Abstract
Background and Aims
The greater diversity of plant clades in the Neotropics compared to their relatives in Africa is a pervasive pattern in biogeography. To better understand the causes of this imbalance, we studied the diversification dynamics of the monocot family Velloziaceae. In addition to being conspicuously richer in the Neotropics compared to the Palaeotropics, many species of Velloziaceae exhibit extreme desiccation tolerance (i.e. ‘resurrection’ behaviour), and other ecological specializations to life on rocky outcrops, poor sandy soils, open vegetation and seasonally dry climates. Velloziaceae is also ecologically dominant in the campos rupestres, a habitat having exceptionally high plant diversity and endemism in Brazil.
Methods
We reconstructed a densely sampled time-calibrated molecular phylogeny and used state-dependent and state-independent models to estimate rates of lineage diversification in relation to continent-scale geographical occurrence and functional traits associated with desiccation tolerance and water storage capacity.
Key Results
Independent shifts to faster diversification occurred within two Neotropical lineages, Vellozia and Barbacenia. The Vellozia radiation was associated with the presence of conspicuous aerial stems, and was followed by decreasing diversification rates during the Oligocene, a time of rising global temperatures and expanding open areas around the world. The Barbacenia radiation was faster and more recent, occurring during the cooling conditions of the Miocene, and associated with the acquisition of aquiferous parenchyma on the leaves.
Conclusions
High species richness of Velloziaceae in South America has been driven by faster diversification in lineages predominantly occurring in the campos rupestres, putatively by the evolution of adaptive strategies in response to independent climatic events. The radiation of Vellozia in particular might have played a key role in the assembly of the campos rupestres vegetation.
Keywords: Adaptive radiation, Barbacenia, ecological strategies, Pandanales, mountain diversity, Neotropics, Vellozia
INTRODUCTION
The unevenness of biodiversity across geographical regions is famously exemplified by the latitudinal gradient of species diversity (Wiens and Donoghue, 2004; Mittelbach et al., 2007), but other disparities are similarly compelling. Within the tropics, a conspicuous biogeographical pattern is the depauperate species richness of Palaeotropical lineages compared to their Neotropical relatives, as seen in palms, bromeliads, Chrysobalanaceae, Malpighiaceae and Vochysiaceae (Hughes et al., 2013; Linder, 2014; Couvreur, 2015). Most of these groups are found in tropical rainforests and the origins of extant species are concentrated in the Quaternary (Hughes et al., 2013; Couvreur, 2015). By contrast, alternative habitat-specific diversification patterns have also been documented in seasonally dry and open habitats (Pennington et al., 2006; Simon et al., 2009; Hughes et al., 2013; Koenen et al., 2013; Pennington and Lavin, 2016). In addition, open upland and montane habitats in both the Palaeo- and Neotropics display astonishing plant diversity and endemism, as exemplified by the Páramos in the Andes (Madriñan et al., 2013) and the Eastern Afromontane biodiversity hotspot in Africa (Mittermeier et al., 2004).
The distinctive open vegetation of campos rupestres (literally, ‘rocky fields’) that occur in the highlands along the Espinhaço Range in eastern Brazil is another example of exceptional plant diversity in mountains (Giulietti and Pirani, 1988; Fiaschi and Pirani, 2009). Campos rupestres cover less than 1 % of Brazil’s area (Sano and Almeida, 1998; Supplementary Data Fig. S1), but its flora has 1951 endemic species, 40 % of its total; it is one of the most species-rich vegetation types recognized by the Brazil Flora Group (BFG, 2015). It extends from the transition zone between the Cerrado sensu lato and the Atlantic rainforest vegetation types in the south, to the xeric caatinga in the north, and westward to the Chapada dos Veadeiros as disjunct patches within the Central Brazilian Cerrado sensu stricto (Giulietti and Pirani, 1988; Fiaschi and Pirani, 2009). At broad scales, campos rupestres habitats have often been included within the Cerrado domain rather than in the Atlantic Forest complex based on their herbaceous and shrubby physiognomy (WWF, 2012). However, more precisely it is a distinctive vegetation type within the Cerrado domain (BFG, 2015; Pirani et al., 2015; Rando et al., 2016). This unique flora has been intensively studied from a taxonomic perspective (i.e. BFG, 2015; Pirani et al., 2015), but our understanding of the assembly and evolutionary history of the campos rupestres remains fragmentary (Conceição et al., 2016).
The campos rupestres habitat occurs on high plateaus and isolated mountain peaks separated by lowland vegetation (Giulietti and Pirani, 1988; Giulietti et al., 2005), similar to other montane sky island systems around the world (Hughes and Eastwood, 2006; Mairal et al., 2017). This leads to the expectation of plant radiations frequently associated with mountains, driven by orogeny and isolation of habitat patches (Hughes and Atchison, 2015; Xing and Ree, 2017). Several studies have suggested this kind of vicariant divergence in campos rupestres lineages (e.g. Trovó et al., 2013; Rando et al., 2016), but there is a lack of evidence of a causal link between these geographical patterns and orogeny (see Conceição et al., 2016). The few studies that have inferred time-calibrated phylogenies for campos rupestres lineages found species diversification concentrated in the Miocene (Rapini et al., 2007; Antonelli et al., 2010) or Pleistocene (de Souza et al., 2013; Ribeiro et al., 2014; Rando et al., 2016). These studies focused on specific lineages of Asclepiadoideae, Fabaceae and Orchidaceae that differ in their relative diversity in this habitat. However, many of the most conspicuous and diverse plant lineages in campos rupestres, e.g. Eriocaulaceae, Velloziaceae, Xyridaceae, and some genera within Asteraceae, Bromeliaceae and Melastomataceae (Giulietti and Pirani, 1988), have received little empirical attention (Fiaschi and Pirani, 2009). Specifically, absolute or relative diversification rate estimates for these lineages are still lacking (but see Simon et al., 2009; Trovó et al., 2013). Moreover, because Eriocaulaceae, Velloziaceae and Xyridaceae are notably more diverse in the Neotropics than in Africa (Giulietti and Hensold, 1990; Mello-Silva, 1995), the characterization of species diversification in these clades may thus aid our understanding of the origins and maintenance of differences between Palaeo- and Neotropical plant diversity, particularly for seasonally dry open shrubby or grassy habitats that have received less attention than tropical forests (Fiaschi and Pirani, 2009; Hughes et al., 2013).
In addition to the archipelago-like distribution of the campos rupestres, similarities in habit, life form, leaf shape and pubescence among diverse campos rupestres taxa have generated hypotheses of convergent evolution and replicated adaptive radiations in this habitat (Coile and Jones, 1981; Harley, 1988). The freely drained sandy or rocky soils, together with high daily temperatures and seasonal rain regimes favour the establishment of resurrection and drought-resistant plants (Alcantara et al., 2015; Oliveira et al., 2016). These abiotic conditions have been considered the main drivers of adaptive divergence in campos rupestres (Harley, 1988). According to the traditional adaptive radiation hypothesis, episodes of explosive species accumulation in a lineage are associated with rapid ecological niche divergence followed by decreasing diversification rates through time (Linder, 2008; Gavrilets and Losos, 2009; Yoder et al., 2010). To date, only one study has studied the role of ecological divergence in the diversification of a campos rupestres lineage, Minaria (Asclepiadoideae), but did not find evidence for it (Ribeiro et al., 2014). Support for the hypothesis of adaptive radiation in the campos rupestres thus remains elusive.
In this study, we focus on Velloziaceae (Pandanales), the most abundant and species-rich vascular plant family in the campos rupestres flora (Porembski and Barthlott, 2000; Conceição et al., 2007a, b; Alcantara et al., 2015, BFG, 2015). Its species generally occur on rocky and shallow soils, especially on rocky inselbergs in both Africa and South America. Most are heliophytes, and range from small trees and shrubs to small rosettes, large stem rosettes and graminoid forms (Ayensu, 1973; Mello-Silva, 1995) (Fig. 1). The family includes mat-forming species that can colonize exposed rock and facilitate the establishment of other plants (Porembski and Barthlott, 2000), tree-like species whose stems support specific epiphytes (Werneck and Espírito-Santo, 2002), and species with densely ramified underground stem-systems that facilitate the capture and concentration of nutrients in sandy soils (Oliveira et al., 2015, 2016). It also displays specializations for survival in seasonally dry areas. For example, all the African species are desiccation tolerant, i.e. resurrection plants (Gaff, 1977; Porembski and Barthlott, 2000), and the South American species show seasonally variable drought resistance or desiccation tolerance (Aidar et al., 2010). Several species from the campos rupestres are not desiccation tolerant, but are extremely drought resistant (Alcantara et al., 2015). The diversity of water-related ecological strategies has been suggested as key traits underlying the dominance and distribution of Velloziaceae species in the campos rupestres (e.g. Alcantara et al., 2015). It is also associated with the adaptive divergence and diversification of campos rupestres plants more generally (Harley, 1988; Giulietti and Pirani, 1988).
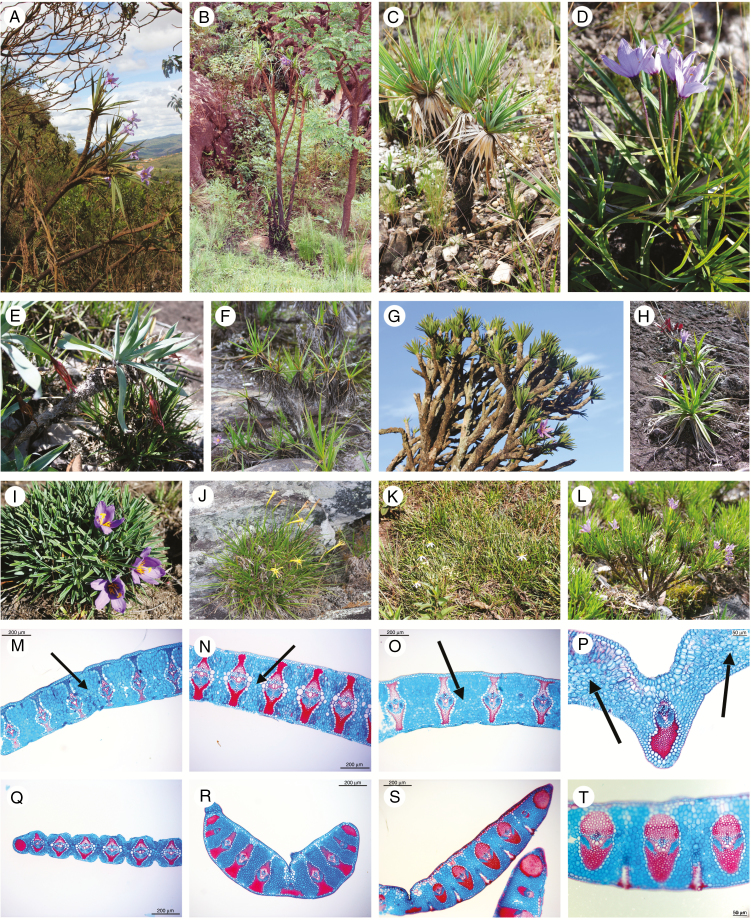
Fig. 1.
Examples of the species and the functional traits analysed. (A–L) Velloziaceae species displaying absence (D, H, I, J and K) or presence (A, B, C, E, F, G and L) of conspicuous aerial stems. (M–T) Anatomical sections of the leaves, showing presence (M, N, O, P) or absence (Q, R, S, T) of the aquiferous parenchyma between the bundles on the leaves (indicated by arrows). Scale bars: 200 µm in M–O and Q–S; 50 µm in P and T. Blue colour indicates tissues with primary cell walls (parenchyma and phloem), red/magenta color indicates tissues with secondary cell walls (fiber and xylem). (A) Vellozia aloifolia Mart. (B) Vellozia glabra J.C.Mikan. (C) Vellozia variabilis Mart. ex Schult. & Schult.f. (D) Vellozia caruncularis Mart. ex Seub. (E) Barbacenia spiralis L.B.Sm. & Ayensu. (F) Vellozia fruticosa L.B.Sm. (G) Vellozia compacta Mart. ex Schult. & Schult.f. (H, M) Barbacenia celiae Maguire. (I) Vellozia cryptanta Seub. (J, Q) Barbacenia riedeliana Goethart & Henrard. (K, S, T) Vellozia gramineae Pohl. (L, R) Vellozia semirii. (N) Barbacenia involucrata L.B.Sm. (O, P) Barbacenia glabra Goethart & Henrard. Credits: A–D, F, G, J–L: S. Alcantara; E, I: R. Mello-Silva; H: J. G. Rando; M–T: K. Drequeceler.
