Abstract
The biting behavior of anophelines is an important determinant of malaria transmission. Understanding the local vector host-seeking behavior, its outdoor/ indoor biting preference, and nocturnal biting periods is essential for effectively applying and improving vector control methods, such as Long Lasting Insecticidal Nets (LLINs) and personal protective measures. To better understand the biting and host-seeking patterns of Anopheles mosquitoes in Northwestern Burkina Faso, we performed biweekly Human Landing Catches (HLC) in six villages during the period of highest mosquito abundance and malaria transmission. We applied a negative binomial regression framework to statistically analyze the host-seeking activities of Anopheles species and test for differences across hours, months, and villages, as well as for differences between indoor and outdoor capture points. Anopheles gambiae s.l. was identified as the main malaria vector in this region, representing about 90% of the total anopheline population. Biting activity was significantly different across hours and showed a peaked plateau between 2000 and 0200 hours. Differences in the pattern of biting cycles were observed between the early and late rainy season. This study shows that anopheline biting activity in Northwest Burkina Faso is high throughout the night, at indoor and outdoor posts alike. Consequently, bed nets alone may not provide sufficient protection against early biting anophelines and should be complemented with additional strategies such as indoor residual spraying (IRS) and larval source management (LSM) to meet the WHO’s ambitious goals that are reflected in the global technical malaria strategy for 2030.
Keywords: malaria, human landing catches, human bait catches, biting time, Anopheles
Solid knowledge of the vector’s biting behavior is an important prerequisite to better understand and reduce malaria transmission. It is known that anopheline species differ regarding their preferred biting time and their favored biting and resting places (Githeko et al. 1996, Sinka et al. 2010). While endophagic species take their blood meal predominantly inside human dwellings, exophagic species feed mostly outdoors. Similarly, with regard to resting behavior, endophilic species commonly prevail indoors while exophilic species rest mostly outdoors. Anopheline species, such as Anopheles gambiae Patton (Diptera: Culicidae) and Anopheles funestus Giles (Diptera: Culicidae), are known to prefer humans for their blood meal (anthropophilic), while others, such as Anopheles quadriannulatus Theobald (Diptera: Culicidae) and Anopheles melas Theobald (Diptera: Culicidae), feed predominantly but not exclusively on animals (zoophilic) (Sylvie et al. 2008).
Understanding the vector’s biting behavior is essential in determining the vectorial capacity. The latter, in its classical form, comprises the parasite’s extrinsic incubation period, the ratio of mosquitoes to humans, mosquito survival through one day, and human biting rates (HBRs) (Brady et al. 2016). Substantial variation in those parameters can be observed across greater regions but also across small-scale geographical features. For instance, hydrography, vegetation, land use, and altitude were found to be important determinants of the absolute and proportional abundance of species (Minakawa et al. 2002, Levine et al. 2004, Sinka et al. 2010). As a response to the adaptation pressure exerted by vector control programs, mosquitoes evolve in their biting behavior and species composition (Moiroux et al. 2012, Gatton et al. 2013). Thus, in addition to the challenges posed by mosquitoes’ increasing resistance to insecticides (Namountougou et al. 2012, Strode et al. 2014), behavioral adaptations of the mosquitoes are a serious impediment for the ongoing success of malaria control. The latter includes a change in both, the peak biting time as well as outdoor and indoor feeding preference as a reaction to control measures (Fornadel et al. 2010, Moiroux et al. 2012, Thomsen et al. 2017). In several regions in sub-Saharan Africa, An. gambiae s.s. Giles (Diptera: Culicidae) was replaced as the primary malaria vector by Anopheles arabiensis Patton (Diptera: Culicidae), as a consequence of bed net distribution (Russell et al. 2011, Kitau et al. 2012, Sougoufara et al. 2016). A previous study within our study region (Diboulo et al. 2015) showed that the most abundant malaria vectors were An. gambiae (80%) and An. funestus (20%), with corresponding Plasmodium falciparum Welch (Haemosporida: Plasmodiidae) sporozoite rates of 9.24 and 2.92%, respectively. Against this background, it is important to collect reliable data on mosquito biting times, host preferences, indoor and outdoor feeding as well as resting behavior for areas with ongoing or planned vector control. Insights from respective assessments can support the targeting of local vector control tools and contribute to an intervention design that deliberately deals with future vector adaptations. Thus, to better understand the biting activity of An. gambiae and An. funestus within the study region, we monitored the biting behavior of the main malaria vectors across multiple villages during the seasonal peak of mosquito activity.
