Abstract
Background and Aims
Evolutionary change in developmental trajectories (heterochrony) is a major mechanism of adaptation in plants and animals. However, there are few detailed studies of the variation in the timing of developmental events among wild populations. We here aimed to identify the climatic drivers and measure selection shaping a genetic-based developmental cline among populations of an endemic tree species complex on the island of Tasmania.
Methods
Seed lots from 38 native provenances encompassing the clinal transition from the heteroblastic Eucalyptus tenuiramis to the homoblastic Eucalyptus risdonii were grown in a common-garden field trial in southern Tasmania for 20 years. We used 27 climatic variables to model the provenance variation in vegetative juvenility as assessed at age 5 years. A phenotypic selection analysis was used to measure the fitness consequences of variation in vegetative juvenility based on its impact on the survival and reproductive capacity of survivors at age 20 years.
Key Results
Significant provenance divergence in vegetative juvenility was shown to be associated with home-site aridity, with the retention of juvenile foliage increasing with increasing aridity. Our results indicated that climate change may lead to different directions of selection across the geographic range of the complex, and in our mesic field site demonstrated that total directional selection within phenotypically variable provenances was in favour of reduced vegetative juvenility.
Conclusions
We provide evidence that heteroblasty is adaptive and argue that, in assessing the impacts of rapid global change, developmental plasticity and heterochrony are underappreciated processes which can contribute to populations of long-lived organisms, such as trees, persisting and ultimately adapting to environmental change.
Keywords: Heteroblasty, heterophylly, ontogeny, heterochrony, neoteny, vegetative juvenility, provenance variation, phenotypic selection, climate change, aridity, Eucalyptus risdonii, Eucalyptus tenuiramis
INTRODUCTION
The developmental (ontogenetic) trajectories of animals and plants are usually under strong genetic control, and involve not only a transition to the reproductive state, but changes in the somatic (vegetative) phenotype (Gould, 1977; Guerrant, 1988). This ontogenetic change in phenotype can be rapid and dramatic, as exemplified by animal metamorphosis (e.g. amphibians and arthropods) or plant heteroblasty/heterophylly, or more subtle and unsynchronized across multiple traits. Evolutionary change in developmental trajectories, termed heterochrony (McNamara, 2012), is believed to be a major mechanism of adaptation in animals and plants (Gould, 1977; Guerrant, 1988; McKinney and McNamara, 1991; Li and Johnston, 2000; Climent et al., 2006; Maherali et al., 2009). It has long been argued that just a few changes in genes which regulate developmental trajectories might produce markedly different phenotypes in descendants and provide a means of rapid adaptive evolution (Goldschmidt, 1940; Gould, 1977; McKinney and McNamara, 1991; Wiltshire et al., 1994). This is particularly significant when the change involves a shift in the timing of reproduction relative to the ontogenetic change in morphology (Wiltshire et al., 1994; Li and Johnston, 2000).
Despite the recognized importance of heterochrony in plant evolution, there have been surprisingly few detailed studies of the variation in the timing of developmental events among wild populations (Franks and Weis, 2008; Santos-del-Blanco et al., 2013), particularly involving heteroblasty (Wiltshire et al., 1998; Jordan et al., 1999, 2000; Climent et al., 2006). The micro-evolutionary processes leading to such heterochronic variation can provide insights into macro-evolutionary processes which have underlain many speciation events (Fernández-Mazuecos and Glover, 2017). Gene flow, drift, phylogenetic/genetic constraints and natural selection all interact to shape the patterns of genetic diversity in wild populations (Endler, 1986; Givnish, 1987; Chenoweth et al., 2010; Wilson and Poissant, 2016). Understanding their relative roles in shaping variation in a focal trait is a major challenge in evolutionary biology (Endler, 1986). Elucidating the role of natural selection, for example, is complicated by the possibility of correlated responses. Indeed, differentiating between direct and indirect selection acting on a trait is important to understand the biological basis of selection occurring within a generation and/or predict the evolutionary response to selection across generations (Lande and Arnold, 1983; Arnold and Wade, 1984; Mitchell-Olds and Shaw, 1987; Rausher, 1992; Chenoweth et al., 2010). Understanding of the role of selection in the evolution of heterochrony in wild populations requires the integration of multiple lines of evidence (Wade and Kalisz, 1990). This is particularly the case with large, long-lived organisms such as trees where opportunities for the application of reverse-genetic approaches and multigeneration selection experiments are limited, and the relevant genetic variation in heterochrony and fitness differentials may take years to be expressed within a generation (Petit and Hampe, 2006; Jaramillo-Correa et al., 2015; Brunner et al., 2016).
Trees of the genus Eucalyptus abound with examples of closely related taxa or intraspecific populations that are differentiated on the basis of the timing of developmental events (Potts and Wiltshire, 1997; Borzak et al., 2015; Brunner et al., 2016). The heteroblastic transition from juvenile to adult leaf types (vegetative phase change) and flowering precocity (reproductive phase change) are the most studied examples. Variation in the onset of these developmental events has been shown to: (1) be under strong genetic control at both the population and intrapopulation levels (Wiltshire et al., 1998; Jordan et al., 1999, 2000; Hamilton et al., 2011), and to exhibit low genotype by environment interaction (Hamilton et al., 2011); (2) reflect expressed variation in multiple quantitative trait loci (Hudson et al., 2014); and (3) be dependent on the number of nodes expanded (Wiltshire and Reid, 1992; James and Bell, 2000; Hudson et al., 2014). In the case of vegetative phase change, there is evidence that microRNAs play an important role in triggering the heteroblastic transition (Wang et al., 2011; Hudson et al., 2014; Brunner et al., 2016), which is associated with major changes in gene methylation (Brunner et al., 2016; Hasbun et al., 2016). In addition to the ontogenetic changes in leaf morphology, there is increasing evidence for multiple physiological (James et al., 1999; Jaya et al., 2010), anatomical (James et al., 1999; James and Bell, 2001; Gras et al., 2005; Loney et al., 2006) and biochemical (Loney et al., 2006; Goodger et al., 2013) differences between ‘juvenile’ and ‘adult’ leaf types. Such differences underlie different biotic interactions involving susceptibility to disease (Park and Keane, 1982; Balmelli et al., 2014), insects (Steinbauer, 2002; Lawrence et al., 2003; Nahrung and Allen, 2003) and vertebrates (Loney et al., 2006), and strongly argue for a functional basis to the ontogenetic variation in heteroblasty. In some eucalypt species, the juvenile phase seems to be more shade tolerant and less drought resistant than the adult phase, whereas in other species it appears to be adapted to better resist frost or high insolation loads and drought stress (Potts and Wiltshire, 1997).
The present study investigates clinal variation in vegetative juvenility in trees from the Eucalyptus risdonii–Eucalyptus tenuiramis complex. This complex is endemic to the southern hemisphere island of Tasmania, Australia, where a cline in increasing retention of the juvenile foliage links the widespread heteroblastic taxon – E. tenuiramis – to its rare sister taxon – E. risdonii – which retains juvenile foliage throughout its life (Wiltshire et al., 1991, 1992, 1998; Turner et al., 2000, 2001). Eucalyptus tenuiramis only bears flowers in the adult leaf stage, whereas its descendant E. risdonii flowers while bearing the juvenile leaves (Fig. 1). The morphological difference between reproductively mature phenotypes of these taxa is striking. The juvenile leaves of E. risdonii are highly glaucous, broad and opposite with nodal pairs fused at the base (i.e. connate). In contrast, while the juvenile leaves of E. tenuiramis are similar to those of E. risdonii, the adult leaves borne by reproductively mature E. tenuiramis are more typical of mature eucalypts, being less glaucous, alternate, petiolate and lanceolate in shape. Eucalyptus risdonii rarely develops this adult leaf form in the wild, and when it does they more resemble the ontogenetically transitional leaves of E. tenuiramis (Wiltshire et al., 1991).
Fig. 1.
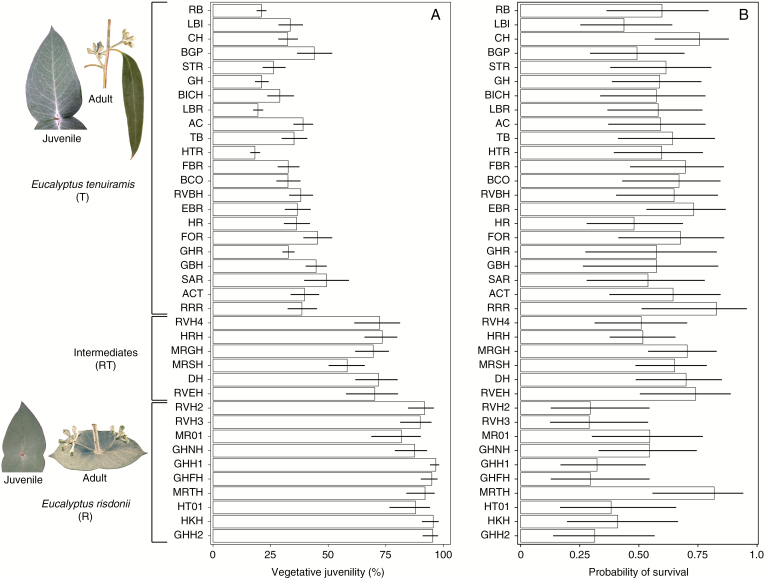
Provenance values for mean effects and associated 95 % confidence intervals for the examined common-garden trial site: (A) vegetative juvenility (i.e. percentage of juvenile foliage retained at age 5 years); and (B) adult survival (i.e. probability of survival at age 20 years). The 38 provenances of the three ontogenetic classes E. tenuiramis (T), E. risdonii (R), and their intermediates (RT) are shown. Provenances have been grouped by ontogenetic class, and then ranked within an ontogenetic class according to the percentage of juvenile foliage previously observed in the wild (Wiltshire et al., 1991, 1998). Ideograms represent the extremes of the phenotypic cline from the heteroblastic E. tenuiramis to the homoblastic E. risdonii ontogenetic classes. The opposite, connate ‘juvenile’ leaf form is retained into reproductive maturity in E. risdonii (bottom), whereas E. tenuiramis is only reproductively mature when bearing the opposite, petiolate ‘adult’ leaf form.