Phylogenetic investigation of traits, geography and diversification rate can be used to assess whether the imbalance between South American and African lineages of Velloziaceae is caused by higher diversification rates in the Neotropics, and to test the specific hypothesis of adaptive radiation in Neotropical clades. Velloziaceae includes approx. 265 species, of which about 80 % are restricted to the campos rupestres (Mello-Silva, 1995), all members of Barbacenia and Vellozia. These two genera each have approx. 120 species, few of which occur outside the campos rupestres (Mello-Silva, 2015). Other members of Velloziaceae include the small genus Barbaceniopis, with 4 species restricted to Andean outcrops (Ibisch et al., 2001), and the genus Xerophyta (approx. 34 species) which occurs on African inselbergs (Mello-Silva et al., 2011). The only East Asian species, Acanthochlamys bracteata, has been consistently recovered as the sister group of the rest of the family (Behnke et al., 2000; Mello-Silva, 2005; Chase et al., 2006; Mello-Silva et al., 2011).
Our first objective in this study is to reconstruct an improved and dated molecular phylogeny of Velloziaceae in order to infer lineage diversification rates through time and across clades. We want to understand whether the species imbalance between African and South American clades can be explained by different rates of speciation and/or extinction, and if changes in rate are temporally associated with changes in climate. We also wish to test for associations between diversification rates and putatively adaptive functional traits, namely the presence of conspicuous aerial stems and the presence of aquiferous parenchyma between vascular bundles in the leaves. These morphological traits are associated with important ecophysiological strategies in this group. The presence of aquiferous parenchyma between vascular bundles in the leaves allows water storage and survival during longer seasonal dry periods (Warming, 1893; Diogo, 1926), while the development of conspicuous aerial stems is a surrogate of desiccation tolerance and plant productivity/competitive ability (Alcantara et al., 2015; Material S2). We hypothesize that these traits played a role in driving the diversification in Velloziaceae. We also evaluate if bursts of species diversification were followed by decreasing rates indicative of density-dependent diversification, i.e. an early burst and rate slowdown trajectory of diversification typical of an evolutionary radiation. In this sense, our second main objective is to test for specific signals of adaptive radiation, based on (1) an association between functional traits and diversification rate shifts and (2) investigation of the diversification rates dynamic itself. Since Velloziaceae is the most conspicuous floristic element in the campos rupestres, we also discuss the implications of our findings in the context of the assembly of the campos rupestres flora more generally.
MATERIAL AND METHODS
Taxon sampling and phylogeny reconstruction
We sampled a total of 150 species of Velloziaceae, represented by 158 accessions, and eight outgroup species, representing six families. We follow the phylogeny-based delimitations of the five genera in the family (Mello-Silva et al., 2011). Sampling included 48 of the 104 species accepted in Barbacenia, 77 of the 122 species accepted in Vellozia, complete sampling of Acanthochlamys and Barbaceniopsis (i.e. one and four species, respectively) and 20 of 34 species in Xerophyta. Estimates of species diversity for Barbacenia and Vellozia are based on the most recent accounts available on the Flora do Brasil website (Mello-Silva, 2015), with inclusion of the only Andean species that does not occur in Brazil (Vellozia andina Ibisch, R.Vásquez & Nowicki) and nine additional species (four of Barbacenia and five of Vellozia) recently described (Mello-Silva and Menezes, 2014; Mello-Silva and Sasaki, 2016). For the three other genera, we used data from The Plant List website (http://www.theplantlist.org, accessed 20 June 2015). In this sense, our sampling of species within genera can be considered phylogenetically random, since we did not focus on specific groups and/or characteristics.
In addition to published sequences of 46 Velloziaceae species and four outgroups from Mello-Silva et al. (2011), we sequenced reproductive specimens collected during extensive fieldwork carried out over approx. 3 years in Velloziaceae-rich areas of Brazil, in addition to cultivated individuals (i.e. most samples of Xerophyta, kindly provided by the Universität Bonn, Germany). This resulted in new molecular sequences for 112 Velloziaceae specimens, representing 104 species, and four additional outgroup taxa; sequences were deposited in GenBank and vouchers were deposited at SPF (Material S3). Fresh leaf tissues were dried in silica gel (Chase and Hills, 1991) and sequenced in the Pritzker Laboratory of Molecular Systematics at The Field Museum, Chicago, IL, USA. We performed total DNA extraction followed by amplification and sequencing of the following molecular markers: (1) trnL-intron – trnL-F spacer (Taberlet et al., 1991); (2) trnH-psbA spacer (Shaw et al., 2005); (3) atpB-rbcL spacer (Chiang et al., 1998); and (4) internal transcribed spacer (ITS) nrDNA (Sun et al., 1994). Laboratory procedures and primers used for amplification are described in Material S3. The phylogenetic utility of these markers was previously demonstrated by Mello-Silva et al. (2011). The raw chromatograms were assembled into contigs in Sequencher 2.0 (Gene Codes Corporation, Ann Arbor, MI, USA), and then were checked by eye and, when necessary, edited using the same software. We created an alignment for each marker using MUSCLE 3.8 (Edgar, 2004) with subsequent manual adjustments, and concatenated them into a single matrix.
We performed Bayesian phylogenetic reconstruction using MrBayes 3.2 (Ronquist et al., 2011), partitioning the combined matrix by gene, setting Acorus gramineus Aiton (Acoraceae) as the outgroup (Chase et al., 2006) and coding alignment indels as missing data. Substitution model parameters were unlinked across partitions. To account for model uncertainty, we used reversible-jump Markov chain Monte Carlo (RJMCMC) in MrBayes to integrate over all sub-models of the GTR class, allowing both among-site rate heterogeneity (Γ) and invariant sites (I), in proportion to their marginal posterior probabilities (Huelsenbeck et al., 2004). Four simultaneous chains were run starting from random trees for 10 million generations, sampling every 500 generations. We used the average standard deviation of split frequencies, Potential Scale Reduction Factor of each parameter (PSFR), average effective sample sizes (ESS) and visual examination of the likelihood trace in the software as provided by Tracer 1.6 (Rambaut et al., 2013) to evaluate stationarity and convergence of the runs, discarding the first 25 % of samples as burn-in. Posterior probabilities of clades were summarized on the majority-rule consensus tree.
Estimation of divergence times
We estimated a time-calibrated phylogeny of Velloziaceae by Bayesian inference using relaxed molecular clock models in BEAST 2.2 (Bouckaert et al., 2014). We based topological priors (i.e. the monophyly of Velloziaceae and of Pandanales) on the results from the non-clock MrBayes analysis. We excluded the two most distant outgroups (Acorus gramineus and Encholirium scrutor), as including them in exploratory runs led to very large confidence intervals for the root age of the tree, and much longer times until MCMC convergence.
There are no known fossils of Velloziaceae, so for calibration purposes we used the oldest fossil known for the order Pandanales, represented in our matrix by members of Velloziaceae and three outgroups: Cyclanthus bipartitus Poit. ex A.Rich. and Thoracocarpus bissectus (Vell.) Harling (Cyclanthaceae), and Pandanus pygmaeus Thouars (Pandanaceae). We used standard procedures (Magallón, 2004; Iles et al., 2015) to assign the oldest fossil of Pandanales to the stem node of the order. To identify this fossil, we carried out an extensive review of the literature on monocot fossils, cross-referenced with a recent review of monocot fossils suitable for molecular dating (Iles et al., 2015). We obtained two candidates: inflorescences of Mabelia and Nuhliantha (Triuridaceae) dating from the Turonian period (93.5–89.3 Mya; Gandolfo et al., 2002), and an infructescence of Pandanaceae, Gruenbachia pandanoides (Herman and Kvacek, 2010), dating from 83.6–72 Mya. The position of Gruenbachia pandanoides within Pandanaceae is still uncertain (Iles et al., 2015), and the Turonian Triuridaceae fossils are older, so we used the latter to establish the minimum age of Pandanales. We specified a lognormal prior with mean M = 15 and standard deviation S = 0.75, resulting in a median of 101 Mya.
As with the MrBayes analysis, we partitioned the data by gene and used RJMCMC to average over substitution models using the BEAST add-on package RBS (Bouckaert et al., 2013). Models and clock rates were unlinked across the four data partitions. We used a birth–death tree prior and a relaxed, uncorrelated lognormal (UCLN) molecular clock (Drummond et al., 2006). All specifications for the analysis are included in the input file (available upon request). We performed four independent runs, each for 50 million generations and a sampling frequency of 5000. To check for stationary and convergence, we examined parameter ESS values and distributions in Tracer 1.6 (Rambaut et al., 2013). Results were combined using LogCombiner (distributed with BEAST 2.2), discarding 20 % as burn-in. We used TreeAnnotator (distributed with BEAST 2.2) and FigTree 1.4.1 (Rambaut, 2009) to produce the maximum clade credibility (MCC) tree, which we used for downstream analyses. We compared node ages recovered by this analysis to those of other studies based on different calibrations (Janssen and Bremer, 2004; Mennes et al., 2013; Hertweck et al., 2015; Magallón et al., 2015).
Diversification analyses
We analysed diversification rates on the MCC tree pruned of all outgroups and seven redundant terminals of six species (two terminals representing different morphological varieties of Barbacenia flava were retained). The final tree included 151 terminals (150 species).
We used this final tree as input to BAMM 2.5, which uses Bayesian RJMCMC to infer heterogeneity in rates of speciation, extinction and trait evolution on phylogenetic trees (Rabosky, 2014). To account for incomplete taxon sampling, we included sampling fractions of each genus of Velloziaceae. We ran BAMM for 10 million generations, sampling every 10 000 generations, and analysed the results using BAMMtools 2.1.6 (Rabosky et al., 2014). We discarded the first 10 % of samples as burn-in and verified chain stationarity by inspecting plots of the likelihood trace and calculating ESS values of parameters.
We used BAMMtools to summarize differences in diversification across the phylogeny of Velloziaceae, and calculated marginal odds ratios for rate shifts on individual branches and Bayes factors (BFs) for the number of distinct rate regimes (Shi and Rabosky, 2015). We recorded the distinct shift configurations in the 95 % credible set and identified the core shifts with BFs exceeding 5. We summarized the number of shifts in the 20 shift configurations that together account for > 50 % of the posterior distribution. We considered a marginal odds ratio > 50 as strong evidence favouring a shift in net diversification rate on a given branch. We performed macroevolutionary cohort analysis (Rabosky et al., 2014), identifying sets of taxa likely to share the same diversification regime. We calculated mean rates of diversification across the Velloziaceae phylogeny and generated rates-through-time plots for specific clades.
To cross-check these results we used MEDUSA, a maximum-likelihood approach of estimating diversification rate shifts (Alfaro et al., 2009), using the R (R Core Team, 2015) package geiger v. 2.0.6 (Harmon et al., 2015). We accounted for incomplete phylogenetic sampling by adding unsampled species to their respective genera (i.e. Barbacenia, Vellozia and Xerophyta).