Methods
Study Site
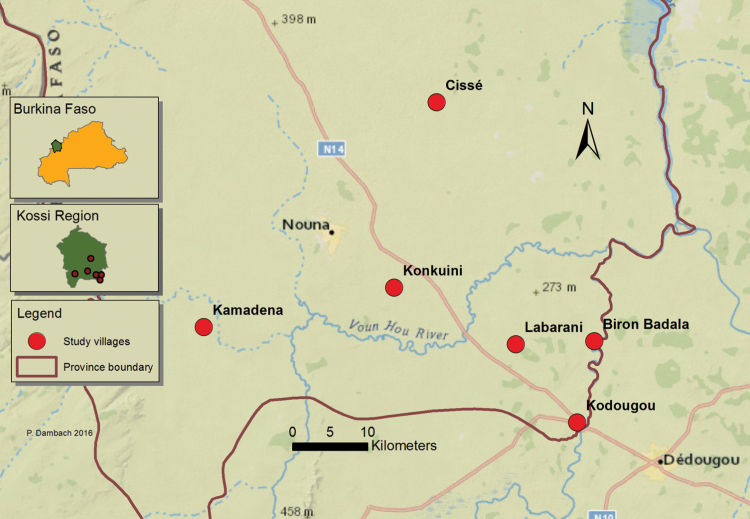
The study was conducted in an area of about 2500 km2 in the rural vicinity of the semi-urban town of Nouna, the capital of the Kossi province. Nouna is located in the North-western part of Burkina Faso near the Mali border at a latitude of 12.73° North and a longitude of 3.86° West (Fig. 1). Characterized as dry savannah with sub-Saharan climate, the annual mean precipitation during the last 30 yr was 817 mm and the mean temperature 27.8°C (data obtained from the Nouna weather station). Precipitation occurs almost exclusively during the rainy season from late June to October. Malaria transmission in the region is holoendemic and peaks in the late rainy season. With up to 131 infective bites per person per year, entomological inoculation rates are high, even though they vary greatly across villages (Diboulo et al. 2015). Pl. falciparum prevalence in Nouna was found to be as high as 58% in children of 6 mo to 4 yr age and 75% in the age group of 5 to 9 yr (Diallo et al. 2017). Malaria control measures in the study area comprise the use of insecticide impregnated bed nets, with a high coverage of more than 90% (Louis et al. 2015). On the other hand, the coverage with artemisinin-based combination therapy (ACT) medication is very low with a range between 0.01 and 0.26%, and indoor residual spraying (IRS) is virtually not performed with 0.02–0.08%, showing little effect in reducing parasitemia risk (Diboulo et al. 2016). During the rainy season we observed people tended to go inside their huts earlier than during the dry season. This is due to cooler temperatures, high mosquito nuisance and the need to rise early to begin fieldwork.
Fig. 1.
Study villages in the Kossi region in Northwestern Burkina Faso in which human landing catches were performed.
Mosquito Collections
Between 21 July and 12 December 2012, Human Landing Catches (HLC) were performed in six villages which were randomly selected from a list of 40 villages in the vicinity of Nouna. In all six villages, collections were carried out every 2 wk with few minor deviances of 1 or 2 d due to inaccessibility of some villages during the peak rainy season. To capture possible intra-village variation in vector abundance, three different zones for performing human landing catches were randomly defined in each village (Fig. 2). One zone was set at the village center while the remaining two zones were allocated towards the villages’ outer limits. Each zone featured three sample locations with one outdoor and one indoor post each, resulting in a total number of 18 simultaneous HLC collections per night. Indoor collections were carried out in the main room of huts where no mosquito net was hung. Most huts in this rural environment only have one bedroom and another main, multi-purpose room. Outdoor catches were performed in the fenced courtyard some 10 to 15 meters away from the hut.
Fig. 2.
Sampling scheme within a study village (Cissé). Each study village featured three zones with three sample points each. Each sample location consisted of one outdoor (blue) and one indoor (red) capture post. Satellite image: Google Maps/ Digital Globe September 2012.
Sample locations within zones were located at least 50 meters apart from each other and were maintained throughout the study duration. At each sample point a local community member was seated with one leg exposed, since the main African malaria vectors are known to feed close to ground level (Braack et al. 2015). The seated person collected landing mosquitoes from the exposed leg using an aspirator. Catches were performed between 1800 and 0600 hours and in two shifts, where the teams’ shifts were exchanged after every capture cycle. Two entomological technicians supervised the setup and the use of aspirators and ensured the correct labeling and timely classification of collected mosquitoes. In addition, each zone featured a local person with basic entomological knowledge who provided supervision and assistance during catches. Mosquitoes were stored in separate plastic tubes with silica gel and cotton balls, and were labeled according to sample location and time. All collected mosquitoes were transported to the laboratory in Nouna the next morning and were analyzed to species level under the microscope using WRBU (Walter Reed Biosystematics Unit) identification keys (Walter Reed Biosystematics Unit 2017).
Data Analysis and Statistical Methods Applied
HBRs for all Anopheles species were calculated as the arithmetic mean of the number of mosquitoes collected by HLC per person per night. Over the collection period from August to November 2012, a total number of 823 HLCs were performed, comprising about 400 HLCs from both outdoor and indoor posts (Table 1). For statistical analysis, HBRs were also calculated by hour and collections from outdoor and indoor posts were considered separately. For graphical illustration, bar charts were applied, depicting arithmetic sample mean values and corresponding 95% confidence intervals. A Generalized Linear Model framework with an assumed negative binomial distribution was applied to test for statistical differences in HBRs across several factors, including evening hours, villages, months, and the placement of the HLC (Fornadel et al. 2010, Van Bortel et al. 2010, Russell et al. 2011, Kenea et al. 2016). Specifically, mosquito counts were assumed to follow a Poisson distribution with an overdispersion parameter. The Incidence Rate Ratio (IRR) was estimated from a negative binomial regression. Where necessary, a Likelihood Ratio test was applied for joint hypothesis testing. The village sites were introduced as covariates. To correct for serial correlation of mosquito biting rates within collection points, standard errors were clustered at the level of collection point. In a final step of the empirical analysis, the average HBRs per person per hour were calculated over weeks and the relationship with lagged precipitation was investigated by estimating Pearson correlation coefficients. Precipitation data are aggregated at the regional level and were obtained from NASA’s Tropical Rainfall Measuring Mission (TRMM/Giovanni). Observations from June and December were excluded since data collection activities did not cover the whole month. For data analysis, STATA version 14, Stata Corp was used.
Table 1.
