Abstract
Background and Aims
Dynamics in branch and leaf growth parameters, such as the phyllochron, duration of leaf expansion, leaf life span and bud mortality, determine tree architecture and canopy foliage distribution. We aimed to estimate leaf growth parameters in adult Arabica coffee plants based on leaf supporter axis order and position along the vertical profile, considering their modifications related to seasonal growth, air [CO2] and water availability.
Methods
Growth and mortality of leaves and terminal buds of adult Arabica coffee trees were followed in two independent field experiments in two sub-tropical climate regions of Brazil, Londrina-PR (Cfa) and Jaguariúna-SP (Cwa). In the Cwa climate, coffee trees were grown under a FACE (free air CO2 enrichment) facility, where half of those had been irrigated. Plants were observed at a 15–30 d frequency for 1 year. Leaf growth parameters were estimated on five axes orders and expressed as functions of accumulated thermal time (°Cd per leaf).
Key Results
The phyllochron and duration of leaf expansion increased with axis order, from the seond to the fourth. The phyllochron and life span during the reduced vegetative seasonal growth were greater than during active growth. It took more thermal time for leaves from the first- to fourth-order axes to expand their blades under irrigation compared with rainfed conditions. The compensation effects of high [CO2] for low water availability were observed on leaf retention on the second and third axes orders, and duration of leaf expansion on the first- and fourth-order axes. The second-degree polynomials modelled leaf growth parameter distribution in the vertical tree profile, and linear regressions modelled the proportion of terminal bud mortality.
Conclusions
Leaf growth parameters in coffee plants were determined by axis order. The duration of leaf expansion contributed to phyllochron determination. Leaf growth parameters varied according the position of the axis supporter along the vertical profile, suggesting an effect of axes age and micro-environmental light modulations.
Keywords: Axis order, bud mortality, Coffea arabica, dynamic multiscale tree graphs, FACE, growing degree-days, irrigation, leaf expansion, life span, metamer, phyllochron, polynomial function
INTRODUCTION
Plant development and growth are characterized by the repeated formation, expansion and continuous senescence of the phytomer (metamer), a basic vegetative architectural unit (White, 1979). In this regard, the position and fate of each metamer will define the plant form. The leaf growth parameters (leaf appearance rate, phyllochron, duration of leaf expansion, life span and leaf fall) contribute to the construction of the whole plant architecture (Harmer, 1992) in combination with shoot bud mortality (Alla et al., 2013).
The leaf appearance rate represents the number of leaves emerging in a unit of time (Xue et al., 2004), while the phyllochron was originally defined as the time interval between the appearance of two successive leaves on the same shoot (Erickson and Michelini, 1957). It means that one metamer is added to the growing shoot during each phyllochron (Wilhelm and McMaster, 1995).
The phyllochron is modified by soil moisture, temperature (Baker et al., 1992; Eggers et al., 2004), water stress (Cutforth et al., 1992), light quality (Zhu et al., 2014), light quantity and daylength (Rosa et al., 2011; Baldissera et al., 2014), or air CO2 supply (Baker et al., 1992). The phyllochron may change with plantation management, e.g. plant population density in potato (Dellai et al., 2005) or sowing dates in oat (Chaves et al., 2017). The effect of temperature on the phyllochron can be calculated as a function of thermal time, expressed in growing degree days [GDDs (°Cd per leaf)] (Garcia-Huidobro et al., 1982) rather than chronological time (days or hours).
Leaf expansion is the central process in which plants colonize space, and it is controlled by water and carbon availability (Pantin et al., 2011). It represents the phase of the leaf’s life span where the construction cost is paid (Jurik and Chabot, 1986). Leaf life span is a property of individual leaves (Kikuzawa, 1991). Despite low variation between short and long daylengths in the tropics, leaf fall in diverse species of this region is signalled by photoperiod (Garcia et al., 2017), and its rate in those species is related to water availability (Borchert, 1998).
The values of leaf growth parameters and their susceptibility to environmental impacts vary with plant ontogeny (Lemaire et al., 2009; Rosa et al., 2011; Pantin et al., 2011). The phyllochron can change between genotypes in salak palm (Ashari, 2002). In peach, the phyllochron can vary over the growing season (Davidson et al., 2015) and can differ within the same tree, relative to branch morphism and branch position (Kervella et al., 1995).
Due to their close relationship to plant architecture, leaf area formation and distribution, leaf growth parameters are widely used in mechanistic models, such as in CANON (Hargreaves and McMaster, 2009), GreenLab (Kang et al., 2008; Jullien et al. 2011), SIRIUS (Jamieson et al., 1998), SORKAM (Narayanan et al., 2014), L-PEACH (Da Silva et al., 2011) and other functional–structural and growth models.
Coffea arabica L. (Arabica coffee) is currently the most important tropical tree in the agronomic sense. In Brazil, the largest worldwide coffee producer, Arabica coffee is cultivated mainly in plantations, with density varying from low (approx. 800 plants ha–1) to high (up to 14 000 plants ha–1; Androcioli-Filho, 2002). It is an evergreen, short-day species, characterized by continuous growth. Its architecture is described as a Roux’s model (Hallé et al., 1978), characterized by the existence of branch dimorphism. The main axis is orthotropic, i.e. erect, with opposite leaves in decussate phyllotaxy. The lateral branches are plagiotropic, i.e. tending to a horizontal orientation. The first level of plagiotropic branches are called the ‘primaries’ in the coffee research community (Bustos et al., 2008). According to the concept of plant architecture, ‘primaries’ are considered second-order axes. The second-order axes have great longevity. From these are born plagiotropic axes of the third to fifth order, (i.e. secondaries, tertiaries and quaternaries). Usually, only the dynamics of first-order axes (orthotropic axes) and growth dynamics of sampled second-order axes are observed in order to evaluate the growth of whole adult Arabica coffee trees (Silva et al., 2004; Ghini et al., 2015). The synchronized growth among first- and second-order axes is defined as a linear regression, where the equation parameters vary according to plant age, planting density and arrangement (Matsunaga et al., 2016).
About 78 % of the length of second-order axes in adult coffee plants is produced in the warm, rainy season (October to March, active growth), while 22 % is produced during the cold-dry season (April to September, reduced growth) in South-east Brazil (Silva et al., 2004), with flowering taking place from September to December and berry harvesting from May to July (Camargo and Camargo, 2001).
Despite the huge agronomical importance of Arabica coffee, the leaf growth parameters and the dynamics in bud mortality have never been quantified in this species. The first hypothesis of our study was that the phyllochron, leaf expansion and leaf life span could vary within the hierarchy of the order of axes in Arabica coffee trees, and those leaf growth parameters could be modified by growth season and climate conditions. The second hypothesis was that air CO2 concentration and water availability would cause modifications of leaf parameters and bud mortality, considering the metabolic and hydraulic roles of those environmental factors. Thus, we aimed to estimate the phyllochron, duration of leaf expansion, and leaf and bud life span in adult field-grown Arabica coffee plants depending on growth season, axis order and position of emerged leaves in coffee trees, with and without water limitations, and under actual and elevated [CO2] to permit accurate leafy branch growth reconstructions.
MATERIALS AND METHODS
Location and agronomic aspects of experiments
Two independent field experiments were carried out on initially 3- and 4-year-old Arabica coffee trees in two sub-tropical Brazilian regions, Southern and South-eastern.
The first experiment was conducted at the Agronomic Institute of Paraná (IAPAR), Londrina (23°18ʹS, 51°17ʹW, altitude 620 m, Köpen-Geiger climate type Cfa, sub-tropical without occurrence of a long dry period; Supplementary Data Fig. S1), Paraná. In the Londrina region, the minimum and maximum daylight durations over the year are 10 h 42 min and 13 h 34 min, respectively. Our experiment was managed during the period from October 2013 to November 2014 and considered an Ethiopian accession, ‘E083’. Ethiopian accessions were seeded in the nursery in 2009, and seedlings were transplanted to the field in March 2010, in a planting design 2 m × 0.5 m. The rows were East–West orientated. The limiting factors for Arabica coffee growth in the Cfa climate are defined by low autumn and winter temperatures (Meireles et al., 2009).
The second experiment was conducted at Embrapa Environment, Jaguariúna (22º42ʹS, 46º59ʹW, altitude 570 m, Köpen-Geiger climate type Cwa, sub-tropical with occurrence of dry winters; Supplementary Data Fig. S2), São Paulo. In the Jaguariúna region, the minimum and maximum daylight durations over the year are 10 h 43 min and 13 h 32 min, respectively. The limiting factor for Arabica coffee vegetative growth in the Cwa climate of South-east Brazil is defined by low autumn and winter precipitations (Chapa and Rao, 2004). Our experiment was managed during the period from July 2015 to July 2016 and considered the cultivar ‘Catuaí Vermelho IAC 144’. Seedlings of ‘Catuaí Vermelho IAC 144’ were transplanted to the field in March 2011, in a planting design 3.5 m × 0.60 m. The plants were grown under a free air CO2 enrichment (FACE) facility (Ghini et al., 2015). The studied plants were delimited by 10 m diameter octagon plots (rings), with each ring containing four North–South-oriented rows, with a total of 44 plants for each ring. The addition of CO2 to air began on 25 August 2011 and stopped on 30 June 2016. The actual air CO2 concentration (a[CO2]) at the beginning of the experiment was about 390 μL CO2 L–1. The direct injection of pure CO2 allowed the elevation of the air [CO2] to 200 μL CO2 L–1 above the a[CO2] during the daylight hours, which represented a treatment named e[CO2]. The dynamics of [CO2] during our experiment are shown in Supplementary Data Fig. S2C.
