Abstract
Aedes albopictus (Skuse) (Diptera: Culicidae) is a vector of several arboviruses impacting human health, including dengue, chikungunya, and potentially Zika. Vector control strategies that deploy modified males into the field are in use or under development and require a solid understanding of male biology; unfortunately, there has been limited effort to understand male Ae. albopictus reproductive biology, including sperm production and capacity. We tested whether body size and age affect spermatogenesis in Ae. albopictus. In general, older and larger males produced more sperm than their younger or smaller counterparts. Large males continued spermatogenesis well after 10-d post-eclosion (dpe), augmenting their reserves by 39%. By contrast, small males stopped producing sperm at 10 dpe. These results contribute to a deeper understanding of Ae. albopictus reproductive physiology. We discuss the usefulness of these findings in the context of Ae. albopictus life history and their utility in optimizing male mosquito release strategies.
Keywords: larval diet, spermatogenesis, modified male release, mating, male reproductive success
Aedes albopictus (Skuse) (Diptera: Culicidae) is a competent vector of more than 20 arboviruses (reviewed in Gratz 2004, Paupy et al. 2009), most notably dengue, chikungunya (Delatte et al. 2008), and Zika (Wong et al. 2013, Grard et al. 2014). While Aedes aegypti (Linnaeus) (Diptera: Culicidae) is responsible for a majority of arbovirus transmission in the tropics, Ae. albopictus also contributes to epidemics (Gratz 2004, Borgherini et al. 2007), has been solely responsible for several arbovirus outbreaks (Effler et al. 2005, Reiter et al. 2006, Tsetsarkin et al. 2007), and may act as a bridge vector that sparks new epidemics (Mondet et al. 1996, Gratz 2004). Being more cold tolerant than Ae. aegypti, Ae. albopictus has expanded its range considerably in recent years, threatening temperate regions that once had no risk of transmission (reviewed in Shragai et al. 2017). Improved methods of controlling this mosquito are imperative.
Some tools for mosquito population suppression involve the release of modified male mosquitoes to interfere with reproduction (reviewed in Macias et al. 2017). The oldest of these approaches, sterile insect technique (SIT), deploys males sterilized by radiation (Klassen and Curtis 2005). Because most females mate only once (Boyer et al. 2012, Helinski et al. 2012a), those females that mate to an SIT male experience reproductive failure (Lees et al. 2015). A variation on traditional SIT uses males that harbor Wolbachia endosymbionts (Walker et al. 2011); wild females that mate with released males are unable to fertilize eggs due to cytoplasmic incompatibility (O’Connor et al. 2012; Zhang et al. 2015a,b; Mains et al. 2016). Finally, released males may be genetically modified so that they pass lethal genes to their offspring, preventing them from reaching adulthood (Alphey et al. 2010). This technique has the advantage of reducing adult mosquito populations while also producing larvae that compete for resources in containers with wild larvae. Regardless of the strategy, the success of each approach hinges on laboratory reared, altered males competing for and successfully inseminating wild females.
Results of modified male mosquito releases have been mixed (Reisen 2003, Harris et al. 2011), and failures could be due to factors of male biology that should not be overlooked (Helinski and Harrington 2013). To assist in designing effective releases of mosquitoes, other authors have examined male vigor, insemination capacity, mating compatibility, and survival (Oliva et al. 2012; reviewed in Helinski and Harrington 2013; Oliva et al. 2013a,b). We propose that sperm quantity is another parameter that may be optimized in future releases. While females are normally monogamous, up to 26% of Ae. albopictus females in the wild mate with and produce progeny from multiple males (Boyer et al. 2012). Thus, released males that transfer more sperm to a polyandrous female may more effectively reduce offspring production by a wild mate. In addition, the capacity to produce excess sperm likely represents a surplus of nutrition that will allow males in a release scenario to be reproductively competitive. Therefore, in this study, we quantify the effect of body size and age on sperm quantity in male Ae. albopictus.
Materials and Methods
Mosquito Rearing
We established a laboratory colony of Ae. albopictus with field-collected eggs from Mercer County, NJ. All mosquitoes in this study originated from this colony within one year of its inception. At all times, we maintained mosquitoes in environmental conditions described in Degner and Harrington (2016). We submerged eggs in deionized water for 30 min with a pinch of pulverized fish food (crushed Cichlid Gold fish food pellets; Hikari, Himeji, Japan) and subsequently placed them under vacuum pressure for 30 min to induce hatching. Larvae grew for one day before transfer to mass rearing trays with different feeding regimes according to treatment. We isolated pupae to ensure virginity; after eclosion, we transferred males to 8.4-liter bucket cages at densities of 200 males per cage with a separate cage for each size class. Adults were offered 10% sucrose ad libitum.