We also tested directly whether speciation rates differ between African and South American clades of Velloziaceae by the Hidden-State Speciation and Extinction model (HiSSE) (Beaulieu and O’Meara, 2016). HiSSE extends previous state-dependent diversification models (e.g. BiSSE; Maddison et al., 2007) by allowing an unobserved binary character to influence diversification dynamics in addition to the observed character, mitigating the risk of Type I error (Rabosky and Goldberg, 2015; Beaulieu and O’Meara, 2016). We coded geography as a binary trait (occurrence in Africa vs. South America), pruning the sole Asian species, Acanthochlamys bracteata, from the tree. We used the R package hisse 1.1 (available at https://cran.r-project.org/web/packages/hisse/index.html) to evaluate the fit of alternative models by their average Akaike weight (wi) (Burnham and Anderson, 2002) and estimate confidence intervals for parameters of the best-fit model. We evaluated two models: BiSSE, having no hidden states; and HiSSE-partial, in which only one observed state (i.e. ‘South America occurrence’, which accounts for most species and genera of Velloziaceae) can transition between ‘hidden’ states. Model fit was assessed by wi (Burnham and Anderson, 2002).
Correlates of diversification: climate, morphology and ecology
We placed the rate shifts inferred by BAMM into geological periods and their associated climatic conditions (Jaramillo et al., 2006; Zachos et al., 2008). We also tested if the presence of aquiferous parenchyma between vascular bundles in the leaves and the development of conspicuous aerial stems were linked to diversification rate using the HiSSE model (Beaulieu and O’Meara, 2016) as described in the previous section. We evaluated three models: BiSSE, having no hidden states; HiSSE-partial, in which only one observed state can transition between ‘hidden’ states; and HiSSE-full, with unrestricted transitions. We also performed stochastic character mapping (Huelsenbeck et al., 2003) implemented in phytools (Revell, 2012) to estimate and visualize the phylogenetic history of these functional traits. For SSE analyses, in addition to considering the whole phylogeny, we conducted lineage-specific analyses to account for rate heterogeneity within Velloziaceae. We specifically focused on the two South American lineages of the family: (1) the genus Barbacenia and (2) the genera Barbaceniopis + Vellozia, which emerge as sister clades and constitute the most diverse lineage of Velloziaceae.
RESULTS
Phylogeny and divergence time estimation
In total, the combined matrix used for phylogeny estimation had 166 terminals, representing 158 ingroups and eight outgroups, and 4037 characters, of which 2611 had informative variation. The majority-rule consensus tree from the MrBayes analysis (Fig. S4) strongly supports the monophyly of Velloziaceae genera, as found by Mello-Silva et al. (2011). In general, infra-generic relationships are poorly supported in the two largest genera of Velloziaceae (Barbacenia and Vellozia) and are more strongly supported in the smaller genera Barbaceniopsis and Xerophyta.
The substitution models with highest posterior support for each data partition in the MrBayes and BEAST analyses are shown in Material S5. Heterogeneity in substitution rates estimated by BEAST indicates that the data do not fit a strict molecular clock model (Fig. S6). The BEAST MCC tree (Fig. S8) supports the same major clades as MrBayes (Fig. S4). Comparison with previous divergence time estimation studies (Table 1) shows congruence with the divergence dates obtained here (approx. 101 Mya) for the crown group age of Pandanales (i.e. the node used here for calibration). We estimated an age of 74.3 Mya (97–52 Mya) for the crown node of Velloziaceae (Fig. 2).
Table 1.
Crown group (c.g.) and stem lineage (s.l.) ages (Mya) estimated from the fossil calibration carried out here and from previous literature available, with confidence interval indicated
| Source | Pandanales s.l. |
Pandanales c.g. |
Velloziaceae s.l. |
Velloziaceae c.g. |
N | Study details |
|---|---|---|---|---|---|---|
| This study (UCLN) |
131.1 (93–175) |
101.24 (91–118) |
101.24 (91–118) |
74.3 (52–97) |
150 | Pandanales tree-dating, focus on divergence times of Velloziaceae, use of 1 fossil calibration point |
|
Janssen and Bremer (2004)
(NPRS) |
124 | 114 | 108 | 14 | 2 | Secondary calibration of the monocots tree-root, based on a previous calibration using 8 fossil points |
|
Mennes et al. (2013)
(UCLN) |
110 (96–123) |
92 (69–110) |
NA | NA | 2 | Monocots tree-dating, focus on positioning of Triuridaceae and divergence dates of Pandanales, use of 4 fossil calibration points |
|
Hertweck et al. (2015)
(PL) |
116 (110–122) |
103 (95–111) |
PL: ~90 | NA | 1 | Monocots tree-dating based on 11 fossil calibration points |
|
Magallón et al. (2015)
(UCLN & PL) |
UCLN: 110.5 (96.5–123) PL: 118.31 (114–121) |
UCLN: 72.35 (53–100) PL: 103.44 (99–109) |
UCLN: 72.35 (53–100) PL: 103.44 (99–109) |
NA | 4 | Angiosperms tree-dating, focusing on the ages of angiosperms families and based on 137 fossil calibration points |
N: number of species of Velloziaceae sampled in each study. UCLN: estimations based on BEAST analyses using a UCLN model. PL: point age estimations on a maximum likelihood (ML) tree with penalized likelihood method. NPRS: estimations based on non-parametric rate smoothing. NA: not applicable.
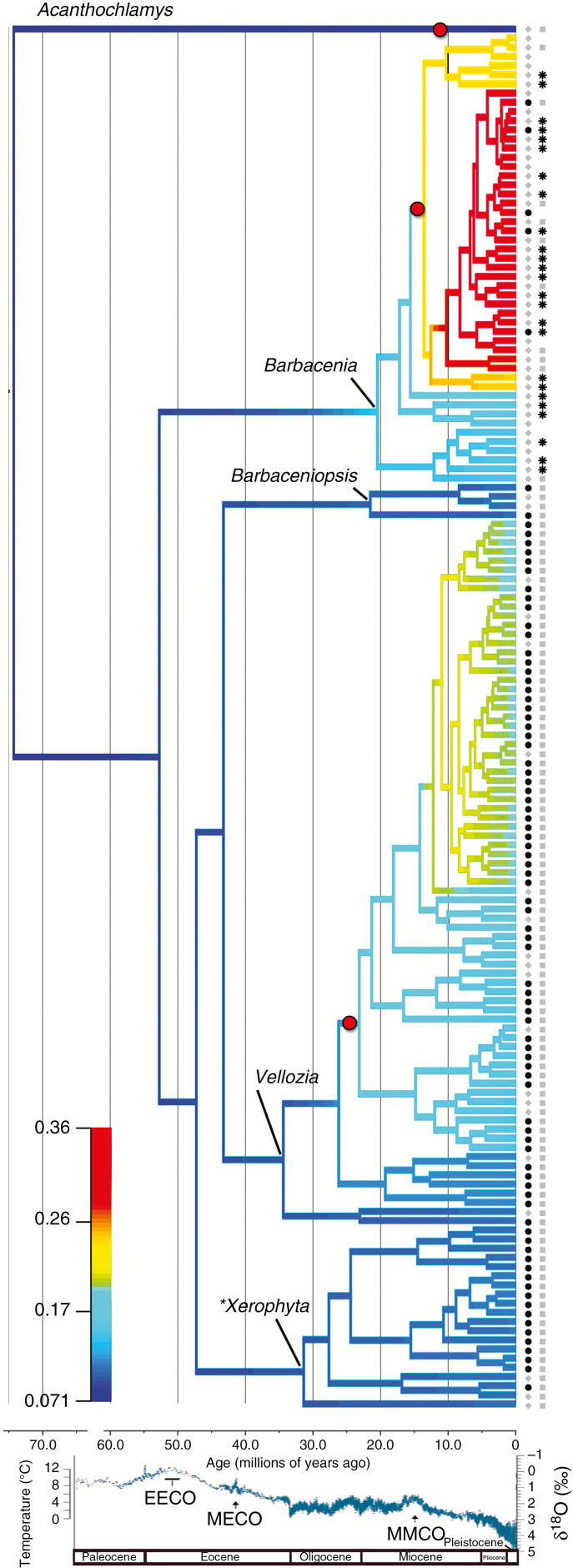
Fig. 2.
Chronogram of Velloziaceae, highlighting net diversification rate (r) simulated by BAMM, with rates increasing from blue to red. Rate values represent new lineages per million years. The African clade is shown by an asterisk (Xerophyta) and states of the traits evaluated for association with diversification rates are displayed in the tips. Trait A: development of conspicuous aerial stems; states: black circles: presence; grey diamonds: absence. Trait B: aquiferous parenchyma between vascular bundles in the leaves; states: black asterisks: presence; grey squares: absence. Bottom graphic shows global mean temperature according to Zachos et al. (2008). EECO: Early Eocene Climatic Optimum; MECO: Mid-Eocene Climatic Optimum; MMCO: Mid-Miocene Climatic Optimum. Age (in million years) and geological epoch are indicated at the bottom of the phylogeny and temperature graphic, respectively.
Diversification rates in Velloziaceae
Both BAMM and MEDUSA indicated heterogeneity in diversification rates across the phylogeny (Fig. 2 and Material S7). There is strong support for two rate shifts (Table 2 and Material S7). Although a three-shift model had the highest posterior probability with BAMM, its BF is only 3.7) over the two-shift model. Examination of the most credible rate shift configurations from BAMM (see Fig. 3A) indicates some uncertainty about the precise number and locations of rate shifts (Material S9), but it is clear that two occur within the two largest Neotropical genera, Barbacenia and Vellozia (Fig. 3B), a result also obtained by MEDUSA (Material S9). We present the mean (model-averaged) diversification rates estimated along every branch of the Velloziaceae tree by BAMM (Fig. 2). In Vellozia, the shift is to a regime with higher initial net diversification that declines through time, while for Barbacenia, the shift is of greater magnitude to a higher constant rate (Fig. 2). We refer to the clades descending from these shifts as core-Vellozia and core-Barbacenia, respectively. Apart from these two clades, rates across the rest of the family are more or less homogeneous, except for weak signals of slowing diversification rates in the monotypic Acanthoclamys (node 1) (Figs 2 and 3; Material S9). This third rate shift has low support (BF = 1.83).
Table 2.
Pairwise Bayes factor matrix computed for models with different rate shift configurations simulated over the Velloziaceae dataset (k = number of rate shifts)
| k | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | p.p. |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1.00 | 0.12 | 0.001 | 0.0004 | 0.0003 | 0.0002 | 0.0003 | 0.0003 | 0.001 |
| 1 | 8 | 1.00 | 0.01 | 0.003 | 0.002 | 0.002 | 0.003 | 0.003 | 0.004 |
| 2 | 696 | 87 | 1.00 | 0.27 | 0.20 | 0.21 | 0.26 | 0.24 | 0.190 |
| 3* | 2576 | 322 | 3.7 | 1.00 | 0.74 | 0.76 | 0.98 | 0.87 | 0.360* |
| 4 | 3504 | 438 | 5.03 | 1.36 | 1.00 | 1.03 | 1.33 | 1.19 | 0.240 |
| 5 | 3392 | 424 | 4.87 | 1.32 | 0.97 | 1.00 | 1.29 | 1.15 | 0.120 |
| 6 | 2624 | 328 | 3.77 | 1.02 | 0.75 | 0.77 | 1.00 | 0.89 | 0.046 |
| 7 | 2944 | 368 | 4.23 | 1.14 | 0.84 | 0.87 | 1.12 | 1.00 | 0.026 |
| 8 | 2816 | 352 | 4.05 | 1.09 | 0.80 | 0.83 | 1.07 | 0.96 | 0.012 |
Values above the diagonal represent the prior probability of the corresponding model with k shifts. Values below the diagonal represent the Bayes factor (BF) evidence in favour of the model with k shifts (indicated by the list number) relative to the simpler model with k shifts (indicated in the column number). Last column indicates the relative posterior distribution (p.p.) of the models with k shifts. Usually, the overall best model from a BAMM analysis is the model with the highest BF relative to the null model, M0 (k = 0). However, model probabilities for rarely sampled models are probably inaccurate (http://bamm-project.org/postprocess.html#bayesfactors), which seems the case of the null model for the Velloziaceae dataset, and the posterior probabilities indicate that the model with three shifts (marked by *) is the most frequent among the 95 % credible set of rate shift configurations sampled with BAMM (see text).
BF > 20: evidence for one model over another; BF > 50: very strong evidence in favour of the numerator model; BF < 5: weak evidence in favour of the numerator model.