The heterochronic variation among populations of the E. risdonii–E. tenuiramis complex has a genetic basis, with the pattern of clinal variation evident in wild populations also evident in a common-garden field trial. The cline, which involves a delay in the nodal transition to adult foliage, is evident in both the height and chronological timing of the vegetative transition, and has been argued to have arisen through neoteny (i.e. retention of the juvenile form of the ancestor) (Wiltshire et al., 1998). It has been hypothesized that vegetative juvenility in this complex is favoured on dry sites (Potts and Wiltshire, 1997), although in other eucalypt species the opposite trend has been reported (Jordan et al., 2000). Using a common-garden trial growing at a mesic site, we test whether: (1) the genetic-based population divergence in the degree of vegetative juvenility is associated with climate aridity at the population origin (i.e. the home site); and (2) developmental differences in vegetative juvenility within phenotypically variable populations have fitness consequences in terms of later-age survival and reproductive capacity.
MATERIALS AND METHODS
Field trial and measurements
The common-garden field trial was established in June 1990 to investigate the genetic basis of variation in the onset of the ontogenetic transition from juvenile to adult foliage types in the E. risdonii–E. tenuiramis complex. The trial is detailed in Wiltshire et al. (1998). In brief, the trial site was located near Franklin in southern Tasmania (latitude 43°04′S, longitude 146°53′E; and elevation of 350 m above sea level) on an ex-forest site that had been cleared and ripped at 3 m intervals for plantation establishment. The site was considered highly productive and suitable to sustain wet sclerophyll forests, with predicted mean annual precipitation of 1030 mm (based on BIOCLIM surfaces, see below). The trial included families (progeny based on open-pollinated seed collection from a single tree) from 40 native populations (hereafter referred to as ‘provenances’) covering the geographic range of E. risdonii (R) and E. tenuiramis (T), as well as geographically and ontogenetically intermediate populations (RT) (with R, T or RT being denoted as a ‘ontogenetic class’; see Wiltshire et al., 1991, 1992, 1998). The geographic location and climatic details of the provenance home sites and the trial site are given in Supplementary Data Table S1. The trial had three replicates into which families (irrespective of provenance) were randomized and represented as single-tree plots at a 3 × 3 m spacing. All provenances were represented in each replicate. However, incomplete replication of some families required the random allocation of additional seedlings from other families to empty positions.
Vegetative juvenility was quantified as the percentage of tree height with juvenile foliage (JUV) at age 5 years, following Jordan et al. (1999, 2000). Trees which had not made the transition by age 5 years were given a value of 100 %. At the provenance level, this relative measure from the field trial is strongly positively correlated with the percentage of the canopy that bears juvenile foliage in wild populations (Wiltshire et al., 1998). For all the trees that had been assessed for JUV at age 5 years, adult survival (SURV) was subsequently determined at age 20 years: an alive plant was scored as 1, and a dead plant was recorded as 0. Reproductive capacity (REPR) was evaluated only in the trees that survived to age 20 years: a plant was recorded as 1 if reproductive (i.e. if capsules or flower buds were observed in the canopy), otherwise it was given a score of 0.
Data analysis
Data sets.
The analyses undertaken in the present study were based on the following data.
Provenance differences in SURV, REPR and JUV were studied by using data from 38 provenances. Thus, from the original 40 provenances planted, two (HF and HRA) were excluded from these analyses due to low sample size in both cases, and also due to an invariant phase change in one of the cases which caused convergence difficulties when modelling JUV. The analyses of provenance differences for SURV and JUV were based on the same data set, including 765 plants from 360 families and comprising all the trees that had been phenotyped for vegetative juvenility at age 5 years. This data set also enabled us to study the association of JUV with home-site climate variables. The analysis of provenance differences for REPR used the sub-set of individuals that survived to age 20 years, and included 437 plants from 273 families. The average number of families per provenance ranged from approximately ten (SURV and JUV) to seven (REPR), with the representation of individuals being sparse across families (i.e. the family sizes varied from one to four plants).
Phenotypic selection on JUV acting within provenances via the examined fitness components (i.e. SURV or REPR) was studied by using data from provenances that were proximal to the intergradation zone between the two taxa and maximized the within-provenance phenotypic diversity for JUV. Thus, more informative data on JUV will be provided by this sub-set of central provenances to study the fitness consequences of within-provenance variation in this focal trait. In this sense, 17 provenances were considered for analysis (i.e. ACT, BCO, DH, EBR, FOR, GHNH, HRH, HT01, MR01, MRGH, MRSH, MRTH, RVBH, RVEH, RVH2, RVH3 and RVH4; Wiltshire et al., 1998), and included six and five provenances from E. risdonii and E. tenuiramis, respectively, as well as six intermediate provenances. The analysis of phenotypic selection on JUV mediated by SURV used 374 individuals, comprising all the plants (from the 17 provenances) that had been measured for JUV at age 5 years; the sample mean and standard deviation for JUV were, respectively, 65.0 % and 27.3 % (range from 17 to 100 %) for these data. A sub-set of 224 plants from these data, and pertaining to those individuals that survived to age 20 years, was used in the analysis of phenotypic selection on JUV occurring through REPR; the sample mean and standard deviation for JUV were, respectively, 59.3 % and 26.9 % (range from 17 to 100 %) for these data.
Provenance differences.
We examined the effect of provenance on SURV, REPR and JUV. Data analyses of SURV and REPR were undertaken using a generalized linear model that included fixed effects for the intercept, provenance and replicate model terms. It was assumed that the SURV and REPR data were generated from a Bernoulli distribution, and the logit link was used as a link function to relate the mean response to the linear predictor. A detailed description on the modelling of SURV and REPR is given in Supplementary Data Methods S1. As a continuous variable with observations bounded at 0 and 100 %, JUV was assumed to follow a standard beta distribution, which can be parameterized as a function of a location parameter µ (i.e. mean response) and a scale parameter ϕ (i.e. precision) (Smithson and Verkuilen, 2006). Prior to analysis, the observed percentage values of JUV were converted into proportions. Then, instead of modelling only µ and fitting ϕ as a nuisance parameter (e.g. Ferrari and Cribari-Neto, 2004), we have applied an extended beta-distributed generalized linear model for joint modelling mean response and precision (and thus dispersion), following the methodology proposed by Smithson and Verkuilen (2006). Within this framework, the parameters µ and ϕ were related to predictor variables (again comprising fixed effects for the intercept, provenance and replicate model terms) via distinct linear predictors, and using the logit and log link functions for modelling µ and ϕ, respectively. Further details on the modelling of JUV (including also rescaling the data to circumvent the presence of some proportions equal to one) are provided in Supplementary Data Methods S2.
The analysis of SURV and JUV used the same set of observations (N = 765), whereas the analysis of REPR was based on a reduced set including only the trees that were alive at age 20 years (N = 437) (see ‘Data sets’ above). Estimates of model parameters and associated standard errors were obtained by maximum likelihood estimation. Wald F-tests (with the denominator degrees of freedom given by N – k, where k denotes the rank of the matrices X or W; for the description of these matrices, see Supplementary Data Methods S1 and S2) were conducted to determine whether differences of mean (all response variables) or dispersion (JUV) effects were statistically significant among provenances or replicates. Least-squares mean estimates were obtained on the link (linear) scale for each provenance, and then provenance-predicted values on the scale of the data (hereafter referred to as ‘provenance values’) were computed by applying the inverse link function to the estimated least-squares means. In this sense, provenance values of mean effects were obtained for SURV and REPR (i.e. probability of surviving or reproducing), and provenance values of mean and dispersion effects were calculated for JUV (i.e. percentage of vegetative juvenility); the respective standard errors were approximated by applying the delta method (Billingsley, 1986; Cox, 1998), and 95 % confidence limits were obtained by inversely linking the corresponding confidence limits on the linear scale. To test the goodness-of-fit of the logistic regression model with categorical predictors used in the analyses of SURV and REPR, the Stukel’s test (Stukel, 1988) was applied. This test found no evidence (P > 0.05) to reject the null hypothesis that the fitted logistic regression model is correct, indicating that the given model was adequately specified for SURV and REPR. In addition, in the analyses of all of the three response variables, the variance of the Pearson residuals was close to one, further suggesting that the applied models fitted the data adequately.
Previous analyses extending the generalized linear model defined above to include random effects were conducted to test whether a random family term, nested within provenances, was statistically significant. A generalized linear mixed model was thus applied, with parameter estimates obtained using adaptive Gauss–Hermite quadrature, which is an integral approximation technique to evaluate the marginal log-likelihood of the data numerically. For the three response variables analysed, the estimated variance for the family effects appeared to be either at the boundary of zero (REPR and JUV) or not statistically significant (P > 0.05) according to a one-tailed likelihood-ratio test (Stram and Lee, 1994) (SURV). Although possibly reflecting a sparse family structure in the data, these insignificant results did not justify the extra model complexity of including a random family term, and thus further analyses treated the individual trees from the same family as being independent observations.
The SAS procedures GLIMMIX, LOGISTIC and NLMIXED (SAS, 2015) were used to undertake the analyses of provenance differences.
Relationship between vegetative juvenility and home-site climate.