Considering the existence of a dry period in the Cwa climate, half of the coffee trees under the FACE facility received drip irrigation (IRR), while all other plants were cultivated under rainfed conditions (NI). The irrigation started in October 2015. The need for irrigation was calculated using the soil water balance method to achieve a soil water storage capacity within the plant’s root zone of about 130 mm m–2 month–1. NPK fertilization was carried out with 1000 kg ha–1 year–1 (20:5:15 NPK formulation) split into four applications in the Cfa region, and 1750 kg ha–1 year–1 (20:5:15 NPK formulation) split into three applications in the Cwa region. Zinc sulphate (0.6 %), potassium chlorite (0.5 %) and boric acid (24 kg ha–1 year–1) were also applied.
Plant abstraction
Coffee plants were observed at a 15–30 d frequency and codified in dynamic multiscale tree graphs (dynamic MTGs; Godin and Caraglio, 1998). The dynamic MTG codification followed the VPlants methodology (Pradal et al., 2008), with three topological scales, i.e. plants, axes and metamers, as in a previous study of coffee plant structure modelling and reconstruction (Rakocevic and Androcioli-Filho, 2010).
A coffee metamer is composed of an internode, two leaves and 4–5 buds, which are present in a linear sequence in axil of each of two leaves in a pair (Majerowicz and Söndahl, 2005). The most developed bud of this series can originate a new lateral branch or an inflorescence. In the coding process, we distinguished orthotropic from plagiotropic metamers. North–South cardinal orientation was considered the x-axis, and East–West orientation was considered the y-axis. Four cardinal orientations of branches and leaves were attributed (N, S, E and W) using the planting lines as the reference. In both experiments, the identification of each fifth metamer on decomposed axes was marked with coloured thread, to help the field identification of the metamer position for data collection. The dynamics of various morphological characteristics were recorded: the length of each internode on orthotropic and plagiotropic axes; the position and orientation of followed axes; length, width and orientation of each leaf; and leaf presence on followed axes. From one date to the next, only the differences in observed characters were recorded.
In the experiment in the Cfa climate, the leaf growth dynamics were followed on four plants, tagging 12 axes of each of second to fifth axis orders, totalling about 135 axes. Some of the plants did not have all 12 axes of the fifth order. All tagged axes were described at the metamer scale, including the four first-order axes. Plants were observed on 26 dates.
In the experiment in the FACE facility in the Cwa climate, plants were codified following the methodology of sampling, considering a detailed description of leaf dynamics of the first-order axes and four second-order axes (each one oriented to one cardinal point) per 50 cm thick layer along a vertical plant profile (Matsunaga et al., 2016). These second-order axes were described at the metamer scale (as detailed in the Cfa experiment), as well as their lateral axes of the third to fifth order. All the other second-order axes were described by their position along the orthotropic trunks, total length of the living branch part (up to the most distant branching), stage of the terminal meristem (active, senescence or dormant) and cardinal orientation. Plants were observed on 22 dates.
As the main aim of this study was to analyse leaf growth parameters, the presence of reproductive components has not been followed. In both experiments, flowering occurred from September–October to November–December, but neither the inflorescence position and intensity, nor berry setting were codified, despite the fact that it has recently been shown that the intensity of branch growth is inversely related to fruit load in coffee (Bote and Vos, 2016).
Phyllochron, duration of leaf expansion, leaf life span and terminal bud senescence
The phyllochron, duration of leaf expansion and life span were expressed as a function of accumulated thermal time [i.e. the sum of growing degree-days (GDDs), °Cd). The growth dynamics of about 400 axes and histories of >4000 leaves were analysed.
Daily minimum and maximum air temperatures and pluviometry for the Cfa experiment were provided by the Meteorological System of Paraná, Simepar (Supplementary Data Fig. S1) and by a local meteorological station for the Cwa experiment, including CO2 concentrations (Supplementary Data Fig. S2). GDDs were calculated as GDD = (Tmean – Tb), where Tmean is the mean air temperature calculated as the average of daily minimum and maximum air temperatures, and Tb is the base temperature for each species. The calculated Tb = 10.2 °C (Pezzopane et al., 2008) was assumed for all calculations.
The phyllochron was defined as the thermal time, expressed in GDDs, required for the emergence of a leaf. It was calculated as 1/b from the linear regression y = bx + a (Streck et al., 2005), where b is the slope coefficient between the number of produced metamers on the plant axis (y-axis of the equation) and accumulated thermal time (x-axis of the equation). The portions of each leafy coffee axis formed during the active and reduced seasonal periods of growth were distinguished. The regular phyllochron was assumed for all leaves on the same axis during each seasonal growth. The R2 values of regressions were always >0.9.
The duration of leaf expansion was calculated as the sum of GDDs from the observed date of metamer appearance to the date of final size acquisition of each leaf in a pair. The life span was calculated as the sum of GDDs from the observed date of metamer appearance to the date of leaf fall, separately for each leaf in a pair.
The proportion of preserved leaves on each observed axis was expressed as a percentage of the observed leaves compared with the maximum potential number [(number of leaves ×100)/(number of metamers × 2)]. During the experimental period, some of the followed axes lost the terminal apex and had not been followed in sequence. New axes of different branching orders appeared and were included in observations.
In the FACE experiment, the fate of terminal buds was followed, considering second- to fourth-order axes. In coffee plants, the branch is not obligatorily completely senesced when the terminal bud is dead, e.g. when the second-order terminal bud mortality occurred (branch top) due to natural causes or trauma by management. The branch can be functional from the insertion on the orthotropic axis (axis bottom) to the metamer bearing the latest third-order branch toward the branch top, preserving the phloem and xylem continuum, and senescing the whole zone from the last branching to the terminal bud.
The average proportion of terminal bud mortality was based on the number of living or dead terminal buds of each axis order on each position over the orthotropic axes, in a population of four plants for each of four treatments. The proportion of terminal bud mortality was determined for each plant observed on the following temporal observation dates: 31 July 2015 (reduced growth), 18 February 2016 (active growth) and 3 July 2016 (active growth). The probability of terminal bud mortality of the second-order axes was modelled as the linear regression related to the position of each second-order axis along the ranks of the orthotropic axis, for the population of four plants for each of four treatments.
Data extraction
The basic data of morphological traits for definition of leaf life dynamics were extracted from dynamic MTGs under AMAPStudio (Griffon and Coligny, 2014), differentiating the period (active and reduced vegetative growth) of metamer appearance, leaf growth and fall, and order of axis supporter (first to fifth). In dynamic MTGs, each axis had the attributed identification that allowed the extraction of its components relative to sequential dates.
The active seasonal growth in the sub-tropical Cfa region occurred in two periods, from October 2013 to March 2014 and from October to November 2014. Those two active periods were pooled to avoid the impact of intercalated reduced growth on phyllochron estimation, leaf expansion rate and life span of active growth. A similar procedure was executed for two periods of reduced growth (from July to September 2015 and from April to July 2016) in the Cwa region experiment, under the FACE facility.
Statistical analyses and modelling of leaf and bud life dynamics
Analysis of variance (ANOVA) of leaf growth parameters (phyllochron, duration of leaf expansion and leaf life span) were estimated by mixed model fit using the ‘lme’ function in R version 3.3.0 (2017), considering plant repetitions, axes repetitions and branch cardinal orientations as fixed effects and analysing the effects of axis order, seasonal growth, irrigation and [CO2].
The rough distributions of phyllochron, duration of leaf expansion, life span and bud fate over the orthotropic rank and different seasonal growth (reduced vs. active), and the impact of irrigation and [CO2] were modelled under SciLab software (2017). The modelled second-degree polynomial functions were estimated by the ordinary least squares method (Burden and Faires, 2003), accepting R2 >0.5. This method consists of the determination of the second-degree polynomial function P(x) = a2 + bx + c to approximate the distance between real f(x) and calculated values of P(x), by minimizing the truncation error determined by |f(x) – P(x)|2, where the P(x) coefficients are calculated by the linear systems equation solved by Gauss elimination. The polynomial equations are more efficient for programming and application in simulation models, and the second degree was chosen to make an efficient numerical approximation of data sets.
RESULTS
Axis order and seasonal growth impacts on leaf growth parameters in two climatic regions
The coffee plants, grown under rainfed conditions in two sub-tropical regions, showed variations in leaf growth parameters, more in Cwa than in the Cfa region, over the axes orders and growth seasons (Figs 1 and 2; Tables 1 and 2). During reduced seasonal growth, the phyllochron and leaf expansion durations were longer than during the active growth season, while the proportion of preserved leaves was higher during the active seasonal growth. The phyllochron and thermal time needed for leaf expansion increased from the second to the fourth axis order in the Cwa region, while in the Cfa region higher GDDs for one leaf to attain its final dimensions were noted only on second-order axes during the active, but not during the reduced seasonal growth. In both regions, the phyllochrons of first-order axes did not differ between two growth seasons, indicating the continuous carbon investment in the plant height components. The leaf life span was highly impacted by the seasonal growth period in the Cwa region and by axis order in the Cfa region. The following sections serve to illustrate these main points.