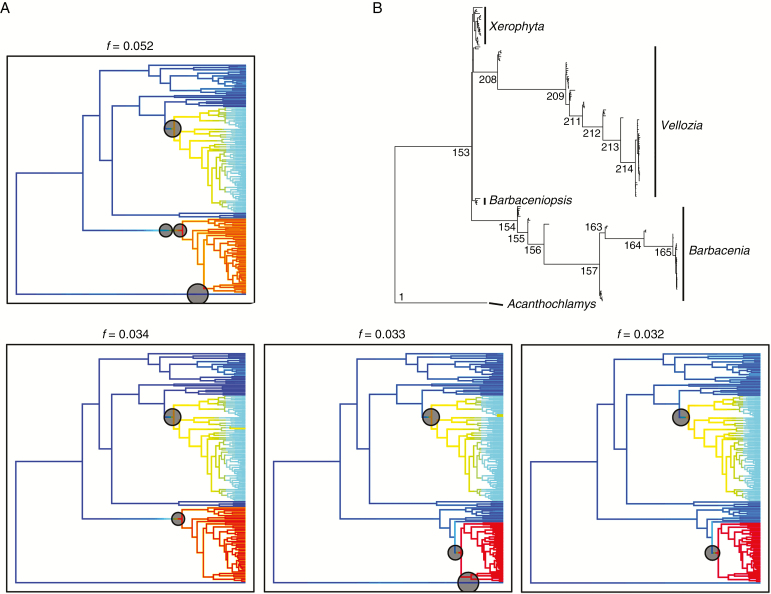
Fig. 3.
BAMM results of rate shifts in Velloziaceae. (A) Top four most credible shift sets recovered by BAMM with associated probabilities (f). Grey circles indicate the location of estimated shifts. Rate values represent new lineages per million years, with rates increasing from blue to red (online version). (B) Phylogeny of Velloziaceae with branch lengths transformed to Bayes-factor support for rate shifts.
MEDUSA shows strongest support for two independent shifts in macroevolutionary regime (we report the results of the analysis including sampling fractions, because there was no difference from the analysis that omitted incomplete sampling). They are inferred to be more recent than those inferred by BAMM (Fig. 2), nesting within the core-Barbacenia and the core-Vellozia clades (Material S7).
Estimates of net diversification by BAMM are higher and more variable (uncertain) in the South American clades of Velloziaceae [lambda: 0.18, quantiles (25–75 %): 0.15–0.25; net diversification: 0.17, quantiles: 0.125–0.195] than in the African clade (lambda: 0.1, quantiles: 0.07–0.14; net diversification: 0.08, quantiles: 0.075–0.081). This is reflected in a relatively complex estimate of macroevolutionary cohorts in the family (Fig. S10), and in comparisons across genera and infra-generic clades of Velloziaceae (Fig. 4), as well as the variable rates of speciation and extinction estimated from BAMM simulations (Fig. S11). The geography-dependent diversification analysis corroborates more variable rates in South America, with the highest support for a model in which the rate of diversification there, in conjunction with a ‘hidden’ binary character (HiSSE-partial model, see Table 3), is about four times higher than the background diversification rates within Velloziaceae (i.e. the rate observed in both the African lineage and the South American representatives that are not associated with the ‘hidden’ state).
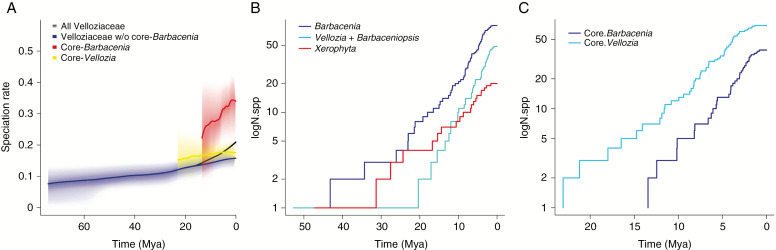
Fig. 4.
(A) Estimated speciation rates in Velloziaceae lineages and (B, C) estimated logarithm of the number of species (logN.spp) accumulation through time (in millions of years) for selected lineages of Velloziaceae. All Velloziaceae: includes all five genera of the family; core-Barbacenia + core-Vellozia: the infra-generic clades within these genera exhibiting the highest probability for having suffered shifts in their diversification rates relative to the background rates simulated by BAMM analyses (delimitated by nodes 157 and 209, respectively).
Table 3.
The fit of alternative models of geography-dependent diversification for the family Velloziaceae (trait states: Africa and South America occurrence, see text)
| Model | Model specification | logL | AICc | ΔAIC | w i |
|---|---|---|---|---|---|
| bisse.null | hidden.states=FALSE, rates=c(1,1,0,0), N=4 | −476.75 | 961.77 | 30.55 | 2.1E-07 |
| bisse | hidden.states=FALSE, rates=(1,2,0,0), N=6 | −471.12 | 954.84 | 19.04 | 6.7E-05 |
| null.hisse | hidden.states=TRUE, rates=c(1,1,2,2), N=5 | −462.70 | 935.8 | 4.58 | 0.0919 |
| hisse.partial | hidden.states=TRUE, rates=c(1,2,0,3), N=10 | −455.82 | 931.22 | 0 | 0.9079 |
The best model fitted, based on ΔAIC and Akaike weights (wi), is denoted in bold. AICc: Akaike information criterion corrected for sample size. N = number of parameters of each model; logL = log-likelihood of the model.
Correlates of diversification: climate, morphology and ecology
The inferred shift to higher diversification rates was more recent in Barbacenia (13.4 Mya; middle/late Miocene: 15–8 Mya) than in Vellozia (23.1 Mya; Oligocene/Miocene transition, 33–13 Mya; Fig. 2). These periods correspond to distinct climate scenarios, with the late Oligocene/early Miocene marked by increasing temperature and humidity culminating in the Middle Miocene Climatic Optimum (approx. 15 Mya), followed by drying/cooling climatic conditions in the late Miocene (Fig. 2).
Ancestral state reconstructions (Figs S12 and S13) indicate that aerial stems and aquiferous parenchyma had already evolved in Vellozia and Barbacenia, respectively, by the time shifts to higher diversification occurred in each clade. The state-dependent diversification analyses give highest support to a model in which the acquisition of aquiferous parenchyma between bundles, in conjunction with a ‘hidden’ binary character, is associated with higher diversification rates in Velloziaceae as a whole (HiSSE-partial model, see Table 4). Our data indicated no variation of this trait in the most diverse lineage (Vellozia + Barbaceniopsis clade), with none of the species sampled in this clade showing the presence of aquiferous parenchyma between bundles.
Table 4.
The fit of alternative models of state-dependent diversification for the family Velloziaceae (traits: aquiferous parenchyma and development of aerial stems) and for the two most diverse subclades of the family (trait development of aerial stems only, see text)
| Lineage - trait | Model specification | logL | AICc | ΔAIC | w i |
|---|---|---|---|---|---|
| Model | |||||
| Velloziaceae - aquiferous parenchyma | |||||
| bisse.null | hidden.states=FALSE, rates=c(1,1,0,0), N=4 | −516.38 | 1041.03 | 11.776 | 0.0027 |
| bisse | hidden.states=FALSE, rates=(1,2,0,0), N=6 | −513.21 | 1039.001 | 9.747 | 0.00756 |
| null.hisse | hidden.states=TRUE, rates=c(1,1,2,2), N=5 | −527.92 | 1066.258 | 37.004 | 9.1E-09 |
| hisse.partial | hidden.states=TRUE, rates=c(1,2,0,3), N=10 | −503.83 | 1029.254 | 0 | 0.9895* |
| hisse.full | hidden.states=TRUE, rates=c(1,2,3,4), N=12 | −509.85 | 1045.98 | 16.726 | 0.0002 |
| Velloziaceae - development of aerial stem | |||||
| bisse.null | hidden.states=FALSE, rates=c(1,1,0,0), N=4 | −543.53 | 1095.34 | 20.57 | 3.4E-05 |
| bisse | hidden.states=FALSE, rates=(1,2,0,0), N=6 | −542.78 | 1098.16 | 23.39 | 8.3E-06 |
| null.hisse | hidden.states=TRUE, rates=c(1,1,2,2), N=5 | −532.17 | 1074.77 | 0 | 0.9999* |
| hisse.partial | hidden.states=TRUE, rates=c(1,2,0,3), N=10 | −538.5 | 1098.59 | 23.82 | 6.7E-06 |
| hisse.full | hidden.states=TRUE, rates=c(1,2,3,4), N=12 | −537.44 | 1101.16 | 26.39 | 1.9E-06 |
| Barbacenia - development of aerial stem | |||||
| bisse.null | hidden.states=FALSE, rates=c(1,1,0,0), N=4 | −149.18 | 307.28 | 0 | 0.8165* |
| bisse | hidden.states=FALSE, rates=(1,2,0,0), N=6 | −149.2 | 312.40 | 5.12 | 0.0632 |
| null.hisse | hidden.states=TRUE, rates=c(1,1,2,2), N=5 | −149.86 | 311.12 | 3.83 | 0.0002 |
| hisse.partial | hidden.states=TRUE, rates=c(1,2,0,3), N=10 | −149.18 | 324.16 | 16.88 | 0.1202 |
| hisse.full | hidden.states=TRUE, rates=c(1,2,3,4), N=12 | −149.19 | 331.04 | 23.76 | 5.7E-06 |
| Vellozia + Barbaceniopis - development of aerial stem | |||||
| bisse.null | hidden.states=FALSE, rates=c(1,1,0,0), N=4 | −292.75 | 594.03 | 10.04 | 0.0045 |
| bisse | hidden.states=FALSE, rates=(1,2,0,0), N=6 | −287.46 | 588.09 | 4.09 | 0.0875 |
| null.hisse | hidden.states=TRUE, rates=c(1,1,2,2), N=5 | −287.79 | 586.38 | 2.38 | 0.2052 |
| hisse.partial | hidden.states=TRUE, rates=c(1,2,0,3), N=10 | −280.93 | 583.99 | 0 | 0.6759* |
| hisse.full | hidden.states=TRUE, rates=c(1,2,3,4), N=12 | −280.93 | 590.44 | 6.44 | 0.0269 |
The best model fitted, based on ΔAIC and Akaike weights (wi), is denoted by *. AICc: Akaike information criterion corrected for sample size. N = number of parameters of each model; logL = log-likelihood of the model.
HiSSE analyses do not support the hypothesis that the development of aerial stems is linked to higher diversification; instead, the best-fit model is one in which variation in diversification is associated with a hidden binary trait, but not associated with aerial stems (HiSSE-null model; Table 4). Similarly, restricting the scope of the analysis to just Barbacenia yields no support for any state-dependent diversification model (BiSSE-null; Table 4). However, for the Vellozia + Barbaceniopsis clade, the best-fit model is HiSSE-partial, followed by HiSSE-full, and average net diversification of lineages with aerial stems is more than twice the rate of those without aerial stems.
DISCUSSION
Our primary results indicate that the disparity of species richness between Africa and South America in Velloziaceae is the result of accelerated species diversification within the two most diverse genera of the family (Fig. 2), which are restricted to the Neotropics. These rate shifts are significantly correlated with transitions in the states of functional traits (Fig. 1; Table 4; Figs S12 and S13), suggesting an adaptive component in these radiations. Diversification rate heterogeneity in the core-Vellozia clade is consistent with the density dependence expected in mature radiations and by traditional hypothesis of adaptive radiation. The timing of the two rate shifts in Velloziaceae, coupled with the signals of adaptive radiation in Vellozia, add new insights into the assembly of the campos rupestres habitat itself, as discussed below.
