Abstract
Background and Aims
In several disciplines, identifying relevant root traits to characterize the root system architecture of species or genotypes is a crucial step. To address this question, we analysed the inter-specific variations of root architectural traits in two contrasting environments.
Methods
We sampled 60 species in natura, at two sites, each presenting homogeneous soil conditions. We estimated for each species and site a set of five traits used for the modelling of the root system architecture: extreme tip diameters (Dmin and Dmax), relative diameter range (Drange), mean inter-branch distance (IBD) and dominance slope between the diameters of parent and lateral roots (DlDm).
Key Results
The five traits presented a highly significant species effect, explaining between 77 and 98 % of the total variation. Dmin, Dmax and Drange were particularly determined by the species, while DlDm and IBD exhibited a higher percentage of environmental variations. These traits make it possible to confirm two main axes of variation: ‘fineness–density’ (defined by Dmin and IBD) and ‘dominance–heterorhizy’ (DlDm and Drange), that together accounted for 84 % of the variations observed.
Conclusions
We confirmed the interest of these traits in the characterization of the root system architecture in ecology and genetics, and suggest using them to enrich the ‘root economic spectrum’.
Keywords: Root branching, phenotyping, modelling, root method, root trait, architecture, root economic spectrum
INTRODUCTION
Plant root systems are essential components of ecosystems and agro-ecosystems, and recent papers have emphasized the importance of their study in the field of genetics (Price et al., 1997; Dorlodot et al., 2007; Courtois et al., 2009; Watt et al., 2013; Kuijken et al., 2015; York and Lynch, 2015) as well as in the field of ecology (Picon-Cochard et al., 2012; Bardgett et al., 2014; De La Riva et al., 2016; Roumet et al., 2016; Iversen et al., 2017), where there is an increasing concern about inter- and intra-specific variations of traits (Mommer and Weemstra, 2012; Siefert et al., 2015). Characterizing the root system architecture (RSA) and its dynamics is particularly important in order to understand root functions and interactions with the soil environment (York and Lynch, 2015), but it is particularly challenging because of the difficulties in accessing growing roots in the soil and because of the plasticity of root systems in this heterogeneous medium. The large samples required by genetic studies in the broad sense exacerbate the difficulty. A common approach in ecology and agricultural sciences is to sample root systems or soil volumes and to evaluate root traits defined at the root system level, such as root length, biomass, depth or specific root length (length per dry mass). All these traits depict various aspects of the root functioning of plants, communities or ecosystems. For example, the distribution of root length density is commonly used as input in uptake models for crops (Nye and Tinker, 1969; Barber and Silberbush, 1984). Specific root length is a favourite trait for the characterization of the acquisitive/conservative behaviour of species in the ‘root economic spectrum’ (Wright et al., 2004; Bardgett et al., 2014; Kramer-Walter et al., 2016). All these root traits, which can be described as ‘integrated traits’, are dependent on time or developmental stages (e.g. Cornelissen et al., 2003; Picon-Cochard et al., 2012), species or genotypes (e.g. Craine et al., 2001; Comas and Eissenstat, 2009; Makita et al., 2012; Matias et al., 2012; Gu et al., 2014; Kong et al., 2014; Valverde-Barrantes et al., 2015, 2017; Roumet et al., 2016), and environmental conditions including soil and climate (e.g. Atkin et al., 2000; Craine et al., 2001). However, these three sources of variation are barely separable in most studies because of the sampling designs.
In order to characterize the RSA more specifically, Pagès (2014, 2016) proposed a set of five traits and a method to evaluate them. These traits are: minimal and maximal tip diameters (Dmin and Dmax); relative range of diameters (Drange); slope of the linear relationship between the tip diameters of lateral roots and the tip diameters of their parent root (DlDm); and inter-branch distance along the parent root (IBD). These traits were conceived to summarize a number of essential architectural attributes of root systems which are connected to the exploration and exploitation capacities of root systems. The minimal diameter (Dmin) reflects the fineness of the numerous roots developed as ultimate branches of root systems, which are usually among the shortest and have a pure absorptive function. Developing very fine roots (low Dmin) is a prime strategy to increase the soil–root exchange surface at a minimal cost (Eissenstat et al., 2000), all the more so because the finest roots tend to be the simplest from a structural viewpoint (e.g. Varney et al., 1991) with a low mass tissue density (Drouet et al., 2005; Picon-Cochard et al., 2012). The Dmax is observed among the longest roots which explore the soil and extend the colonized volume. Thus, the roots with large tips contribute to the determination of the overall amount of available soil resources. The root system extension to depth, for instance, is often used as an indicator of available water for the plant (Cabelguenne and Debaeke, 1998). Large tip diameters were also shown to be favourable for the penetration of strong soils (Materechera et al., 1992; Watt et al., 2013), a decisive advantage in order to achieve this exploration function. In his simulation study, Pagès (2011) showed that not only the extreme diameters considered separately, but also their relative range (Drange), could have a significant and positive impact on the colonized volume. The IBD, i.e. the reciprocal of linear branching density, strongly contributes to defining the root length density per unit of soil volume (Pagès, 2011) and therefore the intensity of soil exploitation. Since diameters are reduced from the mother roots to their laterals through branching, DlDm defines the rate of diameter transition from the thickest to the finest. It is assumed to modulate the topological characteristics between the two extreme figures defined by Fitter (from 1982 onwards): herringbone (strong dominance, low DlDm) and dichotomous (low dominance, high DlDm). Thus, the five traits together are indicative of growth and branching behaviour, and also of the exploration and exploitation functions of the root system. As such, they are associated with a modelling approach, acting as input parameters of a simple architectural model, called Archisimple (Pagès et al., 2014). In this particular model, which was designed to describe and predict the RSA of numerous species in various environments, these traits are the drivers of root elongation and branching. They are thought to depend mainly on genotypes or species and to be stable across environmental conditions. Beyond the significance of each individual trait, model simulations make it possible to combine the proposed trait/parameter values with environmental characteristics to calculate more integrated and dynamic traits, such as root length density profiles or colonized volumes. Thus, the association of the set of traits, the measurement protocol and the dynamic model of the root system representing interactions with the environment is an interesting toolbox. The approach was validated from a theoretical point of view (Pagès, 2011; Zhao et al., 2017). Applied to a set of Poaceae species (Pagès and Picon-Cochard, 2014), it successfully bridged the set of input traits to root depth, root length distribution and specific root length.
