. 2007. The mechanical diversity of stomata and its significance in gas exchange control. Plant Physiology 143: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DLB, Paolillo DJ. 1973. On the functioning of stomates in Funaria.Bryologist 76: 423–427. [Google Scholar]
- Glenny D. 1998. A revised checklist of New Zealand liverworts and hornworts. Tubingia 10: 119–149. [Google Scholar]
- Hartung W. 1983. The site of action of abscisic acid at the guard cell plasmalemma of Valerianella locusta.Plant, Cell and Environment 6: 427–428. [Google Scholar]
- Hartung W. 2010. The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Functional Plant Biology 37: 806–812. [Google Scholar]
- Hartung W, Weiler EW, Volk OH. 1987. Immunochemical evidence that abscisic acid is produced by several species of Anthocerotae and Marchantiales. Bryologist 90: 393–400. [Google Scholar]
- Hartung W, Hellwege EM, Volk OH. 1994. The function of abscisic acid in bryophytes. Journal of the Hattori Botanical Laboratory 76: 59–65. [Google Scholar]
- Haworth M, Killi D, Materassi A, Raschi A. 2015. Coordination of stomatal physiological behaviour and morphology with carbon dioxide determines stomatal control. American Journal of Botany 102: 677–688. [DOI] [PubMed] [Google Scholar]
- Hasegawa J. 1980. Taxonomic studies on Asian Anthocerotae. II. Some Asian species of Dendroceros. Journal of the Hattori Botanical Laboratory 47: 287–309. [Google Scholar]
- Heslop-Harrison JS, Heslop-Harrison Y, Reger BJ. 1987. Anther filament extension in Lilium: potassium ion movement and some anatomical features. Annals of Botany 59: 505–525. [Google Scholar]
- Kaiser H. 2009. The relation between stomatal aperture and gas exchange under consideration of pore geometry and diffusional resistance in the mesophyll. Plant, Cell and Environment 32: 1091–1098. [DOI] [PubMed] [Google Scholar]
- Hõrak H, Kollist H, Ebe Merilo E. 2017. Fern stomatal responses to ABA and CO2 depend on species and growth conditions. Plant Physiology 174: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JA, Walker H, Cameron DC, Lomax BH. 2016. A novel root-to-shoot stomatal response to very high CO2 levels in the soil: electrical, hydraulic and biochemical signalling. Physiologia Plantarum 159: 433–444. [DOI] [PubMed] [Google Scholar]
- Lersten N. 2004. Flowering plant embryology. London: Wiley. [Google Scholar]
- Ligrone R, Fioretto A. 1987. Chloroplast development in dark grown sporophytes of Phaeoceros laevis L. Prosk. New Phytologist 105: 301–308. [Google Scholar]
- Ligrone R, Renzaglia KS. 1990. The sporophyte–gametophyte junction in the hornwort Dendroceros tubercularis Hatt. (Anthocerophyta). New Phytologist 114: 497–505. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. 1993. The gametophyte–sporophyte junction in land plants. Advances in Botanical Research 19: 231–317. [Google Scholar]
- Liu Y, Cox CJ, Wang W, Goffinet B. 2014. Mitochondrial phylogenomics of early land plants: mitigating the effects of saturation, compositional heterogeneity and codon-usage bias. Systematic Biology 63: 862–878. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Renzaglia KS. 2002. Structure and function of hornwort stomata. Microscopy and Microanalysis 8 (Suppl 2): 1090–1091. [Google Scholar]
- McAdam SAM, Brodribb TJ. 2011. Stomatal innovation and the rise of seed plants. Ecology Letters 14: 1–8. [DOI] [PubMed] [Google Scholar]
- McDaniel SF, von Stackelberg M, Richardt S, Quatrano R, Reski R, Rensing SA. 2009. The speciation history of the Physcomitrium–Physcomitrella species complex. Evolution 64: 217–231. [DOI] [PubMed] [Google Scholar]
- Merced A. 2015. Novel insights on the structure and composition of pseudostomata in Sphagnum.American Journal of Botany 102: 329–335. [DOI] [PubMed] [Google Scholar]
- Merced A, Renzaglia KS. 2013. Moss stomata in highly elaborated Oedipodium (Oedipodiaceae) and highly reduced Ephemerum (Pottiaceae) sporophytes are remarkably similar. American Journal of Botany 100: 2318–2327. [DOI] [PubMed] [Google Scholar]
- Merced A, Renzaglia KS. 2014. Developmental changes in guard cell wall structure and pectin composition in the moss Funaria: implications for function and evolution of stomata. Annals of Botany 114: 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merced A, Renzaglia KS. 2017. Structure, function and evolution of stomata from a bryological perspective. Bryophyte Diversity and Evolution 39: 007–020. [Google Scholar]
- Merilo E, Yarmolinsky D, Jalakas P, et al. 2017. Origin and role of ABA in stomatal regulation. Plant Physiology On the Inside doi:10.1104/pp.17.00912. [Google Scholar]
- Morris JL, Puttick MN, Clark J, Edwards D, Kenrick P, Pressel S, Wellman CH, Yang Z, Schneider H, Donoghue PCJ. 2018. The timescale of early land plant evolution. Proceedings of the National Academy of Sciences of the USA 115: E2274–E2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AE, Cox CJ, Duckett JG, Goffinet B, Hedderson TAJ, Mishler BD. 2000. Evolution of the major moss lineages: phylogenetic analyses based on multiple gene sequences and morphology. Bryologist 103: 187–211. [Google Scholar]