African versus South American diversification of Velloziaceae
The diversification analyses clearly support the hypothesis that the imbalance between Palaeo- and Neotropical floristic diversity results from higher speciation rates in South America (e.g. Gentry, 1982; Linder, 2014; Couvreur, 2015). However, occurrence in Africa versus South America per se does not explain much of the variation in diversification of Velloziaceae. Instead, rate heterogeneity is nested within the South American clades, with independent shifts in Vellozia around 23 Mya and in Barbacenia around 13 Mya. Similar patterns of higher speciation in Neotropical lineages have been shown in other plant groups centred in Andean and Amazon regions (e.g. Bell and Donoghue, 2005; Hughes and Eastwood, 2006; Bardon et al., 2013; Koenen et al., 2015). The main biogeographical factors proposed to explain higher speciation in the Neotropics are area, time for speciation and climatic stability (reviewed by Couvreur, 2015). However, climatic instability during the Quaternary resulting in population isolation in refugia has also been invoked to explain recent diversification in the region (Haffer, 1969; Prance, 1974). Here, neither the shifts nor average differences in the diversification rates seem to be related to overall clade ages, since the South American Vellozia–Barbaceniopsis clade and the African Xerophyta are sister clades (i.e. exhibit the same stem lineage ages) but exhibit different diversification dynamics (Fig. 2). In addition, the South American genus Barbacenia, which split from the [Vellozia–Barbaceniopsis]–Xerophyta clade approx. 10 Myr earlier (i.e. showing the older stem age between the three major clades of Velloziaceae), is the clade showing the most recent crown age and the highest diversification rate in the family (Fig. 2). The total geographical range of Xerophyta in Africa is larger than that of the South American genera of Velloziaceae, and range sizes of individual species also tend to be larger in Africa compared to the campos rupestres endemics in South America (Mello-Silva et al., 2011); however, it is difficult to speculate whether small-scale geographical range has influenced diversification rates.
Most diversification in Velloziaceae is concentrated within the Neogene (mainly in the Miocene), although the origin estimated for the stem lineages of the genera occurring in both South America and Africa dates from the early Eocene (approx. 55–40 Mya). This pattern is consistent with expectations of ancient origins and persistence of old lineages under a ‘museum’ hypothesis for the Neotropics. Even considering the alternative scenarios with more recent rate shifts (as reconstructed by MEDUSA), diversification in Neotropical Velloziaceae is still older than that predicted by higher diversification rates caused by Pliocene/Quaternary climatic events. The relatively homogeneous background rates of diversification in Velloziaceae suggest that catastrophic events did not cause higher extinction rates in Africa than in other tropical areas, as proposed by Raven and Axelrod (1974). Indeed, evidence for catastrophic climatic events in Africa is largely lacking (Couvreur, 2015) and many studies have found bursts of diversification since the early Oligocene to the Miocene in African clades (Schnitzler et al., 2011; Linder, 2014), similar to our findings in Velloziaceae. Despite being relatively old, such shifts in diversification linked to environmental changes contradict the ‘museum’ model of tropical biodiversity, which assumes steady diversification under climatic stability (see Koenen et al., 2015).
Current evidence, including our results, suggests that independent bursts of diversification across time, in different lineages, have generated spatial imbalances in tropical diversity, instead of a single factor (Linder, 2008; Hughes et al., 2013; Couvreur, 2015). Likewise, multiple nested rate shifts accounting for the high diversity of angiosperm lineages (Magallón and Sanderson, 2001) appear to be a general pattern, instead of single rate shifts explaining exceptionally species-rich plant groups (Smith et al., 2011; Koenen et al., 2013). This study is one of the first to show that faster diversification underlies higher Neotropical diversity in lineages from open areas, as most studies to date have focused on rain forests or dry forests (Hughes et al., 2013). A notable exception is Leguminosae, in which rate shifts have been documented in Neotropical lineages found in grasslands and savannas (Simon et al., 2009; Koenen et al., 2013), as well as in the campos rupestres and Andean páramos (Drummond et al., 2012; Koenen et al., 2013; de Souza et al., 2013; Rando et al., 2016). Likewise, contrasts in diversity in these legume clades are closely associated with particular distinctive ecologies, and potentially with ecological (biome) shifts, rather than with the strict geographical history of clades (Lavin et al., 2004; Koenen et al., 2013). Our results also imply that continental vicariance did not directly influence Velloziaceae diversification, as the pattern of rate shifts is more complex, independently nested within the two South American lineages, and requiring hidden states unrelated to continental areas of occurrence per se. Vicariance may have been important, however, to the diversification of the Andean representatives of the family (Mello-Silva et al., 2011): the genus Barbaceniopsis and the species Vellozia andina split from their sister lineages around 23–21 Mya, concomitant with the initial South/Central Andean uplift (Hoorn et al., 2010).
Vellozia: adaptive radiation in the campos rupestres?
The core-Vellozia clade shows a trajectory of diversification in line with a ‘mature’ evolutionary radiation (sensu Linder, 2008), i.e. an initial burst followed by declining rates. This could reflect niches filling toward an equilibrium (Benton and Emerson, 2007; Rabosky, 2014), and/or greater extinction as niches become more finely divided among proliferating species (Jansson and Dynesius, 2002; Linder, 2008). A previous study found that diversification of water-related adaptations could be important to niche divergence in Vellozia (Alcantara et al., 2015). The development of conspicuous aerial stems (as a surrogate trait for drought resistance and loss of desiccation tolerance in these species) associated with higher diversification rates in representatives of the Vellozia + Barbaceniopsis clade corroborates this (Fig. 2).
The Oligocene/Miocene transition time inferred for the diversification increase in Vellozia is coincident with a global increase in temperature, fossil angiosperm pollen diversity, particularly of Poaceae and Asteraceae, and areas of C3 grassland dominance (Jaramillo et al., 2006). We hypothesize that in this environment, the development of conspicuous aerial stems (associated with drought resistance) allowed greater plant productivity and size (Alcantara et al., 2015), and enabled Vellozia to escape restricted occurrence in exposed outcrops and expand to open grasslands. It would have allowed the colonization of warmer microhabitats with a deeper soil layer, surrounding the exposed rock outcrop habitats characteristic of campos rupestres. In these soils, seasonal water availability is less restricted, and consequently the competition with other grassland-related species more intense (Alpert, 2006). Thus, this ecological release can be seen as a key opportunity for Vellozia radiation. A better comprehension of the adaptive radiation of Vellozia in the campos rupestres will require a deeper understanding of the origins of alternative drought resistance strategies in the family. It will also be important to consider how other ecological features, such as responses to nutrient soil availability (Ayensu, 1973; Oliveira et al., 2016) and altitude (Mello-Silva, 1995), root specializations (Oliveira et al., 2015), fire resistance (Conceição and Orr, 2012), and interactions with pollinators and herbivores (Sazima and Sazima, 1990, Landau et al., 1999; Jacobi and Sarto, 2007), might have contributed to diversification in Vellozia.
The ecological diversity and endemism of Vellozia and other plant clades of the campos rupestres have been long cited as examples of adaptive radiation (Coile and Jones, 1981; Harley, 1988). Givnish (2015) argues that adaptive radiation does not require explosive diversification in its initial phase; instead, its evolutionary signature is simply closely related to species occupying different ecological niches in sympatry, or to a convergence of adaptive life forms in distantly related species of different lineages occupying similar niches in different locations. The only previous study to test for adaptive diversification in a campos rupestres lineage found no signal of ecological divergence, despite the fact that Minaria (Apocynaceae) radiated only around 1.5 Mya (Ribeiro et al., 2014). Other recent studies have inferred dated molecular phylogenies of campos rupestres lineages and found patterns that suggest evolutionary radiations, but did not explicitly test adaptive hypotheses (Koenen et al., 2013; de Souza et al., 2013; Rando et al., 2016). Our understanding of the evolution and distribution of ecological strategies in this habitat is still in its infancy, preventing a detailed assessment of how ecological divergence has contributed to lineage diversification. Thus, further studies focusing on the evolution and characterization of life forms (e.g. Mello-Silva, 1995; Alves and Kolbek, 2010) and ecological strategies in Vellozia (Oliveira et al., 2005, 2015, 2016; Teodoro, 2014; Alcantara et al., 2015), within this background of diversification rate slowdown, are promising routes toward a better understanding of adaptive radiation in the campos rupestres.
Diversification in Barbacenia: habitat specialization and ongoing high speciation
The rate shift inferred within Barbacenia between 15 and 8 Mya (approx. 13.4 Mya) is consistent with a ‘pull-of-the-present’ effect observed in constant birth–death models (Stadler, 2013) and a high turnover rate characteristic of young radiations (sensu Linder, 2008). It does not exhibit the density-dependent signature of declining rates with the filling of ecological niche space (Morlon et al., 2011). Similar patterns have been observed in South African Aizoaceae (Klak et al., 2004) and South American Lupinus (Hughes and Eastwood, 2006), although the age of the shift in Barbacenia is notable for being relatively old compared to these and similar cases (see Linder, 2008). It coincides with the period following the Middle Miocene Climatic Optimum (approx. 15 Mya), marked by a drying/cooling climate (Zachos et al., 2008). In Hoffmannseggella (Orchidaceae), diversification was associated with the expansion of campos rupestres outcrops during the Miocene cooling, as surrounding forested areas retracted (Antonelli et al., 2010). In that case, populations restricted to the outcrops expanded their geographical range and reached secondary contact before the completion of reproductive isolation in allopatry, leading to speciation by hybridization (Antonelli et al., 2010). We have as yet no evidence that hybridization has contributed to diversification in Barbacenia. Alternatively, based on the restrictive conditions of life in exposed outcrops (e.g. Alpert, 2006; Porembski and Barthlott, 2000), we tested whether a morphofunctional trait associated with such stressing conditions is associated with higher diversification rates.
The acquisition of aquiferous parenchyma between bundles in the leaves, a trait that within Velloziaceae is mostly present in species of Barbacenia, could have improved survival in drier and colder conditions by increasing water accumulation within the plant (Warming, 1893; Menezes, 1973). The ensuing expansion of Barbacenia populations might have led to accelerated diversification by increased habitat specialization and allopatric isolation. Many species of Barbacenia exhibit very small distribution ranges (Mello-Silva, 2009), and several of those are restricted to shadow and colder rocky crevices/caves found near waterfalls, in contrast to the majority of Velloziaceae, which are heliophytes. This habitat specialization strategy might have provided special advantages in the relatively small competition pool in the outcrops (Alpert, 2006).
In summary, contrasting ecological strategies seem to have driven the radiations of Barbacenia and Vellozia. While a preference for rock outcrops generally characterizes Velloziaceae as a whole, Vellozia apparently escaped this ecological restriction by evolving drought resistance (as indicated by aerial stems). Barbacenia, by contrast, evolved the existent desiccation tolerance mixing it with water retention in the leaves (i.e. by the aquiferous parenchyma between bundles) and thus became even more specialized to the outcrops during the colder and drier late Miocene. A better characterization of the habitat and fine-scale distribution of Barbacenia and Vellozia under a phylogenetic perspective is currently underway (S. Alcantara et al., in preparation) and will investigate these hypotheses in more detail.
Velloziaceae and the origin of campos rupestres
The spectacular floristic diversity of the campos rupestres raises compelling questions about its evolutionary origins and ecological dynamics. In this regard, understanding the evolutionary history of the Velloziaceae is tantamount to revealing the time and mode of this assembly. The association between the campos rupestres phytophysiognomy and representatives of Velloziaceae is so emblematic that arborescent and showy flowered species of Vellozia are popularly known as ‘queens of the campos rupestres’. The macroevolutionary evidence for an adaptive Vellozia radiation helps us understand the diversification dynamics that underlie the extant huge species diversity in the campos rupestres.