To determine whether provenance differences in vegetative juvenility at the trial site were associated with home-site climate (i.e. the climate at the native provenance site), the variation in provenance values of mean effects for JUV was modelled by using the random forest algorithm (Breiman, 2001), implemented in the randomForest software package in R (Liaw and Wiener, 2002) (see Supplementary Data Methods S3). To achieve this, we started by obtaining a total of 1089 natural distributional records of E. tenuiramis and E. risdonii from the Natural Values Atlas of Tasmania (https://www.naturalvaluesatlas.tas.gov.au/). Using the latitude and longitude co-ordinates for each of these natural distribution records, 27 climate variables were subsequently derived from baseline climate surfaces (1976–2005; hereafter referred to as ‘contemporary’) obtained from the BIOCLIM module of the software package ANUCLIM (version 6.1; Xu and Hutchinson, 2013). These variables included eleven temperature, eight precipitation and eight radiation variables (Supplementary Data Table S2). We then applied a principal component analysis using the correlation matrix among the 27 bioclimatic variables derived for each of the 1089 records, and the first four principal components explained 94 % of the total variation in the climate data across the native range of the complex (Supplementary Data Figs S1 and S2). The eigenvectors associated with these four principal components were retained, and principal component scores were then calculated for the provenance home sites using the corresponding records for the 27 climate variables (a similar procedure was followed to obtain principal component scores for the trial site and spatial grid cells; see the next paragraph). These principal component scores comprised the values of the four independent variables (i.e. the retained four principal components summarizing the variation in 27 climate variables) that were used in the random forest algorithm to model the relationship between vegetative phase change and home-site climate.
The developed random forest model was then used to predict vegetative juvenility for the trial site and each 30 s (approx. 0.8 km) spatial grid cell across south-eastern Tasmania, based on: (1) the contemporary (1976–2005) BIOCLIM surfaces; and (2) BIOCLIM surfaces projected for 2070–2099 (hereafter denoted as the ‘2080s’ and associated predictions referred to as ‘forecasts’), calculated by the Climate Futures of Tasmania (Corney et al., 2010) using six global circulation models (GCMs) based on the A2 emission scenario (see Supplementary Data Methods S4). While the projections of the six GCMs for the 2080s represent equally plausible future climates (see Supplementary Data Fig. S3 for the 2080s forecasts of the random forest model under each of the GCMs), we restricted the presentation of the results to the ECHAM model, which projects a climate that is nearest to the multimodel mean of all the GCMs used in the current study (see Supplementary Fig. S2 in Harrison et al., 2017). Based on the contemporary surfaces, we assessed the potential fitness impacts of provenance transfer by calculating the difference between the predicted vegetative juvenility for the trial site (‘pseudo-local’) and the corresponding estimate for the home site of each of the 38 translocated provenances (non-local) used to develop the random forest model. To assess the likely risk of maladaptation of native provenances arising from climate change, we calculated for each provenance home site the difference between the vegetative juvenility values based on the projected (2080s) and contemporary climates.
Phenotypic selection analysis.
Phenotypic selection analysis aimed to elucidate the selective importance of JUV occurring at an early age on fitness components manifested at a later life history stage. We thus evaluated the fitness consequences of variation in JUV by performing an analysis of phenotypic selection acting on this trait via SURV or REPR. Although the adaptive value of JUV was the main focus, there may be unmeasured traits influencing SURV and/or REPR that are phenotypically correlated with JUV, which could possibly lead to indirect selection on the focal trait. Consequently, the applied single-trait analysis will measure total selection acting to modify the phenotypic distribution of JUV within the given generation (Lande and Arnold, 1983; Arnold and Wade, 1984). By using least-squares regression to model the relationship between a fitness component and a single target trait, a linear coefficient refers to a selection gradient quantifying the magnitude of total directional selection for higher or lower phenotypic trait values, and a quadratic coefficient pertains to a non-linear selection gradient that describes the effects of total selection in either decreasing (convex or stabilizing selection, when the quadratic coefficient is negative) or increasing (concave or disruptive selection, when the quadratic coefficient is positive) the phenotypic variance of the trait (for example, see Kalisz, 1986; Mitchell-Olds and Shaw, 1987).
As both of the fitness components had dichotomous outcomes, we used a logistic regression model to evaluate the phenotypic selection acting on JUV through either SURV or REPR within provenances. A separate analysis was thus conducted for each fitness component by applying a logistic model with categorical predictors (i.e. using provenance and replicate as fixed effects) and also including linear and quadratic regressor terms for JUV. These analyses of phenotypic selection on JUV were based on data from the provenances that were most variable for this focal trait (see ‘Data sets’ above), and used only the trees effectively contributing to a fitness component (Koenig et al., 1991), i.e. for SURV, all the scored individuals (N = 374); and for REPR, the survivors at age 20 years (N = 224). For each of these analyses, linear and quadratic logistic regression coefficients for JUV were obtained after centring the trait to have a mean of zero. Estimates of model parameters and associated standard errors were obtained by maximum likelihood estimation. Following a preliminary analysis that indicated no empirical support for heterogeneity of selection on JUV among provenances (Supplementary Data Methods S5), a two-tailed likelihood ratio test was used to assess the statistical significance of either the linear or quadratic logistic regression coefficient estimated for JUV. The quadratic logistic regression coefficient was not found to be statistically significant (P > 0.05) in the analysis of either SURV or REPR, possibly reflecting an insufficient sample size, and thus limited statistical power to enable detection of non-linear selection (i.e. requiring N > 500 to detect quadratic selection of a weak magnitude, as indicated by Kingsolver et al., 2001). Therefore, the quadratic term for JUV was not fitted in a final logistic regression model, and only estimates of selection gradients referring to directional selection are reported.
Janzen and Stern (1998) have outlined the advantages of using logistic regression in the context of the analysis of phenotypic selection mediated by a fitness component with only two measured categories. However, a logistic model does not yield regression coefficients that are quantitatively interpretable as selection gradients, and thus they cannot be applied to predict evolutionary responses to selection between generations (Brodie et al., 1995; Janzen and Stern, 1998), unlike the coefficients obtained by least-squares regression (Lande and Arnold, 1983). In this context, the linear logistic regression coefficient was transformed to obtain an approximate directional selection gradient, by calculating either an overall marginal effect of JUV [hereafter denoted as ] or a marginal effect of JUV by setting the value of this variable at its mean and averaging over provenance and replicate effects [hereafter denoted as ]. Details regarding these calculations and their interpretation are given in Supplementary Data Methods S6.
Under the applied final logistic regression model, the statistical significance detected for the untransformed linear logistic regression coefficient estimated for JUV provides an indication of the statistical significance for the corresponding transformations or . The standard error associated with a transformed linear logistic regression coefficient for JUV was approximated using the delta method. Selection gradients estimated by least-squares regression are based on the use of relative fitness (i.e. fitness values scaled to have a mean of one) as the response variable (Lande and Arnold, 1983), whereas a logistic regression model analyses the relationship between absolute fitness (i.e. 0 or 1) and the focal trait(s) (Janzen and Stern, 1998). Consequently, to obtain a directional selection gradient that has an interpretation comparable with a related estimate from the least-squares regression, a transformed linear logistic regression coefficient of JUV was subsequently multiplied by a constant equal to the inverse of the sample mean of the fitness component analysed, so that and refer to the relative fitness scale. The same constant was also multiplied by the approximate standard error of the transformed linear logistic regression coefficient of JUV.
The SAS procedures LOGISTIC and NLMIXED (SAS, 2015) were used to pursue the phenotypic selection analyses.
RESULTS
Provenance differences and relationship between vegetative juvenility and home-site climate
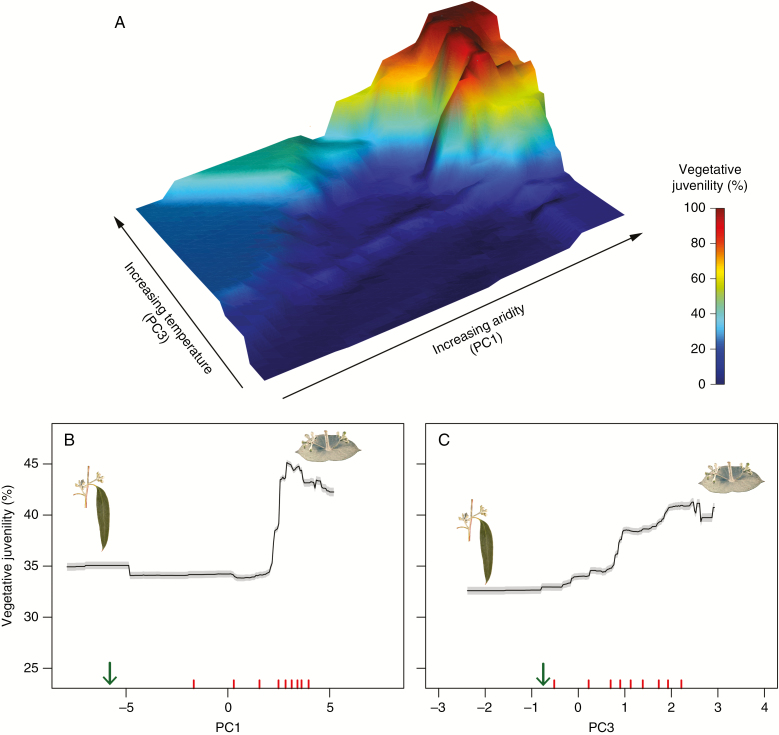
Vegetative juvenility (JUV) differed significantly (P < 0.001; Supplementary Data Table S3) among provenances at the trial site, with continuous variation in the provenance values for mean effects. These differences paralleled the continuous phenotypic variation observed among the wild populations (Fig. 1A). The modelling with the random forest algorithm showed that the variation among provenances for JUV at the trial site was significantly (P < 0.001) associated with variation in the provenance home-site contemporary climate [pseudo-r2 = 0.63, with a bootstrap 95 % confidence interval (CI) of 0.62–0.64]. The first climate principal component (PC1), which reflected an aridity gradient, contributed most to this association, followed by the third principal component (PC3), which reflected a general temperature increase (Supplementary Data Tables S2 and S4). Positive values on the aridity PC1 were associated with decreasing precipitation, increasing radiation and increasing maximum temperature of the warmest week (i.e. reflecting the risk of extreme high temperatures). There was a stepped cline in vegetative juvenility along this aridity gradient, with provenances that predominantly retain the juvenile foliage occurring on more arid home sites (Fig. 2B). In contrast, there was a more gradual cline in vegetative juvenility along PC3 (Fig. 2C). Increasing values of PC3 were associated with a general temperature increase across all seasons (i.e. TANN in Supplementary Data Table S2) at the provenance home site, rather than high temperature extremes (i.e. TMXWW in Table S2). When jointly considered, these climate principal components show that a stepped cline in JUV occurs where there is a combination of both high aridity and high temperatures (Fig. 2A).