In previous papers, Pagès (2014, 2016) demonstrated the feasibility of the measurements of the proposed set of traits in natura on a large number of species and environment combinations, i.e. phenotypes. The large number of phenotypes made it possible to study correlations between traits, revealing underlying trade-offs. A strong positive correlation was shown between Dmin and IBD, leading to an axis called the ‘fineness–density’ axis. Phenotypes with the finest roots (low Dmin) were associated with a high branching density (low IBD), and vice versa: phenotypes with thicker roots (high Dmin) also had spaced branches (high IBD). Another correlation was shown between the relative range of diameters (Drange) and the branching dominance (DlDm). A larger range of diameters was associated with a stronger dominance (‘heterorhizy–dominance’ axis).
To go further into the validation of the approach, with the ultimate aim of accounting for genotype × environment interactions, we now want to evaluate the strength of the inter-specific variations and correlations of the traits, in comparison with their environmental variations. For this study, our strategy was to extend the sampling design of Pagès (2016) in order to obtain pairs of evaluations of the same species within two contrasted environments. To obtain a relevant ranking of the five traits regarding their relative stability to environmental conditions, it was necessary to evaluate them in a large number of species. We obtained 60 pairs for rather widespread species, belonging to common families.
MATERIALS AND METHODS
Sample species and sites
We sampled 60 different species that were found at two contrasting and homogeneous sites. Each species was sampled at both sites between 2013 and 2017. Most species grew spontaneously in kitchen gardens, cultivated fields or meadows, as weeds or regrowth of previous crops. Some were sown or planted in gardens. The list of these species is given in Table 1, using the names of Tela Botanica (http://www.tela-botanica.org/), adapted to the French flora.
Table 1.
List of species and families
| Species name | Family | Biological type |
|---|---|---|
| Amaranthus retroflexus | Amaranthaceae | Therophyte |
| Atriplex hortensis | Amaranthaceae | Therophyte |
| Chenopodium album | Amaranthaceae | Therophyte |
| Allium cepa | Amaryllidaceae | Geophyte |
| Allium porrum | Amaryllidaceae | Geophyte |
| Vinca major | Apocynaceae | Chamephyte |
| Vinca minor | Apocynaceae | Chamephyte |
| Hedera helix | Araliaceae | Panerophyte |
| Artemisia vulgaris | Asteraceae | Hemicryptophyte |
| Lapsana communis | Asteraceae | Therophyte |
| Pilosella officinarum | Asteraceae | Hemicryptophyte |
| Senecio vulgaris | Asteraceae | Therophyte |
| Sonchus asper | Asteraceae | Therophyte/hemicryptophyte |
| Sonchus oleraceus | Asteraceae | Therophyte/hemicryptophyte |
| Taraxacum officinale | Asteraceae | Hemicryptophyte |
| Lycopsis arvensis | Boraginaceae | Therophyte |
| Alliaria petiolata | Brassicaceae | Hemicryptophyte |
| Capsella bursa-pastoris | Brassicaceae | Therophyte |
| Cardamine hirsuta | Brassicaceae | Therophyte |
| Lunaria annua | Brassicaceae | Hemicryptophyte |
| Silene latifolia | Caryophyllaceae | Hemicryptophyte |
| Stellaria media | Caryophyllaceae | Therophyte |
| Euphorbia helioscopia | Euphorbiaceae | Therophyte |
| Lotus corniculatus | Fabaceae | Hemicryptophyte |
| Medicago lupulina | Fabaceae | Therophyte |
| Trifolium pratense | Fabaceae | Hemicryptophyte |
| Trifolium repens | Fabaceae | Hemicryptophyte |
| Geranium molle | Geraniaceae | Therophyte |
| Geranium robertianum | Geraniaceae | Therophyte |
| Ajuga reptans | Lamiaceae | Hemicryptophyte |
| Glechoma hederacea | Lamiaceae | Therophyte |
| Lamium amplexicaule | Lamiaceae | Therophyte |
| Lamium purpureum | Lamiaceae | Therophyte |
| Malva neglecta | Malvaceae | Therophyte |
| Chelidonium majus | Papaveraceae | Therophyte |
| Papaver rhoeas | Papaveraceae | Therophyte |
| Linaria repens | Plantaginaceae | Hemicryptophyte/geophyte |
| Plantago lanceolata | Plantaginaceae | Hemicryptophyte |
| Plantago major | Plantaginaceae | Hemicryptophyte |
| Veronica hederifolia | Plantaginaceae | Therophyte |
| Veronica persica | Plantaginaceae | Therophyte |
| Dactylis glomerata | Poaceae | Hemicryptophyte |
| Holcus lanatus | Poaceae | Hemicryptophyte |
| Hordeum murinum | Poaceae | Therophyte |
| Lolium perenne | Poaceae | Hemicryptophyte |
| Poa annua | Poaceae | Therophyte |
| Fallopia convolvulus | Polygonaceae | Therophyte |
| Polygonum aviculare | Polygonaceae | Therophyte |
| Lysimachia arvensis | Primulaceae | Therophyte |
| Ficaria verna | Ranunculaceae | Geophyte |
| Fragaria vesca | Rosaceae | Hemicryptophyte |
| Potentilla reptans | Rosaceae | Hemicryptophyte |
| Rubus idaeus | Rosaceae | Phanerophyte |
| Rubus ulmifolius | Rosaceae | Phanerophyte |
| Galium aparine | Rubiaceae | Therophyte |
| Verbascum thapsus | Scrophulariaceae | Hemicryptophyte |
| Solanum tuberosum | Solanaceae | Geophyte |
| Urtica dioica | Urticaceae | Hemicryptophyte |
| Viola odorata | Violaceae | Hemicryptophyte |
| Viola tricolor | Violaceae | Therophyte/hemicryptophyte |
The names of species follow the names of Tela Botanica (www.tela-botanica.org). The biological types are adapted from the Raunkiaer’s classification by Tison et al. (2014).