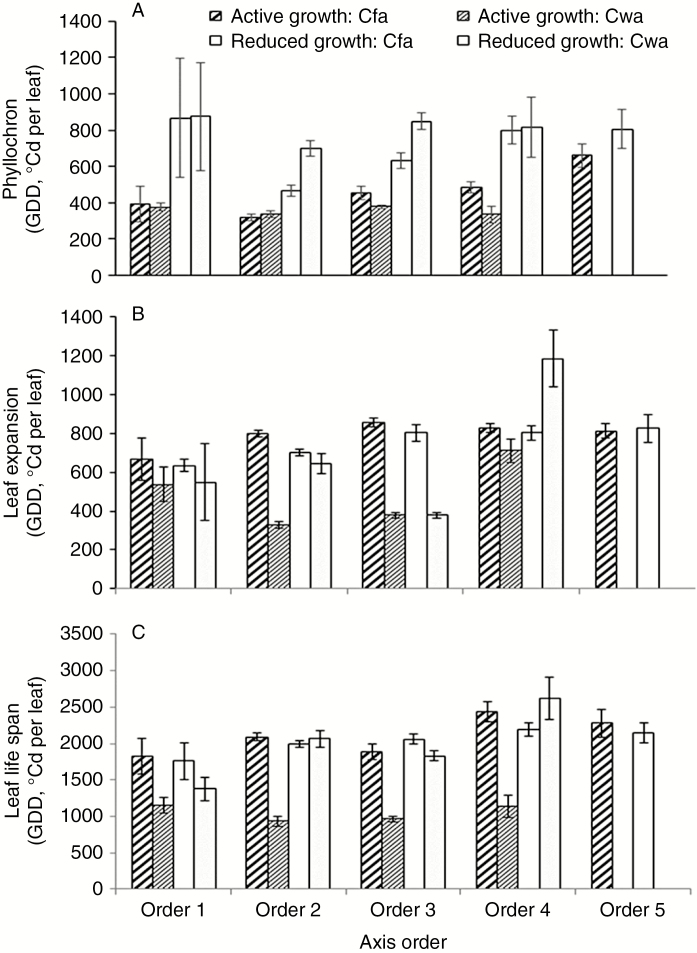
Fig. 1.
Mean and s.e. for leaf growth parameters (GDD, °Cd per leaf) in Coffea arabica estimated from two experiments, one in the sub-tropical Cfa region and the other in the sub-tropical Cwa region, during active and reduced seasonal growth. (A) Phyllochron, (B) duration of leaf expansion and (C) leaf life span of five axes orders.
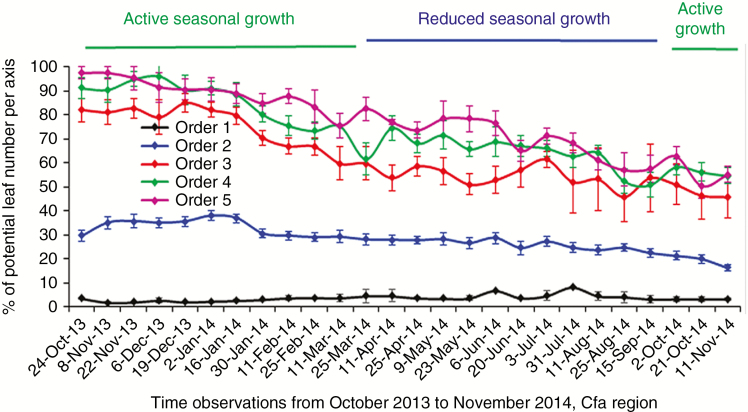
Fig. 2.
The dynamics of preserved leaf proportion of five axes orders in Coffea arabica plants grown in the sub-tropical Cfa region, observed from October 2013 to November 2014, during active and reduced seasonal growth.
Table 1.
ANOVA P-values for effects of five axes orders and two types of seasonal growth (active and reduced) on leaf growth parameters (GDD, °Cd per leaf) in Coffea arabica estimated for two independent experiments
| Effect | Phyllochron | Duration of leaf expansion | Leaf life span | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Order | Season | Order × season | Order | Season | Order × season | Order | Season | Order × season | |
| Order | Sub-tropical Cfa region | ||||||||
| All | <0.0001 | <0.0001 | 0.1588 | 0.0037 | 0.0036 | 0.4319 | 0.0091 | 0.0933 | 0.1978 |
| 1 | 0.2571 | 0.9840 | 0.8557 | ||||||
| 2 | <0.0001 | 0.0050 | 0.1804 | ||||||
| 3 | <0.0001 | 0.5986 | 0.3712 | ||||||
| 4 | <0.0001 | 0.5622 | 0.0096 | ||||||
| 5 | 0.2182 | 0.6244 | 0.0650 | ||||||
| Sub-tropical Cwa region | |||||||||
| All | 0.0163 | <0.0001 | 0.0648 | <0.0001 | 0.0600 | 0.9865 | 0.3079 | <0.0001 | 0.5520 |
| 1 | 0.1758 | 0.9460 | 0.4539 | ||||||
| 2 | <0.0001 | <0.0001 | <0.0001 | ||||||
| 3 | <0.0001 | <0.0001 | <0.0001 | ||||||
| 4 | 0.2023 | 0.0069 | 0.0053 | ||||||
The Cfa sub-tropical region has occurrences of low minimum daily winter temperatures and the Cwa sub-tropical region has an accentuated dry winter period.
P-values <0.05 were considered significant and are marked in bold.
Table 2.
ANOVA P-values for effects of axes orders and seasonal growth (active and reduced) on the proportion of preserved leaves per axis of Coffea arabica grown in the Cfa climate region, observed from October 2013 to November 2014
| Axes order | Effects | ||
|---|---|---|---|
| Order | Season | Order × season | |
| All | <0.0001 | <0.0001 | <0.0001 |
| Order 1 | 0.0072 | ||
| Order 2 | <0.0001 | ||
| Order 3 | <0.0001 | ||
| Order 4 | <0.0001 | ||
| Order 5 | <0.0001 | ||
P-values <0.05 were considered significant and are marked in bold.
Phyllochron variations within the tree structure during active and reduced vegetative seasonal growth
The phyllochron was roughly 30–40 % (Cfa region) to 300 % (Cwa) higher during the reduced compared with the active seasonal growth, on the second- to fourth-order axes (Fig. 1A; Table 1). Among the five axes orders, the first- and second-order axes exhibited the lowest phyllochron (on average 315 °Cd per leaf during active growth in the Cfa region). The phyllochron increased with increasing axis order. The fourth- and fifth-order axes showed the highest phyllochron (about 660 °Cd per leaf in the active seasonal growth); Fig. 1A. In both regions, the phyllochrons of first-order axes did not differ between seasonal growths.
During the active seasonal growth, the phyllochrons of first- and second-order axes did not differ between the two regions; however, they were lower in Cwa than in Cfa for the third- and fourth-order axes. On the other hand, during the reduced seasonal growth, the phyllochrons of second- and fourth-order axes were significantly higher in Cwa than in the Cfa region.
Leaf expansion duration and life span variations within the tree structure during active and reduced vegetative seasonal growth
In the Cfa region, the duration of leaf expansion of first- and second-order axes was similar (about 730 °Cd per leaf during active growth, and about 670 °Cd per leaf during reduced growth), increasing on higher axes orders to 820 °Cd per leaf (Fig. 1B; Table 1). In the Cwa region, the thermal time needed for leaf blade expansion increased from second to fourth axis order.
The first-order axes did not differ in duration of leaf expansion between the active and reduced periods, in both regions (Fig. 1B; Table 1). In the Cfa region, higher GDDs for one leaf to attain its final dimensions were noted on second-order axes during the active, but not during the reduced seasonal growth (Fig. 1B; Table 1), while in the Cwa region the leaf expansion duration was much longer during reduced than during active growth for second- to fourth-order axes (Fig. 1B; Table 1). Throughout the reduced growth in the Cwa region, the average duration of leaf expansion did not differ between the first-, second- and third-order axes, with the highest GDDs recorded on fourth-order axes (about 1085 °Cd per leaf). In the Cwa region, leaves on second-order axes needed the lowest GDD to expand during active growth, but doubled the thermal time for leaf expansion during the reduced growth (Fig. 1B; Table 1).
Life span did not differ between the active and reduced seasonal growth in the Cfa region, except on fourth-order axes (Fig. 1C; Table 1), while life span was higher during the reduced growth within the second- to fourth-order axes in the Cwa region (Fig. 1C; Table 1). The longest life span was observed on fourth- and fifth-order axes in Cfa, on average 2250 °Cd per leaf (Fig. 1C), and the shortest on leaves on first- and second-order axes, on average 1900 °Cd per leaf, in the same region. Under the Cwa conditions, the life span was much shorter during the active than during the reduced seasonal growth (average overall value of 1046 for the active and 1972 °Cd per leaf for the reduced period) and did not differ among the axis orders (Table 1; Fig. 1C).
Proportion of preserved leaves on axes within the tree structure during active and reduced vegetative seasonal growth
The proportion of preserved leaves at each axis compared with the potential number was strongly influenced by the seasonal growth (Fig. 2; Table 2). A higher leaf proportion was preserved during the active than during the reduced seasonal growth (Fig. 2), with the exception of the first-order axes, where this proportion was higher during the reduced seasonal growth (interaction order × period in Table 2). The third-, fourth- and fifth-order axes preserved leaves in a higher proportion than the second-order axes during the whole period of the experiment. The lowest proportion of preserved leaves was found on first-order axes (Fig. 2) related to the greatest total metamer number on this axis order.
Modifications on leaf growth parameters with irrigation and elevated air [CO2]
Between all observed leaf growth parameters, duration of leaf expansion and leaf retention benefited most from irrigation (Figs 3 and 4; Tables 3 and 4). The leaves of first- to fourth-order axes of irrigated plants cultivated under e[CO2] expanded their blades during a longer thermal time than under a[CO2]. The e[CO2] showed the compensation effects for lower water availability (NI) on leaf expansion duration of first- and fourth-order axes; the e[CO2] permitted a longer thermal time for blade expansion than under a[CO2] under rainfed conditions. Similar compensation effects of e[CO2] under low water availability were observed on leaf retention of the second- and third-order axes in the fifth year of growth under FACE. The life span was not impacted by water and carbon supply during this experimental period, representing the most stable leaf growth parameter. The following details illustrate these main points.