Fig. 2.
Partial dependency plots of the random forest model showing the marginal effect (see Supplementary Data Methods S3 for the description of a marginal effect) of the climate principal components 1 (PC1) and 3 (PC3) on the provenance values for mean effects of vegetative juvenility in the examined common-garden field trial. Three- (A) and two-dimensional (B and C) plots are provided for visualization. For plots B and C, the green arrow shows the location of the trial site along each principal component. Red tick marks show the deciles of the data. The grey shading indicates the 95 % confidence intervals derived by iterative bootstrapping (1000 bootstraps). Increasing scores on PC1 correspond to increasing home-site aridity (high maximum summer temperatures, high radiation and low rainfall), and increasing scores on PC3 correspond to increasing home-site temperatures in general (see Supplementary Data Table S2).
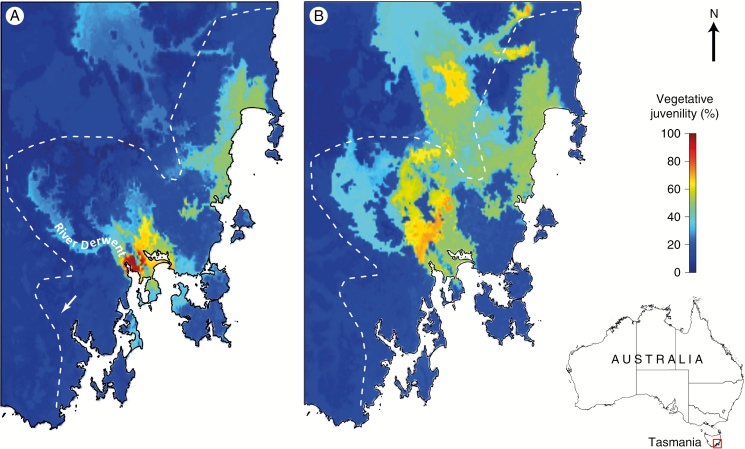
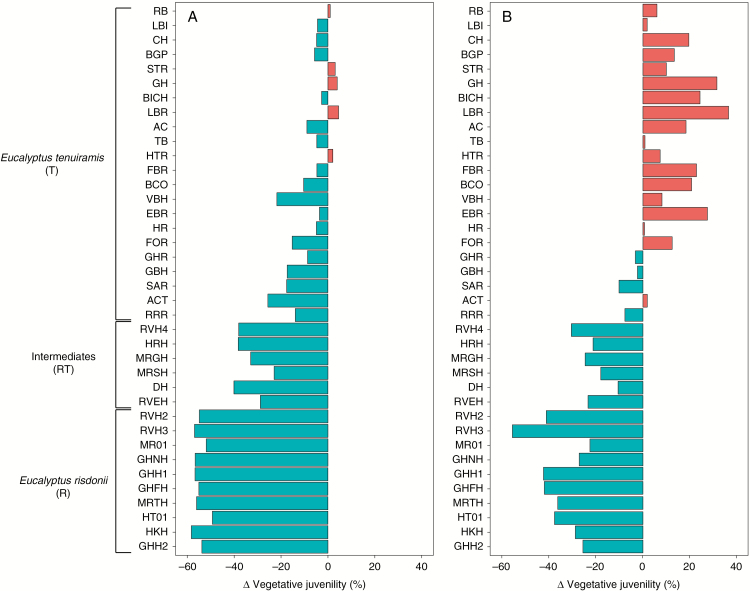
Based on the contemporary climate, the mapping of vegetative juvenility predicted from the developed random forest model shows that the stepped climatic cline is also evident spatially, with a concentration of the late phase change in the central part of the distribution, on the northern shores of the River Derwent (Fig. 3A). In this context, predicted values of vegetative juvenility >90 % were well matched with the current distribution of natural records classified as E. risdonii in the Natural Values Atlas. The 2080s-based forecasts for vegetative juvenility suggest that the steep climatic gradients associated with the present stepped cline between the E. risdonii (R) and E. tenuiramis (T) ontogenetic classes in this region will dissipate under climate change (Fig. 3B; see also Supplementary Data Fig. S3). In this sense, we forecast less vegetative juvenility for provenances of the R class, but more vegetative juvenility in a large portion of the current range of the T class (Figs 3B and 4B; see also Fig. S3).
Fig. 3.
Spatial variation in vegetative juvenility across south-eastern Tasmania, as obtained from the random forest model (see also Supplementary Data Table S4) using climate data based on: (A) the contemporary (1976–2005) BIOCLIM surfaces; and (B) BIOCLIM surfaces projected for 2070–2099 using the ECHAM global circulation model (see Supplementary Data Methods S4 and Fig. S3). Also shown is the location of the common-garden field trial (tip of the arrow head) and the River Derwent. The contemporary distribution limit of the E. risdonii–E. tenuiramis complex is depicted by the dotted white line. The inset map shows the geographic position of the expanded region in Tasmania.
Fig. 4.
Based on the applied random forest model (see Supplementary Data Table S4), the figure shows the differences (Δ) in the vegetative juvenility between: (A) the prediction for the common-garden trial site and the estimates for the provenance home sites, both obtained by using the climate data from the contemporary (1976–2005) BIOCLIM surfaces; and (B) the forecasts for the provenance home sites obtained by using the climate data from the BIOCLIM surfaces projected for 2070–2099 (on the basis of the ECHAM global circulation model; see Supplementary Data Methods S4 and Fig. S3) and the estimates for the provenance home sites obtained by using the climate data from the contemporary (1976–2005) BIOCLIM surfaces. Provenance ranking follows the description provided in the legend of Fig. 1.
As the common-garden trial is at the extreme cold, wet end of the range of the provenance home-site contemporary climates (Fig. 2B, C; see also Supplementary Data Fig. S1), the provenances with more vegetative juvenility would be expected to be poorly adapted under the climate conditions of the trial site (Fig. 4A). Statistically significant differences (P = 0.039; Supplementary Data Table S3) of mean effects among provenances were detected for adult survival (SURV) by age 20 years. As hypothesized, these differences among provenances were mainly associated with a greater probability of mortality for provenances with more vegetative juvenility (Fig. 1B). Indeed, based on testing pairwise contrasts between the ontogenetic classes, the main differences in SURV were observed between either the T or RT classes compared with the R class. These differences between class mean effects on the probability scale (hereafter denoted as Δ) were ΔT–R = 0.20 and ΔRT–R = 0.22 (P < 0.001). No statistically significant difference in SURV occurred between the RT and T classes (ΔRT–T = 0.02; P = 0.644).
The differences of mean effects among provenances were not found to be statistically significant (P = 0.110; Supplementary Data Table S3) for the reproductive capacity of the surviving trees (REPR) at age 20 years. However, there was a significant trend for the R and RT classes to have a higher probability of reproducing than the T class (ΔR–T = 0.29 and ΔRT–T = 0.18; P < 0.001 and P = 0.010, respectively). The difference in REPR between the R and RT classes was, however, not statistically significant (ΔR–RT = 0.11; P = 0.166).
Phenotypic selection on vegetative phase change
Using the sub-set of central provenances that maximized the within-provenance phenotypic diversity in JUV, we measured the effects of total directional selection on this focal trait at the trial site through SURV or REPR. Consistent with directional selection, the within-provenance linear logistic regression coefficient estimated for JUV indicated statistically significant (P < 0.05) fitness consequences of variation in JUV acting via SURV and REPR, although a highly significant (P < 0.001) effect was evident for SURV only (Table 1). As shown in Table 1, for either SURV or REPR, there was a small difference between the two approximate estimates obtained for the total directional selection gradient [i.e. and ]. This indicates a reduced influence of individuals with extreme predicted probabilities on the shape of the expected fitness curve, thus suggesting a reasonable overlap in the phenotypic distributions of JUV for survivors and non-survivors or reproductive and non-reproductive trees. The estimated directional selection gradients were negative for both fitness components, indicating that total phenotypic selection over the 15-year period following the JUV phenotyping favoured individuals within provenances exhibiting less vegetative juvenility. For example, considering SURV (i.e. the most significant fitness component), = –0.0084 indicates that a decrease of 1 % (or 10 %) in vegetative juvenility will increase, on average, the relative fitness by 0.84 % (or 8.4 %) (Table 1). This result is expected from the trial site climate (Fig. 2B, C), and is consistent with the general trend observed among provenances for the probability of surviving (Fig. 1B).
Table 1.
Estimates of directional selection gradients concerning total phenotypic selection acting on vegetative juvenility (JUV) via adult survival (SURV) or reproductive capacity (REPR).
| Fitness component | N | LRT | P-value | ||
|---|---|---|---|---|---|
| SURV | 374 | –0.0084 ± 0.0020 | –0.0099 ± 0.0024 | 18.52 | <0.001 |
| REPR | 224 | –0.0045 ± 0.0023 | –0.0058 ± 0.0029 | 3.87 | 0.049 |
N is the number of observations used in the data analysis.