Average number of HBRs per person per night by village for the major malaria vectors for the months August through November 2012
| Village | An. gambiae s.l. | An. nili | An. coustani | An. funestus | Number of collections | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Total | Outdoor | Indoor | |
| Biron Badala | 17.92 | 8.46 | 2.10 | 2.89 | 0.57 | 1.09 | 0.43 | 1.03 | 92 | 46 | 46 |
| Cisse | 27.81 | 19.08 | 0.06 | 0.31 | 0.27 | 0.57 | 0.07 | 0.39 | 148 | 67 | 81 |
| Kamadena | 21.60 | 9.56 | 0.29 | 1.08 | 0.35 | 0.75 | 0.10 | 0.44 | 153 | 75 | 78 |
| Kodougou | 21.56 | 6.54 | 1.31 | 2.49 | 0.41 | 0.69 | 0.12 | 0.42 | 90 | 45 | 45 |
| Konkuini | 22.42 | 9.15 | 0.04 | 0.25 | 0.32 | 0.74 | 0.10 | 0.51 | 151 | 77 | 74 |
| Labarani | 22.63 | 14.36 | 0.74 | 3.68 | 0.33 | 0.72 | 0.03 | 0.20 | 189 | 91 | 98 |
| Sum | - | - | - | - | 823 | 401 | 422 | ||||
| χ2 | 50.10 | 269.24 | 24.82 | 35.57 | - | - | - | ||||
| P value < | 0.01 | 0.01 | 0.01 | 0.01 | - | - | - | ||||
One collection refers to all mosquito catches performed during one night by one human landing/capture post during. In the bottom rows results from a likelihood ratio test, based on a negative binomial regression, are depicted and indicate significant differences in biting attempts across villages. Specifically, the hypothesis that all village coefficients equal zero is tested and rejected throughout.
Results
Species Composition and Abundance
Between August and November 2012, a total of 10,296 and 9,304 female Anopheles mosquitoes were collected during indoor and outdoor human landing catches, respectively. An. gambiae s.l. represented 95.4% of all Anopheles mosquitoes collected, while other anophelines accounted for less than 5% (Anopheles nili Theobald (Diptera: Culicidae) 2.6%, Anopheles coustani Laveran (Diptera: Culicidae) 1.5%, Anopheles funestus Giles (Diptera: Culicidae) 0.5%). The average HBRs for An. gambiae s.l. per person per night were found to be in the range between 18 and 28. For the other three most abundant anopheline species, nightly HBRs were on average below one (Table 1). Results from a Likelihood Ratio test, depicted at the bottom of Table 1, showing that nightly biting patterns differed significantly across villages for all four species.
Nocturnal Biting Cycles of the Main Anopheles Species
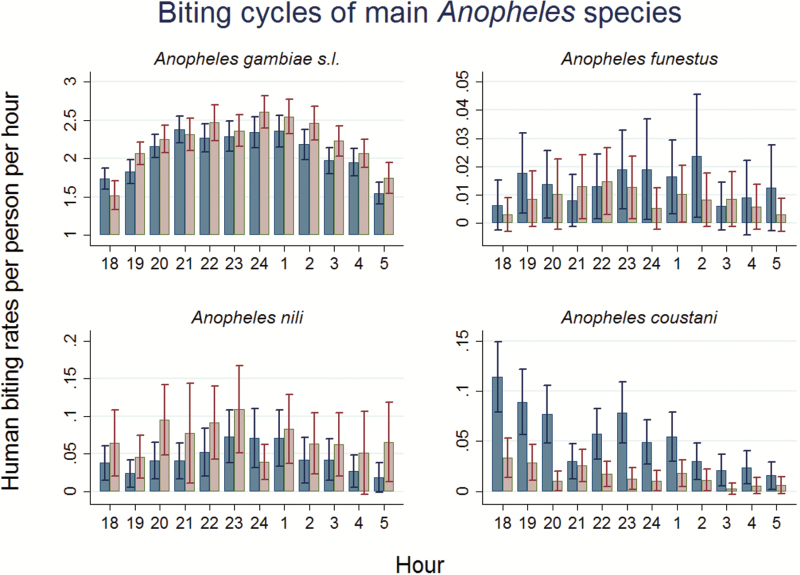
Hourly catches during the peak and ceasing rainy season reflected anopheline activity throughout the night for both, outdoor and indoor HLCs (Fig. 3). The biting behavior of An. gambiae s.l. over time follows a moderate reverse U-shape, resembling a flat bell. At dusk between 1800 and 1900 hours, An. gambiae s.l. biting rates showed around 1.75 bites per person per hour and then gradually increased to reach a plateau of around 2.25 bites per hour between 2000 and 0200 hours. From 0200 hours onwards, biting rates again converged towards the initial levels from the early evening. The confidence intervals suggest statistically significant differences in HBRs between some hour blocks. For An. gambiae s.l., a Likelihood Ratio test strongly rejects the hypothesis of equal biting rates across hours after having controlled for village sites and outdoor versus indoor placement of human landing posts (χ2 = 201.4; P value < 0.01). Similarly, results obtained from a negative binomial regression reveal that in the first quarter of the night HBRs per person per hour were, on average, 13% lower compared to the remaining hours (IRR: 0.87, 95% CI: 0.83–0.92, P value < 0.01), while no significant differences were found between the first and the second half of the night. Overall, outdoor HBRs per person per night for An. gambiae accounted for 96% of indoor HBRs (IRR: 0.96, 95% CI: 0.89–1.00, P value < 0.06), indicating rather small differences in magnitude.
Fig. 3.