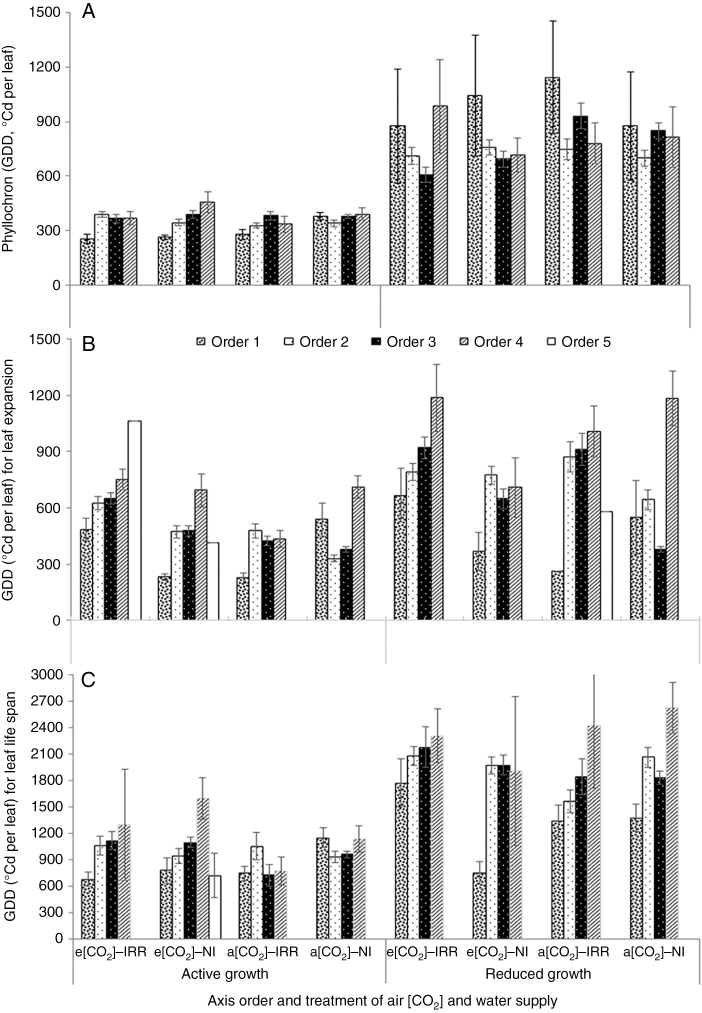
Fig. 3.
Mean and s.e. for leaf growth parameters (GDD, °Cd per leaf). (A) Phyllochron, (B) duration of leaf expansion and (C) leaf life span of four axes orders in Coffea arabica, estimated on plants grown under elevated air [CO2] (e[CO2]) and actual [CO2] (a[CO2]), subjected to drip irrigation (IRR) or rainfed conditions (NI) during active and reduced seasonal growth.
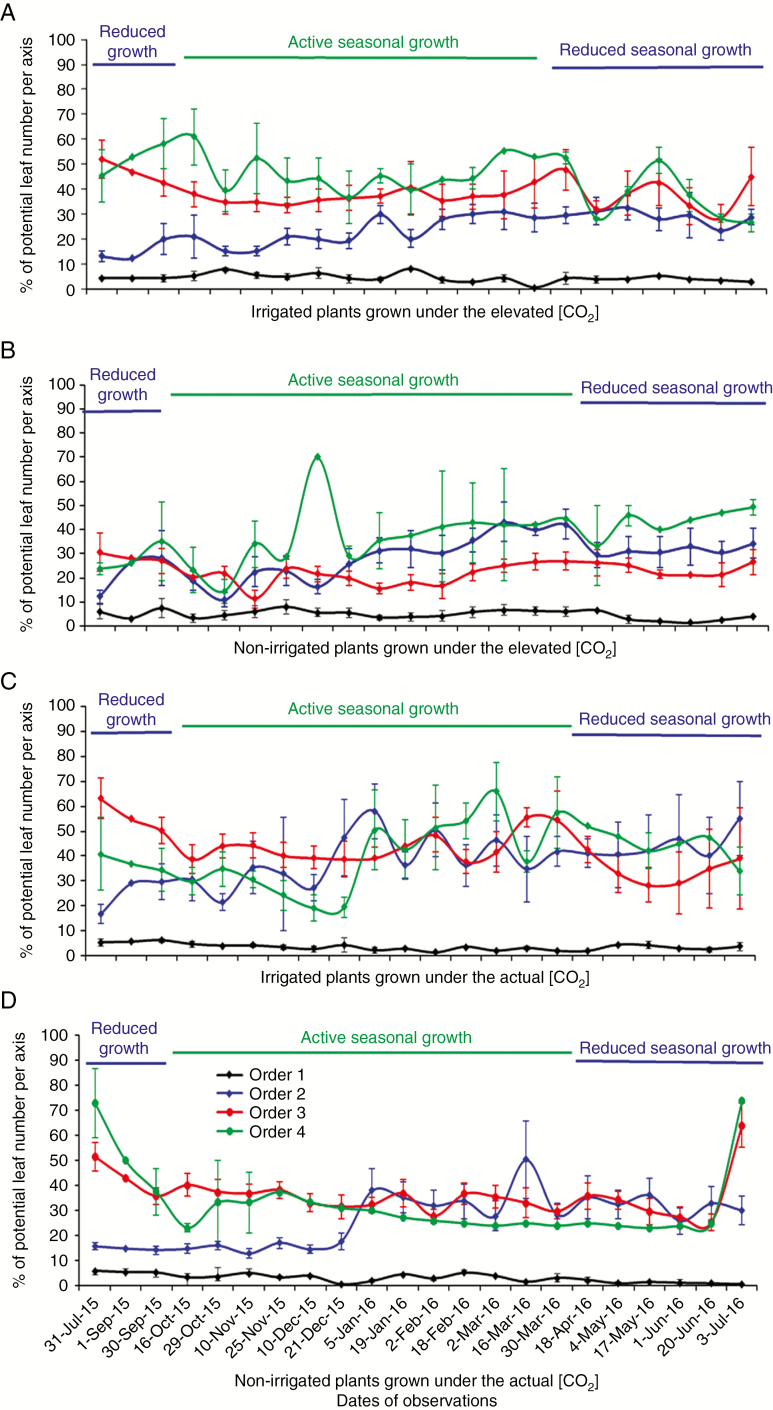
Fig. 4.
The impact of air [CO2] and irrigation on dynamics of the proportion of preserved leaves on four axes orders of Coffea arabica, observed from the end of July 2015 to the beginning of July 2016, during active and reduced seasonal growth. Plants were grown under: (A) elevated [CO2] with irrigation, (B) elevated [CO2] and rainfed conditions, (C) actual [CO2] with irrigation and (D) actual [CO2] and rainfed conditions.
Table 3.
ANOVA P-values for effects of air [CO2] and irrigation (IRR) treatments on leaf growth parameters (GDD, °Cd per leaf) in Coffea arabica estimated during the seasons of active and reduced growth on four axes orders
| Effect | Phyllochron | Duration of leaf expansion | Leaf life span | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Season | <0.0001 | <0.0001 | <0.0001 | ||||||
| Order | 0.0240 | <0.0001 | 0.0003 | ||||||
| Season × order | 0.0008 | 0.0001 | 0.0017 | ||||||
| [CO2] | IRR | [CO2] × IRR | [CO2] | IRR | [CO2] × IRR | [CO2] | IRR | [CO2] × IRR | |
| Order | Season of active growth | ||||||||
| 1 | 0.0120 | 0.0408 | 0.0694 | 0.1471 | 0.4308 | 0.0010 | 0.2063 | 0.1111 | 0.1867 |
| 2 | 0.1012 | 0.2266 | 0.0550 | <0.0001 | <0.0001 | 0.6596 | 0.1891 | 0.2717 | 0.9305 |
| 3 | 0.8779 | 0.8186 | 0.6735 | <0.0001 | 0.0471 | 0.4211 | 0.3825 | 0.1536 | 0.5745 |
| 4 | 0.4933 | 0.7549 | 0.1014 | 0.7099 | 0.0170 | 0.1729 | 0.3771 | 0.1727 | 0.3290 |
| Order | Season of reduced growth | ||||||||
| 1 | 0.8630 | 0.8630 | 0.4656 | 0.7213 | 0.5860 | 0.2116 | 0.1180 | 0.0543 | 0.1641 |
| 2 | 0.6293 | 0.9427 | 0.2496 | 0.3962 | 0.3372 | 0.1269 | 0.5301 | 0.2290 | 0.3486 |
| 3 | 0.0001 | 0.9941 | 0.1655 | 0.0045 | 0.0135 | 0.3194 | 0.1273 | 0.7394 | 0.4253 |
| 4 | 0.1739 | 0.7209 | 0.1940 | 0.1169 | 0.0644 | 0.0011 | 0.6106 | 0.8029 | 0.6578 |
P-values <0.05 were considered significant and are marked in bold.
Table 4.
