Abstract
“Lactation is at one point perilously near becoming a cancerous process if it is at all arrested”, Beatson, 1896. Most breast cancers arise from the milk-producing cells that are characterized by aberrant cellular, molecular, and epigenetic translation. By understanding the underlying molecular disruptions leading to the origin of cancer, we might be able to design novel strategies for more efficacious treatments or, ambitiously, divert the cancerous process. It is an established reality that full-term pregnancy in a young woman provides a lifetime reduction in breast cancer risk, whereas delay in full-term pregnancy increases short-term breast cancer risk and the probability of latent breast cancer development. Hormonal activation of the p53 protein (encode by the TP53 gene) in the mammary gland at a critical time in pregnancy has been identified as one of the most important determinants of whether the mammary gland develops latent breast cancer. This review discusses what is known about the protective influence of female hormones in young parous women, with a specific focus on the opportune role of wild-type p53 reprogramming in mammary cell differentiation. The importance of p53 as a protector or perpetrator in hormone-dependent breast cancer, resistance to treatment, and recurrence is also explored.
Keywords: breast cancer origin, p53 tumor suppressor, latency, treatment, estrogen receptor, pregnancy
1. Introduction
1.1. Overview of Breast Cancer and Pregnancy
Breast cancer is endemic worldwide, with a cumulative lifetime risk of 1:8 by age 70 [1,2,3,4]. About 80% of all breast cancers are late-onset, arising in post-menopausal women, and are mainly estrogen receptor alpha (ERα)-positive (ERα+) and p53 wild-type (p53wt) [5]. The association between female hormone regulation and breast cancer has been known for over a century, since the first pioneering operations by Sir George Beatson in 1895 where he showed that removal of the ovaries, the source of estrogen and progesterone hormones, prevented breast cancer recurrence [6]. The specific mechanisms of the origin of breast cancer are still under intense investigation to date, over 120 years later. The understanding of the mechanisms underlying the origin of breast cancer has important therapeutic implications and is relevant to both prevention and treatment. It is undisputable that fluctuations in female hormones throughout a woman’s lifetime influence the risk of late-onset breast cancer, with substantial evidence that the timing of the first pregnancy has long-term impact on breast cancer risk. In today’s society, there is a global trend for women to have fewer pregnancies at later ages, and this trend is associated with an increased incidence of breast cancer [1,3,4,7,8,9]. Therefore, understanding the molecular changes associated with the reduced breast cancer risk that is observed in young parous females is imperative for the development of effective approaches to reduce breast cancer incidence and to improve treatment outcomes.
1.2. p53 in the Differentiation of the Breast and in Breast Cancer
There is a very strong association between the function of the p53 tumor suppressor (encoded by TP53 gene) and female hormone regulation during the differentiation of the mammary gland in early and late pregnancies, which influences a woman’s susceptibility to breast cancer later in life [10,11,12,13]. This leads to the broader question: is the almost total breast cancer refractoriness in young parous or multiparous women due, in part, to the influence of p53 on the differentiation of the breast in a “critical time window”? In this article, to partly answer this question, we look into early studies of p53 as a key regulator of normal breast cell physiology and its role in hormone-dependent breast pathophysiology. The second most pressing question we address is the role of p53 as a protector or perpetrator in hormone-dependent breast cancer recurrence and resistance to treatment.
This article will review what is known about the protective influence of female hormones in young parous women on the basis of historical and contemporary findings in published studies. We will then focus on the duality of wild-type p53, as a good guy as well as a bad guy, in the origin of breast cancer, as a protector and/or perpetrator. How this may impact on hormone-dependent breast cancer treatment and recurrence will also be discussed.
2. Female Hormones, Pregnancy, and Prevention/Promotion of Breast Cancer
The conundrum that female hormones have a dual effect on breast cancer risk, both protective and causative, has been at the forefront of discussions on the etiology of breast cancer origin for the last century. Seemingly contradictory evidence has demonstrated that administration of estrogen and progesterone, in a ‘window in time’ in young-aged pregnancies may provide a life-long protection against breast cancer [14,15,16,17,18]. Alternatively, the female hormones estrogen and progesterone are well-known mitogens in breast cancer progression [19,20]. The surgery performed by Beatson in 1895 [21] was heralded as the first hormonal therapy for breast cancer. Dating back over 50 years, first-line therapies for hormone-dependent cancers are antiestrogen-based treatments: tamoxifen, blocking ERα, and aromatase inhibitors, namely, anastrozole and letrozole, blocking estrogen production [22,23,24]. However, the very hormones that we target for breast cancer therapy have also been demonstrated to be the very reason why early-aged full-term pregnancy provides long-term protection against tumor formation.
The number of children and the age of the first pregnancy determines a woman’s short-term and long-term risk of breast cancer. It is not surprising that with the flux of hormones during pregnancy there is an enhanced short-term risk of breast cancer compared with nulliparous women, and this imminent risk increases with increasing age at first birth [25,26,27]. This transitory increased risk in young female pregnancies is offset by multiple births and also brings with it a long-term benefit of reduced latent breast cancer. However, the latent protective effect is not observed in women who have children over the age of 35 [25,26,27]. The golden question is: what transformations occur in the developing lactating breast that provide long-term protection against latent breast cancer without increasing the short-term risk? This complex question is partly answered by molecular studies in rodents and is discussed in more detail in Section 4.
Fundamentally, breast cells divide rapidly during pregnancy, so any genetic alterations occurring in these cells at the time of proliferation will be copied. Genetic alterations (multiple DNA mutations) and post-genetic alterations (i.e., methylation and chromatin re-modeling) during pregnancy are strongly implicated in breast cancer development and protection [28].
3. Differentiation in the Mammary Gland during Pregnancy and the Origin of Breast Cancer
Late-onset breast cancers are more likely to originate from ERα-positive luminal epithelial cells, or milk-producing cells, which have been exposed to years of fluctuating hormonal changes from puberty to pregnancy and menopause [29]. As mentioned in Section 2, the best-recognized risk factors for breast cancer are the hormonal changes during pregnancy.
There is irrefutable, reproducible evidence from human studies and in rodent models that full-term and multiple pregnancies have a lifetime protective effect against breast cancer [30,31,32]. Rodent models proved to be well-suited for studying the early development of the mammary gland during puberty and gestation and the effect of a carcinogenic insult in the different stages of breast development [33,34,35,36,37]. In rats, breast tumors can be induced by intravenous or subcutaneous N-methylnitrosurea (MNU) or oral 7,12-dimethylbenz(a) anthracene (DMBA), with a near 100% latency period between 8 and 12 weeks [38]. Pioneering experimental studies in mice and rats (from 1973) provided some insight into the morphological changes occurring in the mammary gland of nulliparous women compared to parous women: undifferentiated or partially differentiated glands in young females were more susceptible to neoplastic transformation upon carcinogenic insult (reviewed in [19,39]). During puberty, under hormonal regulation, terminal lobular units of the breast (lobules type 1) progressively develop and differentiate into type 2 and type 3 lobules [40]. In pregnancy, also under the influence of female hormones, these lobules enlarge progressively to a fully differentiated secretory lobule (type 4). The mammary gland regresses to a different morphology to that of a virgin rat post-involution, and its cells remain more differentiated post-lactation [39,40]. An interesting study by Russo and Russo in 1980, looking at the influence of differentiation on cell cycle kinetics in the parous and non-parous breast of young and older rats [41], found that there was some disparity in cell cycle progression, whereby the cells of non-parous rats were more proliferative. On the basis of these studies, it was suggested that the protective effect of pregnancy was partly due to modifications of the G1 or G0 phases of the cell cycle in such a way to make breast cells refractory to transformation, speculating that the prolonged resting stage of cells in G1 phase allowed for more efficient DNA repair. Later studies showed that DNA repair was more efficient in mammary epithelial cells of parous animals (compared to virgins), and DNA was less susceptible to bind DMBA, a commonly used carcinogen shown to induce tumor formation in rats and mice [38,42,43]. An interesting suggestion from these studies was that the limited proliferation capacity in parous rats was due to commitment of function in pregnancy-induced differentiation. This was associated with specific cell cycle modifications in the G0–G1 phases. Phenotypic studies of histological sections of human breast tissue revealed distinct alterations in the structure of the nuclear compartment of non-parous versus parous breast cells, indicating a shift from non-condensed euchromatin to a more condensed heterochromatin structure, respectively [44]. This observation was supported by transcriptomic studies showing the upregulation of genes involved in chromatin reorganization and epigenetic changes induced during pregnancy [44]. Chromatin remodeling and epigenetic changes were also demonstrated by increased histone methylation, an important posttranscriptional process in gene silencing. As evidenced by these studies, there is a clearly defined stepwise coordinated set of cellular metabolic reprogramming, from undifferentiated to pre-secretory differentiation, to functional differentiation, i.e., lactating breast.
From early studies in rodents, it was unclear whether completion of full differentiation per se (in the absence of estrogen and progesterone) reduced breast cancer risk. A few studies tested whether induction of full differentiation of the mammary gland was indeed the basis of protection against carcinogenicity [10,14,45]. Perphenazine (PPZ) is a dopamine receptor inhibitor known to chemically differentiate mammary glands by inducing prolactin release from the anterior pituitary gland. Using PPZ, fully differentiated mammary glands were actually found to be susceptible to carcinogens [10,14,45], seemingly in contradiction to previous findings, which predicted that functional differentiation of the mammary gland conferred some protection. These studies clearly demonstrated that full differentiation in the absence of hormones did not reduce breast cancer risk, and ‘hormonally induced’ differentiation in young parous rodents was needed for breast cancer prevention.
4. Female Hormones, Pregnancy, and Prevention of Breast Cancer
Early studies, discussed in Section 3, led to the proposal of whether simulating hormonal changes in pregnancy to induce differentiation of the mammary glands as a preventative measure against breast cancer later in life was a feasible and safe preventative strategy. Hence, we propose the controversial question raised by O’Malley and colleagues: can administration of female hormones at a critical stage in reproductive development act as a therapeutic intervention to offer long-term protection against late-onset breast cancer? This poses an interesting challenge, given there is convincing evidence associating estrogen and progesterone in hormone replacement therapy (HRT) with increased breast cancer risk [46] (discussed in Section 6.2). Furthermore, anti-estrogens (tamoxifen) and aromatase inhibitors (blocking estrogen production) are the most common treatment for hormone-related breast cancers [47,48,49]. The identification of molecular changes in the mammary gland of young parous females associated with lifetime protection against breast cancer will provide the knowledge to progress the development of preventative and more efficacious treatments.
4.1. Can Estrogen and Progesterone Mimic Pregnancy-Induced Breast Cancer Protection?
Breast tumors in rodents possess high similarity to ERα-positive and progesterone receptor (PR)-positive human breast tumors. Thus, rodent models have been key in our understanding of breast cancer development [37,43]. As explained by Daniel Medina [50], there are two distinct experimental models of parity/hormone-induced protection. The differences are important in the underlying protective mechanisms and when interpreting the results from these experiments. The “pre-treatment” model implies the administration of carcinogens after the mammary gland has undergone hormonal stimulation, differentiation, and involution. The “post-treatment” model implies exposure to carcinogens and then treatment with hormones for a specified time. In all “pre-treatment” models, full-term pregnancy and combination of estrogen (estradiol-17β) and progesterone conferred protection, leading to a reduction in mammary carcinomas incidence and to a reduction or abrogation of latency. The “post-treatment” model was not as definitive, and the results were more controversial, as will be discussed.