The Oligocene/Miocene diversification burst of the core-Vellozia clade is coincident with the global increase of Poaceae and Asteraceae (Jaramillo et al., 2006), families that also have conspicuous representation in the campos rupestres. A general pattern of temporal congruence in the origin and diversification of other campos rupestres clades with Vellozia would reinforce the importance of the Oligocene increase in global temperature and C3 grasslands to the origin of the campos rupestres in Eastern/Central Brazil. In addition, the Barbacenia radiation during the Miocene cooling corroborates the importance of the expansion of rocky outcrops for the diversification of this flora (Antonelli et al., 2010). Importantly, these results push back the origin of the campos rupestres, which has generally been inferred around the Miocene, based on inferred clade ages in Asclepiadoideae (Rapini et al., 2007). According to Conceição et al. (2016), these Miocene-age lineages would exhibit increasing diversification into the Quaternary, exemplified by recent Pleistocene diversification in Calliandra (Leguminosae) (de Souza et al., 2013), Minaria (Apocynaceae) (Ribeiro et al., 2014) and Chamaecrista (Leguminosae) (Rando et al., 2016). Quaternary diversification is in agreement with the ‘evolutionary pump’ model (Morton, 1972), proposed for West African tropical mountain vegetation and also invoked to explain the high plant diversity in the campos rupestres, as resulting from successive habitat expansion/retraction events in response to Pleistocene climatic oscillations (Harley, 1988, 1995; Alves and Kolbek, 1994; Giulietti et al., 1997; Rapini et al., 2008). It posits that mountaintops were interglacial refugia, accelerating divergence among vicariant populations during warmer and moister interglacial periods. During cooler and drier glacial periods, the campos rupestres would have expanded to lower altitudes, while forests would have retracted. Our results highlight the importance of global climatic oscillations in the diversification of both Barbacenia and Vellozia, although this diversification is much older than the Quaternary glacial cycles predicted by the ‘evolutionary pump’ model.
Habitats with similar vegetation physiognomies (i.e. outcrops inhabited by desiccation-tolerant plants, and surrounded by patches of shallow soil dominated by grasses and xerophytic shrubs) and ecological disturbance (i.e. fire and seasonal rain regimes) around the world have been described as typically old, climatically buffered, infertile landscapes (OCBILs; Hopper, 2009). These habitats, such as the Pantepuis of the Guayana Shield, south-west Australia and the southern African sourvelds, are highly diverse and endemic-rich landscapes that may share ecological and evolutionary patterns with the campos rupestres of the Espinhaço Range (Conceição et al., 2016; Oliveira et al., 2016; Mucina, 2018). The origin of the Espinhaço Range landscapes culminated with crustal flexures and overlap caused by east–west pressure at the end of Neoproterozoic, around 900 Mya (Almeida-Abreu & Pflug, 1994), characterizing the long geological stability predicted for OCBILs (Hopper, 2009). Mucina and Wardell-Johnson (2011) refined this concept by proposing old stable landscapes (OSLs), where soil infertility is a function of landscape age. They also added fire regime predictability as a new dimension of the theory, because it affects the composition and function of biotas and shapes several biomes (Mucina and Wardell-Johnson, 2011). Thus, the OSL highlands would not be cooler and dryer refuges during Pleistocene interglacial periods, but rather refuges for fire-sensitive lineages since the Tertiary (Mucina, 2018). According to this hypothesis, OSL lineages are usually highly specialized, and display low dispersal capability and high phylogenetic conservatism (Mucina and Wardell-Johnson, 2011; Mucina, 2018). The climatic stability of OSLs allows the persistence of these lineages for long periods, characterizing these landscapes as refuges, cradles and/or ‘museums’ of biodiversity that shelter high concentrations of endemic diversity. The Espinhaço Range houses more than 10 % of plant species reported to Brazil and, on average, 40 % of them are endemic (BFG, 2015). In addition, the long-term persistence of South American Velloziaceae lineages (Fig. 2), their high phylogenetic conservatism and habitat specialization, especially in Barbacenia, is consistent with expectations for OSLs.
Based on the ecological similarities between OSL areas in the campos rupestres and in Africa, and the distribution patterns of the monocot families Eriocaulaceae, Xyridaceae and Velloziaceae, these groups have been previously interpreted as Gondwanan in origin (see Mello-Silva et al., 2011). However, the vicariance of South America and Africa (i.e. prior to 110 Mya) pre-dates our inferred crown age of Velloziaceae (i.e. approx. 75 Mya). The divergence of Acanthochlamys has been dated to the Cretaceous, too young to be attributed to the breakup of Gondwana. In addition, the stem ages of the African and South American Velloziaceae genera are concentrated between 55 and 40 Mya (Fig. 2), during the Palaeocene–Eocene Thermal Maximum (PETM; Zachos et al., 2008). All previous studies reporting molecular clock analyses that included Velloziaceae representatives also contradict a Gondwanan origin (see Table 1 and references therein).
Because of the fire-prone nature and open vegetation landscape, the campos rupestres have been considered part of the Cerrado (Simon et al., 2009; Trovó et al., 2013). The flora of the Cerrado was assembled from independent plant lineages from different biomes, mostly over the past 4–5 Myr (Simon et al., 2009), in line with recent estimates for a few campos rupestres lineages, especially Leguminosae genera (de Souza et al., 2013; Rando et al., 2016). However, campos rupestres groups show higher levels of endemism and have higher species turnover than Cerrado flora, suggesting distinct biogeographical processes (see Rando et al., 2016). Although physiognomically similar, the campos rupestres are historically, ecologically and floristically distinct from the Cerrado, with great diversity derived from in situ radiations resulting in microendemic species in campos rupestres (Giulietti and Pirani, 1988; Pirani et al., 2015; Conceição et al., 2016). Our results corroborate the first studies focusing on campos rupestres lineages (Rapini et al., 2007; Antonelli et al., 2010) that suggested older ages than those of most Cerrado lineages (Simon et al., 2009; Hughes et al., 2013).
Despite the differences between Cerrado and campos rupestres, overall biodiversity estimates usually inflate the number of plant species in the Cerrado hotspot by the inclusion of campos rupestres (Alves et al., 2007; Strassburg et al., 2017). It is crucial, however, to recognize the distinct ecological conditions (in particular the edaphic ones) and historical processes that have moulded Cerrado and campos rupestres, especially given current threats to the conservation of both. The campos rupestres might be considered a hotspot within the Cerrado hotspot, similar to what has been proposed for the Páramos within the Tropical Andes hotspot (Madriñan et al., 2013). However, unlike the Páramos, the exceptionally high species diversity of the campos rupestres seems to be derived from generally older evolutionary events. The highest diversification rates in Velloziaceae reaches 0.36 species/Myr since the late Miocene within the core-Barbacenia clade and approx. 0.25 species/Myr within the core-Vellozia clade, while in other groups (e.g. Calliandra, Koenen et al., 2013) highest diversification rates have been observed within the last 5 Mya. Although we still lack average rate estimates for most campos rupestres lineages, the different time of species origins of most clades studied so far indicates a more complex macroevolutionary scenario than that reported for the Páramos and other relatively ‘recent’ species-rich biomes reported by Madriñan et al. (2013) (e.g. the Mediterranean Basin, the Succulent Karoo, the Cerrado, the Cape Floristic Region, the California Floristic Province and south-west Australia).
In this study, we corroborate the hypothesis that adaptive radiation has been crucial to the origin of a dominant lineage in the campos rupestres. Despite the ecological restrictions of Velloziaceae to rock/shallow soils and open vegetation habitats in both Africa and South America, the evolution of diversified water-related strategies in the two genera that occur in the campos rupestres seems to have driven their diversification. We also corroborate the hypothesis of increased diversification in South American lineages associated with transitions in ecological strategies as the main factor that has driven species imbalance between New and Old World Velloziaceae, echoing several other Pantropical plant families and the idiosyncratic origins of different Neotropical biomes.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: map displaying the distribution of campos rupestres vegetation. Material S2: description and codification of the functional traits analysed. Material S3: A, vouchers of the specimens analysed; and B, laboratory procedures and primers used for molecular analyses. Figure S4: majority-rule consensus tree from the Bayesian phylogenetic analysis. Material S5: substitution models prevalent for each data partition from the analyses performed in MrBayes and BEAST. Figure S6: A, coefficient of variation; and B, standard deviation of the UCLD rates estimated from the MCMC runs in BEAST analyses. Material S7: results of the MEDUSA analysis. Figure S8: major clade credibility tree, time-calibrated through the UCLN rate model. Material S9: results of the BAMM simulations. Figure S10: macroevolutionary cohorts in Velloziaceae. Figure S11: A, speciation; B, extinction; and C, net diversification rates estimated by BAMM for African and South American lineages of Velloziaceae. Figure S12: Bayesian reconstruction of ancestral states of the functional trait “development of conspicuous aerial stems”. Figure S13: Bayesian reconstruction of ancestral states of the functional trait “aquiferous parenchyma between the bundles on the leaves”.
ACKNOWLEDGEMENTS
We thank J. R. Pirani for his inspiring work on the flora of campos rupestres and encouragement at the initial steps of this work. D. D. Ackerly, G. S. Teodoro and R. S. Oliveira provided invaluable insights about the functional traits evaluated. J. Lovo, J. Paula-Souza, K. Drequeceler and M. Sousa-Baena provided help for field and lab work; K.D. also provided the pictures of the anatomical sections. J. G. Rando collected and registered the specimens of B. celiae. W. Lobin and M. Weigend provided access to several Xerophyta specimens. IBAMA-SISBIO, IEF-MG and SEMA-BA provided the licences for fieldwork and collection; and the staff of the Parque Nacional da Serra do Cipó, PN das Sempre-Vivas, PN da Serra da Canastra, PN da Chapada Diamantina, PN da Chapada dos Veadeiros, Parque Estadual Pico do Itambé, PE de Grão-Mogol, PE do Rio Preto, PE da Serra do Cabral, PE do Morro do Chapéu and Parque Municipal do Pico das Almas kindly provided assistance and/or authorizations for fieldwork. We also thank staff and students from the Botany departments at USP (São Paulo, Brazil), FMNH (Chicago, EUA), UFSC (Florianópolis, Brazil) and from the Pritzker Laboratory of Molecular Systematics. The Handling editor (Dr G. Petersen), Dr D. Cardoso and one anonymous referee provided invaluable improvements towards the final version of the manuscript. This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo [FAPESP 2010/50923-0 and 2013/01780-0 to S.A. and 2011/52174-4 to R.M.S.] and the Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq Bolsa de produtividade to R.M.S.].
Data archival location: GenBank.