ANOVA P-values for effects of [CO2] and irrigation on the dynamics of the proportion of preserved leaves on four axes orders of Coffea arabica, observed from the end of July 2015 to the beginning of July 2016, during active and reduced seasonal growth
| Effects | Order | Season | Order × season |
|---|---|---|---|
| <0.0001 | 0.3692 | 0.2290 | |
| Effects | [CO2] | IRR | [CO2] × IRR |
| Order | Season of active growth | ||
| Order 1 | <0.0001 | 0.1329 | 0.1983 |
| Order 2 | 0.0148 | 0.0496 | <0.0001 |
| Order 3 | <0.0001 | <0.0001 | 0.0001 |
| Order 4 | 0.6062 | 0.0031 | 0.2116 |
| Order | Season of reduced growth | ||
| Order 1 | 0.7131 | 0.7859 | 0.2712 |
| Order 2 | 0.2398 | 0.2770 | 0.0011 |
| Order 3 | 0.0041 | <0.0001 | 0.2932 |
| Order 4 | 0.2071 | 0.7361 | 0.1126 |
P-values <0.05 were considered significant and are marked in bold.
Phyllochron, leaf expansion and life span modifications under elevated [CO2] and irrigation
During active growth, e[CO2] and irrigation decreased the phyllochron of the first-order axes (Fig. 3A; Table 3). Throughout reduced seasonal growth, the third-order axes of plants grown under e[CO2] showed a decreased phyllochron compared with those grown under a[CO2].
It took more thermal time for leaves of first-order axes to expand their blades in rainfed-grown plants under a[CO2] than under e[CO2] during the active seasonal growth (Fig. 3B; Table 3). The leaves on second- and third-order axes needed a longer thermal time to attain their final size under irrigation than under rainfed conditions, in both active and reduced seasonal growth. It took more thermal time for expansion of leaves on second- and third-order axes with CO2 fertilization than in those under a[CO2] during the active seasonal growth, Finally, the fourth-order axes of IRR plants needed a shorter thermal time for leaf expansion than NI plants, throughout active growth, while CO2 fertilization under rainfed conditions shortened the GDDs throughout the reduced growth of fourth-order axes.
The leaf life span of the investigated plants had not been modified by water and [CO2] treatments (Table 3; Fig. 3C), but was strongly impacted by growth season and axis order (Table 3; Fig. 3).
The proportion of preserved leaves on axes under e[CO2] and irrigation
The first-order axes preserved the lowest proportion of leaves among all axes orders (Table 4; Fig. 4), which was related to the highest metamer number with only a few of the newest metamers bearing pairs of leaves.
During the active growth, e[CO2] promoted the retention of leaves on first-order axes (Table 4; Fig. 4A–D). The preserved leaf proportions on second-order axes in e[CO2] did not differ between two water treatments during the active growth. The e[CO2] compensated the effect of NI on second-order axes during both the active and reduced growth seasons. During the active seasonal growth, the third-order axes retained more leaves under a[CO2] than under e[CO2]; leaf retention of third-order axes was positively impacted by irrigation only in plants grown under e[CO2], but not under a[CO2]. During the reduced seasonal growth, the third-order axes of plants grown under IRR preserved a higher proportion of leaves, and e[CO2] impacted on a lower proportion of retained leaves than a[CO2]. Throughout the active growth, the highest proportion of leaves on fourth-order axes was preserved on plants grown under e[CO2] and irrigation (Table 4; Fig. 4A–D); during the reduced growth, no impact of water and carbon supply was observed on the proportion of retained leaves on fourth -order axes (Table 4).
Modelling the leaf life dynamics in the vertical profile of plants
The distributions of leaf growth parameters on second, third and fourth axes order along the orthotropic rank were estimated by second-degree polynomial functions. The complete list of coefficients for equations defining the distribution of the phyllochron, duration of leaf expansion and leaf life span in the vertical profile and the generated R2 for all treatments and axes orders can be seen in Supplementary Data Tables S1–S3.
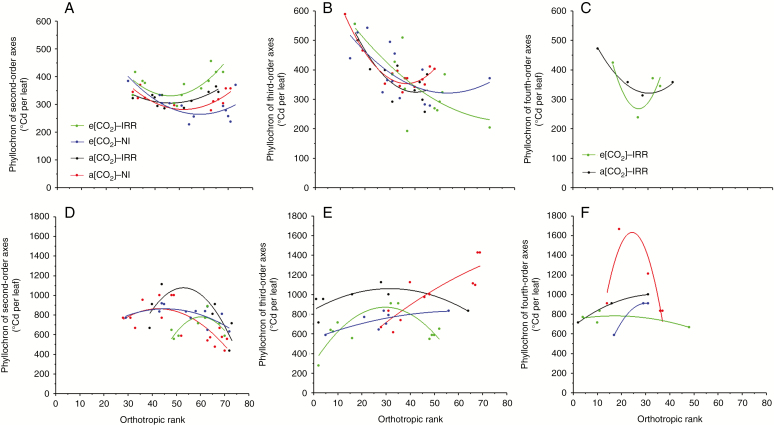
A concave upward parabola modelled the phyllochron distributions along the orthotropic axis during the active growth (Fig. 5A–C) and a concave downward parabola modelled the phyllochrons during the reduced seasonal growth (Fig. 5D–F). This means that during the active growth, the second-order axes in the low and the upper layers were characterized by a higher phyllochron than those in the middle layer (Fig. 5A–C), while the situation among the plant layers was inverted during the reduced seasonal growth.
Fig. 5.
Distribution of phyllochron in the vertical plant profile. (A) Second-, (B) third- and (C) fourth-order axes during active seasonal growth and (D) second-, (E) third- and (F) fourth-order axes during reduced seasonal growth. The phyllochron was expressed in GDD (°Cd per leaf), and four treatments were considered: e[CO2] with irrigation, e[CO2] under rainfed conditions, a[CO2] with irrigation and a[CO2] under rainfed conditions.
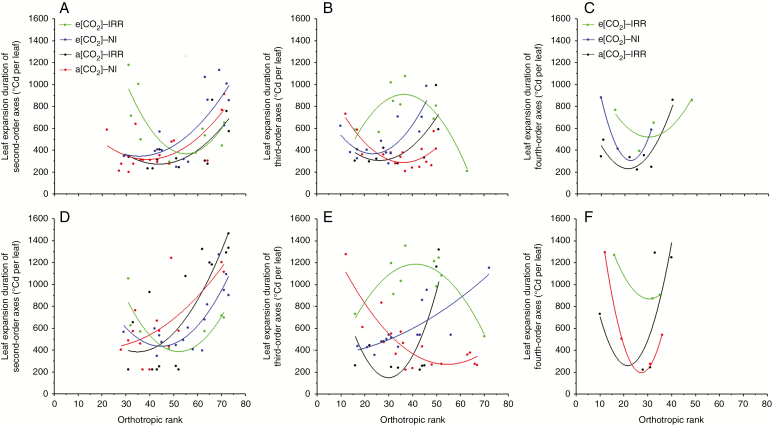
A concave upward parabola modelled the leaf expansions along the orthotropic rank during both active and reduced seasonal growth (Fig. 6), with the exception of leaves of third-order axes under e[CO2]–IRR treatment (Fig. 6B, E). This means that leaves needed higher GDDs to expand when localized in low and upper layers than in the middle layer. Only the third-order axes under high CO2 and water supply expanded with higher GDDs in the middle layer than in the low and upper layers.
Fig. 6.
Distribution of the duration of leaf expansion in the vertical plant profile. (A) Second-, (B) third- and (C) fourth-order axes during active seasonal growth and (D) second-, (E) third- and (F) fourth-order axes during reduced seasonal growth. The duration of leaf expansion was expressed in GDD (°Cd per leaf), and four treatments were considered: e[CO2] with irrigation, e[CO2] under rainfed conditions, a[CO2] with irrigation and a[CO2] under rainfed conditions.
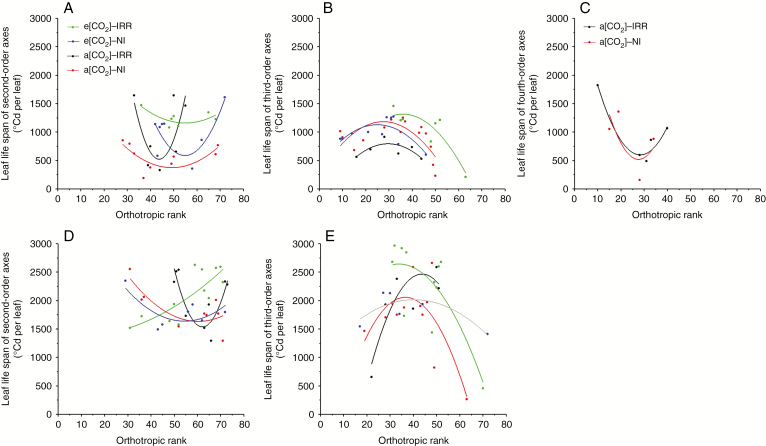
A concave upward parabola modelled the distribution of leaf life span of second-order axes in the vertical profile of coffee plants for both active and reduced seasonal growths (Fig. 7A, D). A concave downward parabola modelled the distribution of leaf life span of third-order axes for both the active and reduced seasonal growths (Fig. 7B, E). This means that leaves on second-order axes had a longer life span when localized in low and upper layers than in the middle layer, while the opposite was observed on leaves of third-order axes, characterized by a lower life span in the low and upper layers. A concave upward parabola modelled the distribution of leaf life span only during the active seasonal growth on fourth-order axes under a[CO2] (Fig. 7C), due to lack of a data set for e[CO2] and reduced seasonal growth. It means that leaves on fourth-order axes situated in low layers had a longer life span than those in the middle layer.
Fig. 7.