Studies, dating back to 1962, demonstrated that the female hormones estradiol-17β and progesterone administered to rats and mice in doses that mimicked pregnancy reduced the incidence of carcinogenicity and conferred long-term cancer protective effects; however, in these studies neither hormone individually was protective [15,16,17,18]. In contrast, a few studies showed that a critical short-term dose of estrogen alone conferred some protection from mammary carcinogenesis [10,14]. The earliest “post-treatment” experiments by Huggins and colleagues demonstrated 100% of Sprague-Dawley (albino) rats treated with the carcinogen DMBA developed latent mammary tumors [17]. Hormone administration of estradiol-17β and progesterone, after chemical induction of DMBA, reduced latency in all rats and eliminated cancer in approximately 50% of treated rats [17]. Conversely, using the same paradigm, pregnancy was found to accelerate tumor growth, indicating that pregnancy per se was not protective after carcinogen insult. Progesterone had a similar effect as estradiol-17β—just delayed the onset of tumors. Similar experiments in the same rat model suggested that full-term pregnancy had to be completed prior to carcinogenetic exposure to be protective [51]. However, it was later demonstrated that pre-treatment with estradiol-17β and progesterone administered for 21 days in sexually mature female rats also reduced mammary adenocarcinoma incidence [14]. Therefore, in summary, these studies indicated that both full or partial differentiation of the mammary gland reduced breast cancer risk, and this risk could be minimized by short-term exposure to hormone treatment [14].
In a study treating rats with estradiol-17β and progesterone for 21 days to mimic the parous protective effects against MNU, a set of 100 markers were identified, displaying persistent changes (upregulated) in the mammary gland compared to virgin rats [52]. One of these changes was RbAp46, a histone-binding protein associated with chromatin remodeling [52]. Other experiments by Yang and colleagues showed that pregnancy and lactation in both in the “pre-treatment” or “post-treatment” model using the MNU carcinogen reduced mammary carcinomas and prolonged latency [53].
In general, the consensus from these studies, and many more not cited, is that short-term administration of estrogen and progesterone, that mimic pregnancy, can reduce malignant tumor formation and breast cancer latency. These changes are accompanied by persistent changes in the cellular and molecular profiles of the mammary cells, making the mammary gland less vulnerable to carcinogens or carcinogenic insults.
4.2. Can hCG Mimic Pregnancy-Induced Breast Cancer Protection?
Early studies by Russo and others suggested that human placental hormone chorionic gonadotropin (hCG) provided an inhibitory action, leading to reduction in breast cancer risk, as reviewed in reference [54]. This long-lasting protective effect could also be reproduced by administering placental hCG to rats for 21 days [28]. The mechanism proposed suggested placental hCG was responsible for imprinting the permanent changes in the genomic signature of the mammary gland [54]. These changes occurred within a time window of pregnancy rendering the cells refractory to malignant transformation [54]. Russo’s laboratory compared gene expression profiles of rats exposed to combined estrogen and progesterone or hCG with untreated or pregnant rats [55]. Only the groups with hCG (pregnancy-induced or hCG treatment groups) underwent complete mammary gland differentiation in these studies, although the combination of estrogen and progesterone, as mentioned previously, has been found to have a protective long-term effect against breast cancer. Regulation of genes common to all treated groups, related to cellular differentiation, maintenance of cellular polarity, tight junction formation, and cellular communication, was observed. These experiments further emphasized that permanent changes in the molecular pathways associated with the female hormones (estrogen, progesterone, and hCG), whether associated with partial or full differentiation, provided protection against breast cancer latency. To support the beneficial effects of hCG treatment as a viable alternative to current anti-hormonal therapies, recombinant hCG treatment in post-menopausal women showed a significant decrease in breast cancer cell proliferation [56]. However, it is noted that not all studies supported hCG as a prospective treatment for breast cancer. In contrast, a large prospective study (1191 women with invasive breast cancer and 2257 controls) completed in Finland showed no correlation between early pregnancy hCG serum concentrations and breast cancer risk [57]. What is coming to light is that there are different forms of hCG, which may have opposing effects on breast cancer development, driving different outcomes in breast cancer risk [54]. The differences in hCG isoform expression may, in part, explain the controversial data surrounding hCG and breast cancer management.
Overall, these studies are illustrative of many other works that explore the influence of the female hormone flux in pregnancy on breast cancer risk. They demonstrate that short-term administration of female hormones, whether it be estrogen and progesterone or hCG to induce mammary gland differentiation, has promise as a therapeutic protective intervention for breast cancer latency. Nevertheless, the challenge is when and how to administer preventative hormones. One of the major questions which still remains unanswered is: what are the molecular changes that govern persistent alterations in hormone-dependent signaling pathways that occur in young parous females and reduce lifetime breast cancer risk? The answer may be in p53’s function, as p53 is believed to be implicated in a number of steps relating to the risk and development of breast cancer.
5. p53 in Pregnancy: Cancer Suppressor Protein and Potent Protector again Latent Breast Cancer
5.1. p53: Looking beyond the Guardian of the Genome
For decades, the p53 protein (encoded by the TP53 gene) has stereotypically been widely researched as the ultimate tumor suppressor protein important in driving cancer cell death through apoptosis or permanent irreversible cell senescence [58,59,60]. It is clear from early and more recent studies that p53 holds a key role in normal cellular programming, differentiation, and survival [61,62,63]. Challenging to p53 importance in normal cellular homeostasis was the observation that that p53-null mice developed no overt defects and were viable, suggesting that p53 was dispensable for normal embryonic development [13,64], albeit a higher percentage of female mice were infertile, and mice were found to be more vulnerable to carcinogens, with increased overall cancer risk [13,64]. The high incidence of cancer in p53-null mice led to the idea that perhaps p53wt was necessary for normal cell differentiation in adult life. On one hand, p53wt maintains a fine balance in normal growth arrest, terminal differentiation, and apoptosis in the undifferentiated, premature adult cell, on the other hand, downregulation of p53wt promotes renewal of stem cell proliferation [61]. In addition, p53-null mice were found to display deregulation of the DNA methylation machinery prior to the increase in tumor formation [65]. In this context, p53 has been shown to act in the surveillance of the epigenetic programming and reprogramming of cells to maintain cell stability [62,66,67].
Today, the actions of p53 extend well beyond its function as the guardian of the genome [68], since it has also been recognized as the guardian of the proteome [69], the guardian of homeostasis [63], the guardian of maternal reproduction [70], and the guardian of cellular respiration and metabolism [71,72,73,74,75,76,77,78,79,80,81]; in addition, it is also a negative regulator of pluripotency and a positive regulator of de-differentiation [82]. The known and ever-increasing emerging roles of p53wt are listed in Table 1.
Table 1.
Known functions of wild-type p53.
| Function | Summary—Key Regulatory Functions | Reference |
|---|---|---|
| Homeostasis regulators | p53 is a key regulator of replication homeostasis within a DNA restart network and is essential for DNA methylation homeostasis in stem cells. It also plays a key role in the regulation of metabolic homeostasis. The function of p53 in cellular energy homeostasis and metabolism is emerging as a critical factor for tumor suppression. | [62,72,83,84,85] |
| Cell cycle arrest | One of the best-understood function of p53 is to promote cell cycle arrest. Cell cycle arrest by p53 is mainly mediated by the transcriptional activation of p21/WAF1 and is reversible after downregulation of p53. | [72,83,86,87,88,89,90] |
| Apoptosis | It has been confirmed in many studies that induction of apoptotic death in nascent neoplastic cells is the principal mechanism by which p53 suppresses tumor development. p53 induces apoptosis in nontransformed cells mostly by direct transcriptional activation of the pro-apoptotic BH3-only proteins PUMA and (to a lesser extent) NOXA. | [86,87,91,92,93] |
| Cellular senescence | Chronic p53 activation can result in senescence of tumor cells. Senescent cells have unique features, such as large cell size, active autophagy, high lysosomal SA-b-gal activity, and secretion of proinflammatory cytokines. Senescence is a unique state of cell cycle arrest that is highly stable but is not completely irreversible. Through the induction of senescence, p53 promotes and achieves permanent inhibition of cell proliferation. | [86,87,93,94,95,96,97,98,99] |
| Cellular quiescence | p53 is activated during both quiescence and senescence. Evidence suggests that p53 activation contributes to the quiescent growth arrest and is a reversible process. | [100,101,102]. |
| Proliferation/survival | There is a strong direct correlation between accumulation of p53 protein and tumor proliferation rate. Expression of mutant p53 protein was associated with high tumor proliferation rate, early recurrence, and death in breast cancer. Recently, it was noted that p53 can also contribute to cell survival. | [86,90,91,99,103,104] |
| Autophagy | In most cases, p53 positively regulates autophagy in tumor cells by inhibiting mTOR pathways via the activation of AMPK. p53 also promotes autophagy by inducing various autophagy-related genes. Autophagy is considered a tumor suppressive mechanism that removes unfolded proteins, damaged cellular components, and damaged organelles to maintain cellular homeostasis. | [96,105,106,107,108,109,110,111] |
| Metabolism | p53 promotes oxidative phosphorylation and dampens glycolysis in cells; disruption of this balance is associated with mutations in p53 and oncogenic transformation. P53 plays a role in alterations seen in glycolysis, gluconeogenesis, and aerobic respiration. Altered metabolism can contribute to malignant transformation, and cancer cells become dependent on these changes. p53 regulates various metabolic pathways, helping to balance glycolysis and oxidative phosphorylation, limiting the production of reactive oxygen species, and contributing to the ability of cells to adapt to and survive mild metabolic stresses. | [72,77,84,87,88,99,112,113] |
| DNA repair | p53 plays a prominent role as a facilitator of DNA repair by halting the cell cycle to allow time for the repair machinery to restore genomic stability; for example, p53 coordinates DNA base excision repair in the cells, and this mechanism is impaired in p53-inactivated cells. Within a DNA restart network, p53 functions as a keystone regulator in DNA replication homeostasis. | [85,114] |
| Oncogenic functions | p53wt is a tumor suppressor gene; mutations in this gene promote oncogenic capacity. Thus, mutant p53 is an actionable target of clinical antitumor therapies. p53 loss of heterozygosity (LOH ) is a critical prerequisite for missense mutant p53 stabilization and gain of function in vivo. | [92,115,116,117] |
| Epigenomic regulator | p53 is not only a pivotal guardian of genomic stability, but also an epigenetic regulator. Epigenomic regulation is a new function of p53, contributing to its tumor suppressor activity. It is thought that the ability of p53 to maintain DNA methylation balance is an important contributor to its tumor suppressor capacity and that loss of p53 may result in cancer initiation by increasing cellular heterogeneity and epigenetic promiscuity. | [62,93,104,118,119] |
| Regulating multiple tumor suppressor genes | Under normal low-stress conditions, p53wt is capable of maintaining the expression of a group of important tumor suppressor genes at baseline, which could contribute to p53-mediated tumor suppression. p53 mutations, with inactivation of multiple tumor suppressor genes in parallel, could lead to the high frequency of p53 mutations in cancer. | [120] |
| Mutant p53 functions | Unidentified mechanisms by which mutp53 confers oncogenic functions by promoting cancer cell adaptation to metabolic stresses. | [88,92,121] |
| Non-canonical cell death | Transcriptional regulation of downstream targets: caspase-independent apoptosis, autophagy, ferroptosis, mitotic catastrophe, paratosis, pyrotosis, efferocytosis (clearing dead cell debris). | [122] |
5.2. A Role for p53 in Breast Cancer Origin
Hormonal activation of the p53 pathway at a critical time in mammary development in full-term pregnancy has been identified as one of the most critical determinants of long-term negative or positive alterations in the mammary gland influencing breast cancer risk. This observation was first determined by mimicking early pregnancies in rodents administered defined hormone treatments of estrogen and progesterone which had been shown to have similar protective properties to early full-term pregnancies (described in Section 4.1) [14]. The p53wt status was ascertained under these defined conditions [10]. In support of p53wt as a mediator of hormone-induced resistance to mammary carcinogenesis in early pregnancy, Sivaraman and colleagues demonstrated that estrogen and progesterone induced sustained p53 nuclear expression and, under these conditions, blocked proliferation in response to a carcinogenetic insult in both mice and rats [10]. The p53 protein was functionally active, as demonstrated by an increase in p21/WAF1, a key downstream target of p53. Hence, the translocation of p53 to the nuclear compartment was essential for the hormone-mediated p53-induced cell cycle arrest. The underlying mechanisms for these discrepancies were unclear but believed to be centered on persistent changes in the hormone-induced p53 signaling pathway outcomes [123], therefore leading to the idea that elevated levels of the p53 protein led to permanent changes in the p53 signaling pathways in these mammary cells. These changes provided protection against DNA damage and reduction in breast cancer risk [10,123]. Conversely, late pregnancy allowed for the accumulation of DNA mutations, leading to late-onset cancer and chronic resistance to breast cancer treatment [10]. Further experiments by Medina and Kittrell proposed that p53 upregulation of p21 blocked cells in the G1 phase of the cell cycle and that both p53 and p21 were hormone-regulated [12]. Thus, p53 was the first gene identified as mediating a hormone protective response against breast cancer and latency. These experiments set the precedence, citing p53 as pivotal in the regulation of hormone-induced mammary gland processes. Thus, O’Malley and colleagues proposed a “cell fate” hypothesis for parity-related changes based on their published results, which stated that, at a critical period in adolescence, the hormonal milieu of pregnancy affected the developmental fate of a ‘subset’ of mammary epithelial cells [10,14]. This ‘cell fate’ model hypothesis laid the foundations for the opposing roles of p53 in the protection against breast cancer or in seeding latent breast cancer cells [124].