For a given fitness component, the linear logistic regression coefficient estimated for JUV was transformed to obtain the following two approximate estimates for the total directional selection gradient: , from computing an overall marginal effect of JUV; and , from calculating a marginal effect of JUV by setting the value of this variable at its mean and averaging over provenance and replicate effects (see Supplementary Data Methods S6). The directional selection gradient estimates (and associated approximate standard errors) presented in the table refer to the relative fitness scale, so that they have an interpretation comparable with related estimates from least-squares regression (i.e. as described by Lande and Arnold, 1983).
For either SURV or REPR, LRT and P-value pertain to the test statistic and significance probability, respectively, corresponding to a two-tailed likelihood ratio test that was conducted to assess the statistical significance of the linear logistic regression coefficient estimated for JUV. In this context, the statistical significance detected for the untransformed linear logistic regression coefficient also provides an indication of the statistical significance for the corresponding transformations or . Wald F-tests, also applied for the same purpose, provided similar significance results (not shown).
DISCUSSION
A marked aridity gradient, defined by increasing maximum summer temperatures (e.g. maximum temperature of the warmest week), increasing radiation (e.g. annual mean radiation) and decreasing rainfall (e.g. precipitation of the coldest quarter) is shown to underlie the genetic-based, heterochronic cline in the E. risdonii–E. tenuriamis complex. The provenances with more vegetative juvenility are confined to a localized area of high aridity in the centre of the geographic distribution of the complex. The reduced survival of the more arid-derived provenances with more vegetative juvenility when grown at the mesic common-garden site argues for some degree of local adaptation. While other traits also vary along the cline (Wiltshire et al., 1991, 1992), when provenances were translocated to a mesic site, the phenotypic selection analysis showed significant total directional selection occurring within provenances on vegetative juvenility, with the corresponding selection gradient indicating that phenotypes with less vegetative juvenility were favoured. While this is evident from the highly significant directional selection gradient acting on vegetative juvenility via adult survival, it is less significant when acting through reproductive capacity (Table 1), and the possibility that trade-offs between growth and reproduction may result in greater future survival of the less reproductive individuals cannot be dismissed. In addition, our evidence for the adaptive value of vegetative juvenility would have been enhanced with a common-garden field trial established in a more arid environment (Blanquart et al., 2013), and with replication of mesic and arid environments (Costa e Silva et al., 2006). In the heteroblastic species Eucalyptus globulus, which is widely grown in plantations in temperate regions of the world, different patterns of genetic correlation between diameter growth and the onset of phase change across sites are also consistent with early transition to adult foliage being advantageous on more mesic sites (Jordan et al., 2000). However, the ecological conditions favouring early phase change are likely to be complex and varied as, for example, precocious ecotypes of E. globulus are also favoured in stressful, wind-exposed coastal areas (Jordan et al., 2000; Hudson et al., 2014).
The stepped nature of the present heterochronic cline in vegetative juvenility with respect to the aridity gradient could arise due to several factors, including an equilibrium condition being reached under a balanced selection/gene flow gradient model (Endler, 1977), steepening of environmental gradients in unmeasured factors such as reduced soil depth enhancing water stress (Harper et al., 2009), or even the co-occurrence of heat and water stress creating an adaptation threshold (Matusick et al., 2013; Mitchell et al., 2014). In the latter case, both summer maximum temperatures and winter rainfall are key predictors of the provenance variation in drought susceptibility in E. globulus (Dutkowski and Potts, 2012), with winter rainfall in these Mediterranean climates probably being a determinant of the potential of the soil water profile to be recharged. Aridity is a major selective gradient shaping provenance divergence in forest tree species, with adaptive changes reported to be genome wide (Steane et al., 2017) and multitrait (Moran et al., 2017). Such adaptation has been associated with constitutive changes in anatomy (David-Schwartz et al., 2016), morphology (Ramirez-Valiente et al., 2009) and physiology (Bradbury et al., 2013) within life history stages; differential phenotypic plasticity (Corcuera et al., 2012; McLean et al., 2013; Ramirez-Valiente et al., 2015a; Martin-Sanz et al., 2016; Ramirez-Valiente and Cavender-Bares, 2017); changes in resource allocation (Li et al., 2000; Ramirez-Valiente et al., 2017), including leaf abscission (Ramirez-Valiente and Cavender-Bares, 2017); and, as suggested by the present and other studies (Climent et al., 2006; Gauli et al., 2015), heterochrony. However, in the case of forest trees, evidence that such changes represent specific functional adaptations is usually dependent on mechanistic interpretations (e.g. traits affecting cavitation resistance; Plomion et al., 2016) and rarely are the fitness gradients associated with specific traits quantified under field conditions (Ramirez-Valiente et al., 2014).
In the case of common-garden trials involving provenance translocations (Franks et al., 2014), there may be multiple traits which differ among provenances (Wiltshire et al., 1991, 1992, 1998; Gauli et al., 2015). In these cases, associations between fitness and functional traits may simply arise through selective covariance (Armbruster and Schwaegerle, 1996) rather than direct causality. By including the fixed effects of provenance as a categorical predictor in the model, our phenotypic selection analysis provided a measure of the fitness consequences of variation in vegetative juvenility that did not confound provenance mean differences in survival or reproductive capacity and variation among individual trees that relates more to a causal link between the focal trait and a fitness component (Heisler and Damuth, 1987; Campbell, 1991). We detected a weak but significant linear component to selection within provenances on vegetative juvenility – signalling directional selection – for both survival and reproductive capacity in favour of phenotypes with less vegetative juvenility. However, we cannot discard the possibility that sample size may have precluded the detection of a non-linear component to selection, particularly for quadratic selection of small magnitude (Kingsolver et al., 2001). Regardless, the single-trait approach that measured total selection on vegetative juvenility is unable to differentiate direct selection on this trait and selection acting indirectly through other (unmeasured) characters, which may be phenotypically correlated with the focal trait. Modelling simultaneously the fitness consequences of variation in multiple traits, chosen to represent the actual targets of selection, allows a distinction to be made between direct and indirect selection acting on them (e.g. by the estimation of partial regression coefficients). Nevertheless, under this multiple trait approach, the possibility that selection is occurring on an observed trait via selection acting on other unmeasured character(s) cannot be dismissed (Lande and Arnold, 1983; Mitchell-Olds and Shaw, 1987). In addition, the form, strength and direction of selection acting on a focal trait may vary with environment (Wade and Kalisz, 1990; Garant et al., 2007; Lau et al., 2014), season (Ramirez-Valiente et al., 2015b) and life history stage (Arnold and Wade, 1984; Kalisz, 1986; Kingsolver et al., 2001; McGlothlin et al., 2005), as well as be mediated through indirect pathways (Lau et al., 2014).
What physiological and anatomical differences between the adult and juvenile leaves could explain the observed fitness impacts of vegetative juvenility in the E. risdonii–E. tenuiramis complex? In other eucalypt species, adult leaves have thicker cuticles and lower specific leaf area than juvenile leaves (Johnson, 1926; James and Bell, 2001; Gras et al., 2005), suggesting better adaptation to water stress, and a role for the juvenile leaves in shaded or more mesic environments. In E. risdonii and E. tenuiramis, however, it is the juvenile leaf forms that are thicker than the adult, especially the epidermal and cuticular layers (Wiltshire, 1991). Their juvenile leaves also have a more isobilateral distribution of photosynthetic tissue and stomata (Wiltshire, 1991) than, for example, the juvenile leaves of the wet sclerophyll species E. fastigata (Cameron, 1970b). The juvenile phase of the dry sclerophyll (open forest) species E. tenuiramis does not appear to be a shade-bearing phase (Wiltshire, 1991), and reduced light interception afforded by the connate leaf arrangement and the glaucous leaves with thickened epidermal layers in the E. risdonii–E. tenuiramis complex may be a factor favouring the persistence of juvenile leaves in the drier, hotter environments due to reduced risk of photoinhibition and heat stress (Ogren and Evans, 1992; Huggins et al., 2018). Certainly, selection to reduce light interception in open environments appears to have been a major factor driving the evolution of differences in many leaf traits among species of the genus Banksia, another major Australian plant genus (Jordan et al., 2005). Other possible advantages of retaining the opposite, sessile and connate juvenile leaf pairs in the more arid environment may relate to better hydraulic properties of the sessile leaves due, for example, to the recently demonstrated greater vulnerability of the petiole to cavitation relative to the stem (Hochberg et al., 2016). In the present case, an eco-physiological mechanism for selection against phenotypes with more vegetative juvenility at the studied cooler, mesic site could be a reduced light interception for photosynthesis due to the waxy glaucousness on the leaf surface (Cameron, 1970a; Close et al., 2007), absence of a petiole preventing ready optimization of leaf orientation (James and Bell, 2000, 2001) and a consequent slower growth rate.
While based on provenance values for mean effects at a mesic site, the extrapolation of the variation in vegetative juvenility across the geographic range of the complex was a reasonable approach to study spatial variation using contemporary or projected climate surfaces, because: (1) the timing of vegetative phase change is under strong genetic control and exhibits little genotype by environment interaction at the provenance level (Hamilton et al., 2011); and (2) the provenance variation in the field trial was strongly correlated with the phenotypic variation observed among wild populations (Wiltshire et al., 1998; see also Fig. 1A). Accordingly, based on the spatial locations studied, we argue that the discrepancies between the contemporary predictions and 2080s forecasts (Fig. 3A, B) are a signal of: (1) changing selection on vegetative juvenility across the geographic range; and (2) variation in the risk of maladaptation of the current generation of trees. Based on the 2080s forecasts, less vegetative juvenility will be favoured in the main core of the E. risdonii (Fig. 4B), reflecting a more mesic climate for a large part of its distribution (Fig. 3B). However, the change towards a less arid climate for the future habitat of the E. risdonii is projected to be less extreme (Fig. 3B) than the difference in contemporary climate involving the translocation of E. risdonii provenances to the trial site (Fig. 3A). Thus, for E. risdonii populations in the wild, selection against vegetative juvenility is likely to be weaker by the 2080s than that currently observed in the trial site (Fig. 4A). In contrast, while translocation of provenances to an arid site was not tested, a large part of the E. tenuiramis range is forecast to become more arid by the 2080s (Fig. 3B), which will favour a greater vegetative juvenility for most of the sampled provenances from this ontogenetic class (Fig. 4B). Such differential impacts of global climate change on selection are expected across species’ ranges (Etterson, 2004; Ramirez-Valiente et al., 2009; Alberto et al., 2013; Drake et al., 2015).