Distribution of leaf life span in the vertical profile. (A) Second-, (B) third- and (C) fourth-order axes during the active seasonal growth and (D) second- and (E) third-order axes during reduced seasonal growth. Leaf life span was expressed in GDD (°Cd per leaf) and four treatments were considered: e[CO2] with irrigation, e[CO2] under rainfed conditions, a[CO2] with irrigation and a[CO2] under rainfed conditions.
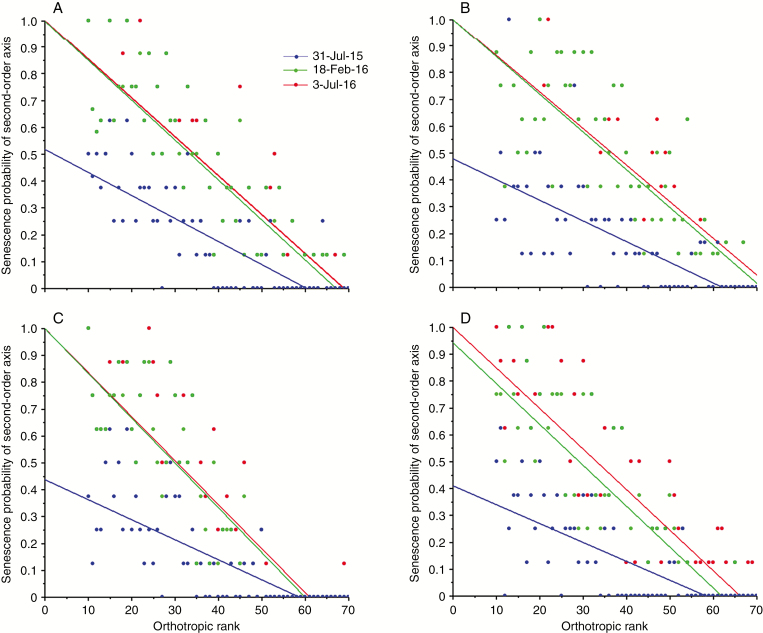
Terminal bud mortality in the vertical profile
The probability of terminal bud mortality of second-order axes in the vertical profile of plants grown under two [CO2] treatments and two water regimes was calculated (Fig. 8). In the beginning of the fifth year under the FACE facility, on 31 July 2015, e[CO2] induced a higher incidence of second-order mortality in the low vertical profile layer (Fig. 8A, B) than a[CO2] (Fig. 8C, D), considering the y-axis intersection. Non-zero probability of the incidence of second-order mortality was encountered more often under e[CO2] than under a[CO2] in the upper layer. In all treatments, the maximum second-order bud mortality occurred between 31 July 2015 and 18 February 2016, because of the previous four dry seasons without irrigation. The mortality of second-order terminal buds significantly diminished between 18 February 2016 and 3 July 2016, from a rainy to a dry period.
Fig. 8.
The probability of mortality of terminal buds in the second-order axes in the vertical profile of plants grown under: (A) e[CO2] with irrigation, (B) e[CO2] and rainfed conditions, (C) a[CO2] with irrigation and (D) a[CO2] and rainfed conditions. Three distinct dates were included: 31 July 2015 (reduced growth), 18 February 2016 (active growth) and 3 July 2016 (reduced growth).
The proportion of terminal bud mortality in relation to the total number of axes was calculated for the third and fourth orders (Supplementary Data Table S4). The percentage of third- and fourth-order terminal bud mortality was lower on 31 July 2015, mainly in a[CO2]–NI treatment. This probability increased from July 2015, when the highest rate of bud mortality occurred, mainly under the e[CO2] treatment. The bud mortality proportion decreased on 3 July 2016, because of the appearance of new living third- and fourth-order axes.
DISCUSSION
Variation in leaf growth parameters over the axes order hierarchy in Arabica coffee trees, and their modification by growth season and climate conditions
The initiation of primordia depends on carbon supply coming from adult leaves and from reserves stored in branch structures (Fanwoua et al., 2014). Shade reduces leaf carbon supply (Felippe and Dale, 1973). In Arabica coffee, the fourth- and fifth-order axes exhibited the highest phyllochron. Those high axes orders were found in more self-shaded positions over the plant canopy. High values of their phyllochrons suggest that the intensity of a botanical event, such as leaf appearance, was modified by low irradiance and the light quality change (lower red:far-red ratio), as noted in maize (Birch et al., 1998; Zhu et al., 2014).
The dry season (reduced growth season) increased the phyllochron of second- and fourth-order axes in the Cwa region much more than in the Cfa region. In spite of idealized continuous growth of coffee plants (Hallé et al., 1978), Amaral et al. (2006) define the seasonal vegetative growth in Arabica coffee for South-east Brazil, attributing the reduction in branch growth to the minimum air temperature and higher stomatal resistance in autumn/winter (reduced growth) than in spring/summer (active growth) season. ‘In situ’ measured leaf photosynthesis rates diminish during the reduced growth season compared with the active growth season, especially in self-shaded leaves of low plant layers (Rakocevic et al., 2015). Thus, the reduced photosynthesis and high stomatal resistance of self-shaded leaves during the season of drought and low minimum daily temperatures would have an impact on lower carbon balance and higher phyllochron of higher axes orders placed in low plant layers under low light conditions.
The leaf growth parameters differed greatly between the two sub-tropical regions, due to climate effects. Two different coffee genotypes, one Ethiopian accession (‘E083’) and ‘Catuaí 144’, used in two experiments, could also contribute to expressed differences. The ‘E083’ accession could have specific genetic responses, as it was the only survivor after one strong frost in 2013, among 63 FAO accessories from Ethiopia and two test cultivars (‘IAPAR 59’ and ‘Catuaí IAC 99’) planted in 2010. Leaves on second-order axes finished their expansion very quickly during the reduced growth season in the Cfa region (‘E083’) and attained a smaller size than during active growth (data not shown), while in the Cwa region the leaf blades of ‘Catuaí IAC 144’ needed comparatively doubled thermal time to attain their final size during the reduced seasonal growth. This means that in the Cfa region the acclimation to temperatures occurred within the same tree of wild-type ‘E083’, in duration of leaf expansion on leading second-order axes, as a response to autumn/winter dry conditions and low minimum daily temperatures. The acclimation to temperatures of leaf growth indicators is observed in maize, comparing some cultivating fields under tropical and temperate climates (Birch et al., 1998).
An increase in leaf expansion duration could shift the time of leaf emergence, and this could contribute to the phyllochron increase (Zhu et al., 2014). The values of the phyllochron and expansion time were very similar in coffee plants, which indicates that leaf expansion contributed to the determination of the phyllochron.
The leaf life span of all axes orders was similar and much higher during the reduced than during the active seasonal growth in the Cwa climate, while small differences between the seasons and the highest life span were noted on fourth- and fifth-order axes in the Cfa climate. High values for life span on high-order axes indicate that it was longer in self-shaded layers, as was observed for the phyllochron. The proportion of preserved leaves per axis also diminished during the reduced compared with the active seasonal growth in Cfa. The proportion of preserved leaves depended on axis order, local shading conditions and metamer number per axis; the first-order axes had about 70–80 metamers at the end of the experiment, the second-order axes were generally shorter, dependent on their insertion rank on the orthotropic axis, while the third- to fifth-order axes had an even lower number of metamers. Finally, the general picture of the proportion of preserved leaves within the plant structure showed an increase with increased axis order, first < second < third = fourth = fifth.
The air [CO2] and water availability modify leaf growth parameters and mortality of buds on the terminal axes
During the active seasonal growth, the e[CO2] diminished the phyllochron of the first-order coffee axes, while during reduced growth the e[CO2] decreased the phyllochron of third-order axes. High [CO2] diminishes the leaf emission rate in rice (Baker et al., 1992), while doubling the ambient CO2 concentration has no effect on the plastochron in young peach trees (Davidson et al., 2016). The lack of any responses in peach could be attributed to the short-term experiment of only 38 d in one tree species.
The coffee leaf expansion was more sensitive to carbon and water variations than the phyllochron. This sensitivity can be related to the fact that during the expansion, new leaves depend on carbon that is assimilated and imported from the older leaves (Fanwoua et al., 2014). Carbon may also be supplied from starch reserves stored in branch structures. This carbon is especially important for initial axis growth and for buffering any deficit in leaf carbon supply (De Schepper et al., 2013). So, leaf expansion is dependent on carbon fluctuations, and vulnerable to water deficit (Van Volkenburgh, 1999). CO2 fertilization showed the compensation effect for a lower water supply in leaf expansion duration of the first- and fourth-order axes allowing a shorter thermal time for blade expansion than under a[CO2]. During the individual leaf ontogeny, the predominant control of leaf expansion switches from metabolic, i.e. carbon and light availability, to hydraulic, i.e. water availability (Pantin et al., 2011). It may be deduced that under CO2 fertilization, the duration of expansion of leaves on first-order axes, localized under high light availability, was shortened during an initial metabolic control, while the expansion of fourth-order leaves, localized in low and shaded layers, was shortened over the later period of expansion, characterized by predominant control of water availability.
The life span of Arabica coffee leaves was not impacted by external water and carbon variations but increased with axis order and showed higher values during the reduced seasonal growth. A longer life span will permit longer assimilation of the same leaf and will decrease the total plant costs. Leaf longevity is related to net photosynthetic rate and construction costs of the leaf (Kikuzawa, 1991). By increasing the life span by one-third, the profit from invested construction costs in leaves can increase by about 80 % in low-light leaves and by about 50 % in high-light leaves in strawberry (Jurik and Chabot, 1986). In this sense, the generally longer leaf life span of second-order coffee axes, together with longer expansion of their blades in the low and upper layers compared with the middle layer, could reflect the coffee tree strategy of higher profit of high- and low-light leaves of this axis order, especially during the reduced growth season.