The sampling sites were chosen because each of them was rather homogeneous (soil origin and climate) and they differed markedly regarding soil and climate. Moreover, their soils were suitable for root excavation because they were rather light (bulk density <1.3) with low levels of clay and stones. The first site is located near Thouzon, in the south-east of France (Provence region: latitude, 43°57’; longitude, 4°59’; altitude, 50 m), with a Mediterranean climate. The soil is a deep calcareous silty soil developed on loess on a geological plain (called ‘Plaine de Thouzon’). The second site is around Nozeyrolles, located in the Massif Central (Auvergne region: latitude, 44°59’; longitude, 3°24’; altitude, 1100 m). Its climate can be succinctly qualified as oceanic/mountainous. The soil was a sandy brown soil developed on the granitic arena of a geological plateau (called ‘Plateau de la Margeride’). The main characteristics of the superficial soils, given by the Laboratory of Soil Analyses (INRA Arras, France), are indicated in Table 2. The main differences concerned pH and soil texture, with more coarse sand and clay in Nozeyrolles and more fine sand and silt in Thouzon.
Table 2.
Main characteristics of the superficial soils – averaged between 5 cm and 40 cm deep – at the two sampling sites
| Sampling site | Thouzon | Nozeyrolles |
|---|---|---|
| Clay (<2 µm) | 129 | 127 |
| Fine silt (2–20 µm) | 299 | 117 |
| Coarse silt (20–50 µm) | 157 | 54 |
| Fine sand (50–200 µm) | 243 | 111 |
| Coarse sand (200–2000 µm) | 173 | 592 |
| pH (water) | 8.9 | 6.7 |
| Carbon | 16.6 | 13.0 |
| Nitrogen | 1.46 | 0.865 |
| Total organic matter | 28.6 | 22.5 |
Values are given in g kg–1, with the exception of pH.
Masses are given on a dry matter basis.
Small variations were noted around these mean characteristics because of local effects mainly due to micro-topography and hydrography. From a climatic point of view, the between-site differences are important, since there is a 7 °C difference in average temperature and a 150 mm difference in average precipitation, with a wetter and more even distribution in Nozeyrolles, due to the oceanic and altitude influences. Moreover, since both sites are distant from each other, submitted to different climatic influences, with shifts of several weeks between the phenological stages of the vegetation, we assumed the independence of weather conditions for each pair of species.
Sampling and excavation procedure
Sampling and measurement methods followed those presented in Pagès (2014). We favoured rather young and vigorous plants at different stages until flowering, especially for dicot species, to obtain a high percentage of healthy and growing roots in the sampled monolith. Sampled plants typically had from eight to 30 unfolded leaves on the main shoot. For Poaceae species, we sampled plants with many tillers, at the flowering stage to ensure their correct determination.
The sampling design was partly dictated by the availability of plants at suitable stages. A total of 2–5 plants per species and site were excavated during the 5 year period of the study. The individual plants were not considered as replicates since all the samples from the same species and site were pooled to measure the root traits as explained below. Isolated plants were preferred to facilitate the subsequent separation of the roots. We used a garden fork to demarcate a monolith around the chosen plant (radius 15–20 cm around the collar, 30–50 cm deep), and to extract it before putting it in a metal mesh in a large bucket filled with water. Then, the monolith was gently washed with running water. Once the root system was nearly free of soil and organic debris, it was moved to a black trough and left for 30 min in salt water (2 g L–1) with liquid soap to complete the cleaning process. The whole study involved the sampling and treatment of >350 monoliths.
Scanning and measurements
Using paintbrushes and mounted needles, root systems were separated in the trough and spread carefully in a several millimetres deep layer of water contained in a transparent plastic tray. The densest root systems were cut into several pieces in order to minimize root overlap in the tray. They were then scanned with flatbed scanners (EPSON perfection V700 and V850) at a resolution of 1200–4800 dpi, using the transparent mode. The resolution was adjusted for each species so as to obtain at least ten pixels transversally to the finest roots, in order to measure them with sufficient accuracy. Previous tests had shown that this adjustment did not introduce any bias, since we obtained the same values (on average) when measuring the same objects at these various resolutions. We also validated the parallel use of several scanners.
Measurements were made on the computer screen by mouse clicking on the displayed images using the measuring tools (i.e. length of a straight line and a segmented line) provided by the ImageJ software (http://rsbweb.nih.gov/ij/). On these images, we identified sub-structures, consisting of young parts of roots together with their laterals, on which we measured the diameter of the parent root, the diameter of the laterals and the distance along the parent root from each lateral to its proximal closest neighbour (from axis to axis). We quantified branching density through the IBD (its reciprocal), because this variable could be measured for each lateral root.
For each sample (species × site), we measured from 80 to 200 lateral roots (total: 15 621 roots).
All diameters (also called ‘apical diameters’ hereafter) were measured on the young part of the roots, as recommended by McCormack et al. (2015), at a location where it is nearly cylindrical and where it exhibits a primary structure. The closeness of the growing apex guarantees that the zone is young. In cases where the apex of the root was broken, it could be certified by the combination of several visual criteria obtained on intact roots from the same sample (e.g. the short length of the laterals if any, colour, tissue transparency and structure, turgescence, integrity of the cortex, presence and state of the root hairs). Short zones of local thickening were sometimes observed along the roots, thought to be due to local mechanical constraints (Konôpka et al., 2009) because they were associated with local curvatures. They were systematically avoided for diameter measurements.
Data analyses
All data treatments, plots and analyses were done with the R software (R Core Team, 2013; http://www.r-project.org/). We used the non-parametric Wilcoxon signed rank test (function ‘wilcox.test’) to test the differences of trait distributions between the two sites. We estimated the parameters of linear models with the ‘lm’ function and performed analyses of variance (ANOVA) with the ‘anova’ function to test the species and site effects on the traits. Principal component analyses (PCAs) were performed with the ‘ade4’ R package (Chessel et al., 2004).