As we forecasted for E. tenuiramis, global climate change is expected to increase aridity across a significant component of the eucalypt woodlands and forests in Australia (Butt et al., 2013). The present study suggests that vegetative juvenility is an adaptive trait, and it is likely to be part of the strategy that eucalypts use to respond to such change in two ways. First, at the individual level, there is phenotypic plasticity in vegetative phase change itself. Most eucalypt species generally exhibit at least some form of vegetative phase change during their development, and often revert to earlier ontogenetic stages when resprouting from lignotubers or stems following the damage of above-ground parts through, for example, fire or drought (Pryor, 1976). Secondly, there is a genetic basis of population-level adaptation through heterochrony. The ontogenetic timing of the heteroblastic transition in eucalypts is also heritable (Wiltshire et al., 1998; Jordan et al., 1999; Hamilton et al., 2011), meaning that selection gradients acting on the phenotypic variation in vegetative phase change will be expected to elicit heterochronic evolution. Together, these processes provide a pathway for species to respond to rapid environmental change through exploitation of their embodied ontogenetic diversity. The general roles of phenotypic plasticity, genetic adaptation and hybridization as mechanisms allowing the in situ persistence of populations in the face of global climate change are well recognized (Nicotra et al., 2010; Hoffmann and Sgro, 2011; Alberto et al., 2013; Franks et al., 2014; Janes and Hamilton, 2017). Given the pace of global climate change relative to the generation intervals and dispersal capacity of many species (Alberto et al., 2013; Franks et al., 2014), the present study highlights developmental plasticity (Diggle, 2002) and heterochrony (Williams et al., 2015) as underappreciated processes which can contribute to populations of long-lived organisms, such as trees, persisting and ultimately adapting to environmental change.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Methods S1: provenance differences – analysis of SURV and REPR. Methods S2: provenance differences – analysis of JUV. Methods S3: details of the random forest procedure used to model the relationship between vegetative juvenility and home-site climate. Methods S4: details of the global circulation models used to project the 2080s climate. Methods S5: phenotypic selection analysis – heterogeneity of selection among provenances. Methods S6: phenotypic selection analysis – calculating marginal effects of JUV. Figure S1: principal components analysis of the bioclimatic variables considered to model the relationship between vegetative juvenility and home-site climate. Figure S2: scree plot for the principal component analysis based on the correlation matrix of the 27 bioclimatic variables considered to model the relationship between vegetative juvenility and home-site climate. Figure S3: forecasted spatial variation in vegetative juvenility, as obtained from the random forest model, across south-eastern Tasmania using BIOCLIM climate surfaces projected for 2070–2099 (2080s) by each of the six global circulation models detailed in Methods S4 and Table S5 under the A2 emission scenario. Table S1: geographic location and climatic details of provenance home sites and the trial site. Table S2: Eigenvectors associated with the first four principal components retained from a principal component analysis based on the correlation matrix of the 27 bioclimatic variables considered to model the relationship between vegetative juvenility and home-site climate. Table S3: results from Wald F-tests conducted to assess whether differences of mean effects were statistically significant among provenances or replicates for vegetative juvenility (JUV), adult survival (SURV) or reproductive capacity (REPR). Table S4: importance of the independent variables in the random forest model. Table S5: details of the six global circulation models used in the current study, showing the contemporary (1976–2005) mean annual temperature (TANN, °C) and mean annual precipitation (RANN, mm) for Tasmania, and the projected mean change in TANN and percentage change in RANN from each global circulation model by the 2080s (2070–2099).
ACKNOWLEDGEMENTS
The contribution of João Costa e Silva to this work was supported by Fundação para a Ciência e a Tecnologia I.P. (FCT), Portugal, through the Programa Operacional Potencial Humano and the European Social Fund. The Centro de Estudos Florestais is a research unit funded by FCT [UID/AGR/00239/2013]. Writing up of this work was also supported by an Australian Research Council (ARC) Discovery Grant [DP160101650] awarded to Brad M Potts. We are thus grateful to the ARC and FCT for their financial support, which provided the opportunity to complete this study. The field trial was established on land owned by Forestry Tasmania (now Sustainable Timber Tasmania), and the contributions of Greening Australia and Forestry Tasmania to the trial establishment are gratefully acknowledged. We thank Paul Tilyard for assistance with data management and the many people who assisted with trial assessment.
Conflicts of Interest: The authors have no conflicts of interest.
LITERATURE CITED
- Alberto FJ, Aitken SN, Alía R, et al. . 2013. Potential for evolutionary responses to climate change – evidence from tree populations. Global Change Biology 19: 1645–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster WS, Schwaegerle KE. 1996. Causes of covariation of phenotypic traits among populations. Journal of Evolutionary Biology 9: 261–276. [Google Scholar]
- Arnold SJ, Wade MJ. 1984. On the measurement of natural and sexual selection: applications. Evolution 38: 720–734. [DOI] [PubMed] [Google Scholar]
- Balmelli G, Simeto S, Torres D, Castillo A, Altier N, Diez JJ. 2014. Susceptibility to Teratosphaeria nubilosa and precocity of vegetative phase change in Eucalyptus globulus and E. maidenii (Myrtaceae). Australian Journal of Botany 61: 583–591. [Google Scholar]
- Billingsley P. 1986. Probability and measure. New York: John Wiley & Sons, Inc. [Google Scholar]
- Blanquart F, Kaltz O, Nuismer SL, Gandon S. 2013. A practical guide to measuring local adaptation. Ecology Letters 16: 1195–1205. [DOI] [PubMed] [Google Scholar]
- Borzak CL, Potts BM, Davies NW, O’Reilly-Wapstra JM. 2015. Population divergence in the ontogenetic trajectories of foliar terpenes of a Eucalyptus species. Annals of Botany 115: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury D, Smithson A, Krauss SL. 2013. Signatures of diversifying selection at EST-SSR loci and association with climate in natural Eucalyptus populations. Molecular Ecology 22: 5112–5129. [DOI] [PubMed] [Google Scholar]
- Breiman L. 2001. Random forests. Machine Learning 45: 5–32. [Google Scholar]
- Brodie ED, Moore AJ, Janzen FJ. 1995. Visualizing and quantifying natural selection. Trends in Ecology and Evolution 10: 313–318. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Varkonyi-Gasic E, Jones RC. 2016. Phase change and phenology in trees. In: Groover A, Cronk Q, eds. Comparative and evolutionary genomics of angiosperm trees. Plant genetics and genomics: crops and models, Vol. 21 Cham: Springer, 227–274. [Google Scholar]
- Butt N, Pollock LJ, McAlpine CA. 2013. Eucalypts face increasing climate stress. Ecology and Evolution 3: 5011–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. 1970a Light intensity and the growth of Eucalyptus seedlings. II. The effect of cuticular waxes on light absorption in leaves of Eucalyptus species. Australian Journal of Botany 18: 275–284. [Google Scholar]
- Cameron RJ. 1970b Light intensity and the growth of Eucalyptus seedlings I. Ontogenetic variation in E. fastigata. Australian Journal of Botany 18: 29–43. [Google Scholar]
- Campbell DR. 1991. Effects of floral traits on sequential components of fitness in Ipomopsis aggregata. American Naturalist 137: 713–737. [Google Scholar]
- Chenoweth SF, Rundle HD, Blows MW. 2010. The contribution of selection and genetic constraints to phenotypic divergence. American Naturalist 175: 186–196. [DOI] [PubMed] [Google Scholar]
- Climent J, Chambel MR, Lopez R, Mutke S, Alia R, Gil L. 2006. Population divergence for heteroblasty in the Canary Island pine (Pinus canariensis, Pinaceae). American Journal of Botany 93: 840–848. [DOI] [PubMed] [Google Scholar]
- Close DC, Davidson NJ, Shields CB, Wiltshire R. 2007. Reflectance and phenolics of green and glaucous leaves of Eucalyptus urnigera. Australian Journal of Botany 55: 561–567. [Google Scholar]
- Corcuera L, Gil-Pelegrin E, Notivol E. 2012. Aridity promotes differences in proline and phytohormone levels in Pinus pinaster populations from contrasting environments. Trees 26: 799–808. [Google Scholar]
- Corney S, Katzfey J, McGregor J, et al. . 2010. Climate futures for Tasmania: climate modelling technical report. Hobart, Tasmania: Antartic Climate & Ecosystem CRC. [Google Scholar]
- Costa e Silva J, Potts BM, Dutkowski G. 2006. Genotype by environment interaction for growth of Eucalyptus globulus in Australia. Tree Genetics and Genomes 2: 61–75. [Google Scholar]
- Cox C. 1998. Delta method. In: Armitage P, Colton T, eds. Encyclopedia of biostatistics. New York: John Wiley & Sons, Inc, 1125–1127. [Google Scholar]