Generally, a decrease in branching intensity of any plant species corresponds to the tuning of growth to resource availability, at the whole-plant scale (Diepenbrock, 2000). In regions with dry winters, the usual drops of leaves of fourth- and fifth-order axes are expected as normal botanic events during the winter in coffee (Camargo and Camargo, 2001), which was expressed through their high terminal bud mortality, and high values of the phyllochron in this season.
Berry load and fruit maturing on productive axes (Bote and Vos, 2016), which happen at the beginning of reduced seasonal growth, could be a biological factor that induced high values of phyllochron and leaf drop. In C. canephora, greater leaf retention and longevity might be partially associated with improved growth of irrigated plants at the end of a dry season (Silveira and Carvalho, 1996), but in our experiment the leaf longevity was not impacted by irrigation.
The new branches appeared at the end of reduced growth and at the beginning of active growth, which explained the elevated proportion of leaves on second- to fourth-order axes. The first- and second-order axes retained more leaves during the active than during the reduced growth, while the third- and fourth-order axes retained more leaves during the reduced seasonal growth. The latter could be a response of plant rejuvenation after fruit collection (in May and the middle of June), when emergence of new third- and fourth-order axes occurred with 100 % leaf presence on a branch, together with the lower phyllochron of those axes orders under e[CO2].
The compensation effects of e[CO2] for low water availability was observed as leaf retention of the second- and third-order axes during the active and reduced seasonal growths. The mitigation effect of e[CO2] under drought in the reduced growth season is observed in the leaf assimilation rate (Ghini et al., 2015; Rakocevic et al., 2018a). Our data could help to paint a more complete picture of possible morphophysiological responses in Arabica coffee under global climate changes.
The phyllochron, duration of leaf expansion and life span of second-order axes were higher in low and upper layers compared with the middle layer during active vegetative growth. Variations in blade elongation duration are also shown to be bell-shaped over the phytomer ranks of maize plants (Zhu et al., 2014). The bell-shaped leaf growth parameter distributions over the orthotropic rank in coffee plants suggest the age effects of the axes and environmental light modulations. The responses in coffee leaf growth parameters could be generalized and applied to younger and older plants, but the modulations with age effects could be expected (Matsunaga et al., 2016). In young plants, less complex synchronization of leaf growth parameters between axes orders could be expected, when only two axes orders are present (Rakocevic et al., 2018b) that share the same phyllochron (Matsunaga et al., 2016).
Our intention is to include the equations regarding leaf growth parameters with water and carbon availability during the seasonal growth in the CoffePlant3D software (Matsunaga et al., 2016). This software provides a reconstruction and visualization of the structure of several varieties of Arabica coffee, using an available data set modelled by mathematical and statistical methods and random algorithms. The inclusion of the equation parameters can provide a first step toward a functional–structural plant modelling of coffee tree growth, similarly to models such as L-PEACH (Da Silva et al., 2011), which considered water transport, carbohydrate allocation and physiological functions at the peach organ level. The integration of polynomial functions into CoffeePlant3D could be facilitated by their generalization as second-order functions, changing only the coefficients, according to the leaf growth parameters, which can be stored in a relational database with GDD information. The phyllochron value resulting from processing of the equation would help in decision-making about metamer emission, the duration of leaf expansion would be associated with the rate of increase in the leaf area considering its initial and final size, while leaf life span would be linked to the leaf drop and the GDD information stored in a database. This application would facilitate the generation of 3-D reconstructions of coffee plants in different growing seasons, without requiring manual, daily and exhaustive measurements.
CONCLUSIONS
The novelty of this work is that the phyllochron, leaf expansion and leaf life span in Arabica coffee plants vary within the tree structure and axes orders and that they were seasonally modified by the environment. This work could raise the question of the impact of branching order in other species. The seasonal environmental impacts on leaf growth parameters distinguished among regions of coffee production could be attributed to differences in minimum daily autumn/winter temperatures and water availability. The e[CO2] showed the compensation of the low water availability effect on duration of leaf expansion and leaf retention, while leaf life span was the least sensitive to carbon and water variations among the observed leaf growth parameters. Furthermore, the probability of occurrence of a terminal bud mortality event in the low layer was higher under e[CO2] than under a[CO2]. On the other hand, in the upper layer the probability of non-occurrence of a terminal bud mortality event was higher under e[CO2] than under a[CO2]. Investments in leaves under high carbon and water availability promoted the leaf permanence of the second- and fourth-order axes in low and upper layers during active seasonal growth, while during the dry period higher dynamics in leaf growth parameters were observed in the upper layer. Those findings could help to paint a more complete picture of possible morphophysiological responses in Arabica coffee under global climate changes. The dynamics and distribution of plant leaf area of Arabica coffee were shown to be the result of synchronization in the phyllochron, duration of leaf expansion, leaf life span, emergence of axes and senescence within the branching structure.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: meteorological data in the Cfa region. Figure S2: meteorological data in the Cwa region. Table S1: coefficients for equations defining the distribution of phyllochron in the vertical profile and the generated R2 for all treatments and axes orders. Table S2: coefficients for equations defining the distribution of leaf expansion duration in the vertical profile and the generated R2 for all treatments and axes orders. Table S3: coefficients for equations defining the distribution of leaf life span in the vertical profile and the generated R2 for all treatments and axes orders. Table S4: proportion of terminal bud mortality of third- and fourth-order axes in the vertical profile related to the total number of analysed axes of respective order.
ACKNOWLEDGEMENTS
This work was supported by the Consórcio Pesquisa Café [02.09.20.008.00.03 and 02.13.02.042.00.03], Agronomic Institute of Paraná (Londrina-PR) and Embrapa Environment (Jaguariúna-SP). M.R. thanks the Consórcio Pesquisa Café for a fellowship. We thank undergraduate students Laís Escorcio Correia and Carolina Antônio Alvim for their technical help, and the editors and three anonymous referees for all helpful criticisms and suggestions.
LITERATURE CITED
- Alla AQ, Camarero JJ, Palacio S, Montserrat-Martí G. 2013. Revisiting the fate of buds: size and position drive bud mortality and bursting in two coexisting Mediterranean Quercus species with contrasting leaf habit. Trees 27: 1375–1386. [Google Scholar]
- Amaral JAT, Rena AB, Amaral do JFT. 2006. Crescimento vegetativo sazonal do cafeeiro e sua relação com fotoperíodo, frutificação, resistência estomática e fotossíntese. Pesquisa Agropecuaria Brasileira 41: 377–384. [Google Scholar]
- Androcioli-Filho A. 2002. Café adensado – Espaçamentos e cuidados no manejo da lavoura, Circular No. 121. Londrina: IAPAR. [Google Scholar]
- Ashari S. 2002. On the agronomy and botany of Salak (Salacca zalacca). PhD Thesis, Wageningen University, The Netherlands. [Google Scholar]
- Baker JT, Allen LH Jr, Boote KJ. 1992. Temperature effects on rice at elevated CO2 concentration. Journal of Experimental Botany 43: 959–964. [Google Scholar]
- Baldissera TC, Frak E, Carvalho PF, Louarn G. 2014. Plant development controls leaf area expansion in alfalfa plants competing for light. Annals of Botany 113: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch CJ, Vos J, Kiniry J, Bos HJ, Elings A. 1998. Phyllochron responds to acclimation to temperature and irradiance in maize. Field Crops Research 59: 187–200. [Google Scholar]
- Borchert R. 1998. Responses of tropical trees to rainfall seasonality and its long-term changes. Climatic Change 39: 381–393. [Google Scholar]
- Bote AD, Vos J. 2016. Branch growth dynamics, photosynthesis, yield and bean size distribution in response to fruit load manipulation in coffee trees. Trees 30: 1275–1285. [Google Scholar]
- Burden RL, Faires JD. 2003. Numerical analysis. Pioneira Thomson Learning. [Google Scholar]
- Bustos PA, Pohlan HAJ, Schulz M. 2008. Interaction between Coffee (Coffea arabica L.) and intercropped herbs under field conditions in the Sierra Norte of Puebla, Mexico. Journal of Agriculture and Rural Development in the Tropics and Subtropics 109: 85–93. [Google Scholar]
- Camargo AP, Camargo MBP. 2001. Definição e esquematização das fases fenológicas do cafeeiro arábica nas condições tropicais do Brasil. Bragantia 60: 65–68. [Google Scholar]
- Chapa SR, Rao VB. 2004. Annual cycle of precipitation and moisture characteristics over Brazil Available at http://mtcm15.sid.inpe.br/col/cptec.inpe.br/walmeida/2004/10.14.16.