5.3. Hormonal Activation of P19ARF–p53 in Mammary Development
In support of the role of p53 function in normal mammary development, deletion of the upstream p53 regulator p19 alternative reading frame (p19ARF), the homolog of the human p14ARF protein, led to immortalization of mammary epithelial cells [125]. p19ARF was shown to be regulated during pregnancy by progesterone, and activation of the p19ARF–p53 pathway was necessary for normal proliferation and cell death during mammary gland development in pregnancy. Activation of the p19ARF/p14ARF–p53 pathway has been shown to block cells in both the G1 and G2 phases of the cell cycle, initiating a rapid cell cycle arrest in breast cancer cells [90,98,126]. The cell cycle is dynamic and, depending on the phase of cell cycle arrest (G1, G2, or S phase), different genes We proteins are be switch on or off, hence activating distinct molecular signaling pathways [127]. In speculate that discordance in p53 regulation of the cell cycle pathways could, in part, have negative repercussions in p53-associated differentiation and de-differentiation of breast cells during development.
5.4. Lessons from the p53-Null Mammary Gland Transplantations
A major breakthrough in supporting the role of p53 in governing parity-related changes in the mammary gland came from p53-null mammary gland transplantation studies [13]. By transplanting p53-null mammary tissue into the cleared fat pad of p53wt mice, researchers were able to show a direct correlation between loss of p53 function in the mammary gland and spontaneous development of mammary tumors. Interestingly, the absence of p53 did not alter hormone -responsiveness or normal mammary development in p53-null mammary gland transplants [13]. However, these transplants were more susceptible to tumorigenesis compared to mammary glands harboring p53wt [13]. Exposure of p53null mammary glands to carcinogens (p53null+carcinogen compared to p53null), slightly increased tumor incidence and decreased the latency onset period. The most interesting finding was that exogenous hormone stimulation increased tumor incidence in p53null mammary glands, contrary to the protective effect of hormone stimulation in p53wt mammary tissue. In addition, multiple pregnancies in mice carrying the p53null mammary gland transplants showed no protection against tumor formation. Latent tumors in p53null mice were aneuploid, suggesting that pathogenicity associated with loss of p53 function was not necessarily due to genetic instability. The results from these p53-null transplantation studies are an important milestone in demonstrating the importance of p53 function in parity-related latent breast cancer protection [13].
5.5. p53 and Mammary Stem Cell Proliferation
A quote from Beatson’s seminal paper on carcinoma of the mamma (1896) states: “a reasonable ground for thinking the active processes seen in a cancerous tumor are best explained by regarding the epithelium of the part as having taken on the powers of the germinal epithelium” [128]. A likely function proposed for p53 is the control of mammary stem cell renewal [82,129]. In the mammary gland, bipotent mammary stem cells exist [130,131,132]. Progenitor or stem cells possess little proliferation activity and remain dormant until triggered into activity. Potentially, p53 controls the balance between self-renewal, differentiation, and de-differentiation of epithelial progenitor cells. This assertion is based on the observation that deletion of p53 in pluripotent cells showed improvement in the reprogramming efficiency of these cells, and reactivation of p53 initiated cell cycle arrest and stem cell differentiation, as reviewed in reference [82]. Stem cells have been characterized as having the ability to divide asymmetrically, i.e., the two daughter cells produced in cell division possess different proliferative capacity: one daughter retains proliferative capacity, whilst the second daughter cell remains in a quiescent state (progenitor cell). This allows cells to simultaneously generate more stem cells (self-renew) and to produce cells that differentiate [133]. Alternatively, stem cells also use symmetrical division to self-renew, in which both daughter cells have the same fate, i.e., remain in the proliferative state [133]. Stem cell fate is believed to be switched by developmental or environmental factors. Cicalese and colleagues demonstrated that p53 presence controlled the asymmetrical division of self-renewing mammary stem cells: in p53-null mammary glands, stem cells underwent symmetrical division, producing two identical daughter cells, whereas when p53 was present, two daughter cells were produced with different proliferative capacities [134]. This explanation supports a fundamental functional role for p53 in mammary gland differentiation during pregnancy.
5.6. p53 and Mammary Cancer Stem Cell Theory
The mammary cancer stem cell theory is based on a model whereby transformation of early progenitor cells or stem cells eventuate in latent tumorigenesis. A central event in the development of cancer is the deregulation of normal stem cell self-renewal, eventually leading to uncontrolled proliferation, aberrant differentiation, and generation of a heterogeneous breast cancer cell population [135,136]. Although this mammary stem cell theory is not proven, there are similarities between the mammary progenitor cell and mammary cancer stem cell phenotypic characteristics, specifically, the plasticity of both cell types, that is, the ability to self-renew or remain in a resting state (dormancy or quiescence), which makes this theory feasible. In general, most breast tumors have lost p53 function, although the majority of post-menopausal breast tumors retain p53wt, making reactivation of p53 a viable target for breast cancer treatments. In light of the findings that p53 is a key player in mammary stem cell regulation, as determined in rodent models, by deduction, dysregulation of p53 function has been cited as a plausible primary origin of latent breast cancer [136].
5.7. p53 and Methylation
Wild-type p53 protein regulates p53 transcriptional programs in the repair of genetic alterations and also by preventing epigenetic abnormalities [66,137]. Tovy and colleagues showed that p53 was important in balancing DNA methylation in embryonic stem cells and in maintaining DNA homeostasis [62]. Cells lacking p53 responded poorly to differentiation signals [62]. These observations were important, given the growing evidence that one of the drivers of cancer is epigenetic aberration. It has been suggested that, under the influence of estrogen and progesterone, p53 participates in chromatin remodeling and initiates epigenetic reprogramming of the parous cell [138]. Epigenetic regulation (DNA methylation, chromatin remodeling, and histone modification) is an important process in development and provides a lasting genetic imprint, restricting gene expression patterns for many years after the modification effector has been removed [139]. As proposed by Ginger and Rosen, these persistent changes may determine mammary cell fate, providing a lasting “memory” and preventing cell lineage anomalies that lead to breast cancer [123]. The p53 gene itself is a prime target for epigenetic alterations. Specific chromatin alterations in the mammary parous glands have been associated with a persistent increase in p53 activity [138]. As mentioned, parous breast cells contain higher levels of heterochromatin compared to breast cells of nulliparous postmenopausal women, which predominantly contain euchromatin. This suggests that, as the nulliparous breast cells did not reach full differentiation, they are more susceptible to transformation and insult from carcinogenic agents. As a consequence, latent breast cancer cells retain the ability to re-acquire the potential to self-renew [28]. The remodeling of the chromatin has been proposed as a decisive step in the protection against breast cancer latency in post-menopausal years [28].
6. The Connection between p53 Status and Responsiveness to Female Hormones in Breast Cancer
6.1. “Paracrine to Autocrine” Hormonal Response in Normal to Breast Cancer Transition
A major difference in the molecular characteristics of normal breast epithelial cells and breast cancer cells is that the majority (approximately 75–85%) of normal breast cells do not express ERα and PR, whereas the majority of breast cancer cells contain high levels of both hormone receptors (ERα+PR+ cells) [140]. Normal breast cells expressing ERα and PR do not self-renew in response to estrogen treatment; these cells send paracrine signals to neighboring cells to proliferate [140]. In contrast, ERα+PR+ breast cancer cells are self-renewing when exposed to estrogen treatment and display an autocrine response. The fundamental switch, or transition, in the molecular response to estrogen provides a survival advantage to breast cancer cells. When and why these changes occur is an enigma. However, it is evident that this switch, or dysregulation of ERα signaling, is most likely to contribute to the early stages of breast cancer development [141].
In this section, we review the mounting evidence that p53 and ER activities are mutually regulated in breast cancer. Conceptually, a question worth asking is: is the loss of, or change in, p53 function associated with the signature of “paracrine-to-autocrine” response in the transition from normal to breast cancer cells? In Section 5, we provided an overview of the current thinking about the contribution of p53wt in early pregnancy and protection against latent breast cancer. Here, we summarize the current knowledge on p53 and its perceived role in breast cancer resistance and recurrence.
6.2. Replacement of Female Hormones and Breast Cancer Risk
This review would be amiss if we did not comment on the seminal findings of the epic Million Women Study by Beral and colleagues, investigating the use of hormone replacement therapy (HRT) in postmenopausal women [46]. In this epidemiological study, the authors investigated the breast cancer risk of using estrogen plus progestin (HRT) compared to estrogen only (HRT). Contrary to the protective effects that estrogen plus progesterone display during pregnancy (Section 4.1), there is, paradoxically, overwhelming evidence to suggest that estrogen plus progestin given to postmenopausal women increase breast cancer incidence [46]. The findings revealed that women given estrogen alone in HRT were comparatively less likely to be diagnosed with breast cancer than women taking HRT containing both progestin and estrogen [142,143,144]. Subsequent studies also revealed that women using HRT were more likely to have increased breast cancer incidence, node-positive, metastatic breast cancers and a higher mortality rate [143,145,146]. These seminal studies exemplify the changing role of hormones: increasing breast cancer risk in postmenopausal women and exerting a protective effect in early pregnancy.
6.3. p53 Status in Hormone-Responsive Breast Cancer
The p53 tumor suppressor is intimately associated with inhibition of cancer and, in general, mutations in the TP53 gene that disrupt cell cycle inhibition are associated with the onset of more than 80% of all cancers [147,148,149]. Nevertheless, somatic mutations and deletions only occur in 20–25% of breast cancers [150]. This observation suggests that mutations in the TP53 gene are not the main culprits in breast cancer development and progression. The majority of breast cancers occurring later in life (in postmenopausal women) are hormone-responsive (ERα-positive) and, interestingly, harbor functional wild-type TP53 [5,151]. In many breast cancers, the p53 protein is continuously degraded by the process of ubiquitination, and, therefore, p53 is non-functional [152,153]. Restoring p53 function by reactivation of p53 through therapeutic intervention, including radiation, chemotherapy, or mimetics of p14ARF, rely mainly on p53 promoting cancer cell death [58,153,154,155,156,157].