Nightly outdoor (blue) and indoor (red) biting cycles of Anopheles species within the survey region for the period August to November 2012. Bars show the average HBRs per person per hour and 95 % confidence intervals. Values are averaged over the six study villages.
While biting rates of the remaining species occurred at substantially lower levels, An. funestus (IRR: 1.67, 95% CI: 0.92–3.05, P value < 0.09), An. nili (IRR: 0.55, 95% CI: 0.34–0.88, P value < 0.01) and An. coustani (IRR: 3.55, 95% CI: 2.62–4.82, P value < 0.01) showed statistically significantly higher biting rates at outdoor capture posts. A Likelihood Ratio test rejected the hypothesis of equal biting rates across hours for An. nili (χ2 = 33.6, P value < 0.01) and An. coustani (χ2 = 113.5, P value < 0.01) but not for An. funestus (χ2 = 11.5, P value < 0.40). The bar chart for An. nili suggests a biting pattern that is similar to An. gambiae s.l., with more pronounced differences between outdoor and indoor catches. In comparison to other species, the biting pattern of An. coustani showed substantial differences. HBRs in the first 6 h were 2.2 times higher than in the second half of the night (IRR: 2.2, 95% CI: 1.71–2.86; P value < 0.01) and there was a 3.6 times higher preference to feed outdoors than indoors.
Monthly Variations in Nightly Biting Cycles
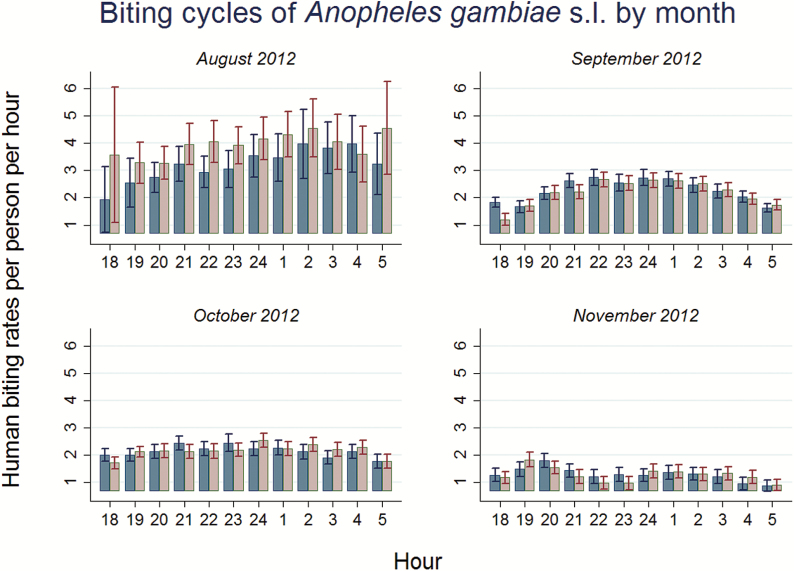
For An. gambiae s.l., biting cycles were depicted separately for each month to test for potential differences in biting activity levels and patterns (Fig. 4). Overall, bar charts suggest a decreasing trend in biting rates over months and a Likelihood ratio test, based on a negative binomial regression function, strongly rejects the null hypothesis that monthly biting rates are equal across all 4 mo (χ2 = 69.63; P value < 0.01). An. gambiae s.l. had its highest abundance during the early peak rainy season in August. During this period, show a much less pronounced reversed U-shaped pattern when compared to Fig. 3. In addition, this month showed a relatively high indoor feeding preference. In September, the pattern of nightly biting activity was similar to the observed overall pattern (Fig. 3). November featured the lowest biting rates with two minor peaks of activity around 2000 and 0100 hours. Overall, the negative binomial regressions that were estimated by month did not indicate a clear preference of An. gambiae s.l. to feed outdoors or indoors.
Fig. 4.
Monthly variation in HBRs per person per hour of An. gambiae s.l. from August to November 2012. Month fixed effects in our negative binomial regression are significantly different from zero (P value < 0.01) and a Likelihood Ratio test rejects the hypothesis that all month fixed effects equal zero (χ2 = 69.63; P value < 0.01).
Weekly Biting Dynamics of An. gambiae s.l.
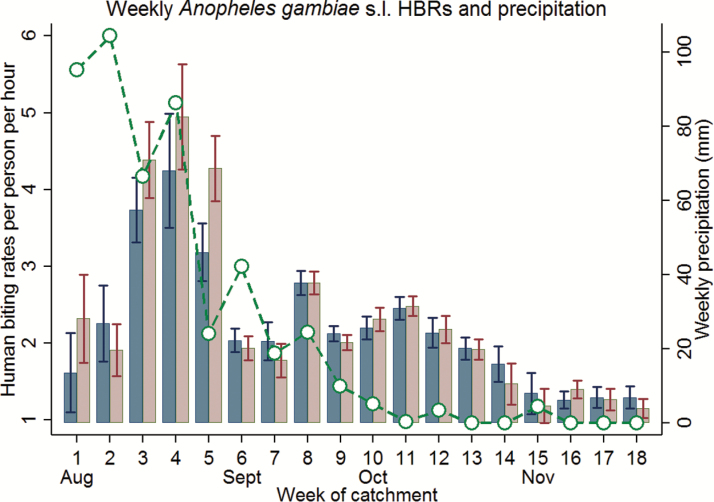
In Fig. 5, biting rates of An. gambiae s.l. are averaged over sampling week to provide a more disaggregated illustration of how HBRs per person per night evolve over time. In addition, the region’s weekly precipitation is included into the picture, to investigate the nexus between Anopheles abundance and precipitation. Overall, the average An. gambiae s.l. biting activity over weeks accounted for about 2.25 bites per person per hour with substantial variation across weeks but little variation between outdoor and indoor posts. In conjunction with heavy lagged precipitations, HBRs peaked in late August and early September (week 3 to 5), amounting to about 4.5 and 3.7 bites per person per hour for outdoor and indoor posts, respectively. This time-lagged relationship between precipitation and biting activity can be observed throughout the first half of the period. It translates into a substantial positive correlation between mean weekly HBRs and 2-wk-lagged precipitation of 0.84 (P value < 0.01) and 0.83 (P value < 0.01) for outdoor and indoor HLCs, respectively. Although there were only minor rainfalls after End-September, An. gambiae s.l. activity stayed above 1.5 bites per person per hour until the end of the rainy season.