In this study, we considered five traits (Dmin, Dmax, Drange, IBD and DlDm) which were presented and justified in detail in Pagès (2014). One value was estimated per species and per site for each trait. Dmin is the ‘minimal’ tip diameter developed by the given species on the given site. It was estimated by the 2 % quantile of the diameter distribution of all measured lateral roots. Dmax is the maximal tip diameter developed by the species. It was estimated by the maximal value of the diameter distribution of all the measured roots. IBD is the inter-branch distance, calculated as the average value of the distances between neighbouring lateral roots located on mother roots with a diameter above the central value of the diameter range [i.e. >0.5 × (Dmin + Dmax)]. DlDm is the slope of the linear regression of the diameter of lateral roots vs. that of their mother, this linear regression being forced to pass through the co-ordinates (Dmin, Dmin). The lowest is DlDm and the highest is the diameter dominance between mother and daughter roots. Drange, the relative range of diameters, is calculated using extreme diameter values such as: 2 × (Dmax – Dmin)/(Dmax + Dmin).
RESULTS
Distribution of traits and overall effects of species and site
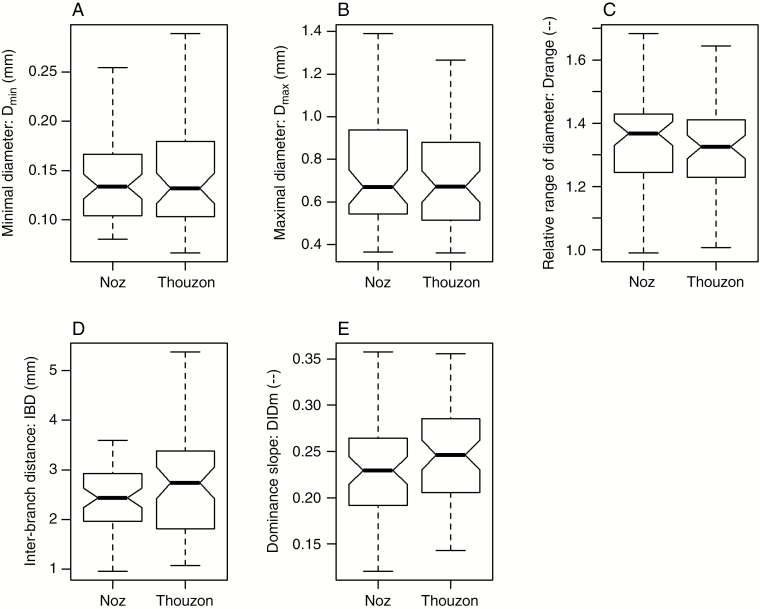
Figure 1 presents the distribution of traits in the population split according to the observation site. It shows that the trait variations were large, especially for Dmin and IBD, with a ratio of 6.7 between the maximal and minimal value. Visually, the distributions of these variables were rather similar, except for IBD where the distribution was wider in Thouzon and for DlDm where it was slightly higher. A Wilcoxon signed rank test confirmed the significance of these differences only for these two traits (P = 0.031 for IBD; P = 0.037 for DlDm).
Fig. 1.
Boxplots showing the distributions of the traits for the two sites (Nozeyrolles and Thouzon). The 0.05 and 0.95 quantiles are indicated on these boxplots by the extreme lower and upper horizontal segments. The central thick segments are the medians, which are statistically different when the notches do not overlap. The two other segments are the 0.25 and 0.75 quartiles.
In addition, we performed an ANOVA for each trait (response variable), studying the effects of species (60 level factor) and site (two level factor) in an additive model (Table 3). The absence of replicates at each site prevented the study of an interaction effect between the two factors. The analysis showed a strong and highly significant effect of the species on all traits (the highest P-value only reached 4.47e-7, for DlDm). According to this ANOVA, the species factor explained between 77 (for DlDm) and 98 % (for Dmin) of the total variation of the traits. The site effect was significant only for DlDm (P = 0.015), for which the Nozeyrolles site tended to give lower values (i.e. stronger dominance in diameters). For IBD, the P-value of the site effect was close to the 5 % threshold (P = 0.055).
Table 3.
Analyses of variance to test the effects of species and sites on the five traits
| Trait | Effect | d.f. | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| Dmin | Species | 59 | 0.0075193 | 51.4 | 0.000*** |
| Site | 1 | 0.0000752 | 0.514 | 0.476n.s. | |
| Residuals | 59 | 0.0001462 | |||
| Dmax | Species | 59 | 0.194415 | 23.4 | 0.000*** |
| Site | 1 | 0.009937 | 1.19 | 0.279n.s. | |
| Residuals | 59 | 0.008326 | |||
| Drange | Species | 59 | 0.043693 | 8.10 | 0.000*** |
| Site | 1 | 0.004429 | 0.821 | 0.369n.s. | |
| Residuals | 59 | 0.005395 | |||
| IBD | Species | 59 | 2.59652 | 9.28 | 0.000*** |
| Site | 1 | 1.07721 | 3.8491 | 0.0545 | |
| Residuals | 59 | 0.27986 | |||
| DlDm | Species | 59 | 0.0046375 | 3.78 | 0.000*** |
| Site | 1 | 0.0077038 | 6.27 | 0.0151* | |
| Residuals | 59 | 0.0012275 |
Relationships between sites
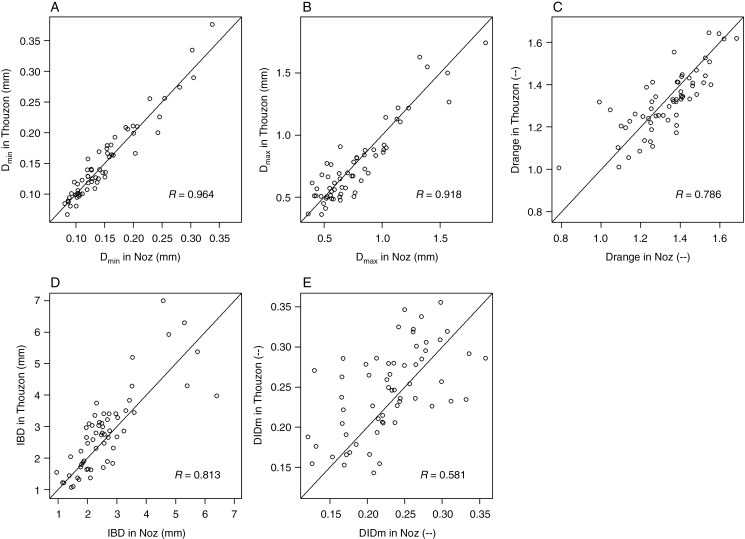
The relationships between the different trait values at the two sites are presented in Fig. 2. The bisecting lines were drawn on the graphs to facilitate the visual location of the points.