As mentioned in Section 6.1, the “paracrine-to-autocrine” hormonal response is a major change in the “normal-to-breast-cancer” transition; hence, breast cancer cells proliferate in response to estrogen. ERα positivity is used as a diagnosis tool for designing breast cancer treatment strategies and predicting prognosis. Therefore, in ERα-positive breast cancers, anti-estrogens, such as tamoxifen, are primary therapies used in treatment and prevention. The most pressing problems with hormonal therapies are resistance and recurrence. Despite an apparent initial success of treatment, many ERα-positive breast cancers have intrinsic or acquired resistance to endocrine therapy [48,158,159]. Latent recurrence is prevalent, particularly in ERα-positive breast cancers, and is associated with cell dormancy, rather than with death, after treatment, as reviewed in reference. Recurrence usually manifests as metastatic cancer and can occur months or decades after the initial therapy. These metastatic breast cancers are more difficult to treat and have higher mortality rates. The mechanisms underlying breast cancer recurrence and metastasis and how we can intervene in these processes are the major challenges faced by breast cancer researchers.
6.4. Molecular Basis for p53 and ERα Association in Resistance and Recurrence in Breast Cancer
Although reactivation of the p53 signaling pathway has been considered as a good breast cancer treatment strategy, there is growing evidence at the molecular level to suggest an association between ERα and p53, which protects breast cancer cells from dying [90,93,98,160,161,162,163]. Studies by Konduri and colleagues, exploring the role of p53 in breast cancer, found that ligand-bound ERα physically stabilized p53 and prevented p53 degradation [93,98,160,162,163,164]. Furthermore, this interaction stopped the accumulation of DNA damage in breast cancer cells and strongly supported the idea that the binding of p53 to ERα–estrogen protects breast cancer cells from apoptosis, hence providing a base for the resistant phenotype. Liu et al. reported that ERα altered p53’s function via direct binding, and this suppression was achieved by the activation of the ERα function-2 domain and the C-terminal regulatory domain of p53 [162]. ERα has also been shown to bind to p53 and inhibit its transcriptional repression of anti-apoptotic genes, thus contributing to the ERα anti-apoptotic function in ERα-positive breast cancer cells [163]. ERα-positive breast cancers with p53wt were more likely to be responsive to anti-estrogens than ERα-positive breast cancer with mutated p53 [160]. This response was due not only to the inhibition of estrogen binding to ERα and of ERα-dependent transcription, but also to the reactivation of p53wt by disrupting the p53–ERα complex [160]. p53 activation by estrogen in postmenopausal breast cancer has been associated with prevention of apoptosis [165], and high levels of estrogen in ERα-positive breast cancers have been shown to correlate with high levels of p53 [166]. Recently, Berger et al. showed that estrogen binding to ERα enhanced p53 activation, whereas anti-estrogens reduced p53 activation [167].
Our laboratory has demonstrated that activation of the p53 pathway in ERα-positive breast cancer cells rapidly, within hours, shuts down DNA replication, downregulates proteins involved in apoptosis, and increases the levels of proteins that are involved in normal breast cell metabolism [90,97,98]. Other studies in the Myles Brown laboratory used a genome-wide approach to understand the underlying mechanism by which ERα protects breast cancer cells from the p53 apoptotic function and identified the modulation of a specific subset of p53 and ERα target genes [93]. These studies question the canonical role of p53 as a tumor suppressor in normal breast physiology.
6.5. p53 and ERβ Association in Breast Cancer Prevention
In 1997, a second ER was identified, named ERβ [168,169]. The two receptors are transcribed from different chromosomes; however, they have high homology in their DNA-binding and carboxyl-terminal ligand-binding domains. Both receptors bind estrogen (17β-estradiol), albeit with different binding affinities and responsiveness to selective estrogen receptor modulators (SERMs), such as tamoxifen and raloxifene [170]. The major differences between the two receptors lie in their amino-terminal domains, which bear little similarity [171]. Each of the ER transcripts have different downstream functions, as identified in ERα and ERβ knockout (KO) mouse models [172]. In selective ERα and ERβ KO mouse models, ERα was shown to be essential for normal reproductive development [173]. In contrast, although ERβ-KO mice had decreased side branching and partial alveolar development, the mammary glands secreted milk normally, and mothers were able to nurse their young [173]. Unlike ERα, which is associated with breast cancer proliferation, ERβ has an opposite effect in hormone-dependent cancers and is associated with an inhibitory role in tumorigenesis and metastasis [174]. Conventional ERβ-KO mice alone did not lead to tumorigenesis [175]. Alternatively, in conditional ERβ and p53 KO mice, Bado and colleagues demonstrated that a concomitant loss of ERβ and p53 induced early onset of basal-like mammary tumors [176]. In two recent independent publications, ERβ was shown to interact with p53, reduce p53–ERα binding affinity, and antagonize transcription regulation by p53–ERα [177,178]. Thus, if there is competitive binding between the ER proteins with p53, it has been suggested that the ratio of ERβ and ERα may affect estrogen responsiveness in breast cancer [178]. The pro-apoptosis response of ERβ in breast cancer cells also involved epigenetic changes in the methylation of histones affecting gene regulation and, hence, cellular activities of the cells [177]. Thus, these studies elucidated a novel anti-proliferative and pro-apoptotic mechanism in breast cancer involving the ERβ–p53 interaction.
In essence, the role of p53 as a protector or perpetrator in breast cancer may very well be dependent on the ratio of ERα and ERβ in breast cancer. In turn, the crosstalk between ERα, ERβ, and p53 has the potential to affect hormone-dependent breast cancers responsiveness to endocrine and other breast cancer therapies [177,178].
6.6. Hypothesis of p53 Activation Changing Metabolism and Breast Cancer Survival
Beatson’s theory from his doctorate dissertation was that lactation was very close to being a cancerous process and, if arrested in its progression, could become carcinogenic, as cited in reference [179]. In other words, a breast cancer cell is a normal mammary cell “lost in translation” during the re-programming process in pregnancy.
MCF-7 cells are derived from the luminal breast epithelial, which consists of cells that differentiate to become milk-producing cells. This cell line has provided vital information on hormone regulation of breast cancer and treatments to combat this disease. We have recently demonstrated that activation of p53 in MCF-7 breast epithelial cells initiated changes in cell morphology and cellular metabolism consistent with a differentiated phenotype and was most likely to be important in cell survival and recurrence in ERα-positive breast cancer cells [90,97,98]. The potential functional role of p53 in breast cancer metabolism is reviewed in our recent article [180]. We used global proteomic labeling to determine changes occurring in ERα-positive breast cancer cells after p53 activation. As part of the global protein changes, we described a unique snapshot profile analysis of the differential regulation of the annexin and S100A calcium-binding-associated protein family [97]. The annexin family comprises proteins that are important regulators of normal cellular function, and tight regulation of calcium is critical in breast homeostasis during pregnancy and lactation, the primary function of the breast [181]. A tight reprogramming of calcium regulators during pregnancy is vital to avoid aberrant cell proliferation and death during the lactation process. Alternatively, aberrations in calcium regulation are key features of breast cancer [182,183]. Aberrant expression of individual annexins and S100A proteins has been associated with malignant transformation [184,185,186,187,188], tumor invasion [189,190,191], metastasis, angiogenesis, and drug resistance [192,193,194], depending on breast cancer sub-type. The hypothesis resulting from these studies is that, although MCF-7 is a cancer cell line, it has a functional memory of self, that is, of lactation. Understanding the changes that occur after the reactivation of p53 in these cells in association with hormone exposure should provide useful clues to defining the role of p53 in the development of breast cancer, and help gain insights into the process leading to breast cancer recurrence.
6.7. Problems Raised with p53-Based Treatments in ERα-positive Breast Cancer
Our studies and the work of other groups have strongly suggested that re-activation of the p53 signaling pathway is strongly implicated in chemotherapy- and endocrine therapy-resistant breast cancers, especially in the most prevalent breast cancer sub-type (ERα+/p53+) [93,98]. Activation of the p53 pathway in ERα-positive breast cancer cells rapidly induces cell cycle arrest; however, the cells remain viable, show changes in morphology and metabolic functions (increase in mitochondria biomass and readout function), can remain in a dormant state, and have the potential to reproduce by endoreplication in cell culture [90,98]. Although the activation of the p53 axis has been shown to rapidly stop cell division, this treatment does not necessarily lead to cell death [90,97,98]. More recently, a role of p53 in energy metabolism and survival has been proposed, providing cancer cells with a survival advantage; however, the mechanisms are less well understood [77,99,195]. Therefore, understanding the basic metabolic processes that lead to chemo- and endocrine resistance and relapse in breast cancer and that are based on the activation of the p53 tumor suppressor survival pathway is of high importance.
The theory that the p53 protein suppresses cell cycle progression, induces resistance to apoptosis, and maintains the breast cancer cells viable through metabolic reprogramming [90,98] is an important concept underlying breast cancer endocrine resistance and recurrence and needs to be explored in more detail.
7. Summary and Conclusions
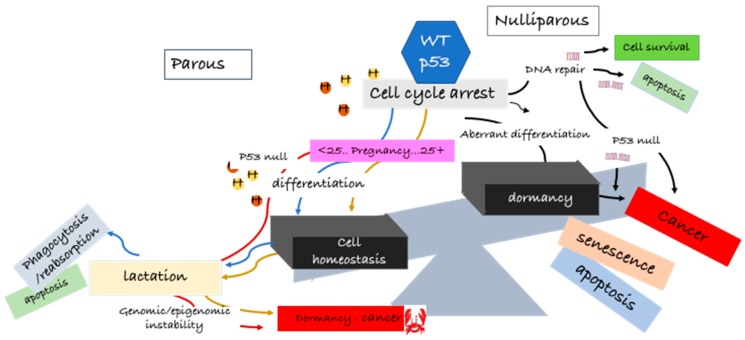
In summary, we do not know the extent of the function of p53 in the origin of latent hormone-responsive breast cancers, and this review does not delve into the many complexities of the normal p53 function. What is emerging from these cited reports and others is that p53 expression at a critical timing in young age pregnancy is pivotal in preventing mammary gland tumorigenesis. The underlying associated molecular changes could be permanent reprogramming of cellular events and/or DNA methylation, imprinting a protective signature and maintaining normal cell homeostasis. With older age pregnancies or in nulliparous women, accumulation of DNA mutations or other aberrations in the mammary gland abrogate this protective signature, and the cells are more susceptible to carcinogenic agents. Hormonal activation of the p53 in the mammary gland is implicated in the development of latent breast cancer. Confidence in the protective effect of p53 comes from p53null rodents, where, in the absence of p53, spontaneous tumors are found in the mammary glands, independent of age, as summarized in Figure 1.
Figure 1.
A role for p53wt in breast cancer origin and latency—p53wt “good guy–bad guy” hypothesis. During pregnancy (before 25 years of age), under the influence of the female hormones (estrogen and progesterone), p53 participates in stepwise chromatin remodeling and epigenetic reprogramming. These reprogramming events imprint a lasting protective signature on mammary cells, maintaining homeostasis (blue line). After lactation, wasted cells are phagocytosed and reabsorbed or undergo apoptosis. Mammary glands in females over the age of 25 years undergo the same process; however, because of the potential genomic and epigenetic instability, resulting from the continuous pre-pregnancy hormonal flux over the years, the mammary cells are not protected against latent breast cancer. The mammary cells may remain in a pre-cancerous dormant state for decades until stimulated by mitogens to proliferate (gold line). In nulliparous females, p53null mammary cells also produce active lactating cells but are highly vulnerable to spontaneous cancer (red line). p53 tries to repair the damaged cells for survival (black line). If DNA damage cannot be repaired, cells undergo apoptosis, senescence, or remain in a pre-cancerous dormant state (black line). In aberrant differentiation, 53wt tries to repair the DNA mismatches. If unable to complete the repair, the cells undergo apoptosis or become precancerous cells (black lines). Aberrant differentiation can also lead to dormancy and, thereafter, apoptosis, senescence, or emerging of latent breast cancer cells (black line). Hypothetically, recurrence of breast cancer may follow the process of dormancy, i.e., cells do not die but remain in a vulnerable pre-cancerous state.