Fig. 5.
Weekly HBRs of female Anopheles s.l. outdoors (blue) and indoors (red), August through November 2012. Averages of HBRs per person per hour were calculated by week. The dotted line indicates averaged cumulated weekly precipitations for the study region. Precipitation data was obtained from NASA’s Tropical Rainfall Measuring Mission (TRMM/Giovanni).
Discussion
An. gambiae s.l. is by far the most abundant vector of malaria within the study region. Its high abundance, its consistent biting pattern, and high presence at both outdoor and indoor posts make it omnipresent during the rainy season. An. gambiae biting activity was moderate within the first 2 h after dusk and increased quickly towards a 6-h period of high activity. This finding is novel and contrasts with many other African research sites, where the respective main vectors showed higher variation in nocturnal biting activity, featuring pronounced biting peaks and periods of lower activity. Sande et al. (Sande et al. 2016) reported two biting peaks of An. funestus for Zimbabwe, between 2200 and 2300 hours, and around 0300 hours. Kenea et al. (Kenea et al. 2016) observed an early biting peak between 1900 and 2000 hours for the most important vector Anopheles ziemanni Grunberg (Diptera: Culicidae) in Ethiopia, while An. arabiensis showed two peaks, from 2000 to 2200 hours and around midnight. Similarly, a study from Ethiopia (Taye et al., 2016) found An. gambiae s.l. and mosquitoes from the An. coustani complex to have two activity peaks, one between dusk and 2200 hours and a smaller in the early morning around 0400 hours. Research from Zimbabwe (Dandalo 2007) found evidence that variability in biting peak levels for An. merus largely depends on the year’s season. A study from South Africa, similarly, described multiple biting peaks with the highest activity of An. arabiensis in the predawn period (Braack et al. 1994). For two sites in Uganda, An. gambiae s.l. and An. funestus were found to have much higher biting activity in the second half of the night (Kabbale et al. 2013). The results presented here also contrast with earlier findings from the same region and season in 2004, when Yé (Yé et al. 2008) observed very low activity of An. gambiae s.l. in the early evening until 2100 hours, followed by two biting peaks. He reported a first peak around 2000–2300 hours and a second, higher and more extended one between midnight and 0200 hours. This significant change in the nightly biting cycle with a shift of biting activity towards the early evening hours might, at least partially, be attributable to the introduction of LLINs. In 2004, only 50% of the population was using bed nets, which were mostly non-impregnated (Traore 2003). After a mass distribution campaign in 2010, LLIN coverage increased to more than 95% (Louis et al. 2015). However, natural variation in vector species composition or activity can occur during different years and may bias a generalization of such deductions (Fontenille et al. 1997). The differences in outdoor and indoor biting activity of An. gambiae s.l. might be partly attributable to host seeking preferences of different sibling species as it is known from other studies (Kitau et al. 2012, Sougoufara et al. 2016).
Despite the observed extension of biting activity to the early evening hours, biting rates were still high during the time when people would normally sleep under their bed nets. Compared to previous years, this would represent an increase in vector abundance, for which possible origins could be found in the species composition. Different member species of the An. gambiae s.l. complex may have contributed to the continuously high biting activities at specific times at indoor and outdoor posts. Different population dynamics and breeding times amongst An. gambiae sibling species might contribute to an explanation of the different monthly biting patterns.
Research on the seasonal differences in nocturnal biting behavior of anopheline species is limited for sub-Saharan Africa (Shililu et al. 1998, Dandalo 2007, Oyewole et al. 2007, Cooke et al. 2015, Sande et al. 2016). For the most abundant malaria vector in the region, An. gambiae s.l., we observed different activity patterns between the peak rainy season in August and the two following months. Heavy rainfalls, a drop in temperature and strong winds prior to rainfalls have a hampering effect on mosquito abundance and mobility (Gillies and Wilkes 1981, Sharp 1983, Costantini et al. 1996). The high number of An. gambiae s.l. collected indoor in August might not have been caused by the vector’s reaction to environmental conditions alone but could be an adaptation to human behavior. It was observed that people in the study villages tended to go inside houses and huts earlier during the rainy season and An. gambiae s.l. might follow humans into their dwellings in the search for a blood meal.
Implications for Malaria Control
The risk for contact with the vector is mainly a function of abundance and host-seeking preference of the mosquitoes. While the abundance describes the general vector load in the environment, attempted bites, their timely distribution and the vector’s outdoor or indoor feeding preference define the risk of being bitten depending on personal behavior. Such personal behavior includes the use of bed nets, repellents, the type of clothing or the time people go to bed. The main malaria vector within the region, An. gambiae s.l., showed high abundance throughout the night for all observed periods during the rainy season. Although biting peaks were observed in the early morning (August), around midnight (September, October), the nightly patterns do not show any period with notably low vector abundance. An. gambiae s.l. biting activity already occurred at 1800 hours when HLCs started and rapidly increased towards high mosquito activity with almost three landings per person per hour.