- David-Schwartz R, Paudel I, Mizrachi M, et al. . 2016. Indirect evidence for genetic differentiation in vulnerability to embolism in Pinus halepensis. Frontiers in Plant Science 7: 768. doi: 10.3389/fpls.2016.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle PK. 2002. A developmental morphologist’s perspective on plasticity. Evolutionary Ecology 16: 267–283. [Google Scholar]
- Drake JE, Aspinwall MJ, Pfautsch S, et al. . 2015. The capacity to cope with climate warming declines from temperate to tropical latitudes in two widely distributed Eucalyptus species. Global Change Biology 21: 459–472. [DOI] [PubMed] [Google Scholar]
- Dutkowski GW, Potts BM. 2012. Genetic variation in the susceptibility of Eucalyptus globulus to drought damage. Tree Genetics and Genomes 8: 757–773. [Google Scholar]
- Endler JA. 1977. Geographical variation, speciation and clines. Princeton, NJ: Princeton University Press. [Google Scholar]
- Endler JA. 1986. Natural selection in the wild. Princeton, NJ: Princeton University Press. [Google Scholar]
- Etterson JR. 2004. Evolutionary potential of Chamaecrista fasciculata in relation to climate change. I. Clinal patterns of selection along an environmental gradient in the Great Plains. Evolution 58: 1446–1456. [DOI] [PubMed] [Google Scholar]
- Fernández-Mazuecos M, Glover BJ. 2017. The evo-devo of plant speciation. Nature Ecology and Evolution 1: 0110. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Cribari-Neto F. 2004. Beta regression for modelling rates and proportions. Journal of Applied Statistics 31: 799–815. [Google Scholar]
- Franks SJ, Weis AE. 2008. A change in climate causes rapid evolution of multiple life-history traits and their interactions in an annual plant. Journal of Evolutionary Biology 21: 1321–1334. [DOI] [PubMed] [Google Scholar]
- Franks SJ, Weber JJ, Aitken SN. 2014. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evolutionary Applications 7: 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garant D, Kruuk LEB, McCleery RH, Sheldon BC. 2007. The effects of environmental heterogeneity on multivariate selection on reproductive traits in female great tits. Evolution 61: 1546–1559. [DOI] [PubMed] [Google Scholar]
- Gauli A, Vaillancourt RE, Bailey TG, Steane DA, Potts BM. 2015. Evidence for local climate adaptation in early-life traits of Tasmanian populations of Eucalyptus pauciflora. Tree Genetics and Genomes 11: 104. [Google Scholar]
- Givnish TJ. 1987. Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytologist 106: 131–160. [Google Scholar]
- Goldschmidt R. 1940. The material basis of evolution. New Haven, CT: Yale University Press. [Google Scholar]
- Goodger JQD, Heskes AM, Woodrow IE. 2013. Contrasting ontogenetic trajectories for phenolic and terpenoid defences in Eucalyptus froggattii. Annals of Botany 112: 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. 1977. Ontogeny and phylogeny. Cambridge, MA: Harvard University Press. [Google Scholar]
- Gras EK, Read J, Mach CT, Sanson GD, Clissold FJ. 2005. Herbivore damage, resource richness and putative defences in juvenile versus adult Eucalyptus leaves. Australian Journal of Botany 53: 33–44. [Google Scholar]
- Guerrant E., Jr 1988. Heterochrony in plants: the intersection of evolution, ecology and ontogeny. In: McKinney M, ed. Heterochrony in evolution. New York: Plenum Press, 111–133. [Google Scholar]
- Hamilton MG, Tilyard PA, Williams DR, Vaillancourt RE, Wardlaw TJ, Potts BM. 2011. The genetic variation in the timing of heteroblastic transition in Eucalyptus globulus is stable across environments. Australian Journal of Botany 59: 170–175. [Google Scholar]
- Harper RJ, Smettem KRJ, Carter JO, McGrath JF. 2009. Drought deaths in Eucalyptus globulus (Labill.) plantations in relation to soils, geomorphology and climate. Plant and Soil 324: 199–207. [Google Scholar]
- Harrison PA, Vaillancourt RE, Harris RMB, Potts BM. 2017. Integrating climate change and habitat fragmentation to identify candidate seed sources for ecological restoration. Restoration Ecology 25: 524–531. [Google Scholar]
- Hasbun R, Iturra C, Bravo S, Rebolledo-Jaramillo B, Valledor L. 2016. Differential methylation of genomic regions associated with heteroblasty detected by M&M algorithm in the nonmodel species Eucalyptus globulus Labill. International Journal of Genomics 2016: 4395153. doi: 10.1155/2016/4395153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler I, Damuth J. 1987. A method for analyzing selection in hierarchically structured populations. American Naturalist 130: 582–602. [Google Scholar]
- Hochberg U, Albuquerque C, Rachmilevitch S, et al. . 2016. Grapevine petioles are more sensitive to drought induced embolism than stems: evidence from in vivo MRI and microcomputed tomography observations of hydraulic vulnerability segmentation. Plant, Cell and Environment 39: 1886–1894. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Sgro CM. 2011. Climate change and evolutionary adaptation. Nature 470: 479–485. [DOI] [PubMed] [Google Scholar]
- Hudson CJ, Freeman JS, Jones RC, et al. . 2014. Genetic control of heterochrony in Eucalyptus globulus. G3-Genes Genomes Genetics 4: 1235–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins TD, Mohammed S, Sengodon P, Ibrahim AMH, Tilley M, Hays DB. 2018. Changes in leaf epicuticular wax load and its effect on leaf temperature and physiological traits in wheat cultivars (Triticum aestivum L.) exposed to high temperatures during anthesis. Journal of Agronomy and Crop Science 204: 49–61. [Google Scholar]
- James SA, Bell DT. 2000. Influence of light availability on leaf structure and growth of two Eucalyptus globulus ssp globulus provenances. Tree Physiology 20: 1007–1018. [DOI] [PubMed] [Google Scholar]
- James SA, Bell DT. 2001. Leaf morphological and anatomical characteristics of heteroblastic Eucalyptus globulus ssp globulus (Myrtaceae). Australian Journal of Botany 49: 259–269. [Google Scholar]
- James SA, Smith WK, Vogelmann TC. 1999. Ontogenetic differences in mesophyll structure and chlorophyll distribution in Eucalyptus globulus ssp. globulus (Myrtaceae). American Journal of Botany 86: 198–207. [PubMed] [Google Scholar]
- Janes J, Hamilton J. 2017. Mixing it up: the role of hybridization in forest management and conservation under climate change. Forests 8: 237. [Google Scholar]
- Janzen FJ, Stern HS. 1998. Logistic regression for empirical studies of multivariate selection. Evolution 52: 1564–1571. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Correa JP, Prunier J, Vázquez-Lobo A, Keller SR, Moreno-Letelier A. 2015. Molecular signatures of adaptation and selection in forest trees. In: Plomion C, Adam-Blondon A-F, eds. Advances in botanical research, Vol. 74 Academic Press, 265–306. [Google Scholar]
- Jaya E, Kubien DS, Jameson PE, Clemens J. 2010. Vegetative phase change and photosynthesis in Eucalyptus occidentalis: architectural simplification prolongs juvenile traits. Tree Physiology 30: 393–403. [DOI] [PubMed] [Google Scholar]
- Johnson ED. 1926. A comparison of the juvenile and adult leaves of Eucalyptus globulus. New Phytologist 26: 202–212. [Google Scholar]
- Jordan G, Potts BM, Wiltshire R. 1999. Strong, independent quantitative genetic control of vegetative phase change and first flowering in Eucalyptus globulus ssp. globulus. Heredity 83: 179–187. [DOI] [PubMed] [Google Scholar]
- Jordan GJ, Potts BM, Chalmers P, Wiltshire RJE. 2000. Quantitative genetic evidence that the timing of vegetative phase change in Eucalyptus globulus ssp globulus is an adaptive trait. Australian Journal of Botany 48: 561–567. [Google Scholar]
- Jordan GJ, Dillon RA, Weston PH. 2005. Solar radiation as a factor in the evolution of scleromorphic leaf anatomy in Proteaceae. American Journal of Botany 92: 789–796. [DOI] [PubMed] [Google Scholar]
- Kalisz S. 1986. Variable selection on the timing of germination in Collinsia verna (Scrophulariaceae). Evolution 40: 479–491. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Hoekstra HE, Hoekstra JM, et al. . 2001. The strength of phenotypic selection in natural populations. American Naturalist 157: 245–261. [DOI] [PubMed] [Google Scholar]
- Koenig WD, Albano SS, Dickinson JL. 1991. A comparison of methods to partition selection acting via components of fitness: do larger male bullfrogs have greater hatching success?Journal of Evolutionary Biology 4: 309–320. [Google Scholar]
- Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37: 1210–1226. [DOI] [PubMed] [Google Scholar]
- Lau JA, Shaw RG, Reich PB, Tiffin P. 2014. Indirect effects drive evolutionary responses to global change. New Phytologist 201: 335–343. [DOI] [PubMed] [Google Scholar]
- Lawrence R, Potts BM, Whitham TG. 2003. Relative importance of plant ontogeny, host genetic variation, and leaf age for a common herbivore. Ecology 84: 1171–1178. [Google Scholar]
- Li CY, Berninger F, Koskela J, Sonninen E. 2000. Drought responses of Eucalyptus microtheca provenances depend on seasonality of rainfall in their place of origin. Australian Journal of Plant Physiology 27: 231–238. [Google Scholar]
- Li P, Johnston MO. 2000. Heterochrony in plant evolutionary studies through the Twentieth Century. Botanical Review 66: 57–88. [Google Scholar]
- Liaw A, Wiener M. 2002. Classification and regression by randomForest. R News 2: 18–22. [Google Scholar]