We manipulated larval density to produce two different adult size classes. For large body sizes, we placed 75 first-instar larvae in plastic trays with 1 liter of DI water; for small males, we transferred 750 larvae per tray. Each tray received four fish food pellets (as above). As a proxy for body size, we measured wings of all 240 males in this study, as in Ponlawat and Harrington (2007). Both sizes had normally distributed wing lengths (Shapiro–Wilk normality test, W = 0.99, P > 0.05), with large males’ wings (2.48 ± 0.08 mm; mean ± SD) significantly longer than small males’ wings (2.11 ± 0.09 mm; t = 34.6; df = 237, P < 0.001).
Sperm Quantification
We obtained total sperm counts using the methods of Ponlawat and Harrington (2007) with slight modification. Briefly, we dissected the testes, vas deferentia, and seminal vesicle, transferred them to 200-µl deionized water, and used minutien pins to release all sperm and homogeneously mix the solution. In a pilot study, we verified that no sperm remained on the dissecting tools with this method by washing with 1% Triton and viewing the wash solution under a compound microscope with darkfield illumination. After transferring ten 5-µl aliquots of sperm to a glass slide, we fixed and stained sperm with Giemsa dye. We counted nuclei using darkfield illumination at 100× magnification and calculated the total number of individualized sperm from this subsample. A pilot study demonstrated that extrapolating total sperm counts from one quarter of the sperm homogenate accurately represents the true number of sperm (Supp Table 1 [Online only]).
We enumerated sperm at 1-, 5-, 10-, and 20-d posteclosion (dpe) for each size class, including fifteen males of each size at each time point. We stored some mosquitoes at −20°C prior to counting; a pilot experiment demonstrated that freezing does not hinder accurate sperm enumeration. We repeated this experiment twice with independent cohorts.
Data Analysis
After verifying that residuals of sperm counts from both cohorts were normally distributed and had homoscedastic variance, we tested the effects of age and body size on sperm quantity using a univariate general linear model (UGLM) with body size, age, and cohort as fixed factors. We constructed our model iteratively, beginning with a fully factorial design and an intercept and removing the least significant term in each iteration until all remaining terms were significant. Post-hoc pairwise comparisons were based on estimated marginal means and Bonferroni corrected. All statistics were conducted using SPSS (IBM SPSS Statistics for Windows, v24). Raw data can be accessed at https://doi.org/10.7298/X4P26W7B.
Results
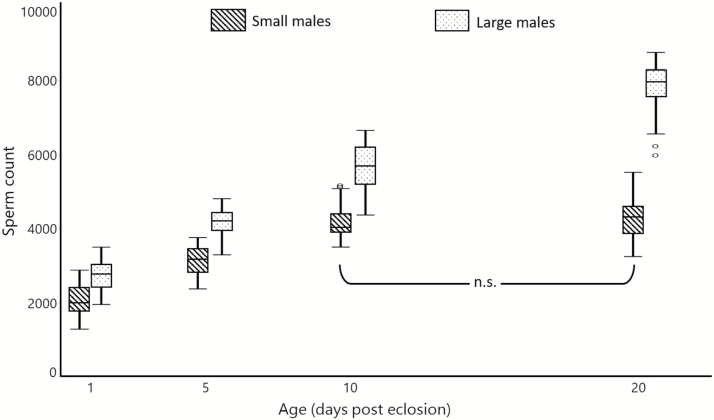
Our final corrected model included four terms (excluding the intercept) that significantly predicted sperm quantity in Ae. albopictus (UGLM; df = 8,231; F = 388.8; P < 0.001; r2 = 0.931; Supp Table 2 [Online only]). Both body size and age influenced sperm quantity (UGLM; dfage = 3,231; dfbody size = 1,231; Fage = 665.3, Fbody size = 769.8, Page,body size < 0.001; Fig. 1). At each age up to 10 dpe, large males had significantly more sperm (30–35%) than small males (P < 0.001). Likewise, males produced more sperm as they aged; in all but one comparison, older males had significantly more sperm than their younger counterparts of the same body size (P < 0.001). The only exception was the comparison of 10 and 20 dpe small males, which had similar sperm quantity (P = 1); this was accounted for in our model by a significant interaction between body size and age (UGLM; df = 3,231; F = 112.5; P < 0.001).