The results presented here indicate that the use of Long Lasting Insecticidal Nets (LLINs) might provide only limited protection against the major malaria vectors within the region, despite high LLIN utilization rates in the region, with 98% of young children sleeping under a LLIN during the rainy season (Louis et al. 2015). Biting rates were already high during the early evening, when people were still active outdoors and not yet protected by bed nets.
The presented findings suggest that additional vector control methods are necessary to effectively provide protection for periods when people do not rest under bed nets, particularly in the light of possible future adaptations of the vector (Fornadel et al. 2010, Moiroux et al. 2012). Supplementary measures could include the modification and treatment of mosquito larval habitats which has proven efficient with current and future formulations of biological larvicides such as Bacillus thuringiensis israelensis and Bacillus sphaericus (Fillinger and Lindsay 2006, Nyarango et al. 2006, Fillinger et al. 2009, Geissbühler et al. 2009). In particular, in areas with high biting activities throughout the night, an additional treatment with larvicides could deliver effective additional protection at relatively low cost (White et al. 2011, Dambach et al. 2016). In extremely remote areas or regions, where larviciding would not be cost effective (Majambere et al. 2007), the use of personal repellents (Lupi et al. 2013, Hodson et al. 2016) or IRS might be an appropriate additional measure to bring down vector host contact for endophilic and endophagic species.
There are several strengths and limitations of this study. Strengths include the high number of collection places per village and the biweekly collection cycle during and after a typical rainy season. The total number of nine sample locations per village, featuring one outdoor and one indoor capture post each, provide a total number of 18 sample posts per village. The collection period of mosquitoes throughout a complete rainy season with extension into the low transmission dry season allows for detection of seasonal patterns. There are limitations regarding the comparability of collected mosquitoes between villages, arising from the sampling scheme. Human landing catches were not performed the same day in all six study villages but instead followed a rotating scheme, roughly with a 14-d interval, depending on village accessibility. Human landing catches were not performed in the sleeping room but in the main living room of huts, which may have slightly influenced the recorded biting rates. On the other hand, most of the huts do not have doors between different rooms, some only have a single room. A considerable number of mosquitoes resting in the sleeping room is likely to have found their way to the HLC post in the adjacent room. Species determination was performed under relatively simple conditions by experienced entomologists but without the opportunity to distinguish sibling species. We are aware of the importance of detection of possible shifts in the composition of sibling species within the An. gambiae s.l. complex as an adaptation to the performed vector control activities.
Conclusions
In the study villages of the greater Nouna region there was high An. gambiae s.l. indoor and outdoor biting activity throughout the night with no marked single or multiple peaks. These biting patterns could be the consequence of mosquito adaptation to the recently achieved high LLLIN coverage in the population. Given the observed high activity of anopheline mosquitoes throughout the night, vector contact at any time during the night is high when not sleeping under a bed net. Pursuing the goal of further reducing malaria transmission might raise the need for additional vector control methods that either diminish vector populations or reduce human-vector contact.
Acknowledgments
We are deeply grateful to the research unit at the CRSN (Centre de Recherche en Santé de Nouna) for their cooperation, interest and personal commitment in the underlying PaluClim study. We thank the ‘Programme Gestion et Impacts du Changement Climatique’ established by the French ministry of ecology, energy, sustainable development and oceans for supporting this research project. We acknowledge financial support by Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding program Open Access Publishing for publishing this article. We want to thank Christophe Rogier, Jean-Pierre Lacaux, Vanessa Machault, Cécile Vignolles, Till Bärnighausen and Rainer Sauerborn for their valuable ideas and contribution to the design of this study. P.D. and N.B. developed the concept and design of the study. S.O., P.D., and N.B. supervised and performed the collection of the data. P.D. and M.S. performed the analysis and interpretation of the data. M.S. ran the statistical analyses in STATA. M.D., P.K., and J.D. contributed to the entomological and ecological interpretation of the data and to the discussion. P.D., M.S., P.K., A.S., J.D., M.D., and N.B. wrote the paper. All authors contributed to writing of the paper and the interpretation of the data. All authors read and approved the final manuscript. The study was approved by the national ethics board of Burkina Faso in Ouagadougou, the local ethics committee at the research site in Nouna and the ethics committee of the University of Heidelberg under the certificate number S-438/2013. We collected informed consent for the human landing catches from each participant. Malaria prophylaxis was provided free of charge to all participants during and up to 1 mo after the study. All participants were vaccinated against yellow fever. There are no case presentations that require disclosure of respondents’ confidential data/information in this study. The datasets supporting the conclusions of this article are available at the Health Research Center in Nouna, Burkina Faso and will be made easily available on request, when required. Funding was received through Universitaet Heidelberg, no external funding was received for performing this study. The authors declare that they have no competing interests.