- Chaves GG, Cargnelutti AF, Alves BM, et al. 2017. Phyllochron and leaf appearance rate in oat. Bragantia 76: 73–81. http://www.scielo.br/pdf/brag/v76n1/0006-8705-brag-1678-4499090.pdf [Google Scholar]
- Cutforth HW, Jame YW, Jefferson PG. 1992. Effect of temperature, vernalization and water stress on phyllochron and final main-stem leaf number of HY320 and Neepawa spring wheats. Canadian Journal of Plant Science 72: 1141–1151. [Google Scholar]
- Da Silva D, Favreau R, Auzmendi I, Dejong TM. 2011. Linking water stress effects on carbon partitioning by introducing a xylem circuit into L-PEACH. Annals of Botany 41: 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AM, Da Silva D, Quintana B, DeJong TM. 2015. The phyllochron of Prunus persica shoots is relatively constant under controlled growth conditions but seasonally increases in the field in ways unrelated to temperature or radiation. Scientia Horticulturae 184: 106–113. [Google Scholar]
- Davidson AM, Da Silva D, Saa S, Mann P, DeJong TM. 2016. The influence of elevated CO2 on the photosynthesis, carbohydrate status, and plastochron of young peach (Prunus persica) trees. Horticulture, Environment and Biotechnology 57: 364–370. [Google Scholar]
- Dellai J, Trentin G, Bisognin DA, Streck NA. 2005. Phyllochron at different plant densities in potato. Ciência Rural 35: 1269–1274. [Google Scholar]
- De Schepper V, De Swaef T, Bauweraerts I, Steppe K. 2013. Phloem transport: a review of mechanisms and controls. Journal of Experimental Botany 64: 4839–4850. [DOI] [PubMed] [Google Scholar]
- Diepenbrock W. 2000. Yield analysis of winter oilseed rape (Brassica napus L.): a review. Field Crops Research 67: 35–49. [Google Scholar]
- Eggers L, Cadenazzi M, Boldrini II. 2004. Phyllochron of Paspalum notatum Fl. and Coelorhachis selloana (Hack.) Camus in natural pasture. Scientia Agricola 61: 353–357. [Google Scholar]
- Erickson RO, Michelini FJ. 1957. The plastochron index. American Journal of Botany 44: 297–305. [Google Scholar]
- Fanwoua J, Bairam E, Delaire M, Buck-Sorlin G. 2014. The role of branch architecture in assimilate production and partitioning: the example of apple (Malus domestica). Frontiers in Plant Science 5: 338. doi: 10.3389/fpls.2014.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felippe GM, Dale JE. 1973. Effects of shading the first leaf of barley plants on growth and carbon nutrition of the stem apex. Annals of Botany 37: 45–56. [Google Scholar]
- Garcia LC, Barros FV, Lemos-Filho JP. 2017. Environmental drivers on leaf phenology of ironstone outcrops species under seasonal climate. Annals of the Brazilian Academy of Sciences 89: 131–143. [DOI] [PubMed] [Google Scholar]
- Garcia-Huidobro J, Monteith JL, Squire GR. 1982. Time, temperature and germination of pearl millet (Pennisetum typhoides S & H). I. Constant temperature. Journal of Experimental Botany 33: 288–296. [Google Scholar]
- Ghini R, Torre-Neto A, Dentzien AFM, et al. 2015. Coffee growth, pest and yield responses to free-air CO2 enrichment. Climatic Change 132: 307–320. [Google Scholar]
- Godin C, Caraglio Y. 1998. A multiscale model of plant topological structures. Journal of Theoretical Biology 191: 1–46. [DOI] [PubMed] [Google Scholar]
- Griffon S, de Coligny F. 2014. AMAPstudio: an editing and simulation software suite for plants architecture modeling. Ecological Modelling 290: 3–10. [Google Scholar]
- Hallé F, Oldeman RAA, Tomlinson PB. 1978. Tropical trees and forests: an architectural analysis. Berlin: Springer. [Google Scholar]
- Hargreaves JNG, McMaster GS. 2009. CANON: a canonical composition for building plant shoots from the bottom up. In: Cao W, White JW, Wang E, eds. Crop modeling and decision support. Dordrecht: Springer, 59–70. [Google Scholar]
- Harmer R. 1992. Relationships between shoot length, bud number and branch production in Quercus petraea (Matt.). Liebl. Forestry 65: 61–72. [Google Scholar]
- Jamieson PD, Semenov MA, Brooking IR, Francis GS. 1998. Sirius: a mechanistic model of wheat response to environmental variation. European Journal of Agronomy 8: 161–179. [Google Scholar]
- Jullien A, Mathieu A, Allirand JM, et al. 2011. Characterization of the interactions between architecture and source–sink relationships in winter oilseed rape (Brassica napus) using the GreenLab model. Annals of Botany 107: 765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurik TW, Chabot BF. 1986. Leaf dynamics and profitability in wild strawberries. Oecologia 69: 296–304. [DOI] [PubMed] [Google Scholar]
- Kang MZ, Cournède PH, de Reffye P, Auclair D, Hu BG. 2008. Analytical study of a stochastic plant growth model: application to the GreenLab model. Mathematics and Computers in Simulation 78: 57–75. [Google Scholar]
- Kervella J, Pagès L, Génard M. 1995. Growth context and fate of axillary meristems of young peach trees. Influence of parent shoot growth characteristics and of emergence date. Annals of Botany 76: 559–567. [Google Scholar]
- Kikuzawa K. 1991. A cost–benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. American Naturalist 138: 1250–1263. [Google Scholar]
- Lemaire S, Maupas F, Cournède PH, de Reffye P. 2009. A morphogenetic crop model for sugar-beet (Beta vulgaris L.). In: Cao W, White JW, Wang E, eds. Crop modeling and decision support. Dordrecht: Springer, 116–129. [Google Scholar]
- Majerowicz N, Söndahl MR. 2005. Induction and differentiation of reproductive buds in Coffeea arabica L. Brazilian Journal of Plant Physiology 17: 247–254. [Google Scholar]
- Matsunaga FT, Tosti JB, Androcioli-Filho A, Brancher JD, Costes E, Rakocevic M. 2016. Strategies to reconstruct 3D Coffea arabica L. plant structure. SpringerPlus 5: 2075. doi: 10.1186/s40064-016-3762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles EJL, Camargo MBP, Pezzopane JRM, et al. 2009. Fenologia do Cafeeiro: condições agrometeorológicas e balanço hídrico do ano agrícola 2004–2005. Embrapa Informação Tecnológica. [Google Scholar]
- Narayanan S, Aiken RM, Prasad PVV, Xin Z, Paul G, Yu J. 2014. A simple quantitative model to predict leaf area index in sorghum. Agronomy Journal 106: 219–226. [Google Scholar]
- Pantin F, Simonneau T, Rolland G, Dauzat M, Muller B. 2011. Control of leaf expansion: a developmental switch from metabolics to hydraulics. Plant Physiology 156: 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzopane JRM, Júnior MJP, de Camargo MBP, Fazuoli LC. 2008. Heat requirements of Mundo Novo coffee for the flowering-harvest phenological stage. Ciência e Agrotecnologia 32: 1781–1786. [Google Scholar]
- Pradal C, Boudon F, Nouguier C, Chopard J, Godin C. 2008. PlantGL: a Python-based geometric library for 3D plant modelling at different scales. Graphical Models 71: 1–21. [Google Scholar]
- Rakocevic M, Androcioli-Filho A. 2010. Morphophysiological characteristics of Coffea arabica L in different arrangements: lessons from a 3D virtual plant approach. Coffee Science 5: 154–166. [Google Scholar]
- Rakocevic M, Scholz MBS, Charmetant P. 2015. Leaf photosynthesis in four coffee genotypes as response to the irrigation during the biennial period. In: IX Simpósio de Pesquisa dos Cafés do Brasil, Curitiba-PR, 89: 1–6. [Google Scholar]
- Rakocevic M, Ribeiro RV, Marchiori PER, Filizola HF, Batista ER. 2018a Structural and functional changes in coffee trees after 4 years under free air CO2 enrichment. Annals of Botany 121: 1065–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakocevic M, Scholz MBS, Kitzberger CSG. 2018b Berry distributions on coffee trees cultivated under high densities modulate the chemical composition of respective coffee beans during one biannual cycle. International Journal of Fruit Science 18: 117–137. [Google Scholar]
- Rosa HT, Walter LC, Streck NA, Andriolo JL, da Silva MR, Langner JA. 2011. Base temperature for leaf appearance and phyllochron of selected strawberry cultivars in a subtropical environment. Bragantia 70: 939–945. [Google Scholar]
- Scilab Enterprises 2017. SciLab, open source software for numerical computation Last accessed 15 May 2017.
- Silva EA, DaMatta FM, Ducatti C, Regazzi AJ, Barros RS. 2004. Seasonal changes in vegetative growth and photosynthesis of arabica coffee trees. Field Crops Research 89: 349–357. [Google Scholar]
- Silveira JSM, Carvalho CHS. 1996. Efeito da época de irrigação sobre o crescimento do ramo plagiotrópico e da longevidade foliar do café conilon. In: Anais de 22º Congresso Brasileiro de Pesquisas Cafeeiras, PROCAFÉ, Água de Lindóia, 99–100. [Google Scholar]
- Streck NA, Tibola T, Lago I, et al. 2005. Estimating the plastochron in muskmelon (Cucumis melo L.) grown inside plastic greenhouse at different planting dates. Ciência Rural 35: 1448–1450. [Google Scholar]
- Van Volkenburgh E. 1999. Leaf expansion – an integrating plant behavior. Plant, Cell and Environment 22: 1463–147. [Google Scholar]
- White J. 1979. The plant as a metapopulation. Annual Review of Ecology and Systematics 10: 109–145. [Google Scholar]
- Wilhelm WW, McMaster GS. 1995. Importance of the phyllochron in studying development and growth in grasses. Crop Science 35: 1–3. [Google Scholar]
- Xue Q, Weiss A, Baenziger PS. 2004. Predicting leaf appearance in field-grown winter wheat: evaluating linear and non-linear models. Ecological Modelling 175: 261–270. [Google Scholar]
- Zhu J, Vos J, Werf van der W, Putten van der PEL, Evers JB. 2014. Early competition shapes maize whole-plant development in mixed stands. Journal of Experimental Botany 65: 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.