Fig. 1.
Sperm quantity of large and small male Ae. albopictus at different ages. Cross-hashed and dotted boxes indicate small and large males, respectively. Age and body size both significantly predicted sperm quantity (univariate general linear model; dfage = 3,231; dfbody size = 1,231; Fage = 665.3, Fbody size = 769.8, Page,body size < 0.001). In addition, there was a significant interaction between these terms (univariate general linear model; df = 3,231; F = 112.5; P < 0.001). All pairwise comparisons between groups of the same age or the same body size are significantly different except for small males at 10 and 20 dpe (α = 0.05). Figure includes data from two independent cohorts. Boxes indicate inner quartiles, and whiskers and outliers are drawn using the Tukey method. n.s. = not significant.
This experiment was replicated twice with separate cohorts of mosquitoes. We tested ‘cohort’ as a factor in our model to verify the repeatability of our experiment, and our final model included it as a significant predictor (UGLM; df = 1,231; F = 7.105; P = 0.008). Further analysis revealed that males in the first cohort had slightly more sperm than those in the second cohort (average of 4%, or 162 more sperm). However, wing lengths did not differ between cohorts for either size class (large males: t = 1.221; df = 116.9, P = 0.22; small males: t = 0.747, df = 118, P = 0.46). Despite this difference, our statistical and biological conclusions remain the same whether we analyze the first, second, or both cohorts together.
Discussion
We found that older and larger Ae. albopictus males produced more sperm than their younger and smaller counterparts. Old males in our small body treatment were an exception to this trend, with sperm counts that plateaued at 10 dpe. In contrast, large males continued to produce sperm beyond this age. Although we did not quantify sperm at ages between 10 and 20 dpe, based on the rate of spermatogenesis between 5 and 10 dpe, it is likely that males produced sperm at least until 17 dpe (Supp Analysis [Online only])—the oldest recorded sperm production in a mosquito to date (Hausermann and Nijhout 1975, Clements 1992). The fact that sperm production plateaus at different ages depending on size suggests a divergence in resource availability or allocation; large Ae. albopictus males are able to invest more in sex than small males. A similar age- and size-dependent pattern of spermatogenesis exists in Ae. aegypti (Ponlawat and Harrington 2007), with one key difference: Ae. aegypti males’ sperm count peaked at 10 dpe, regardless of size. This interspecific difference in gamete production may reflect nuances in the biology of Ae. aegypti and Ae. albopictus, or it may be an artifact of slightly different larval diets in the two experiments.
Sperm counts in this study should be interpreted with two caveats in mind. First, males were not allowed to mate or cohabit with females. This allowed precise and accurate enumeration of total sperm production, but under natural conditions, it is possible that mating or a natural sex ratio would accelerate sperm production. While this has not been documented in mosquitoes, plasticity in the rate of spermatogenesis has been demonstrated in Drosophila bifurca (Bjork et al. 2007) and Drosophila melanogaster (Moatt et al. 2014). Second, our methods do not identify sperm that are dead or incompetent to fertilize. However, sperm are efficiently maintained for months in a females’ spermathecae (Styer et al. 2007, Shaw et al. 2014), and it is likely that males similarly protect their gametic investment. Future work should investigate factors that influence the rate of spermatogenesis and the viability of sperm maintained in old males.
Producing and transferring excess sperm may benefit males by reducing the likelihood of sharing paternity with a second mate. While female Ae. albopictus are primarily monandrous, one field study found that as many as 26% of Ae. albopictus females produced progeny with mixed paternity (Boyer et al. 2012). Low levels of polyandry have also been noted in several other mosquitoes, including Ae. aegypti (Bullini et al. 1976, Tripet et al. 2003, Helinski et al. 2012b, Richardson et al. 2015, Degner and Harrington 2016). In such cases, the transfer of excess sperm may mitigate the proportion of offspring a male loses to a second mate by augmenting his sperm’s representation in the female’s storage organs. In contrast, abundant sperm may not directly increase the number of females a male can sterilize in a modified male strategy; semen, rather than sperm, contains the behavior-modulating compound(s) that make a female monogamous (Craig 1967, Helinski et al. 2012a). While other authors have quantified the number of females an Aedes male can inseminate and the effects of depleted ejaculate components in the laboratory (Helinski and Harrington 2011, Oliva et al. 2013a, Alfonso-Parra et al. 2014), the respective contributions of sperm and semen limitation on phenomena such as polyandry, sperm competition, and sperm precedence remain poorly understood. Furthermore, whether and how frequently males become limited by ejaculate components in the field has not, to our knowledge, been investigated.