How can we utilize this knowledge for more efficacious treatments and to prevent recurrence and metastasis? While the tumor suppressor role of p53 in breast cancer treatment is well recognized, the evidence supporting an opposite action of p53 in treatment resistance and recurrence in breast cancer is not as clear. Breast cancer recurrence may well be a consequence of the heterogeneous nature of the initial tumor, confounded by the change in hormone signaling from a paracrine to an autocrine proliferative response upon estrogen exposure. Breast cancer cells have a “memory of self”, and investigations in hormone-responsive breast cancer cells after p53 activation show characteristics of aberrant mammary differentiation, followed by dormancy, mediated by p53 activation. Further studies into how p53 protects breast cancer cells from cell death by switching cellular metabolism, downregulating apoptotic proteins, and initiating a cellular differentiation phenotype reminiscent of the normal mammary function is key to our understanding of recurrence in latent breast cancer development and a basis for the design of more efficacious treatments.
We need to look to the past to move on to the future and we now have the technology to do so.
Acknowledgments
D.H. was the recipient of an Australian Postgraduate Award and Cancer Institute of NSW (CINSW) scholarships (Sydney Vital and Translational Cancer Research Network (TCRN)). E.M.M was the recipient of travel awards from the CINSW TCRN.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zheng R., Zeng H., Zhang S., Chen W. Estimates of cancer incidence and mortality in China, 2013. Chin. J. Cancer. 2017;36:66. doi: 10.1186/s40880-017-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A., Ward E.M., Johnson C.J., Cronin K.A., Ma J., Ryerson B., Mariotto A., Lake A.J., Wilson R., Sherman R.L., et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J. Natl. Cancer Inst. 2017;109 doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis C.E., Ma J., Goding Sauer A., Newman L.A., Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J. Clin. 2017;67:439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 4.Ginsburg O., Bray F., Coleman M.P., Vanderpuye V., Eniu A., Kotha S.R., Sarker M., Huong T.T., Allemani C., Dvaladze A., et al. The global burden of women’s cancers: A grand challenge in global health. Lancet. 2017;389:847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benz C.C. Impact of aging on the biology of breast cancer. Crit. Rev. Oncol. Hematol. 2008;66:65–74. doi: 10.1016/j.critrevonc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatson G.T. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Lancet. 1896;148:162–165. doi: 10.1016/S0140-6736(01)72384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troisi R., Bjorge T., Gissler M., Grotmol T., Kitahara C.M., Myrtveit Saether S.M., Ording A.G., Skold C., Sorensen H.T., Trabert B., et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: A review of the evidence. J. Intern. Med. 2018 doi: 10.1111/joim.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carioli G., Malvezzi M., Rodriguez T., Bertuccio P., Negri E., La Vecchia C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast. 2017;36:89–95. doi: 10.1016/j.breast.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Fan L., Strasser-Weippl K., Li J.J., St Louis J., Finkelstein D.M., Yu K.D., Chen W.Q., Shao Z.M., Goss P.E. Breast cancer in China. Lancet Oncol. 2014;15:e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 10.Sivaraman L., Conneely O.M., Medina D., O’Malley B.W. P53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis. Proc. Natl. Acad. Sci. USA. 2001;98:12379–12384. doi: 10.1073/pnas.221459098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin T., Chao C., Saito S., Mazur S.J., Murphy M.E., Appella E., Xu Y. P53 induces differentiation of mouse embryonic stem cells by suppressing nanog expression. Nat. Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- 12.Medina D., Kittrell F.S. P53 function is required for hormone-mediated protection of mouse mammary tumorigenesis. Cancer Res. 2003;63:6140–6143. [PubMed] [Google Scholar]
- 13.Jerry D.J., Kittrell F.S., Kuperwasser C., Laucirica R., Dickinson E.S., Bonilla P.J., Butel J.S., Medina D. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 2000;19:1052–1058. doi: 10.1038/sj.onc.1203270. [DOI] [PubMed] [Google Scholar]
- 14.Sivaraman L., Stephens L.C., Markaverich B.M., Clark J.A., Krnacik S., Conneely O.M., O’Malley B.W., Medina D. Hormone-induced refractoriness to mammary carcinogenesis in wistar-furth rats. Carcinogenesis. 1998;19:1573–1581. doi: 10.1093/carcin/19.9.1573. [DOI] [PubMed] [Google Scholar]
- 15.Swanson S.M., Whitaker L.M., Stockard C.R., Myers R.B., Oelschlager D., Grizzle W.E., Juliana M.M., Grubbs C.J. Hormone levels and mammary epithelial cell proliferation in rats treated with a regimen of estradiol and progesterone that mimics the preventive effect of pregnancy against mammary cancer. Anticancer Res. 1997;17:4639–4645. [PubMed] [Google Scholar]
- 16.Grubbs C.J., Juliana M.M., Whitaker L.M. Short-term hormone treatment as a chemopreventive method against mammary cancer initiation in rats. Anticancer Res. 1988;8:113–117. [PubMed] [Google Scholar]
- 17.Huggins C., Moon R.C., Morii S. Extinction of experimental mammary cancer. I. Estradiol-17β and progesterone. Proc. Natl. Acad. Sci. USA. 1962;48:379–386. doi: 10.1073/pnas.48.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina D., Peterson L.E., Moraes R., Gay J. Short-term exposure to estrogen and progesterone induces partial protection against n-nitroso-n-methylurea-induced mammary tumorigenesis in wistar–furth rats. Cancer Lett. 2001;169:1–6. doi: 10.1016/S0304-3835(01)00507-9. [DOI] [PubMed] [Google Scholar]
- 19.Russo I.H., Russo J. Role of hormones in mammary cancer initiation and progression. J. Mammary Gland Biol. Neoplasia. 1998;3:49–61. doi: 10.1023/A:1018770218022. [DOI] [PubMed] [Google Scholar]
- 20.Russo I.H., Russo J. Hormonal approach to breast cancer prevention. J. Cell. Biochem. Suppl. 2000;34:1–6. doi: 10.1002/(SICI)1097-4644(2000)77:34+<1::AID-JCB2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Horn J., Vatten L.J. Reproductive and hormonal risk factors of breast cancer: A historical perspective. Int. J. Womens Health. 2017;9:265–272. doi: 10.2147/IJWH.S129017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansouri S., Teymourzadeh A., Farahmand L., Majidzadeh-A K. Cancer Genetics and Psychotherapy. Springer; Cham, Switzerland: 2017. Challenges of endocrine therapy in breast cancer. [Google Scholar]
- 23.Palmieri C., Patten D.K., Januszewski A., Zucchini G., Howell S.J. Breast cancer: Current and future endocrine therapies. Mol. Cell. Endocrinol. 2014;382:695–723. doi: 10.1016/j.mce.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Early Breast Cancer Trialists’ Collaborative Group Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 25.Albrektsen G., Heuch I., Hansen S., Kvale G. Breast cancer risk by age at birth, time since birth and time intervals between births: Exploring interaction effects. Br. J. Cancer. 2005;92:167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambe M., Hsieh C., Trichopoulos D., Ekbom A., Pavia M., Adami H.O. Transient increase in the risk of breast cancer after giving birth. N. Engl. J. Med. 1994;331:5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh C., Pavia M., Lambe M., Lan S.J., Colditz G.A., Ekbom A., Adami H.O., Trichopoulos D., Willett W.C. Dual effect of parity on breast cancer risk. Eur. J. Cancer. 1994;30A:969–973. doi: 10.1016/0959-8049(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 28.Barton M., Santucci-Pereira J., Russo J. Molecular pathways involved in pregnancy-induced prevention against breast cancer. Front. Endocrinol. 2014;5:213. doi: 10.3389/fendo.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson W.F., Pfeiffer R.M., Dores G.M., Sherman M.E. Comparison of age distribution patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol. Biomark. Prev. 2006;15:1899–1905. doi: 10.1158/1055-9965.EPI-06-0191. [DOI] [PubMed] [Google Scholar]
- 30.Rosner B., Colditz G.A., Willett W.C. Reproductive risk factors in a prospective study of breast cancer: The nurses’ health study. Am. J. Epidemiol. 1994;139:819–835. doi: 10.1093/oxfordjournals.aje.a117079. [DOI] [PubMed] [Google Scholar]
- 31.MacMahon B., Cole P., Lin T.M., Lowe C.R., Mirra A.P., Ravnihar B., Salber E.J., Valaoras V.G., Yuasa S. Age at first birth and breast cancer risk. Bull. World Health Organ. 1970;43:209–221. [PMC free article] [PubMed] [Google Scholar]
- 32.Mustacchi P. Ramazzini and rigoni-stern on parity and breast cancer. Clinical impression and statistical corroboration. Arch. Intern. Med. 1961;108:639–642. doi: 10.1001/archinte.1961.03620100131018. [DOI] [PubMed] [Google Scholar]
- 33.Russo J. Significance of rat mammary tumors for human risk assessment. Toxicol. Pathol. 2015;43:145–170. doi: 10.1177/0192623314532036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha D.K., Pazik J.E., Dao T.L. Prevention of mammary carcinogenesis in rats by pregnancy: Effect of full-term and interrupted pregnancy. Br. J. Cancer. 1988;57:390–394. doi: 10.1038/bjc.1988.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grubbs C.J., Farnell D.R., Hill D.L., McDonough K.C. Chemoprevention of n-nitroso-n-methylurea-induced mammary cancers by pretreatment with 17 β-estradiol and progesterone. J. Natl. Cancer Inst. 1985;74:927–931. [PubMed] [Google Scholar]
- 36.Russo J., Wilgus G., Tait L., Russo I.H. Influence of age and parity on the susceptibility of rat mammary gland epithelial cells in primary cultures to 7,12-dimethylbenz(a)anthracene. In Vitro. 1981;17:877–884. doi: 10.1007/BF02618283. [DOI] [PubMed] [Google Scholar]
- 37.Swanson S.M., Guzman R.C., Collins G., Tafoya P., Thordarson G., Talamantes F., Nandi S. Refractoriness to mammary carcinogenesis in the parous mouse is reversible by hormonal stimulation induced by pituitary isografts. Cancer Lett. 1995;90:171–181. doi: 10.1016/0304-3835(95)03712-6. [DOI] [PubMed] [Google Scholar]
- 38.Russo I.H., Russo J. Mammary gland neoplasia in long-term rodent studies. Environ. Health Perspect. 1996;104:938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo J., Hu Y.F., Silva I.D., Russo I.H. Cancer risk related to mammary gland structure and development. Microsc. Res. Tech. 2001;52:204–223. doi: 10.1002/1097-0029(20010115)52:2<204::AID-JEMT1006>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Russo J., Russo I.H. Hormonally induced differentiation: A novel approach to breast cancer prevention. J. Cell. Biochem. Suppl. 1995;22:58–64. doi: 10.1002/jcb.240590809. [DOI] [PubMed] [Google Scholar]
- 41.Russo J., Russo I.H. Influence of differentiation and cell kinetics on the susceptibility of the rat mammary gland to carcinogenesis. Cancer Res. 1980;40:2677–2687. [PubMed] [Google Scholar]
- 42.Russo J., Russo I.H. Toward a physiological approach to breast cancer prevention. Cancer Epidemiol. Biomark. Prev. 1994;3:353–364. [PubMed] [Google Scholar]