- Parlange J-Y, Waggoner PE. 1970. Stomatal dimensions and resistance to diffusion. Plant Physiology 46: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JA, Pearce JV. 1957. The occurrence, structure and functions of the stomata in British bryophytes. II. Functions and physiology. Transactions of the British Bryological Society 3: 242–259. [Google Scholar]
- Peterson KM, Rychel AL, Torii KU. 2010. Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. The Plant Cell 22: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressel S, Goral T, Duckett JG. 2014. Stomatal differentiation and abnormal stomata in hornworts. Journal of Bryology 36: 87–103. [Google Scholar]
- Puttick MN, Morris J, Williams TA, Cox CJ, Edwards D, Kernick P, Pressel S, Wellman CH, Schneider H, Pisani D, Donoghue PCJ. 2018. The interrelationships of land plants and the nature of the ancestral embryophyte. Current Biology 28: 733–745. [DOI] [PubMed] [Google Scholar]
- Qiu Y-L, Li L, Wang B, et al. 2006. The deepest divergences in land plants inferred from phylogenomic evidence. Proceedings of the National Academy of Sciences, USA 103: 15511–15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y-L, Li L, Wang B, et al. 2007. Nonflowering land plant phylogeny inferred from nucleotide sequences of seven chloroplast, mitochondrial, and nuclear genes. International Journal of Plant Science 168: 691–708. [Google Scholar]
- Raven JA. 2002. Selection pressures on stomatal evolution. New Phytologist 153: 371–386. [DOI] [PubMed] [Google Scholar]
- Rensing SA. 2018. Plant evolution: phylogenetic relationships between the earliest land plants. Current Biology 28: R208–R231. [DOI] [PubMed] [Google Scholar]
- Renzaglia KS, Villarreal JC, Piatkowski BT, Lucas JR, Merced A. 2017. Hornwort stomata: architecture and fate shared with 400 million year old fossil plants without leaves. Plant Physiology 174: 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, et al. 2011. Land plants acquired active stomatal control early in their evolutionary history. Current Biology 21: 1030–1035. [DOI] [PubMed] [Google Scholar]
- Rychel AL, Peterson KM. 2010. Plant twitter: ligands under 140 amino acids enforcing stomatal patterning. Journal of Plant Research 123: 275–280. [DOI] [PubMed] [Google Scholar]
- Sack F, Paolillo DJ. 1983a. Stomatal pore and cuticle formation in Funaria. Protoplasma 116: 1–13. [Google Scholar]
- Sack F, Paolillo DJ. 1983b. Protoplasmic changes during stomatal development in Funaria.Canadian Journal of Botany 61: 2515–2526. [Google Scholar]
- Sack F, Paolillo DJ. 1983c. Structure and development of walls in Funaria stomata. American Journal of Botany 70: 1019–1030. [Google Scholar]
- Schmid R. 1976. Filament histology and anther dehiscence. Botanical Journal of the Linnean Society 73: 303–315. [Google Scholar]
- Schuster RM. 1984. Morphology, phylogeny and classification of the hornworts. In: Schuster RM, ed. New manual of bryology, Vol. 2 Nichinan, Japan: Hattori Botanical Laboratory, 1071–1092. [Google Scholar]
- Shaw AJ, Renzaglia KS. 2004. Phylogeny and diversification of bryophytes. American Journal of Botany 10: 1557–1581. [DOI] [PubMed] [Google Scholar]
- Shtein I, Shelef Y, Marom Z, et al. 2017a. Stomatal cell wall composition: distinctive structural patterns associated with different phylogenetic groups. Annals of Botany 119: 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtein I, Popper ZA, Harpaz-Saad S. 2017b. Permanently open stomata of aquatic angiosperms display modified cellulose crystallinity patterns. Plant Signaling and Behavior 12: e1339858. doi: 10.1080/15592324.2017.1339858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK. 2002. Notothylaceae of India and Nepal: a morpho-taxonomic revision. Dehradun, India: Bishen Singh Mahendrapal Singh. [Google Scholar]
- Stevens ABP. 1956. The structure and development of the hydathodes of Caltha palustris L. New Phytologist 55: 339–345. [Google Scholar]
- Sussmilch FC, Brodribb TJ, McAdam SAM. 2017. What are the evolutionary origins of stomatal responses to abscisic acid (ABA) in land plants?Journal of Integrative Plant Biology 59: 240–260. [DOI] [PubMed] [Google Scholar]
- Takeda F, Wisniewski ME, Glenn DM. 1991. Occlusion of water pores prevents guttation in older strawberry leaves. Journal of the American Society of Horticultural Science 116: 1122–1125. [Google Scholar]
- Villarreal JC, Renzaglia KS. 2015. The hornworts: important advancements in early land plant evolution. Journal of Bryology 37: 157–170. [Google Scholar]
- Wickett NJ, Mirarab S, Nguyen N, et al. 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proceedings of the National Academy of Sciences, USA 111: E4859–E4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer CM, Fricker M. 1996. Stomata, 2nd edn London: Chapman & Hall. [Google Scholar]
- Woodward FI. 1987. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327: 617–618. [Google Scholar]
- Xu Z, Jiang Y, Jia B, Zhou G. 2016. Elevated-CO2 response of stomata and its dependence on environmental factors. Frontiers in Plant Science 7: 657. doi:10.3389/fpls.2016.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.