Fig. 2.
Comparison of the trait values for the two sites. On each graph, the bisecting line was drawn as a landmark. Correlation coefficients were also indicated on each graph.
Analyses showed several tight correlations, especially for Dmin and Dmax. The correlation was still highly significant but looser for DlDm. Correlation coefficient values confirmed that for all five traits, the species effect was high in comparison with the site effect. Unlike other traits, the points regarding DlDm (Fig. 2E) were not distributed equally above and under the bisecting line, confirming the weak effect of the site on this trait.
Principal component analysis)
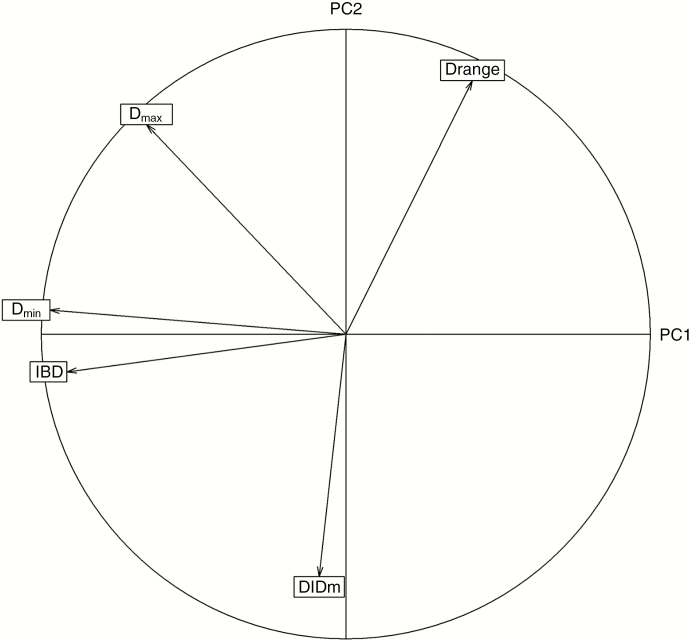
The first plane of the PCA is presented in Fig. 3. The first two principal components accounted for 48 and 36 % of the variations, respectively. All variables were close to the correlation circle, which means that they were all very well represented on this first plane. Dmin and IBD were highly negatively correlated with the first principal component (PC1) whereas the second principal component (PC2) was mostly correlated with Drange (positively) and with DlDm (negatively). Dmax had an intermediate position between the two components.
Fig. 3.
Trait values and correlation circle projected on the first plane of the PCA (defined by the two first components).
Analyses of variance were carried out on these two principal components (Table 4). The ‘species’ effects were highly significant for both components. They explained 96 and 91 % of the total variations for PC1 and PC2, respectively. Conversely, the ‘site’ effect was not significant for PC1, and significant but weak for PC2 (P = 0.01).
Table 4.
Analyses of variance to test the effects of species and site on the first and second principal components of the PCA
| Principal component | Effect | d.f. | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| PC1 | Species | 59 | 4.699 | 28.5 | 0.000*** |
| Site | 1 | 0.460 | 2.79 | 0.1n.s. | |
| Residuals | 59 | 0.165 | |||
| PC2 | Species | 59 | 3.363 | 11.1 | 0.000*** |
| Site | 1 | 2.097 | 6.91 | 0.0109* | |
| Residuals | 59 | 0.304 |
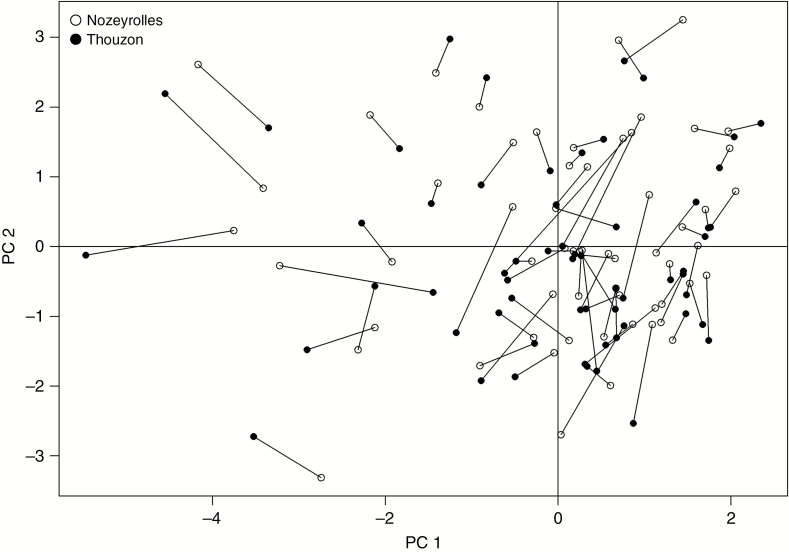
In Fig. 4, individual observations (each corresponding to one species at one site) were projected on this PC1–PC2 plane. The points were scattered without any visible cluster, showing that the co-ordinates on this plane displayed continuous variations. The two points from the same species at the two sites, linked by a segment, were close to each other, confirming the dominant species effect. Moreover, these segments did not exhibit any directional structure, attesting to the weak site effect.
Fig. 4.
Individuals (species observed at sites) projected on the first plane of the PCA. Segments join the points of the same species.