- 43.Alvarado A., Lopes A.C., Faustino-Rocha A.I., Cabrita A.M.S., Ferreira R., Oliveira P.A., Colaco B. Prognostic factors in mnu and dmba-induced mammary tumors in female rats. Pathol. Res. Pract. 2017;213:441–446. doi: 10.1016/j.prp.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Russo J., Santucci-Pereira J., de Cicco R.L., Sheriff F., Russo P.A., Peri S., Slifker M., Ross E., Mello M.L., Vidal B.C., et al. Pregnancy-induced chromatin remodeling in the breast of postmenopausal women. Int. J. Cancer. 2012;131:1059–1070. doi: 10.1002/ijc.27323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guzman R.C., Yang J., Rajkumar L., Thordarson G., Chen X., Nandi S. Hormonal prevention of breast cancer: Mimicking the protective effect of pregnancy. Proc. Natl. Acad. Sci. USA. 1999;96:2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beral V. Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–427. doi: 10.1016/S0140-6736(03)14596-5. [DOI] [PubMed] [Google Scholar]
- 47.Jensen E.V., Jordan V.C. The estrogen receptor: A model for molecular medicine. Clin. Cancer Res. 2003;9:1980–1989. [PubMed] [Google Scholar]
- 48.Ali S., Coombes R.C. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer. 2002;2:101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 49.Ali S., Buluwela L., Coombes R.C. Antiestrogens and their therapeutic applications in breast cancer and other diseases. Annu. Rev. Med. 2011;62:217–232. doi: 10.1146/annurev-med-052209-100305. [DOI] [PubMed] [Google Scholar]
- 50.Medina D. Mammary developmental fate and breast cancer risk. Endocr. Relat. Cancer. 2005;12:483–495. doi: 10.1677/erc.1.00804. [DOI] [PubMed] [Google Scholar]
- 51.Russo J., Wilgus G., Russo I.H. Susceptibility of the mammary gland to carcinogenesis: I differentiation of the mammary gland as determinant of tumor incidence and type of lesion. Am. J. Pathol. 1979;96:721–736. [PMC free article] [PubMed] [Google Scholar]
- 52.Ginger M.R., Gonzalez-Rimbau M.F., Gay J.P., Rosen J.M. Persistent changes in gene expression induced by estrogen and progesterone in the rat mammary gland. Mol. Endocrinol. 2001;15:1993–2009. doi: 10.1210/mend.15.11.0724. [DOI] [PubMed] [Google Scholar]
- 53.Yang J., Yoshizawa K., Nandi S., Tsubura A. Protective effects of pregnancy and lactation against n-methyl-n-nitrosourea-induced mammary carcinomas in female lewis rats. Carcinogenesis. 1999;20:623–628. doi: 10.1093/carcin/20.4.623. [DOI] [PubMed] [Google Scholar]
- 54.Schuler-Toprak S., Treeck O., Ortmann O. Human chorionic gonadotropin and breast cancer. Int. J. Mol. Sci. 2017;18:1587. doi: 10.3390/ijms18071587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santucci-Pereira J., George C., Armiss D., Russo I.H., Vanegas J.E., Sheriff F., de Cicco R.L., Su Y., Russo P.A., Bidinotto L.T., et al. Mimicking pregnancy as a strategy for breast cancer prevention. Breast Cancer Manag. 2013;2:283–294. doi: 10.2217/bmt.13.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janssens J.P., Russo J., Russo I., Michiels L., Donders G., Verjans M., Riphagen I., Van den Bossche T., Deleu M., Sieprath P. Human chorionic gonadotropin (hcg) and prevention of breast cancer. Mol. Cell. Endocrinol. 2007;269:93–98. doi: 10.1016/j.mce.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 57.Fortner R.T., Schock H., Kaaks R., Lehtinen M., Pukkala E., Lakso H.A., Tanner M., Kallio R., Joensuu H., Korpela J., et al. Human chorionic gonadotropin does not correlate with risk for maternal breast cancer: Results from the finnish maternity cohort. Cancer Res. 2017;77:134–141. doi: 10.1158/0008-5472.CAN-16-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vousden K.H., Lu X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 59.Vousden K.H. P53: Death star. Cell. 2000;103:691–694. doi: 10.1016/S0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 60.Vousden K.H., Vande Woude G.F. The ins and outs of p53. Nat. Cell Biol. 2000;2:E178–E180. doi: 10.1038/35036427. [DOI] [PubMed] [Google Scholar]
- 61.Rotter V., Foord O., Navot N. In search of the functions of normal p53 protein. Trends Cell Biol. 1993;3:46–49. doi: 10.1016/0962-8924(93)90151-P. [DOI] [PubMed] [Google Scholar]
- 62.Tovy A., Spiro A., McCarthy R., Shipony Z., Aylon Y., Allton K., Ainbinder E., Furth N., Tanay A., Barton M., et al. P53 is essential for DNA methylation homeostasis in naive embryonic stem cells, and its loss promotes clonal heterogeneity. Genes Dev. 2017;31:959–972. doi: 10.1101/gad.299198.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aylon Y., Oren M. The paradox of p53: What, how, and why? Cold Spring Harb. Perspect. Med. 2016;6:a026328. doi: 10.1101/cshperspect.a026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Medina D., Kittrell F.S., Shepard A., Contreras A., Rosen J.M., Lydon J. Hormone dependence in premalignant mammary progression. Cancer Res. 2003;63:1067–1072. [PubMed] [Google Scholar]
- 65.Park I.Y., Sohn B.H., Choo J.H., Joe C.O., Seong J.K., Lee Y.I., Chung J.H. Deregulation of DNA methyltransferases and loss of parental methylation at the insulin-like growth factor ii (igf2)/h19 loci in p53 knockout mice prior to tumor development. J. Cell. Biochem. 2005;94:585–596. doi: 10.1002/jcb.20263. [DOI] [PubMed] [Google Scholar]
- 66.Levine A.J. The p53 protein plays a central role in the mechanism of action of epigentic drugs that alter the methylation of cytosine residues in DNA. Oncotarget. 2017;8:7228–7230. doi: 10.18632/oncotarget.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.West L.E., Gozani O. Regulation of p53 function by lysine methylation. Epigenomics. 2011;3:361–369. doi: 10.2217/epi.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lane D.P. Cancer. P53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 69.Ryan K.M. P53 and autophagy in cancer: Guardian of the genome meets guardian of the proteome. Eur. J. Cancer. 2011;47:44–50. doi: 10.1016/j.ejca.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 70.Levine A.J., Tomasini R., McKeon F.D., Mak T.W., Melino G. The p53 family: Guardians of maternal reproduction. Nat. Rev. Mol. Cell Biol. 2011;12:259–265. doi: 10.1038/nrm3086. [DOI] [PubMed] [Google Scholar]
- 71.Gomes A.S., Ramos H., Soares J., Saraiva L. P53 and glucose metabolism: An orchestra to be directed in cancer therapy. Pharmacol. Res. 2018;131:75–86. doi: 10.1016/j.phrs.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 72.Itahana Y., Itahana K. Emerging roles of p53 family members in glucose metabolism. Int. J. Mol. Sci. 2018;19:776. doi: 10.3390/ijms19030776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parrales A., Iwakuma T. P53 as a regulator of lipid metabolism in cancer. Int. J. Mol. Sci. 2016;17:2074. doi: 10.3390/ijms17122074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hainaut P. P53 in metabolism, aging and cancer. Ann. Dermatol. Venereol. 2014;141:S200–S201. doi: 10.1016/j.annder.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Jiang P., Du W., Yang X. P53 and regulation of tumor metabolism. J. Carcinog. 2013;12:21. doi: 10.4103/1477-3163.122760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sen N., Satija Y.K., Das S. P53 and metabolism: Old player in a new game. Transcription. 2012;3:119–123. doi: 10.4161/trns.20094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vousden K.H., Ryan K.M. P53 and metabolism. Nat. Rev. Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 78.Fields J., Hanisch J.J., Choi J.W., Hwang P.M. How does p53 regulate mitochondrial respiration? IUBMB Life. 2007;59:682–684. doi: 10.1080/15216540601185021. [DOI] [PubMed] [Google Scholar]
- 79.Ma W., Sung H.J., Park J.Y., Matoba S., Hwang P.M. A pivotal role for p53: Balancing aerobic respiration and glycolysis. J. Bioenerg. Biomembr. 2007;39:243–246. doi: 10.1007/s10863-007-9083-0. [DOI] [PubMed] [Google Scholar]
- 80.Matoba S., Kang J.G., Patino W.D., Wragg A., Boehm M., Gavrilova O., Hurley P.J., Bunz F., Hwang P.M. P53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 81.Cheung E.C., Vousden K.H. The role of p53 in glucose metabolism. Curr. Opin. Cell Biol. 2010;22:186–191. doi: 10.1016/j.ceb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 82.Lin T., Lin Y. P53 switches off pluripotency on differentiation. Stem Cell Res. Ther. 2017;8:44. doi: 10.1186/s13287-017-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roy S., Tomaszowski K.H., Luzwick J.W., Park S., Li J., Murphy M., Schlacher K. P53 orchestrates DNA replication restart homeostasis by suppressing mutagenic rad52 and poltheta pathways. eLife. 2018;7:e31723. doi: 10.7554/eLife.31723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berkers C.R., Maddocks O.D., Cheung E.C., Mor I., Vousden K.H. Metabolic regulation by p53 family members. Cell Metab. 2013;18:617–633. doi: 10.1016/j.cmet.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poletto M., Legrand A.J., Fletcher S.C., Dianov G.L. P53 coordinates base excision repair to prevent genomic instability. Nucleic Acids Res. 2016;44:3165–3175. doi: 10.1093/nar/gkw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016;6:a026104. doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aubrey B.J., Kelly G.L., Janic A., Herold M.J., Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25:104–113. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tran T.Q., Lowman X.H., Reid M.A., Mendez-Dorantes C., Pan M., Yang Y., Kong M. Tumor-associated mutant p53 promotes cancer cell survival upon glutamine deprivation through p21 induction. Oncogene. 2017;36:1991–2001. doi: 10.1038/onc.2016.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bieging K.T., Mello S.S., Attardi L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGowan E.M., Alling N., Jackson E.A., Yagoub D., Haass N.K., Allen J.D., Martinello-Wilks R. Evaluation of cell cycle arrest in estrogen responsive mcf-7 breast cancer cells: Pitfalls of the mts assay. PLoS ONE. 2011;6:e20623. doi: 10.1371/journal.pone.0020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ou W.B., Lu M., Eilers G., Li H., Ding J., Meng X., Wu Y., He Q., Sheng Q., Zhou H.M., et al. Co-targeting of fak and mdm2 triggers additive anti-proliferative effects in mesothelioma via a coordinated reactivation of p53. Br. J. Cancer. 2016;115:1253–1263. doi: 10.1038/bjc.2016.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu J., Zhang C., Hu W., Feng Z. Tumor suppressor p53 and its mutants in cancer metabolism. Cancer Lett. 2015;356:197–203. doi: 10.1016/j.canlet.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bailey S.T., Shin H., Westerling T., Liu X.S., Brown M. Estrogen receptor prevents p53-dependent apoptosis in breast cancer. Proc. Natl. Acad. Sci. USA. 2012;109:18060–18065. doi: 10.1073/pnas.1018858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jackson J.G., Pant V., Li Q., Chang L.L., Quintas-Cardama A., Garza D., Tavana O., Yang P., Manshouri T., Li Y., et al. P53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell. 2012;21:793–806. doi: 10.1016/j.ccr.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rufini A., Tucci P., Celardo I., Melino G. Senescence and aging: The critical roles of p53. Oncogene. 2013;32:5129–5143. doi: 10.1038/onc.2012.640. [DOI] [PubMed] [Google Scholar]