- Loney PE, McArthur C, Potts BM, Jordan GJ. 2006. How does ontogeny in a Eucalyptus species affect patterns of herbivory by Brushtail Possums?Functional Ecology 20: 982–988. [Google Scholar]
- Maherali H, Caruso CM, Sherrard ME. 2009. The adaptive significance of ontogenetic changes in physiology: a test in Avena barbata. New Phytologist 183: 908–918. [DOI] [PubMed] [Google Scholar]
- Martin-Sanz RC, Santos-Del-Blanco L, Notivol E, Chambel MR, San-Martin R, Climent J. 2016. Disentangling plasticity of serotiny, a key adaptive trait in a Mediterranean conifer. American Journal of Botany 103: 1582–1591. [DOI] [PubMed] [Google Scholar]
- Matusick G, Ruthrof KX, Brouwers NC, Dell B, Hardy GSJ. 2013. Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. European Journal of Forest Research 132: 497–510. [Google Scholar]
- McGlothlin JW, Parker PG, Nolan V, Ketterson ED, Benkman C. 2005. Correlational selection leads to genetic integration of body size and an attractive plumage trait in dark-eyed juncos. Evolution 59: 658–671. [PubMed] [Google Scholar]
- McKinney M, McNamara K. 1991. Heterochrony: the evolution of ontogeny. New York: Plenum Press. [Google Scholar]
- McLean EH, Prober SM, Stock WD, et al. . 2013. Plasticity of functional traits varies clinally along a rainfall gradient in Eucalyptus tricarpa. Plant, Cell and Environment 37: 1440–1451. [DOI] [PubMed] [Google Scholar]
- McNamara KJ. 2012. Heterochrony: the evolution of development. Evolution: Education and Outreach 5: 203–218. [Google Scholar]
- Mitchell PJ, O’Grady AP, Hayes KR, Pinkard EA. 2014. Exposure of trees to drought-induced die-off is defined by a common climatic threshold across different vegetation types. Ecology and Evolution 4: 1088–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell-Olds T, Shaw RG. 1987. Regression analysis of natural selection: statistical-inference and biological interpretation. Evolution 41: 1149–1161. [DOI] [PubMed] [Google Scholar]
- Moran E, Lauder J, Musser C, Stathos A, Shu M. 2017. The genetics of drought tolerance in conifers. New Phytologist 216: 1034–1048. [DOI] [PubMed] [Google Scholar]
- Nahrung HF, Allen GR. 2003. Intra-plant host selection, oviposition preference and larval survival of Chrysophtharta agricola (Chapuis) (Coleoptera: Chrysomelidae: Paropsini) between foliage types of a heterophyllous host. Agricultural and Forest Entomology 5: 155–162. [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. . 2010. Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15: 684. [DOI] [PubMed] [Google Scholar]
- Ogren E, Evans JR. 1992. Photoinhibition of photosynthesis in situ in six species of Eucalyptus. Australian Journal of Plant Physiology 19: 223–232. [Google Scholar]
- Park RF, Keane PJ. 1982. Leaf diseases of Eucalyptus associated with Mycosphaerella species. Transactions of the British Mycological Society 79: 101–115. [Google Scholar]
- Petit RJ, Hampe A. 2006. Some evolutionary consequences of being a tree. Annual Review of Ecology, Evolution, and Systematics 37: 187–214. [Google Scholar]
- Plomion C, Bartholomé J, Bouffier L, et al. . 2016. Understanding the genetic bases of adaptation to soil water deficit in trees through the examination of water use efficiency and cavitation resistance: maritime pine as a case study. Journal of Plant Hydraulics 3: e008. doi: 10.20870/jph.2016.e008. [Google Scholar]
- Potts BM, Wiltshire RJE. 1997. Eucalypt genetics and genecology. In: Williams J, Woinarski J, eds. Eucalypt ecology: individuals to ecosystems. Cambridge: Cambridge University Press, 56–91. [Google Scholar]
- Pryor LD. 1976. Biology of eucalypts. Studies in biology. London: Edward Arnold. [Google Scholar]
- Ramirez-Valiente JA, Cavender-Bares J. 2017. Evolutionary trade-offs between drought resistance mechanisms across a precipitation gradient in a seasonally dry tropical oak (Quercus oleoides). Tree Physiology 37: 889–901. [DOI] [PubMed] [Google Scholar]
- Ramirez-Valiente JA, Valladares F, Gil L, Aranda I. 2009. Population differences in juvenile survival under increasing drought are mediated by seed size in cork oak (Quercus suber L.). Forest Ecology and Management 257: 1676–1683. [Google Scholar]
- Ramirez-Valiente JA, Valladares F, Sanchez-Gomez D, Delgado A, Aranda I. 2014. Population variation and natural selection on leaf traits in cork oak throughout its distribution range. Acta Oecologica-International Journal of Ecology 58: 49–56. [Google Scholar]
- Ramirez-Valiente JA, Koehler K, Cavender-Bares J. 2015a Climatic origins predict variation in photoprotective leaf pigments in response to drought and low temperatures in live oaks (Quercus series Virentes). Tree Physiology 35: 521–534. [DOI] [PubMed] [Google Scholar]
- Ramirez-Valiente JA, Valladares F, Delgado A, Nicotra AB, Aranda I. 2015b Understanding the importance of intrapopulation functional variability and phenotypic plasticity in Quercus suber. Tree Genetics and Genomes 11: 35. [Google Scholar]
- Ramirez-Valiente JA, Center A, Sparks JP, et al. . 2017. Population-level differentiation in growth rates and leaf traits in seedlings of the neotropical Live Oak Quercus oleoides grown under natural and manipulated precipitation regimes. Frontiers in Plant Science 8: 585. doi: 10.3389/fpls.2017.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausher MD. 1992. The measurement of selection on quantitative traits – biases due to environmental covariances between traits and fitness. Evolution 46: 616–626. [DOI] [PubMed] [Google Scholar]
- Santos-del-Blanco L, Bonser SP, Valladares F, Chambel MR, Climent J. 2013. Plasticity in reproduction and growth among 52 range-wide populations of a Mediterranean conifer: adaptive responses to environmental stress. Journal of Evolutionary Biology 26: 1912–1924. [DOI] [PubMed] [Google Scholar]
- SAS 2015. SAS/STAT® 14.1. user’s guide. Cary, NC: SAS Institute Inc. [Google Scholar]
- Smithson M, Verkuilen J. 2006. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychological Methods 11: 54–71. [DOI] [PubMed] [Google Scholar]
- Steane DA, Potts BM, McLean EH, et al. . 2017. Genomic scans across three eucalypts suggest that adaptation to aridity is a genome-wide phenomenon. Genome Biology and Evolution 9: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbauer MJ. 2002. Oviposition preference and neonate performance of Mnesampela privata in relation to heterophylly in Eucalyptus dunnii and E. globulus. Agricultural and Forest Entomology 4: 245–253. [Google Scholar]
- Stram DO, Lee JW. 1994. Variance components testing in the longitudinal mixed effects model. Biometrics 50: 1171–1177. [PubMed] [Google Scholar]
- Stukel T. 1988. Generalized logistic models. Journal of the American Statistical Association 83: 426–431. [Google Scholar]
- Turner C, Wiltshire RJE, Potts BM, Vaillancourt RE. 2000. Allozyme variation and conservation of the Tasmanian endemics, Eucalyptus risdonii, E. tenuiramis and E. coccifera. Conservation Genetics 1: 209–216. [Google Scholar]
- Turner C, Wiltshire RJE, Potts BM, Vaillancourt RE. 2001. Variation in seedling morphology in the Eucalyptus risdonii Hook. f – E. tenuiramis Miq. complex. Australian Journal of Botany 49: 43–54. [Google Scholar]
- Wade MJ, Kalisz S. 1990. The causes of natural selection. Evolution 44: 1947–1955. [DOI] [PubMed] [Google Scholar]
- Wang J-W, Park MY, Wang L-J, et al. . 2011. miRNA control of vegetative phase change in trees. PLoS Genetics 7: e1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL, Jacquemyn H, Ochocki BM, Brys R, Miller TE. 2015. Life history evolution under climate change and its influence on the population dynamics of a long-lived plant. Journal of Ecology 103: 798–808. [Google Scholar]
- Wilson AJ, Poissant J. 2016. Quantitative genetics in natural populations. In: Kliman R, ed. Encyclopedia of evolutionary biology. Oxford: Academic Press, 361–371. [Google Scholar]
- Wiltshire RJE. 1991. Heterochrony and heteroblasty in the Eucalyptus risdonii Hook.F./E. tenuiramis Miq. complex, PhD Thesis, University of Tasmania. [Google Scholar]
- Wiltshire RJE, Reid JB. 1992. The pattern of juvenility within Eucalyptus tenuiramis Miq. saplings. In: Mass production technology for genetically improved fast growing forest tree species (AFOCEL-IUFRO Symposium 1992), Bordeaux. Nangis, France: Association Forêt Cellulose, 37–49. [Google Scholar]
- Wiltshire RJE, Potts BM, Reid JB. 1991. A paedomorphocline in Eucalyptus: natural variation in the E. risdonii/E. tenuiramis complex. Australian Journal of Botany 39: 545–566. [Google Scholar]
- Wiltshire RJE, Potts BM, Reid JB. 1992. A paedomorphocline in Eucalyptus: II. Variation in seedling morphology in the E. risdonii/tenuiramis complex. Australian Journal of Botany 40: 789–805. [Google Scholar]
- Wiltshire RJE, Murfet IC, Reid JB. 1994. The genetic control of heterochrony: evidence from developmental mutants of Pisum sativum L. Journal of Evolutionary Biology 7: 447–465. [Google Scholar]
- Wiltshire RJE, Potts BM, Reid JB. 1998. The genetic control of reproductive and vegetative phase change in the Eucalyptus risdonii/E. tenuiramis complex. Australian Journal of Botany 46: 45–63. [Google Scholar]
- Xu T, Hutchinson MF. 2013. New developments and applications in the ANUCLIM spatial climatic and bioclimatic modelling package. Environmental Modelling & Software 40: 267–279. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.