DISCUSSION
Unexpectedly large genetic variations in root system architecture were evidenced among common species
For all traits, we observed large relative variations: the lowest for Drange and the highest for IBD. The species effect was dominant for all traits. It explained between 77 and 98 % of the total variations. We did not expect such tightness in the correlation between the two sites for four traits: Dmin, Dmax, Drange and IBD. The definition of these traits and the procedure established to measure them clearly emphasized the genetic capacities of the plant. Our results for diameter traits are in accordance with the recent findings of several teams (Gu et al., 2014; Kong et al., 2014; Valverde-Barrantes et al., 2017) who pointed out the importance of the species effect and of the phylogenetic signal that exists in several root traits, particularly in the root diameter. Our work refines this result for several aspects of the root diameter (extreme and range) and extends it to other architectural traits (IBD and DlDm) for which the importance of inter-specific variations is more controversial (Kong et al., 2014). Dmin exhibited the largest inter-specific variations in comparison with total variations. This probably indicates that the capacity to produce thin roots is genetically determined, and that thin roots with similar diameters are always produced by a plant belonging to a given species, whatever the environmental conditions, provided that the plant has developed sufficiently. The percentage of variations explained by the species was slightly lower for Dmax. Several reasons may explain this: (1) the characteristics of the thickest roots may be more dependent on environmental conditions than those of the thinnest roots; (2) the number of thick roots is much lower than the number of thin roots, the sample to estimate Dmax is therefore reduced; and (3) Dmax is less variable than Dmin among species.
Dmin and Dmax were not very strongly correlated. In addition, Drange was also largely dependent on species, showing that differences of Dmin and Dmax could not be summarized by a scaling effect. Beyond the theoretical consideration that the same average diameter could be obtained in species with large differences in extreme diameters, this justifies considering extreme diameters instead of average values as done in most studies (Cornelissen et al., 2003). The IBD values were in keeping with previously published data (e.g. Johnson and Aguirre, 1991; Pagès and Pellerin, 1994; Fita et al., 2008; Arredondo and Johnson, 2011; Adu et al., 2014; Colombi and Walter, 2016; Wu et al., 2016). The recommendations for IBD measurements, i.e. measuring IBDs on the young branched zones of several thick roots and discarding zones where the roots have encountered strong soils (Pagès, 2014, 2016), were effective in reducing variations due to local heterogeneity reported by Malamy (2005). Although sensitive to global and local environmental conditions, branching intensity also clearly depends on plant species. For DlDm, the species was also the main source of variation. The method proposed for determining DlDm provides a way to quantify, at least partially, the dominance between mother and daughter roots (alternative method to that of Fitter, 1982).
The first two principal components corresponded to the previously (Pagès, 2016) identified ‘fineness–density’ axis determined by Dmin and IBD and the ‘dominance–heterorhizy’ axis determined by Drange and DlDm. They were highly controlled by species effects, which explained 96 and 91 % of the total variations on these axes. These results confirm that these two axes may correspond to important characteristics of root system architecture and that the corresponding trade-offs are under genetic control. The ‘fineness–density’ axis would mean that species can produce high-density branching only if they are able to produce thin roots. The ‘dominance–heterorhizy’ axis indicates that differences between mother and daughter are greater in species producing diverse roots. These two axes are orthogonal, which suggests that branching density (measured with IBD) and branching dominance (measured with DlDm) are independent features.
Possible use for species characterization
The set of traits we propose could help characterize inter-specific RSA variations in natura and be included in developing databases (Iversen et al., 2017). The species studied were chosen because they were present on both sites (Nozeyrolles and Thouzon) in spite of the large differences in environmental conditions at these sites. Further investigations revealed that most of them had spread throughout world temperate zones [US National Plant Germplasm System https://npgsweb.ars-grin.gov USDA, NRCS, Plants database http://plants.usda.gov). Thus, large genetic variations for all the traits studied were observed among very common species. Even larger variations could be expected for species living in more extreme environments. The large range observed for all traits can be seen as an advantage from a phenotyping perspective (de Dorlodot et al., 2007), because it makes it possible to include measurement uncertainty, and it defines a large scale to quantify and compare species. The main difficulty for studies in natura would be the excavation of plants. In some species, Dmax is only observed on long and deep roots, which are not easily obtained. Care is also necessary because such roots with the thickest tips are not abundant. The finest roots, close to the Dmin diameter, are very fragile and easily desiccated, but they are also short and numerous. Thus, it is rather easy to include a large number of them in the measured samples. Their measurements also require very careful excavation and high-resolution images (between 1200 and 4800 dpi depending on the species). The excavation of whole root systems is not required, however, provided that one can make sure that the roots belong to the targeted species. In cases where the tracking of roots would be very difficult, DNA fingerprints of the samples could be used.
Possible use for intra-specific phenotyping
Besides specific characterization, there is an increasing demand for the study of intra-specific variations, especially in the field of genetics and breeding (Colombi et al., 2015; Kuijken et al., 2015; Walter et al., 2015). Seeing the large inter-specific variations we observed, intra-specific variations are likely to affect the traits studied, as observed for other traits (Dorlodot et al., 2007). Indeed, intra-specific variations for these traits have already been observed in three Solanaceae species by Bui et al. (2015). For genetics studies, numerous genotypes have to be characterized and observation methods must be simple and fast. Growing plants in sifted soil in individual pots would free the method from the main difficulties faced in field studies, i.e. the disentangling of roots from other root systems, organic debris and strong soil. In keeping with other results (Watt et al., 2013; Zhao et al., 2017), our observations suggest, however, that the characterization of very young seedlings can only give a restricted view of the RSA. Both the ‘fineness–density axis’ and the ‘dominance–heterorhizy’ axis involve traits that can only be reliably observed on well-developed plants. The minimal diameter (Dmin) must be estimated on plants which have already expressed all their branching orders. The maximal diameter (Dmax) may be observed very early on during the ontogenesis in young radicles such as in Pisum sativum, or much later on, e.g. on late nodal roots as in Zea mays, to take the example of two well-known species (not included in the present study). For some species, the thickest root tips were found at the very periphery of the monolith, relatively deep in the soil. Therefore, phenotyping procedures must include plants with a sufficient level of development, and monoliths or containers must be sufficiently deep. Based on the experience gained by these numerous plant observations, it is also important to study plants of sufficient vigour to obtain the right (very maximal) Dmax, which can be seen as the genetic potential of the species.
In order to simplify the measurements further, the close association of Dmin and IBD on the PC1 and the opposition of Drange (calculated from Dmin and Dmax) and DlDm on the PC2 suggested that, as a first approach, one could only measure Dmin and Dmax. A PCA based on Dmax, Dmin and Drange (not shown) gave similar results to those with the whole set of traits. The correlation coefficients between these two analyses were 0.9 and 0.84 for PC1 and PC2, respectively.