References Cited
- Braack L. E., Coetzee M., Hunt R. H., Biggs H., Cornel A., and Gericke A.. 1994. Biting pattern and host-seeking behavior of Anopheles arabiensis (Diptera: Culicidae) in northeastern South Africa. j. Med. Entomol. 31: 333–339. [DOI] [PubMed] [Google Scholar]
- Braack, L., Hunt R., Koekemoer L. L., Gericke A., Munhenga G., Haddow A. D., Becker P., Okia M., Kimera I., and Coetzee M.. 2015. Biting behaviour of African malaria vectors: 1. where do the main vector species bite on the human body?Parasit. Vectors. 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady O. J., Godfray H. C., Tatem A. J., Gething P. W., Cohen J. M., McKenzie F. E., Perkins T. A., Reiner R. C. Jr, Tusting L. S., Sinka M. E., et al. . 2016. Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg. 110: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, M. K., Kahindi S. C., Oriango R. M., Owaga C., Ayoma E., Mabuka D., Nyangau D., Abel L., Atieno E., Awuor S.,, et al. 2015. ‘A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar. J. 14: 259. doi:10.1186/s12936-015-0766-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini, C., Li S.-G., Torre A. D., Sagnon N., Coluzzi M., Taylor C. E.. 1996. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan savanna village. Med. Vet. Entomol. 10: 203–219. doi:10.1111/j.1365-2915.1996.tb00733.x [DOI] [PubMed] [Google Scholar]
- Dambach, P., Schleicher M., Stahl H. C., Traoré I., Becker N., Kaiser A., Sié A., and Sauerborn R.. 2016. Routine implementation costs of larviciding with Bacillus thuringiensis israelensis against malaria vectors in a district in rural Burkina Faso. Malar. J. 15: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandalo, L. C. 2007. The abundance and biting behaviour of Anopheles merus (Dönitz) in Gokwe South District, Zimbabwe. University of Zimbabwe. [Google Scholar]
- Diallo, A., Sié A., Sirima S., Sylla K., Ndiaye M., Bountogo M., Ouedraogo E., Tine R., Ndiaye A., Coulibaly B., et al. . 2017. An epidemiological study to assess Plasmodium falciparum parasite prevalence and malaria control measures in Burkina Faso and Senegal. Malar. J. 16: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diboulo, E., Sié A., Diadier D. A., Karagiannis Voules D. A., Yé Y., and Vounatsou P.. 2015. Bayesian variable selection in modelling geographical heterogeneity in malaria transmission from sparse data: an application to Nouna Health and Demographic Surveillance System (HDSS) data, Burkina Faso. Parasit. Vectors. 8: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diboulo, E., Sié A., and Vounatsou P.. 2016. Assessing the effects of malaria interventions on the geographical distribution of parasitaemia risk in Burkina Faso. Malar. J. 15: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger, U., and Lindsay S. W.. 2006. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop. Med. Int. Health. 11: 1629–1642. [DOI] [PubMed] [Google Scholar]
- Fillinger, U., Ndenga B., Githeko A., and Lindsay S. W.. 2009. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull. World Health Organ. 87: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenille, D., Lochouarn L., Diagne N., Sokhna C., Lemasson J. J., Diatta M., Konate L., Faye F., Rogier C., and Trape J. F.. 1997. High annual and seasonal variations in malaria transmission by anophelines and vector species composition in Dielmo, a holoendemic area in Senegal. Am. J. Trop. Med. Hyg. 56: 247–253. [DOI] [PubMed] [Google Scholar]
- Fornadel, C. M., Norris L. C., Glass G. E., and Norris D. E.. 2010. Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am. J. Trop. Med. Hyg. 83: 848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatton, M. L., Chitnis N., Churcher T., Donnelly M. J., Ghani A. C., Godfray H. C., Gould F., Hastings I., Marshall J., Ranson H., et al. . 2013. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 67: 1218–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissbühler, Y., Kannady K., Chaki P. P., Emidi B., Govella N. J., Mayagaya V., Kiama M., Mtasiwa D., Mshinda H., Lindsay S. W., et al. . 2009. Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS One. 4: e5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies, M. T., Wilkes T. J.. 1981. Field experiments with a wind tunnel on the flight speed of some west African mosquitoes (Diptera: Culicidae). Bull. Entomol. Res. 71: 65–70. doi: 10.1017/S0007485300051038 [Google Scholar]
- Githeko, A. K., Adungo N. I., Karanja D. M., Hawley W. A., Vulule J. M., Seroney I. K., Ofulla A. V., Atieli F. K., Ondijo S. O., Genga I. O., et al. . 1996. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp. Parasitol. 82: 306–315. [DOI] [PubMed] [Google Scholar]
- Hodson, C. N., Yu Y., Plettner E., and Roitberg B. D.. 2016. New repellent effective against African malaria mosquito Anopheles gambiae: implications for vector control. Med. Vet. Entomol. 30: 369–376. [DOI] [PubMed] [Google Scholar]
- Kabbale, F. G., Akol A. M., Kaddu J. B., and Onapa A. W.. 2013. Biting patterns and seasonality of Anopheles gambiae sensu lato and Anopheles funestus mosquitoes in Kamuli District, Uganda. Parasit. Vectors. 6: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenea, O., Balkew M., Tekie H., Gebre-Michael T., Deressa W., Loha E., Lindtjørn B., and Overgaard H. J.. 2016. Human-biting activities of Anopheles species in south-central Ethiopia. Parasit. Vectors. 9: 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitau, J., Oxborough R. M., Tungu P. K., Matowo J., Malima R. C., Magesa S. M., Bruce J., Mosha F. W., and Rowland M. W.. 2012. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis?PLoS One. 7: e31481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, R. S., Peterson A. T., and Benedict M. Q.. 2004. Geographic and ecologic distributions of the Anopheles gambiae complex predicted using a genetic algorithm. Am. j. Trop. Med. Hyg. 70: 105–109. [PubMed] [Google Scholar]