This study demonstrates that larval diet and density affect the number of sperm a male produces. This is logical, given that critical periods for spermatogenesis occur during late larval and pupal stages (Clements 1992). Furthermore, resources obtained as larvae likely limit males’ lifetime potential sperm production. The adult Ae. albopictus male diet is primarily comprised of carbohydrates (reviewed in Foster 1995, Muller et al. 2011), and thus, protein is probably a limiting nutrient in spermatogenesis. We suspect that large males in our study were able to store more nutritional reserves from their larval diet than small males, and thus were able to continue investing in sperm late into adulthood.
Future investigations should identify a larval diet that is optimized for male reproductive success, and we propose that quantifying sperm production may help assist in the development of such a diet. While much of the power of modified male releases lies in seminal fluid, measuring seminal production is time consuming and requires quantification of semen in all of a male’s mates. To date, ejaculate volume has been estimated either by Western blots requiring custom made antibodies (Alfonso-Parra et al. 2014) or by measuring dimensions of the bursa (Oliva et al. 2013a). In contrast, sperm are easily and precisely quantified. Furthermore, because spermatogenesis continues into old age, even in the absence of mating, analysis of a single old male may reveal how well equipped he is to allocate nutritional reserves to reproductive efforts. Thus, sperm capacity is a straight-forward means of quantifying male reproductive investment and may be used to fine-tune rearing protocols for male releases.
Supplementary Data
Supplementary data are available at Journal of Medical Entomology online.
Acknowledgments
We thank Sylvie Pitcher for technical expertise and Dr. Alongkot Ponlawat for experimental guidance. This work was funded in part by a Cornell Graduate School fellowship (to E.C.D.) and NIH/NIAID grant R01AI095491.
References Cited
- Alfonso-Parra C., Avila F. W., Deewatthanawong P., Sirot L. K., Wolfner M. F., and Harrington L. C.. 2014. Synthesis, depletion and cell-type expression of a protein from the male accessory glands of the dengue vector mosquito Aedes aegypti. J. Insect Physiol. 70: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey, L., Benedict M., Bellini R., Clark G. G., Dame D. A., Service M. W., and Dobson S. L.. 2010. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 10: 295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork, A., Dallai R., and Pitnick S.. 2007. Adaptive modulation of sperm production rate in Drosophila bifurca, a species with giant sperm. Biol. Lett. 3: 517–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgherini, G., Poubeau P., Staikowsky F., Lory M., Le Moullec N., Becquart J. P., Wengling C., Michault A., and Paganin F.. 2007. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin. Infect. Dis. 44: 1401–1407. [DOI] [PubMed] [Google Scholar]
- Boyer, S., Toty C., Jacquet M., Lempérière G., and Fontenille D.. 2012. Evidence of multiple inseminations in the field in Aedes albopictus. PLoS One. 7: e42040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullini, L., Coluzzi M., and Bianchibullini A. P.. 1976. Biochemical variants in study of multiple insemination in Culex pipiens L (Diptera, Culicidae). Bull. Entomol. Res. 65: 683–685. [Google Scholar]
- Clements, A. N. 1992. The biology of mosquitoes: development, nutrition, and reproduction. vol. 1, CABI Publishing, New York. [Google Scholar]
- Craig, G. B., Jr. 1967. Mosquitoes: female monogamy induced by male accessory gland substance. Science. 156: 1499–1501. [DOI] [PubMed] [Google Scholar]
- Degner, E. C., and Harrington L. C.. 2016. Polyandry depends on postmating time interval in the dengue vector Aedes aegypti. Am. J. Trop. Med. Hyg. 94: 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte, H., Paupy C., Dehecq J. S., Thiria J., Failloux A. B., and Fontenille D.. 2008. [Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control]. Parasite. 15: 3–13. [DOI] [PubMed] [Google Scholar]