Use of the traits to characterize environmental conditions
Although clearly dependent on species, Dmax, Drange, DlDm and IBD were all affected by environmental conditions. According to our observations, the general vigour of the plant or local strong soil conditions might influence the expression of these traits. Thus, these traits could also be used to characterize the plasticity of the RSA, with other adapted sampling strategies. On the basis of PCA results, it is now possible to choose a sub-set of species that would encompass most of the variations for the root traits. One could describe the traits in a larger set of environmental and controlled conditions that can be obtained in other laboratory experiments (such as in the work of Colombi and Walter, 2016 or Moreau et al., 2017) and thus get a better insight into the key environmental determinants of these trait variations.
CONCLUSIONS
The present study validates the use of the five traits as model parameters to characterize species or genotypes, since, for all the traits measured, the site effect was much lower than the species effect. Combining the evaluation of the proposed traits with the use of the ‘Archisimple’ model would provide a dynamic vision of the RSA for the species studied in natura, which could be difficult to obtain otherwise, as recommended by Dunbabin et al. (2013). The model could also be used to establish the link between these analytical traits and desirable agronomic traits (as shown by Pagès and Picon-Cochard, 2014).
A reduced sample of species could now be chosen to determine the environmental factors which contribute to modulate the expression of Dmax, Drange and, above all, DlDm and IBD which exhibited larger site and residual variations, in order to improve our understanding of root system plasticity.
ACKNOWLEDGEMENTS
We thank César Kunasz, Héloïse Pagès, Emilie Perrousset and Valérie Serra for their help in scanning and measuring the roots. This work was financially supported by the ANR (Agence Nationale de la Recherche) project COSAC (ANR-2014-CE18-0007-04).
LITERATURE CITED
- Adu MO, Chatot A, Wiesel L. 2014. A scanner system for high-resolution quantification of variation in root growth dynamics of Brassica rapa genotypes. Journal of Experimental Botany 65: 2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo JT, Johnson DA. 2011. Allometry of root branching and its relationship to root morphological and functional traits in three range grasses. Journal of Experimental Botany 62: 5581–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin OK, Edwards EJ, Loveys BR. 2000. Response of root respiration to changes in temperature and its relevance to global warming. New Phytologist 147: 141–154. [Google Scholar]
- Barber SA, Silberbush M. 1984. Plant root morphology and nutrient uptake. In: Roots, nutrients and water influx and plant growth. Madison, WI: Soil Science Society of America, 65–87. [Google Scholar]
- Bardgett RD, Mommer L, de Vries FT. 2014. Going underground: root traits as drivers of ecosystem processes. Trends in Ecology and Evolution 29: 692–699. [DOI] [PubMed] [Google Scholar]
- Bui HH, Serra V, Pagès L. 2015. Root system development and architecture in various genotypes of the Solanaceae family. Botany 93: 465–474. [Google Scholar]
- Cabelguenne M, Debaeke P. 1998. Experimental determination and modelling of the soil water extraction capacities of crops of maize, sunflower, soya bean, sorghum and wheat. Plant and Soil 202: 175–192. [Google Scholar]
- Chessel D, Dufour AB, Thioulouse J. 2004. The ade4 package. 1. One-table methods. R News 4: 5–10. [Google Scholar]
- Colombi T, Walter A. 2016. Root responses of triticale and soybean to soil compaction in the field are reproducible under controlled conditions. Functional Plant Biology 43: 114–128. [DOI] [PubMed] [Google Scholar]
- Colombi T, Kirchgessner N, Le Marie CA, York LM, Lynch JP, Hund A. 2015. Next generation shovelomics: set up a tent and REST. Plant and Soil 388: 1–20. [Google Scholar]
- Comas LH, Eissenstat DM. 2009. Patterns in root trait variation among 25 co-existing North American forest species. New Phytologist 182: 919–928. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. . 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. [Google Scholar]
- Courtois B, Ahmadi N, Khowaja F, et al. . 2009. Rice root genetic architecture: meta-analysis from a drought QTL database. Rice 2: 115–128. [Google Scholar]
- Craine JM, Froehle J, Tilman GD, et al. . 2001. The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 93: 274–285. [Google Scholar]
- De La Riva EG, Pérez-Ramos IM, Tosto A, et al. . 2016. Disentangling the relative importance of species occurrence, abundance and intraspecific variability in community assembly: a trait-based approach at the whole-plant level in Mediterranean forests. Oikos 125: 354–363. [Google Scholar]
- de Dorlodot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X. 2007. Root system architecture: opportunities and constraints for genetic improvement of crops. Trends in Plant Science 12: 474–482. [DOI] [PubMed] [Google Scholar]
- Drouet JL, Pagès L, Serra V. 2005. Dynamics of leaf mass per unit leaf area and root mass per unit root volume of young maize plants: implications for growth models. European Journal of Agronomy 22: 185–193. [Google Scholar]
- Dunbabin VM, Postma JA, Schnepf A, et al. . 2013. Modelling root–soil interactions using three-dimensional models of root growth, architecture and function. Plant and Soil 372: 93–124. [Google Scholar]
- Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL. 2000. Building roots in a changing environment: implications for root longevity. New Phytologist 147: 33–42. [Google Scholar]
- Fita A, Pico B, Monforte AJ. 2008. Genetics of root system architecture using near-isogenic lines of melon. Journal of the American Society for Horticultural Science 133: 448–458. [Google Scholar]
- Fitter AH. 1982. Morphometric analysis of root systems: application of the technique and influence of soil fertility on root system development in two herbaceous species. Plant, Cell and Environment 5: 313–322. [Google Scholar]
- Gu J, Xu Y, Dong X, Wang H, Wang Z. 2014. Root diameter variations explained by anatomy and phylogeny of 50 tropical and temperate tree species. Tree Physiology 34: 415–425 [DOI] [PubMed] [Google Scholar]