LITERATURE CITED
- Aidar ST, Meirelles ST, Pocius O, Delitti WBC, Souza GM, Gonçalves AN. 2010. Desiccation tolerance in Pleurostima purpurea (Velloziaceae). Plant Growth Regulation 62: 193–202. [Google Scholar]
- Alcantara S, Mello-Silva R, Teodoro GS, Drequeceler K, Ackerly DD, Oliveira RS. 2015. Carbon assimilation and habitat segregation in resurrection plants: Comparison between desiccation and non-desiccation-tolerant species of Neotropical Velloziaceae (Pandanales). Functional Ecology 29: 1499–1512. [Google Scholar]
- Alfaro ME, Santini F, Brock C, et al. . 2009. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proceedings of the National Academy of Sciences USA 106: 13410–13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Abreu PA, Pflug R. 1994. The geodynamic evolution of the southern Serra do Espinhaço, Minas Gerais, Brazil. Zentrallblatt für Geologie und Paläontologie 1: 21–44. [Google Scholar]
- Alpert P. 2006. Constraints of tolerance: Why are desiccation-tolerant organisms so small or rare?Journal of Experimental Biology 209: 1575–1584. [DOI] [PubMed] [Google Scholar]
- Alves RJV, Kolbek J. 1994. Plant species endemism in savanna vegetation on table mountains (Campo rupestre) in Brazil. Vegetation 113: 125–139. [Google Scholar]
- Alves RJV, Kolbek J. 2010. Vegetation strategy of Vellozia crinita (Velloziaceae). Biologia 65: 254–264. [Google Scholar]
- Alves RJV, Cardin L, Kropf MS. 2007. Angiosperm disjunction ‘Campos rupestres-restingas’: a re-evaluation. Acta Botanica Brasilica 21: 675–685. [Google Scholar]
- Antonelli A, Verola CF, Parisod C, Gustafsson ALS. 2010. Climate cooling promoted the expansion and radiation of a threatened group of South American orchids (Epidendroideae: Laeliinae). Biological Journal of the Linnean Society 100: 597–607. [Google Scholar]
- Ayensu ES. 1973. Biological and morphological aspects of the Velloziaceae. Biotropica 5: 135–149. [Google Scholar]
- Bardon L, Chamagne J, Dexter KG, Sothers CA, Prance GT, Chave J. 2013. Origin and evolution of Chrysobalanaceae: insights into the evolution of plants in the Neotropics. Botanical Journal of the Linnean Society 171: 19–37. [Google Scholar]
- Beaulieu JM, O’Meara BC. 2016. Detecting hidden diversification shifts in models of trait-dependent speciation and extinction. Systematic Biology 65: 583–601. [DOI] [PubMed] [Google Scholar]
- Behnke HD, Treutlein J, Wink M, Klamer K. 2000. Systematics and evolution of Velloziaceae, with special reference to sieve-element plastids and rbcL sequence data. Botanical Journal of the Linnean Society 134: 93–129. [Google Scholar]
- Bell CD, Donoghue MJ. 2005. Phylogeny and biogeography of Valerianaceae (Dipsacales) with special reference to the South American valerians. Organisms Diversity and Evolution 5: 147–159. [Google Scholar]
- Benton MJ, Emerson BC. 2007. How did life become so diverse? The dynamics of diversification according to the fossil record and molecular phylogenetics. Palaeontology 50: 23–40. [Google Scholar]
- BFG 2015. Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguesia 66: 1085–1113. [Google Scholar]
- Bouckaert R, Alvarado-Mora M, Rebello Pinho J. 2013. Evolutionary rates and hbv: Issues of rate estimation with Bayesian molecular methods. Antiviral Therapy 18: 497–503. [DOI] [PubMed] [Google Scholar]
- Bouckaert R, Heled J, Kühnert D, et al. . 2014. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Computational Biology 10: e1003537. doi:10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: A practical information-theoretic approach. New York: Springer. [Google Scholar]
- Chase MW, Hills HH. 1991. Silica gel: An ideal material for field preservation of leaf samples for DNA studies. Taxon 40: 215–220. [Google Scholar]
- Chase MW, Fay MF, Devey DS, et al. . 2006. Multigene analyses of monocot relationships: A summary. Aliso 22: 63–75. [Google Scholar]
- Chiang T, Schaal BA, Peng C. 1998. Universal primers for sequencing a noncoding spacer between the atpB and rbcL genes of chloroplast DNA. Botanical Bulletin of Academia Sinica 39: 245–250. [Google Scholar]
- Coile NC, Jones Jr. SB. 1981. Lychnophora (compositae: Vernonieae), a genus endemic to the Brazilian Planalto. Brittonia 33: 528–542. [Google Scholar]
- Conceição AA, Orr BJ. 2012. Post-fire flowering and fruiting in Vellozia sincorana, a caulescent rosette plant endemic to Northeast Brazil. Acta Botanica Brasilica 26: 94–100. [Google Scholar]
- Conceição AA, Giulietti AM, Meirelles ST. 2007. a Ilhas de vegetação em afloramentos de quartzito-arenito no Morro do Pai Inácio, Chapada Diamantina, Bahia, Brasil. Acta Botanica Brasilica 21: 335–347. [Google Scholar]
- Conceição AA, Pirani JR, Meirelles ST. 2007. b Floristics, sctructure and soil of insular vegetation in four quartzite-sandstone outcrops of “Chapada Diamantina”, northeast Brazil. Brazilian Journal of Botany 30: 641–655. [Google Scholar]
- Conceição AA, Rapini A, do Carmo FF, et al. . 2016. Rupestrian grassland vegetation, diversity, and origin. In Fernandes GW, ed. Ecology and conservation of mountaintop grasslands in Brazil. Basel: Springer International Publishing, 105–127. [Google Scholar]
- Couvreur TLP. 2015. Odd man out: why are there fewer plant species in African rain forests?Plant Systematic and Evolution 301: 1299–1313. [Google Scholar]
- Diogo JC. 1926. As folhas das vellozias e seu aparelho regulador da transpiração. Arquivos do Museu Nacional 28: 19–46. [Google Scholar]
- Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biology 4: e88. doi:10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond CS, Eastwood RJ, Miotto ST, Hughes CE. 2012. Multiple continental radiations and correlates of diversification in Lupinus (Leguminosae): testing for key innovation with incomplete taxon sampling. Systematic Biology 61: 443–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi P, Pirani JR. 2009. Review of plant biogeographic studies in Brazil. Journal of Systematic and Evolution 47: 477–496. [Google Scholar]
- Gaff DF. 1977. Desiccation-tolerant vascular plants of Southern Africa. Oecologia 31: 95–109. [DOI] [PubMed] [Google Scholar]
- Gandolfo MA, Nixon KC, Crepet WL. 2002. Triuridaceae fossil flowers from the Upper Cretaceous of New Jersey. American Journal of Botany 89: 1940–1957. [DOI] [PubMed] [Google Scholar]
- Gentry AH. 1982. Neotropical floristic diversity: phytogeographical connections between Central and South America, Pleistocene climatic fluctuations, or an accident of the Andean orogeny?Annals of the Missouri Botanical Garden 69: 557–593. [Google Scholar]
- Giulietti AM, Hensold N. 1990. Padrões de distribuição geográfica dos gêneros de Eriocaulaceae. Acta Botanica Brasilica 4: 133–159. [Google Scholar]
- Giulietti AM, Pirani JR. 1988. Patterns of geographic distribution of some plant species from the Espinhaço Range, Minas Gerais and Bahia, Brazil. In Vanzolini PE, Heyer WR, eds. Proceedings of a workshop on neotropical distribution patterns. Rio de Janeiro: Academia Brasileira de Ciências, 39–69. [Google Scholar]
- Giulietti AM, Pirani JR, Harley RM. 1997. Espinhaço range region, eastern Brazil. In: Davis SD, ed. Centres of plant diversity: A guide and strategy for their conservation, vol. 3 Cambridge: WWF/IUCN, 397–404. [Google Scholar]
- Giulietti AM, Harley RM, de Queiroz LP, Wanderley MGL, van den Berg C. 2005. Biodiversity and conservation of plants in Brazil. Conservation Biology 19: 632–639. [Google Scholar]
- Givnish TJ. 2015. Adaptive radiation versus ‘radiation’ and ‘explosive diversification’: Why conceptual distinctions are fundamental to understanding evolution. New Phytologist 207: 297–303. [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Losos JB. 2009. Adaptive radiation: Contrasting theory with data. Science 323: 732–737. [DOI] [PubMed] [Google Scholar]
- Haffer J. 1969. Speciation in Amazonian forest birds. Science 165: 131–137. [DOI] [PubMed] [Google Scholar]
- Harley RM. 1988. Evolution and distribution of Eriope (Labiatae), and its relatives, in Brazil. In: Vanzolini PE, Heyer WR, eds. Proceedings of a workshop on neotropical distribution patterns. Rio de Janeiro: Academia Brasileira de Ciências, 71–120. [Google Scholar]
- Harley RM. 1995. Introduction. In: Stannard BL, ed. Flora of the Pico das Almas, Chapada Diamantina, Brazil. London: Kew Royal Botanic Garden, 1–40. [Google Scholar]
- Harmon LJ, Weir J, Brock C, et al. . 2015. geiger: A package for macroevolutionary simulation and estimating parameters related to diversification from comparative phylogenetic data http://www.webpages.uidaho.edu/~lukeh/software.html. Accessed 16 February 2015.
- Herman AB, Kvacek J. 2010. Late Cretaceous Grünbach Flora of Austria. Vienna: Naturhistorisches Museum Wien. [Google Scholar]
- Hertweck KL, Kinney MS, Stuart SA, et al. . 2015. Phylogenetics, divergence times and diversification from three genomic partitions in monocots. Botanical Journal of the Linnean Society 178: 375–393. [Google Scholar]
- Hoorn C, Wesselingh FP, ter Steege H, et al. . 2010. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330: 927–931. [DOI] [PubMed] [Google Scholar]
- Hopper SD. 2009. OCBIL theory: Towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322: 49–86. [Google Scholar]
- Huelsenbeck JP, Nielsen R, Bollback JP. 2003. Stochastic mapping of morphological characters. Systematic Biology 52: 131–158. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Larget B, Alfaro ME. 2004. Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Molecular Biology and Evolution 21: 1123–1133. [DOI] [PubMed] [Google Scholar]
- Hughes CE, Atchison GW. 2015. The ubiquity of alpine plant radiations: From the Andes to the Hengduan Mountains. New Phytologist 207: 275–282. [DOI] [PubMed] [Google Scholar]
- Hughes CE, Eastwood RJ. 2006. Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences USA 103: 10334–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CE, Pennington RT, Antonelli A. 2013. Neotropical plant evolution: Assembling the big picture. Botanical Journal of the Linnean Society 171: 1–18. [Google Scholar]
- Ibisch PL, Nowicki C, Vásquez R, Koch K. 2001. Taxonomy and biology of Andean Velloziaceae: Vellozia andina sp. nov. and notes on Barbaceniopsis (including Barbaceniopsis castillonii comb. nov.). Systematic Botany 26: 5–16. [Google Scholar]
- Iles WJD, Smith SY, Gandolfo MA, Graham SW. 2015. Monocot fossils suitable for molecular dating analyses. Botanical Journal of the Linnean Society 178: 346–374. [Google Scholar]
- Jacobi CM, Sarto MCL. 2007. Pollination of two species of Vellozia (Velloziaceae) from high-altitude quartzitic grasslands, Brazil. Acta Botanica Brasilica 21: 325–333. [Google Scholar]
- Janssen T, Bremer K. 2004. The age of major monocot groups inferred from 800+ rbcL sequences. Botanical Journal of the Linnean Society 146: 385–398. [Google Scholar]
- Jansson R, Dynesius M. 2002. The fate of clades in a world of recurrent climatic change: Milankovitch oscillations and evolution. Annual Review of Ecology and Systematics 33: 741–777. [Google Scholar]
- Jaramillo C, Rueda MJ, Mora G. 2006. Cenozoic plant diversity in the Neotropics. Science 311: 1893–1896. [DOI] [PubMed] [Google Scholar]
- Klak C, Reeves G, Hedderson T. 2004. Unmatched tempo of evolution in Southern African semi-desert ice plants. Nature 427: 63–65. [DOI] [PubMed] [Google Scholar]
- Koenen EJM, de Vos JM, Atchison GW, et al. . 2013. Exploring the tempo of species diversification in legumes. South African Journal of Botany 89: 19–30. [Google Scholar]
- Koenen EJM, Clarkson JJ, Pennington TD, Chatrou LW. 2015. Recently evolved diversity and convergent radiations of rainforest mahoganies (Meliaceae) shed new light on the origins of rainforest hyperdiversity. New Phytologist 207: 327–339. [DOI] [PubMed] [Google Scholar]
- Landau EC, Gonçalves-Alvim SJ, Fagundes M, Fernandes GW. 1999. Riqueza e abundância de herbívoros em flores de Vellozia nivea (Velloziaceae). Acta Botanica Brasilica 12: 403–409. [Google Scholar]