- Effler, P. V., Pang L., Kitsutani P., Vorndam V., Nakata M., Ayers T., Elm J., Tom T., Reiter P., Rigau-Perez J. G., et al. . 2005. Hawaii Dengue Outbreak Investigation Team. 2005. Dengue fever, Hawaii, 2001–2002. Emerg. Infect. Dis. 11: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, W. A. 1995. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40: 443–474. [DOI] [PubMed] [Google Scholar]
- Grard, G., Caron M., Mombo I. M., Nkoghe D., Mboui Ondo S., Jiolle D., Fontenille D., Paupy C., and Leroy E. M.. 2014. Zika virus in Gabon (Central Africa)–2007: a new threat from Aedes albopictus?PLoS Negl. Trop. Dis. 8: e2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz, N. G. 2004. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18: 215–227. [DOI] [PubMed] [Google Scholar]
- Harris, A. F., Nimmo D., McKemey A. R., Kelly N., Scaife S., Donnelly C. A., Beech C., Petrie W. D., and Alphey L.. 2011. Field performance of engineered male mosquitoes. Nat. Biotechnol. 29: 1034–1037. [DOI] [PubMed] [Google Scholar]
- Hausermann, W., and Nijhout H. F.. 1975. Permanent loss of male fecundity following sperm depletion in Aedes aegypti (L.). J. Med. Entomol. 11: 707–715. [DOI] [PubMed] [Google Scholar]
- Helinski, M. E. and Harrington L. C.. 2011. Male mating history and body size influence female fecundity and longevity of the dengue vector Aedes aegypti. J. Med. Entomol. 48: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski, M. E. H., and Harrington L. C.. 2013. Considerations for male fitness in successful genetic vector control programs, pp. 221–244. InTakken W. and Koenraadt C. J. (eds.), Ecology and control of vector-borne diseases: olfaction in vector-host interactions, vol. 3 Wageningen Academic Publishers, Wageningen, the Netherlands. doi:10.3920/978-90-8686-744-8 [Google Scholar]
- Helinski, M. E., Deewatthanawong P., Sirot L. K., Wolfner M. F., and Harrington L. C.. 2012a. Duration and dose-dependency of female sexual receptivity responses to seminal fluid proteins in Aedes albopictus and Ae. aegypti mosquitoes. J. Insect Physiol. 58: 1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski, M. E., Valerio L., Facchinelli L., Scott T. W., Ramsey J., and Harrington L. C.. 2012b. Evidence of polyandry for Aedes aegypti in semifield enclosures. Am. J. Trop. Med. Hyg. 86: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM 2016. IBM SPSS statistics 24 core system user’s guide. IBM Corporation, Armonk, NY. [Google Scholar]
- Klassen, W., and Curtis C. F.. 2005. History of the Sterile Insect Technique, pp. 3–36. InDyck V. A., Hendrichs J., and Robinson A. S. (eds.), Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, the Netherlands [Google Scholar]
- Lees, R. S., Gilles J. R. L., Hendrichs J., Vreysen M. J. B., and Bourtzis K.. 2015. Back to the future: the sterile insect technique against mosquito disease vectors. Curr. Opin. Ins. Sci. 10: 156–162. [Google Scholar]
- Macias, V. M., Ohm J. R., and Rasgon J. L.. 2017. Gene drive for mosquito control: where did it come from and where are we headed?Int. J. Environ. Res. Public Health. 14: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains, J. W., Brelsfoard C. L., Rose R. I., and Dobson S. L.. 2016. Female adult Aedes albopictus Suppression by Wolbachia-infected male mosquitoes. Sci. Rep. 6: 33846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatt, J. P., Dytham C., and Thom M. D.. 2014. Sperm production responds to perceived sperm competition risk in male Drosophila melanogaster. Physiol. Behav. 131: 111–114. [DOI] [PubMed] [Google Scholar]
- Mondet, B., da Rosa A. P., and Vasconcelos P. F.. 1996. The risk of urban yellow fever outbreaks in Brazil by dengue vectors. Aedes aegypti and Aedes albopictus. Bull. Soc. Pathol. Exot. 89: 107–113; discussion 114. [PubMed] [Google Scholar]
- Müller, G. C., Xue R. D., and Schlein Y.. 2011. Differential attraction of Aedes albopictus in the field to flowers, fruits and honeydew. Acta Trop. 118: 45–49. [DOI] [PubMed] [Google Scholar]