- 96.Riley T., Sontag E., Chen P., Levine A. Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 97.Hatoum D., Yagoub D., Ahadi A., Nassif N.T., McGowan E.M. Annexin/s100a protein family regulation through p14arf-p53 activation: A role in cell survival and predicting treatment outcomes in breast cancer. PLoS ONE. 2017;12:e0169925. doi: 10.1371/journal.pone.0169925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McGowan E.M., Tran N., Alling N., Yagoub D., Sedger L.M., Martiniello-Wilks R. P14arf post-transcriptional regulation of nuclear cyclin d1 in mcf-7 breast cancer cells: Discrimination between a good and bad prognosis? PLoS ONE. 2012;7:e42246. doi: 10.1371/journal.pone.0042246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kruiswijk F., Labuschagne C.F., Vousden K.H. P53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y., Elf S.E., Miyata Y., Sashida G., Liu Y., Huang G., Di Giandomenico S., Lee J.M., Deblasio A., Menendez S., et al. P53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Itahana K., Dimri G.P., Hara E., Itahana Y., Zou Y., Desprez P.Y., Campisi J. A role for p53 in maintaining and establishing the quiescence growth arrest in human cells. J. Biol. Chem. 2002;277:18206–18214. doi: 10.1074/jbc.M201028200. [DOI] [PubMed] [Google Scholar]
- 102.Korotchkina L.G., Leontieva O.V., Bukreeva E.I., Demidenko Z.N., Gudkov A.V., Blagosklonny M.V. The choice between p53-induced senescence and quiescence is determined in part by the mtor pathway. Aging. 2010;2:344–352. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allred D.C., Clark G.M., Elledge R., Fuqua S.A., Brown R.W., Chamness G.C., Osborne C.K., McGuire W.L. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J. Natl. Cancer Inst. 1993;85:200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- 104.Zhang E.B., Yin D.D., Sun M., Kong R., Liu X.H., You L.H., Han L., Xia R., Wang K.M., Yang J.S., et al. P53-regulated long non-coding rna tug1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating hoxb7 expression. Cell Death Dis. 2014;5:e1243. doi: 10.1038/cddis.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levine B., Abrams J. P53: The janus of autophagy? Nat. Cell Biol. 2008;10:637–639. doi: 10.1038/ncb0608-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tasdemir E., Chiara Maiuri M., Morselli E., Criollo A., D’Amelio M., Djavaheri-Mergny M., Cecconi F., Tavernarakis N., Kroemer G. A dual role of p53 in the control of autophagy. Autophagy. 2008;4:810–814. doi: 10.4161/auto.6486. [DOI] [PubMed] [Google Scholar]
- 107.Tasdemir E., Maiuri M.C., Galluzzi L., Vitale I., Djavaheri-Mergny M., D’Amelio M., Criollo A., Morselli E., Zhu C., Harper F., et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 2008;10:676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh S.S., Vats S., Chia A.Y., Tan T.Z., Deng S., Ong M.S., Arfuso F., Yap C.T., Goh B.C., Sethi G., et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37:1142–1158. doi: 10.1038/s41388-017-0046-6. [DOI] [PubMed] [Google Scholar]
- 109.Crighton D., Wilkinson S., O’Prey J., Syed N., Smith P., Harrison P.R., Gasco M., Garrone O., Crook T., Ryan K.M. Dram, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 110.Feng Z., Zhang H., Levine A.J., Jin S. The coordinate regulation of the p53 and mtor pathways in cells. Proc. Natl. Acad. Sci. USA. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Green D.R., Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clarke R., Tyson J.J., Dixon J.M. Endocrine resistance in breast cancer—An overview and update. Pt 3Mol. Cell. Endocrinol. 2015;418:220–234. doi: 10.1016/j.mce.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gottlieb E., Vousden K.H. P53 regulation of metabolic pathways. Cold Spring Harb. Perspect. Biol. 2010;2:a001040. doi: 10.1101/cshperspect.a001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williams A.B., Schumacher B. P53 in the DNA-damage-repair process. Cold Spring Harb. Perspect. Med. 2016;6:a026070. doi: 10.1101/cshperspect.a026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mantovani F., Walerych D., Sal G.D. Targeting mutant p53 in cancer: A long road to precision therapy. FEBS J. 2017;284:837–850. doi: 10.1111/febs.13948. [DOI] [PubMed] [Google Scholar]
- 116.Escoll M., Gargini R., Cuadrado A., Anton I.M., Wandosell F. Mutant p53 oncogenic functions in cancer stem cells are regulated by wip through yap/taz. Oncogene. 2017;36:3515–3527. doi: 10.1038/onc.2016.518. [DOI] [PubMed] [Google Scholar]
- 117.Alexandrova E.M., Mirza S.A., Xu S., Schulz-Heddergott R., Marchenko N.D., Moll U.M. P53 loss-of-heterozygosity is a necessary prerequisite for mutant p53 stabilization and gain-of-function in vivo. Cell Death Dis. 2017;8:e2661. doi: 10.1038/cddis.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laptenko O., Prives C. P53: Master of life, death, and the epigenome. Genes Dev. 2017;31:955–956. doi: 10.1101/gad.302364.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kastenhuber E.R., Lowe S.W. Putting p53 in context. Cell. 2017;170:1062–1078. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pappas K., Xu J., Zairis S., Resnick-Silverman L., Abate F., Steinbach N., Ozturk S., Saal L.H., Su T., Cheung P., et al. P53 maintains baseline expression of multiple tumor suppressor genes. Mol. Cancer Res. 2017;15:1051–1062. doi: 10.1158/1541-7786.MCR-17-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gurpinar E., Vousden K.H. Hitting cancers’ weak spots: Vulnerabilities imposed by p53 mutation. Trends Cell Biol. 2015;25:486–495. doi: 10.1016/j.tcb.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Ranjan A., Iwakuma T. Non-canonical cell death induced by p53. Int. J. Mol. Sci. 2016;17:2068. doi: 10.3390/ijms17122068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Medina D., Sivaraman L., Hilsenbeck S.G., Conneely O., Ginger M., Rosen J., Omalle B.W. Mechanisms of hormonal prevention of breast cancer. Ann. N. Y. Acad. Sci. 2001;952:23–35. doi: 10.1111/j.1749-6632.2001.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 124.Ginger M.R., Rosen J.M. Pregnancy-induced changes in cell-fate in the mammary gland. Breast Cancer Res. 2003;5:192–197. doi: 10.1186/bcr603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yi Y., Shepard A., Kittrell F., Mulac-Jericevic B., Medina D., Said T.K. P19arf determines the balance between normal cell proliferation rate and apoptosis during mammary gland development. Mol. Biol. Cell. 2004;15:2302–2311. doi: 10.1091/mbc.e03-11-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharpless N.E., Bardeesy N., Lee K.H., Carrasco D., Castrillon D.H., Aguirre A.J., Wu E.A., Horner J.W., DePinho R.A. Loss of p16ink4a with retention of p19arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 127.Salazar-Roa M., Malumbres M. Fueling the cell division cycle. Trends Cell Biol. 2017;27:69–81. doi: 10.1016/j.tcb.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 128.Beatson G.T. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Trans. Med. Chir. Soc. Edinb. 1896;15:153–179. [PMC free article] [PubMed] [Google Scholar]
- 129.Vousden K.H., Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 130.Van de Moosdijk A.A., Fu N.Y., Rios A.C., Visvader J.E., van Amerongen R. Lineage tracing of mammary stem and progenitor cells. Methods Mol. Biol. 2017;1501:291–308. doi: 10.1007/978-1-4939-6475-8_15. [DOI] [PubMed] [Google Scholar]
- 131.Fu N.Y., Rios A.C., Pal B., Law C.W., Jamieson P., Liu R., Vaillant F., Jackling F., Liu K.H., Smyth G.K., et al. Identification of quiescent and spatially restricted mammary stem cells that are hormone responsive. Nat. Cell Biol. 2017;19:164–176. doi: 10.1038/ncb3471. [DOI] [PubMed] [Google Scholar]
- 132.Stingl J., Raouf A., Emerman J.T., Eaves C.J. Epithelial progenitors in the normal human mammary gland. J. Mammary Gland Biol. Neoplasia. 2005;10:49–59. doi: 10.1007/s10911-005-2540-7. [DOI] [PubMed] [Google Scholar]
- 133.Morrison S.J., Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 134.Cicalese A., Bonizzi G., Pasi C.E., Faretta M., Ronzoni S., Giulini B., Brisken C., Minucci S., Di Fiore P.P., Pelicci P.G. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 135.Dontu G., Al-Hajj M., Abdallah W.M., Clarke M.F., Wicha M.S. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl. S1):S59–S72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Magee J.A., Piskounova E., Morrison S.J. Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Levine A.J., Berger S.L. The interplay between epigenetic changes and the p53 protein in stem cells. Genes Dev. 2017;31:1195–1201. doi: 10.1101/gad.298984.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jerry D.J., Dunphy K.A., Hagen M.J. Estrogens, regulation of p53 and breast cancer risk: A balancing act. Cell. Mol. Life Sci. 2010;67:1017–1023. doi: 10.1007/s00018-009-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muller C., Leutz A. Chromatin remodeling in development and differentiation. Curr. Opin. Genet. Dev. 2001;11:167–174. doi: 10.1016/S0959-437X(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 140.Clarke R.B., Howell A., Potten C.S., Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 141.Anderson E. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res. 2002;4:197–201. doi: 10.1186/bcr452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chlebowski R.T., Anderson G., Manson J.E., Pettinger M., Yasmeen S., Lane D., Langer R.D., Hubbell F.A., McTiernan A., Hendrix S., et al. Estrogen alone in postmenopausal women and breast cancer detection by means of mammography and breast biopsy. J. Clin. Oncol. 2010;28:2690–2697. doi: 10.1200/JCO.2009.24.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chlebowski R.T., Anderson G.L. Menopausal hormone therapy and breast cancer mortality: Clinical implications. Ther. Adv. Drug Saf. 2015;6:45–56. doi: 10.1177/2042098614568300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gurney E.P., Nachtigall M.J., Nachtigall L.E., Naftolin F. The women’s health initiative trial and related studies: 10 years later: A clinician’s view. J. Steroid Biochem. Mol. Biol. 2014;142:4–11. doi: 10.1016/j.jsbmb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 145.Chlebowski R.T., Anderson G.L., Gass M., Lane D.S., Aragaki A.K., Kuller L.H., Manson J.E., Stefanick M.L., Ockene J., Sarto G.E., et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chlebowski R.T., Manson J.E., Anderson G.L., Cauley J.A., Aragaki A.K., Stefanick M.L., Lane D.S., Johnson K.C., Wactawski-Wende J., Chen C., et al. Estrogen plus progestin and breast cancer incidence and mortality in the women’s health initiative observational study. J. Natl. Cancer Inst. 2013;105:526–535. doi: 10.1093/jnci/djt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Muller P.A., Vousden K.H. P53 mutations in cancer. Nat. Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 148.Bykov V.J.N., Eriksson S.E., Bianchi J., Wiman K.G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer. 2018;18:89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 149.Petitjean A., Mathe E., Kato S., Ishioka C., Tavtigian S.V., Hainaut P., Olivier M. Impact of mutant p53 functional properties on tp53 mutation patterns and tumor phenotype: Lessons from recent developments in the iarc tp53 database. Hum. Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 150.Pharoah P.D., Day N.E., Caldas C. Somatic mutations in the p53 gene and prognosis in breast cancer: A meta-analysis. Br. J. Cancer. 1999;80:1968–1973. doi: 10.1038/sj.bjc.6690628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lacroix M., Toillon R.A., Leclercq G. P53 and breast cancer, an update. Endocr. Relat. Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- 152.Agrawal A., Yang J., Murphy R.F., Agrawal D.K. Regulation of the p14arf-mdm2-p53 pathway: An overview in breast cancer. Exp. Mol. Pathol. 2006;81:115–122. doi: 10.1016/j.yexmp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 153.Haupt S., Vijayakumaran R., Miranda P.J., Burgess A., Lim E., Haupt Y. The role of mdm2 and mdm4 in breast cancer development and prevention. J. Mol. Cell Biol. 2017;9:53–61. doi: 10.1093/jmcb/mjx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Burgess A., Chia K.M., Haupt S., Thomas D., Haupt Y., Lim E. Clinical overview of mdm2/x-targeted therapies. Front. Oncol. 2016;6:7. doi: 10.3389/fonc.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ozaki T., Nakagawara A. Role of p53 in cell death and human cancers. Cancers (Basel) 2011;3:994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Turpin E., Bieche I., Bertheau P., Plassa L.F., Lerebours F., de Roquancourt A., Olivi M., Espie M., Marty M., Lidereau R., et al. Increased incidence of erbb2 overexpression and tp53 mutation in inflammatory breast cancer. Oncogene. 2002;21:7593–7597. doi: 10.1038/sj.onc.1205932. [DOI] [PubMed] [Google Scholar]