- Louis, V. R., Schoeps A., Tiendrebéogo J., Beiersmann C., Yé M., Damiba M. R., Lu G. Y., Mbayiha A. H., De Allegri M., Jahn A., et al. . 2015. An insecticide-treated bed-net campaign and childhood malaria in Burkina Faso. Bull. World Health Organ. 93: 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi, E., Hatz C., and Schlagenhauf P.. 2013. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp. - a literature review. Travel Med. Infect. Dis. 11: 374–411. [DOI] [PubMed] [Google Scholar]
- Majambere, S., Lindsay S. W., Green C., Kandeh B., and Fillinger U.. 2007. Microbial larvicides for malaria control in The Gambia. Malar. J. 6: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakawa, N., Seda P., and Yan G.. 2002. Influence of host and larval habitat distribution on the abundance of African malaria vectors in western Kenya. Am. J. Trop. Med. Hyg. 67: 32–38. [DOI] [PubMed] [Google Scholar]
- Moiroux, N., Gomez M. B., Pennetier C., Elanga E., Djènontin A., Chandre F., Djègbé I., Guis H., and Corbel V.. 2012. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 206: 1622–1629. [DOI] [PubMed] [Google Scholar]
- Namountougou, M., Simard F., Baldet T., Diabaté A., Ouédraogo J. B., Martin T., and Dabiré R. K.. 2012. Multiple insecticide resistance in Anopheles gambiae s.l. populations from Burkina Faso, West Africa. PLoS One. 7: e48412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyarango, P. M., Gebremeskel T., Mebrahtu G., Mufunda J., Abdulmumini U., Ogbamariam A., Kosia A., Gebremichael A., Gunawardena D., Ghebrat Y., et al. . 2006. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar. J. 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyewole, I. O., Awolola T. S., Ibidapo C. A., Oduola A. O., Okwa O. O., Obansa J. A.. 2007. Behaviour and population dynamics of the major anopheline vectors in a malaria endemic area in southern Nigeria. J. Vector Borne Dis. 44: 56–64. [PubMed] [Google Scholar]
- Russell, T. L., Govella N. J., Azizi S., Drakeley C. J., Kachur S. P., and Killeen G. F.. 2011. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande, S., Zimba M., Chinwada P., Masendu H. T., and Makuwaza A.. 2016. Biting behaviour of Anopheles funestus populations in Mutare and Mutasa districts, Manicaland province, Zimbabwe: implications for the malaria control programme. J. Vector Borne Dis. 53: 118–126. [PubMed] [Google Scholar]
- Sharp, B. L. 1983. Anopheles merus (Donitz), its biting cycle in relation to environmental parameters. J. Entomol. Soc. South. Afr. [Google Scholar]
- Shililu, J. I., Maier W. A., Seitz H. M., Orago A. S.. 1998. Seasonal density, sporozoite rates and entomological inoculation rates of anopheles gambiae and Anopheles funestus in a high-altitude sugarcane growing zone in Western Kenya. Trop. Med. Int. Health. 3: 706–710. [DOI] [PubMed] [Google Scholar]
- Sinka, M. E., Bangs M. J., Manguin S., Coetzee M., Mbogo C. M., Hemingway J., Patil A. P., Temperley W. H., Gething P. W., Kabaria C. W., et al. . 2010. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit. Vectors. 3: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sougoufara, S., Harry M., Doucouré S., Sembène P. M., and Sokhna C.. 2016. Shift in species composition in the Anopheles gambiae complex after implementation of long-lasting insecticidal nets in Dielmo, Senegal. Med. Vet. Entomol. 30: 365–368. [DOI] [PubMed] [Google Scholar]
- Strode, C., Donegan S., Garner P., Enayati A. A., and Hemingway J.. 2014. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: systematic review and meta-analysis. Plos Med. 11: e1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvie, M., Pierre C., and Jean M.. 2008. Biodiversity of malaria in the world. John Libbey Eurotext, Montrouge, France. [Google Scholar]
- Taye, B., Lelisa K., Emana D., Asale A., and Yewhalaw D.. 2016. Seasonal dynamics, longevity, and biting activity of anopheline mosquitoes in southwestern Ethiopia. J. Insect Sci. Online. 16: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen, E. K., Koimbu G., Pulford J., Jamea-Maiasa S., Ura Y., Keven J. B., Siba P. M., Mueller I., Hetzel M. W., and Reimer L. J.. 2017. Mosquito behavior change after distribution of bednets results in decreased protection against malaria exposure. J. Infect. Dis. 215: 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore, C. 2003. Epidemiology of malaria in a holoendemic area of rural burkina faso. Dissertation, Institute of Public Health, University of Heidelberg, Germany. [Google Scholar]
- Van Bortel, W., Trung H. D., Hoi L. E. X., Van Ham N., Van Chut N., Luu N. D., Roelants P., Denis L., Speybroeck N., D’Alessandro U., et al. . 2010. Malaria transmission and vector behaviour in a forested malaria focus in central Vietnam and the implications for vector control. Malar. J. 9: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter Reed Biosystematics Unit 2017. http://www.wrbu.org/index.html Walter reed biosystematics unit.
- White, M. T., Conteh L., Cibulskis R., and Ghani A. C.. 2011. Costs and cost-effectiveness of malaria control interventions-a systematic review. Malar J. 10: 1475–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yé, Y., Sankoh O., Kouyaté B., Sauerborn R.. 2008. Environmental factors and malaria transmission risk: modelling the risk in a Holoendemic area of Burkina Faso. Ashgate Publishing, Ltd, Farnham, United Kingdom. [Google Scholar]