- O’Connor, L., Plichart C., Sang A. C., Brelsfoard C. L., Bossin H. C., and Dobson S. L.. 2012. Open release of male mosquitoes infected with a Wolbachia biopesticide: field performance and infection containment. PLoS Negl. Trop. Dis. 6: e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva, C. F., Jacquet M., Gilles J., Lemperiere G., Maquart P. O., Quilici S., Schooneman F., Vreysen M. J., and Boyer S.. 2012. The sterile insect technique for controlling populations of Aedes albopictus (Diptera: Culicidae) on Reunion Island: mating vigour of sterilized males. PLoS One. 7: e49414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva, C. F., Damiens D., Vreysen M. J., Lemperière G., and Gilles J.. 2013a. Reproductive strategies of Aedes albopictus (Diptera: Culicidae) and implications for the sterile insect technique. PLoS One. 8: e78884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva, C. F., Maier M. J., Gilles J., Jacquet M., Lemperiere G., Quilici S., Vreysen M. J., Schooneman F., Chadee D. D., and Boyer S.. 2013b. Effects of irradiation, presence of females, and sugar supply on the longevity of sterile males Aedes albopictus (Skuse) under semi-field conditions on Reunion Island. Acta Trop. 125: 287–293. [DOI] [PubMed] [Google Scholar]
- Paupy, C., Delatte H., Bagny L., Corbel V., and Fontenille D.. 2009. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 11: 1177–1185. [DOI] [PubMed] [Google Scholar]
- Ponlawat, A., and Harrington L. C.. 2007. Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 44: 422–426. [DOI] [PubMed] [Google Scholar]
- Reisen, W. K. 2003. Lessons from the past: an overview of studies by the University of Maryland and the University of California, Berkeley, pp. 25–32. InTakken W. and Scott T. W. (eds.), Ecological Aspects for Application of Genetically Modified Mosquitoes, vol. 2. Wag Ur Fron, Wageningen UR Frontis Series, Wageningen, the Netherlands: https://library.wur.nl/ojs/index.php/frontis/issue/view/194 [Google Scholar]
- Reiter, P., Fontenille D., and Paupy C.. 2006. Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem?Lancet. Infect. Dis. 6: 463–464. [DOI] [PubMed] [Google Scholar]
- Richardson, J. B., Jameson S. B., Gloria-Soria A., Wesson D. M., and Powell J.. 2015. Evidence of limited polyandry in a natural population of Aedes aegypti. Am. J. Trop. Med. Hyg. 93: 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, W. R., Teodori E., Mitchell S. N., Baldini F., Gabrieli P., Rogers D. W., and Catteruccia F.. 2014. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A. 111: 5854–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shragai, T., Tesla B., Murdock C., and Harrington L. C.. 2017. Zika and chikungunya: mosquito-borne viruses in a changing world. Ann. N. Y. Acad. Sci. 1399: 61–77. [DOI] [PubMed] [Google Scholar]
- Styer, L. M., Minnick S. L., Sun A. K., and Scott T. W.. 2007. Mortality and reproductive dynamics of Aedes aegypti (Diptera: Culicidae) fed human blood. Vector Borne Zoonotic Dis. 7: 86–98. [DOI] [PubMed] [Google Scholar]
- Tripet, F., Touré Y. T., Dolo G., and Lanzaro G. C.. 2003. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am. J. Trop. Med. Hyg. 68: 1–5. [PubMed] [Google Scholar]
- Tsetsarkin, K. A., Vanlandingham D. L., McGee C. E., and Higgs S.. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. Plos Pathog. 3: e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, T., Johnson P. H., Moreira L. A., Iturbe-Ormaetxe I., Frentiu F. D., McMeniman C. J., Leong Y. S., Dong Y., Axford J., Kriesner P., et al. . 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 476: 450–453. [DOI] [PubMed] [Google Scholar]
- Wong, P. S., Li M. Z., Chong C. S., Ng L. C., and Tan C. H.. 2013. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl. Trop. Dis. 7: e2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D., Lees R. S., Xi Z., Gilles J. R., and Bourtzis K.. 2015a. Combining the sterile insect technique with Wolbachia-based approaches: II–A safer approach to Aedes albopictus population suppression programmes, designed to minimize the consequences of inadvertent female release. PLoS One. 10: e0135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D., Zheng X., Xi Z., Bourtzis K., and Gilles J. R.. 2015b. Combining the sterile insect technique with the incompatible insect technique: I—Impact of Wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS One. 10: e0121126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.