- Lavin M, Schrire BD, Lewis G, et al. . 2004. Metacommunity processes rather than continental tectonic history better explain geographically structured phylogenies in legumes. Philosophical Transactions of the Royal Society 359: 1509–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder HP. 2008. Plant species radiations: Where, when, why?Philosophical Transactions of the Royal Society 363: 3097–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder HP. 2014. The evolution of African plant diversity. Frontiers of Ecology and Evolution 2: 38. doi:10.3389/fevo.2014.00038. [Google Scholar]
- Maddison WP, Midford PE, Otto SP. 2007. Estimating a binary character’s effect on speciation and extinction. Systematic Biology 56: 701–710. [DOI] [PubMed] [Google Scholar]
- Madriñan S, Cortés AJ, Richardson JE. 2013. Páramo is the world’s fastest evolving and coolest biodiversity hotspot. Frontiers in Genetics 4: 192. doi:10.3389/fgene.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magallón S. 2004. Dating lineages: molecular and paleontological approaches to the temporal framework of clades. International Journal of the Plant Sciences 165 (Suppl. 4): S7–S24. [Google Scholar]
- Magallón S, Sanderson MJ. 2001. Absolute diversification rates in angiosperm clades. Evolution 55: 1762–1780. [DOI] [PubMed] [Google Scholar]
- Magallón S, Gómez-Acevedo S, Sánches-Reyes LL, Hernández-Hernández T. 2015. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytologist 207: 437–53. [DOI] [PubMed] [Google Scholar]
- Mairal M, Sanmartín I, Herrero A, et al. . 2017. Geographic barriers and Pleistocene climate change shaped patterns of genetic variation in the Eastern Afromontane biodiversity hotspot. Scientific Reports 7: 45749. doi:10.1038/srep45749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello-Silva R. 1995. Aspectos taxonômicos, biogeográficos, morfológicos e biológicos das Velloziaceae de Grão-Mogol, Minas Gerais, Brasil. Boletim de Botânica da USP 14: 49–79. [Google Scholar]
- Mello-Silva R. 2005. Morphological analysis, phylogenies and classification in Velloziaceae. Botanical Journal of the Linnean Society 148: 157–173. [Google Scholar]
- Mello-Silva R. 2009. Velloziaceae raras do Brasil. In: Giulietti AM, Rapini A, Andrade MJG, Queiroz LP, Silva JMC, eds. Plantas Raras do Brasil. Belo Horizonte: International Conservation, 392–398. [Google Scholar]
- Mello-Silva R. 2015. Velloziaceae in Lista de Espécies da Flora do Brasil Jardim Botânico do Rio de Janeiro: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB245. Accessed 26 June 2016. [Google Scholar]
- Mello-Silva R, Menezes NL. 2014. Velloziaceae in honorem appellatae. Phytotaxa 175: 85–96. [Google Scholar]
- Mello-Silva R, Sasaki D. 2016. Revision of the Brazilian hemispheric ovary clade of Vellozia (Velloziaceae) with the new V. religiosa. Annals of the Missouri Botanical Garden 101: 553–567. [Google Scholar]
- Mello-Silva R, Santos DYAC, Salatino MLF, et al. . 2011. Five vicariant genera from Gondwana: The Velloziaceae as shown by molecules and morphology. Annals of Botany 108: 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes NL. 1973. Natureza dos apêndices petalóides em Barbacenioideae (Velloziaceae). Boletim de Zoologia & Biologia Marinha, N. S. 30: 713–755. [Google Scholar]
- Mennes CB, Smets EF, Moses SN, Merckx VSFT. 2013. New insights in the long-debated evolutionary history of Triuridaceae (Pandanales). Molecular Phylogenetics and Evolution 69: 994–1004. [DOI] [PubMed] [Google Scholar]
- Mittelbach GG, Schemske DW, Cornell HV, et al. . 2007. Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecology Letters 10: 315–331. [DOI] [PubMed] [Google Scholar]
- Mittermeier RA, Gil PR, Hoffman M, et al. . 2004. Hotspots revisited: Earth’s biologically richest and most endangered terrestrial ecoregions. Mexico City: Conservation International. [Google Scholar]
- Morlon H, Parsons T, Plotkin H. 2011. Reconciling molecular phylogenies with the fossil record. Proceedings of the National Academy of Sciences USA 108: 16327–16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JK. 1972. Phytogeography of the West African mountains. In: Moore DM, Valentine DH, eds. Taxonomy, phytogeography and evolution. London: Academic Press, 221–236. [Google Scholar]
- Mucina L. 2018. Vegetation of Brazilian campos rupestres on siliceous substrates and their global analogues. Flora 238: 11–23. [Google Scholar]
- Mucina L, Wardell-Johnson GW. 2011. Landscape age and soil fertility, climatic stability, and fire: Beyond the OCBIL framework. Plant Soil 341: 1–23. [Google Scholar]
- Oliveira RS, Dawson TE, Burgess SSO. 2005. Evidence for direct water absorption by the shoot of the desiccation-tolerance plant Vellozia flavicans in the savannas of central Brazil. Journal of Tropical Ecology 21: 585–588. [Google Scholar]
- Oliveira RS, Galvão HC, De Campos MCR, Eller CB, Pearse SJ, Lambers H. 2015. Mineral nutrition of campos rupestres plant species on contrasting nutrient-impoverished soil types. New Phytologist 205: 1183–1194. [DOI] [PubMed] [Google Scholar]
- Oliveira RS, Abrahão A, Pereira C, et al. . 2016. Ecophysiology of campos rupestres plants. In: Fernandes GW, ed. Ecology and conservation of mountaintop grasslands in Brazil. Basel: Springer International Publishing, 227–272. [Google Scholar]
- Pennington RT, Lavin M. 2016. The contrasting nature of woody plant species in different neotropical forest biomes reflects differences in ecological stability. New Phytologist 210: 25–36. [DOI] [PubMed] [Google Scholar]
- Pennington RT, Ratter JA, Lewis GP. 2006. Neotropical savannas and seasonally dry forests: Plant diversity, biogeography and conservation. Boca Raton: CRC Press. [Google Scholar]
- Pirani JR, Sano PT, Mello-Silva R, et al. . 2015. (orgs.) Flora da Serra do Cipó, Minas Gerais Available in: http://www.ib.usp.br/botanica/serradocipo. Accessed 1 April 2017.
- Porembski S, Barthlott W. 2000. Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecology 151: 19–28. [Google Scholar]
- Prance GT. 1974. Phytogeographic support for the theory of Pleistocene forest refuges in the Amazon Basin, based on evidence from distribution patterns in Caryocaraceae, Chrysobalanaceae, Dichapetalaceae and Lecythidaceae. Acta Amazonica 3: 5–28. [Google Scholar]
- Rabosky DL. 2014. Automatic detection of key innovations, rate shifts, and diversity dependence on phylogenetic trees. PLoS One 9: e89543. doi:10.1371/journal.pone.0089543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky DL, Goldberg EE. 2015. Model inadequacy and mistaken inferences of trait-dependent speciation. Systematic Biology 64: 340–355. [DOI] [PubMed] [Google Scholar]
- Rabosky DL, Grundler MC, Anderson CJR, et al. . 2014. BAMMtools: An R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods in Ecology and Evolution 5: 701–707. [Google Scholar]
- Rambaut A. 2009. FigTree 1.4.1 Available from: http://tree.bio.ed.ac.uk/software/figtree. Accessed 13 January 2015.
- Rambaut A, Suchard M, Drummond AJ. 2013. Tracer v1.6 Edinburgh (United Kingdom): Institute of Evolutionary Biology, University of Edinburgh; Available from: http://beast.bio.ed.ac.uk/Tracer. Accessed 13 January 2015. [Google Scholar]
- Rando JG, Zuntini AR, Conceição AS, van den Berg C, Pirani JR, Queiroz LP. 2016. Phylogeny of Chamaecrista ser. Coriaceae (Leguminosae) unveils a lineage recently diversified in Brazilian campo rupestre vegetation. International Journal of Plant Sciences 177: 3–17. [Google Scholar]
- Rapini A, Berg C, Liede-Schumann S. 2007. Diversification of Asclepiadoideae (Apocynaceae) in the New World. Annals of the Missouri Botanical Garden 94: 407–422. [Google Scholar]
- Rapini A, Ribeiro PL, Lambert S, Pirani JR. 2008. A flora dos campos rupestres da Cadeia do Espinhaço. Megadiversidade 4: 16–24. [Google Scholar]
- Raven PH, Axelrod DI. 1974. Angiosperm biogeography and past continental movements. Annals of the Missouri Botanical Garden 61: 539–673. [Google Scholar]
- R Core Team 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Available from: http://www.R-project.org/. Accessed 20 July 2015. [Google Scholar]
- Revell LJ. 2012. phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Ribeiro PL, Rapini A, Damascena LS, van den Berg C. 2014. Plant diversification in the Espinhaço Range: insights from the biogeography of Minaria (Apocynaceae). Taxon 63: 1253–1264. [Google Scholar]
- Ronquist F, Huelsenbeck J, Teslenko M. 2011. MrBayes version 3.2 Manual. Available in: http://mrbayes.sourceforge.net. Accessed 17 April 2017. [Google Scholar]
- Sano SM, Almeida SP. 1998. Cerrado: ambiente e flora Planaltina. Brasília: EMBRAPA-CPAC. [Google Scholar]
- Sazima M, Sazima I. 1990. Hummingbird pollination in two species of Vellozia (Liliiflorae: Velloziaceae) in Southeastern Brazil. Botanica Acta 103: 83–86. [Google Scholar]
- Schnitzler J, Barraclough TG, Boatwright JS, et al. . 2011. Causes of plant diversification in the Cape biodiversity hotspot of South Africa. Systematic Biology 60: 343–357. [DOI] [PubMed] [Google Scholar]
- Shaw J, Lickey EB, Beck JT, et al. . 2005. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany 92: 142–166. [DOI] [PubMed] [Google Scholar]
- Shi JJ, Rabosky DL. 2015. Speciation dynamics during the global radiation of extant bats. Evolution 69: 1528–1545. [DOI] [PubMed] [Google Scholar]
- Simon MF, Grether R, Queiroz LP, Skema C, Pennington RT, Hughes CE. 2009. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proceedings of the National Academy of Sciences USA 106: 20359–20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Beaulieu JM, Stamatakis A, Donoghue MJ. 2011. Understanding angiosperm diversification using small and large phylogenetic trees. American Journal of Botany 98: 404–414. [DOI] [PubMed] [Google Scholar]
- de Souza ER, Lewis GP, Forest F, Schnadelbach AS, Van den Berg C, de Queiroz LP. 2013. Phylogeny of Calliandra (Leguminosae: Mimosoideae) based on nuclear and plastid molecular markers. Taxon 62: 1200–1219. [Google Scholar]
- Stadler T. 2013. Recovering speciation and extinction dynamics based on phylogenies. Journal of Evolutionary Biology 26: 1203–1219. [DOI] [PubMed] [Google Scholar]
- Strassburg B, Brooks T, Feltran-Barbieri R, et al. . 2017. Moment of truth for the Cerrado hotspot. Nature Ecology & Evolution 1: 1–3. [DOI] [PubMed] [Google Scholar]
- Sun Y, Skinner DZ, Liang GH, Hulbert SH. 1994. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theoretical Application Genetics 89: 26–32. [DOI] [PubMed] [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105–1109. [DOI] [PubMed] [Google Scholar]
- Teodoro GS. 2014. Extreme drought effects on the phenology, growth and ecophysiology performance of campos rupestres species. PhD Thesis, Universidade Estadual de Campinas, Brazil. [Google Scholar]
- Trovó M, De Andrade MJG, Sano PT, Ribeiro PL, Van den Berg C. 2013. Molecular phylogenetics and biogeography of Neotropical Paepalanthoideae with emphasis on Brazilian Paepalanthus (Eriocaulaceae). Botanical Journal of the Linnean Society 171: 225–243. [Google Scholar]
- Warming E. 1893. Note sur la biologie et l’anatomie de la feuille des Vellosiacées. Oversigt over det Kongelige Danske Videnskabernes Selskabs Forhandlinger og dets Medlemmers Arbeider 2: 57–100. [Google Scholar]
- Werneck MS, Espírito-Santo MM. 2002. Species diversity and abundance of vascular epiphytes on Vellozia piresiana in Brazil. Biotropica 34: 51–57. [Google Scholar]
- Wiens JJ, Donoghue MJ. 2004. Historical biogeography, ecology and species richness. Trends in Ecology and Evolution 19: 639–644. [DOI] [PubMed] [Google Scholar]
- WWF – World Wide Fund for Nature 2012. Wildfinder Database Available from: https://www.worldwildlife.org/publications/wildfinder-database. Accessed 1 June 2017.
- Yoder JB, Clancey E, Des Roches S, et al. . 2010. Ecological opportunity and the origin of adaptive radiations. Journal of Evolutionary Biology 23: 1581–1596. [DOI] [PubMed] [Google Scholar]
- Xing Y, Ree RH. 2017. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proceedings of the National Academy of Sciences USA 114: E3444–E3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos JC, Dickens GR, Zeebe RE. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451: 279–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.