- 157.Xia L., Paik A., Li J.J. P53 activation in chronic radiation-treated breast cancer cells: Regulation of mdm2/p14arf. Cancer Res. 2004;64:221–228. doi: 10.1158/0008-5472.CAN-03-0969. [DOI] [PubMed] [Google Scholar]
- 158.Musgrove E.A., Sutherland R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 159.Turner N.C., Neven P., Loibl S., Andre F. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet. 2017;389:2403–2414. doi: 10.1016/S0140-6736(16)32419-9. [DOI] [PubMed] [Google Scholar]
- 160.Konduri S.D., Medisetty R., Liu W., Kaipparettu B.A., Srivastava P., Brauch H., Fritz P., Swetzig W.M., Gardner A.E., Khan S.A., et al. Mechanisms of estrogen receptor antagonism toward p53 and its implications in breast cancer therapeutic response and stem cell regulation. Proc. Natl. Acad. Sci. USA. 2010;107:15081–15086. doi: 10.1073/pnas.1009575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu W., Ip M.M., Podgorsak M.B., Das G.M. Disruption of estrogen receptor α-p53 interaction in breast tumors: A novel mechanism underlying the anti-tumor effect of radiation therapy. Breast Cancer Res. Treat. 2009;115:43–50. doi: 10.1007/s10549-008-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu W., Konduri S.D., Bansal S., Nayak B.K., Rajasekaran S.A., Karuppayil S.M., Rajasekaran A.K., Das G.M. Estrogen receptor-α binds p53 tumor suppressor protein directly and represses its function. J. Biol. Chem. 2006;281:9837–9840. doi: 10.1074/jbc.C600001200. [DOI] [PubMed] [Google Scholar]
- 163.Sayeed A., Konduri S.D., Liu W., Bansal S., Li F., Das G.M. Estrogen receptor α inhibits p53-mediated transcriptional repression: Implications for the regulation of apoptosis. Cancer Res. 2007;67:7746–7755. doi: 10.1158/0008-5472.CAN-06-3724. [DOI] [PubMed] [Google Scholar]
- 164.Gudas J.M., Nguyen H., Li T., Sadzewicz L., Robey R., Wosikowski K., Cowan K.H. Drug-resistant breast cancer cells frequently retain expression of a functional wild-type p53 protein. Carcinogenesis. 1996;17:1417–1427. doi: 10.1093/carcin/17.7.1417. [DOI] [PubMed] [Google Scholar]
- 165.Holst F., Stahl P.R., Ruiz C., Hellwinkel O., Jehan Z., Wendland M., Lebeau A., Terracciano L., Al-Kuraya K., Janicke F., et al. Estrogen receptor α (esr1) gene amplification is frequent in breast cancer. Nat. Genet. 2007;39:655–660. doi: 10.1038/ng2006. [DOI] [PubMed] [Google Scholar]
- 166.Fernandez-Cuesta L., Anaganti S., Hainaut P., Olivier M. Estrogen levels act as a rheostat on p53 levels and modulate p53-dependent responses in breast cancer cell lines. Breast Cancer Res. Treat. 2011;125:35–42. doi: 10.1007/s10549-010-0819-x. [DOI] [PubMed] [Google Scholar]
- 167.Berger C.E., Qian Y., Liu G., Chen H., Chen X. P53, a target of estrogen receptor (ER) α, modulates DNA damage-induced growth suppression in ER-positive breast cancer cells. J. Biol. Chem. 2012;287:30117–30127. doi: 10.1074/jbc.M112.367326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kuiper G.G., Gustafsson J.A. The novel estrogen receptor-β subtype: Potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/S0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- 169.Tremblay G.B., Tremblay A., Copeland N.G., Gilbert D.J., Jenkins N.A., Labrie F., Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol. Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 170.Katzenellenbogen B.S., Katzenellenbogen J.A. Estrogen receptor transcription and transactivation: Estrogen receptor α and estrogen receptor β: Regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res. 2000;2:335–344. doi: 10.1186/bcr78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Delaunay F., Pettersson K., Tujague M., Gustafsson J.A. Functional differences between the amino-terminal domains of estrogen receptors α and β. Mol. Pharmacol. 2000;58:584–590. doi: 10.1124/mol.58.3.584. [DOI] [PubMed] [Google Scholar]
- 172.Lee H.R., Kim T.H., Choi K.C. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab. Anim. Res. 2012;28:71–76. doi: 10.5625/lar.2012.28.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hewitt S.C., Korach K.S. Oestrogen receptor knockout mice: Roles for oestrogen receptors α and β in reproductive tissues. Reproduction. 2003;125:143–149. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- 174.Thomas C., Gustafsson J.A. The different roles of er subtypes in cancer biology and therapy. Nat. Rev. Cancer. 2011;11:597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 175.Forster C., Makela S., Warri A., Kietz S., Becker D., Hultenby K., Warner M., Gustafsson J.A. Involvement of estrogen receptor β in terminal differentiation of mammary gland epithelium. Proc. Natl. Acad. Sci. USA. 2002;99:15578–15583. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bado I., Nikolos F., Rajapaksa G., Wu W., Castaneda J., Krishnamurthy S., Webb P., Gustafsson J.A., Thomas C. Somatic loss of estrogen receptor β and p53 synergize to induce breast tumorigenesis. Breast Cancer Res. 2017;19:79. doi: 10.1186/s13058-017-0872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lu W., Katzenellenbogen B.S. Estrogen receptor-β modulation of the ERα-p53 loop regulating gene expression, proliferation, and apoptosis in breast cancer. Horm. Cancer. 2017;8:230–242. doi: 10.1007/s12672-017-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bado I., Pham E., Soibam B., Nikolos F., Gustafsson J.A., Thomas G. Erβ alters the chemosensitivity of luminal breast cancer cells by regulating p53 function. Oncotarget. 2018;9:22509–22522. doi: 10.18632/oncotarget.25147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Goldenberg I.S. Hormones and breast cancer: Historical perspectives. Surgery. 1963;53:285–288. [PubMed] [Google Scholar]
- 180.Moulder D.E., Hatoum D., Tay E., Lin Y., McGowan E.M. The roles of p53 in mitochondrial dynamics and cancer metabolism: The pendulum between survival and death in breast cancer? Cancers. 2018:accepted. doi: 10.3390/cancers10060189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cross B.M., Breitwieser G.E., Reinhardt T.A., Rao R. Cellular calcium dynamics in lactation and breast cancer: From physiology to pathology. Am. J. Physiol. Cell Physiol. 2013;306:C515–C526. doi: 10.1152/ajpcell.00330.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Monteith G.R., McAndrew D., Faddy H.M., Roberts-Thomson S.J. Calcium and cancer: Targeting Ca2+ transport. Nat. Rev. Cancer. 2007;7:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 183.Stewart T.A., Yapa K.T., Monteith G.R. Altered calcium signaling in cancer cells. Biochimica et Biophysica Acta. 2014;1848:2502–2511. doi: 10.1016/j.bbamem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 184.Yom C.K., Han W., Kim S.W., Kim H.S., Shin H.C., Chang J.N., Koo M., Noh D.Y., Moon B.I. Clinical significance of annexin a1 expression in breast cancer. J. Breast Cancer. 2011;14:262–268. doi: 10.4048/jbc.2011.14.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ang E.Z., Nguyen H.T., Sim H.L., Putti T.C., Lim L.H. Annexin-1 regulates growth arrest induced by high levels of estrogen in mcf-7 breast cancer cells. Mol. Cancer Res. 2009;7:266–274. doi: 10.1158/1541-7786.MCR-08-0147. [DOI] [PubMed] [Google Scholar]
- 186.Cao Y., Li Y., Edelweiss M., Arun B., Rosen D., Resetkova E., Wu Y., Liu J., Sahin A., Albarracin C.T. Loss of annexin a1 expression in breast cancer progression. Appl. Immunohistochem. Mol. Morphol. 2008;16:530–534. doi: 10.1097/PAI.0b013e31817432c3. [DOI] [PubMed] [Google Scholar]
- 187.Bhardwaj A., Ganesan N., Tachibana K., Rajapakshe K., Albarracin C.T., Gunaratne P.H., Coarfa C., Bedrosian I. Annexin a1 preferentially predicts poor prognosis of basal-like breast cancer patients by activating mtor-s6 signaling. PLoS ONE. 2015;10:e0127678. doi: 10.1371/journal.pone.0127678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sobral-Leite M., Wesseling J., Smit V.T., Nevanlinna H., van Miltenburg M.H., Sanders J., Hofland I., Blows F.M., Coulson P., Patrycja G., et al. Annexin a1 expression in a pooled breast cancer series: Association with tumor subtypes and prognosis. BMC Med. 2015;13:156. doi: 10.1186/s12916-015-0392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Okano M., Kumamoto K., Saito M., Onozawa H., Saito K., Abe N., Ohtake T., Takenoshita S. Upregulated annexin a1 promotes cellular invasion in triple-negative breast cancer. Oncol. Rep. 2015;33:1064–1070. doi: 10.3892/or.2015.3720. [DOI] [PubMed] [Google Scholar]
- 190.Sharma M.R., Koltowski L., Ownbey R.T., Tuszynski G.P., Sharma M.C. Angiogenesis-associated protein annexin ii in breast cancer: Selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp. Mol. Pathol. 2006;81:146–156. doi: 10.1016/j.yexmp.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 191.Wang T., Yuan J., Zhang J., Tian R., Ji W., Zhou Y., Yang Y., Song W., Zhang F., Niu R. Anxa2 binds to stat3 and promotes epithelial to mesenchymal transition in breast cancer cells. Oncotarget. 2015;6:30975–30992. doi: 10.18632/oncotarget.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mussunoor S., Murray G.I. The role of annexins in tumour development and progression. J. Pathol. 2008;216:131–140. doi: 10.1002/path.2400. [DOI] [PubMed] [Google Scholar]
- 193.Hu H., Zhao J., Zhang M. Expression of annexin a2 and its correlation with drug resistance and recurrence of bladder cancer. Technol. Cancer Res. Treat. 2015;15:NP61–NP68. doi: 10.1177/1533034615617078. [DOI] [PubMed] [Google Scholar]
- 194.Jaiswal J.K., Nylandsted J. S100 and annexin proteins identify cell membrane damage as the achilles heel of metastatic cancer cells. Cell Cycle. 2015;14:502–509. doi: 10.1080/15384101.2014.995495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Puzio-Kuter A.M. The role of p53 in metabolic regulation. Genes Cancer. 2011;2:385–391. doi: 10.1177/1947601911409738. [DOI] [PMC free article] [PubMed] [Google Scholar]