- Iversen CM, McCormack ML, Powell AS, et al. . 2017. A global fine-root ecology database to address below-ground challenges in plant ecology. New Phytologist 215: 15–26. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Aguirre L. 1991. Effect of water on morphological development in seedlings of 3 range grasses. Branching patterns. Journal of Range Management 44: 355–360. [Google Scholar]
- Konôpka B, Pagès L, Doussan C. 2009. Soil compaction modifies morphological characteristics of seminal maize root. Plant, Soil and Environment 55: 1–10. [Google Scholar]
- Kong D, Ma C, Zhang Q, et al. . 2014. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytologist 203: 863–872. [DOI] [PubMed] [Google Scholar]
- Kramer-Walter KR, Bellingham PJ, Millar TR, et al. . 2016. Root traits are multidimensional: specific root length is independent from root tissue density and the plant economic spectrum. Journal of Ecology 104: 1299–1310. [Google Scholar]
- Kuijken RCP, van Eeuwijk FA, Marcelis LFM, Bouwmeester HJ. 2015. Root phenotyping: from component trait in the lab to breeding. Journal of Experimental Botany 66: 5389–5401. [DOI] [PubMed] [Google Scholar]
- Makita N, Kosugi Y, Dannoura M, et al. . 2012. Patterns of root respiration rates and morphological traits in 13 tree species in a tropical forest. Tree Physiology 32: 303–312. [DOI] [PubMed] [Google Scholar]
- Malamy JE. 2005. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment 28: 67–77. [DOI] [PubMed] [Google Scholar]
- Materechera SA, Alston AM, Kirby JM, Dexter AR. 1992. Influence of root diameter on the penetration of seminal roots into a compacted subsoil. Plant and Soil 144: 297–303. [Google Scholar]
- Matias L, Quero JL, Zamora R, Castro J. 2012. Evidence for plant traits driving specific drought resistance. A community field experiment. Environmental and Experimental Botany 81: 55–61. [Google Scholar]
- McCormack ML, Dickie IA, Eissenstat DM, et al. . 2015. Redefining fine roots improves understanding of belowground contributions to terrestrial biosphere processes. New Phytologist 207: 505–518. [DOI] [PubMed] [Google Scholar]
- Mommer L, Weemstra M, 2012. The role of roots in the resource economics spectrum. New Phytologist 195: 725–727. [DOI] [PubMed] [Google Scholar]
- Moreau D, Abiven F, Busset H, Matejicek A, Pagès L. 2017. Effects of species and soil-nitrogen availability on root system architecture traits – study on a set of weed and crop species. Annals of Applied Biology 117: 103–116. [Google Scholar]
- Nye PH, Tinker PB. 1969. The concept of a root demand coefficient. Journal of Applied Ecology 6: 293–300 [Google Scholar]
- Pagès L. 2011. Links between root developmental traits and foraging performance. Plant, Cell and Environment 34: 1749–1760. [DOI] [PubMed] [Google Scholar]
- Pagès L. 2014. Branching patterns of root systems. Analysis of the diversity among dicotyledonous species. Annals of Botany 114: 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès L. 2016. Branching patterns of root systems. Comparison of dicotyledonous and monocotyledonous species. Annals of Botany 118: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès L, Pellerin S. 1994. Evaluation of parameters describing the root system architecture of field grown maize plants (Zea mays L.). II. Density, length, and branching of first-order lateral roots. Plant Soil 164: 169–176. [Google Scholar]
- Pagès L, Picon-Cochard C. 2014. Modelling the root system architecture of Poaceae. Can we simulate integrated traits from morphological parameters of growth and branching?New Phytologist 204: 149–158. [DOI] [PubMed] [Google Scholar]
- Pagès L, Bécel C, Boukcim H, et al. . 2014. Calibration and evaluation of ArchiSimple, a parsimonious model of the root system architecture. Ecological Modelling 290: 76–84. [Google Scholar]
- Picon-Cochard C, Pilon R, Tarroux E, et al. . 2012. Effect of species, root branching order and season on the root traits of 13 perennial grass species. Plant and Soil 353: 47–57. [Google Scholar]
- Price AH, Tomos AD, Virk DS. 1997. Genetic dissection of root growth in rice (Oryza sativa L).1. a hydroponic screen. Theoretical and Applied Genetics 95: 132–142. [Google Scholar]
- R Core Team 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; URL http://www.R-project.org/ [Google Scholar]
- Roumet C, Birouste M, Picon-Cochard C, et al. . 2016. Root structure–function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytologist 210: 815–826. [DOI] [PubMed] [Google Scholar]
- Siefert A, Violle C, Chalmandrier L, et al. . 2015. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecological Letters 18: 1406–1419. [DOI] [PubMed] [Google Scholar]
- Tison JM, Jauzein P, Michaud H. 2014. Flore de la France méditerraéenne continentale. Turriers, France: Naturalia publications. [Google Scholar]
- Valverde-Barrantes OJ, Smemo KA, Blackwood CB. 2015. Fine root morphology is phylogenetically structured, but nitrogen is related to the plant economics spectrum in temperate trees. Functional Ecology 29: 796–807. [Google Scholar]
- Valverde-Barrantes OJ, Freschet GT, Roumet C, Blackwood CB. 2017. A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytologist 215: 1562–1573. [DOI] [PubMed] [Google Scholar]
- Varney GT, Canny MJ, Wang XL, McCully ME. 1991. The branch roots of Zea. I. First order branches, their number, sizes and division into classes. Annals of Botany 67: 357–364. [Google Scholar]
- Walter A, Liebisch F, Hund A. 2015. Plant phenotyping: from bean weighing to image analysis. Plant Methods 11: 14. doi:10.1186/s13007-015-0056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M, Moosavi S, Cunningham SC, Kirkegaard JA, Rebetzke GJ, Richards RA. 2013. A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Annals of Botany 112: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. . 2004. The worldwide leaf economic spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Wu Q, Pagès L, Wu J. 2016. Relationships between root diameter, root length and root branching along lateral roots in adult, field-grown maize. Annals of Botany 117: 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York LM, Lynch JP. 2015. Intensive field phenotyping of maize (Zea mays L.) root crowns identifies phenes and phene integration associated with plant growth and nitrogen acquisition. Journal of Experimental Botany 66: 5493–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bodner G, Rewald B, Leitner D, Nagel KA, Nakhforoosh A. 2017. Root architecture simulation improves the inference from seedling root phenotyping towards mature root systems. Journal of Experimental Botany 68: 965–982. [DOI] [PMC free article] [PubMed] [Google Scholar]