Abstract
Background: The aim of this study was to identify potential variables influencing the clinical presentation of breakthrough cancer pain (BTP). Methods: Cancer patients with a diagnosis of BTP were enrolled. Demographic and clinical characteristics, as well as background pain and BTP characteristics were collected. Multivariate analyses were conducted to assess the correlation between BTP characteristics and the variables examined. Results: Data of 4016 patients were analysed. Average daily number of BTP episodes was 2.4, mean intensity was 7.5, and a mean duration was 43.3 min. A short onset BTP was observed in 68.9% of patients. In 30.5% of patients BTP was predictable. There were 86.0% of participants who reported a marked interference of BTP with their daily activities. Furthermore, 86.8% of patients were receiving opioids for the management of BTP. The average time to meaningful pain relief was 16.5 min and 70.9% of patients were satisfied with their BTP medications. Age, head and neck cancer, Karnofsky, background pain intensity, predictable and fast onset BTP were independently associated with the number of BTP episodes. BTP pain intensity was independently associated with background pain intensity, fast onset BTP, and Karnofsky. Neuropathic pain mechanism was independently associated with unpredictable BTP. Variables independently associated with a longer duration of BTP were age, place of visit, cancer diagnosis, disease-oriented therapy, background pain intensity and mechanism, and unpredictable BTP. Age, Karnofsky, background pain intensity, fast onset, and long duration of BTP were independently associated with interference with daily activity. Conclusions: BTP has a variable presentation depending on interdependent relationships among its different characteristics.
Keywords: breakthrough pain, cancer, palliative care, supportive care
1. Introduction
Pain is a frequent symptom in cancer patients [1], mainly controlled by available analgesics [2]. However, transient flares of pain may occur. This phenomenon has been defined as breakthrough cancer pain (BTP) [3], which is associated with a negative impact on quality of life [4]. Many studies have assessed the epidemiology of BTP during the last 25 years [5], reporting variable data due to different definitions, assessment tools, and methodologies [6,7,8,9]. Although BTP has been better characterized in recent years [7,10,11,12], possible factors interfering with BTP presentation have never been explored [11,13,14].
The aim of this study was to assess the main characteristics of BTP to find potential factors influencing its presentation in a large number of patients [15].
2. Methods
This was a national, multicenter study that involved 32 centers. The local ethical committees approved the protocol, and written informed consent was obtained from each patient. Patients were enrolled during a period of 24 months in different settings (oncology, pain therapy, palliative care, and radiotherapy) and in different places (inpatient units, day hospitals, outpatient clinics, or palliative care).
Inclusion criteria were: age ≥18 years; diagnosis of cancer at any stage; well-controlled and stable background pain with an intensity ≤4 on a 0–10 numerical scale; and presence of BTP episodes of moderate-severe intensity, clearly distinguished from background pain. The definition of BTP was: a transitory pain exacerbation of moderate to severe intensity that occurs spontaneously or predictably [7,8,10,11], well distinguished from background pain [9,11,12,16]. Exclusion criteria were: no cancer diagnosis; unstable and/or uncontrolled background pain (>4/10); no relevant peaks in pain intensity (<5/10); and incapability to be assessed. Patients meeting the inclusion criteria were consecutively surveyed.
Age, sex, setting and place of the visit, primary tumor, extent of the disease (loco regional or metastatic), type of ongoing anticancer treatments, presence and grade of mucositis [17]; presence of oral candidiasis, presence and duration of xerostomia, and Karnofsky status were recorded. For background pain, average pain intensity (0–10) in the last week, current analgesic therapy, site and mechanism of pain; were recorded. The presence of a prevalent neuropathic pain mechanism was based on patients’ description and clinical examination. Daily opioid doses were expressed as oral morphine equivalents (OME) [2]. For BTP, mean daily number of episodes in the last week, mean intensity of pain (0–10), predictability and triggering factors, site and mechanism of pain, time to maximum pain intensity (≤10 min or >10 min), mean duration of untreated episodes, relieving factors, interference with daily activities on a 0 (none) to 3 (very much) scale, who firstly made BTP diagnosis, type and dosage of medications currently used for BTP treatment, type, intensity, duration and therapy of drug adverse reactions, and time to meaningful pain relief after taking medication, were recorded.
3. Statistical Analysis
Descriptive statistics have been provided both for outcomes and explanatory variables. Frequency distributions as well as explorative univariate tests have been performed to detect feasible association and correlation pattern among variables. Accordingly, x2 tests were used for categorical variables, spearman correlations where due and point biserial correlations if dummies and continuous variables were involved. This preliminary part of the analysis used a 5% statistical significance level with no adjustment for multiple testing. Multivariate generalized linear models, i.e., Poisson, logistic and linear, have been built up to model the primary end points responses, Holmes adjusted for multiple testing. The univariate examination guided the models’ covariates choice. The count Poissonian and logistic count models underwent omnibus likelihood ratio tests. Overdispersion concern with Poisson models has dealt with carrying out x2 goodness of fit tests comparing the model performance with the corresponding negative binomial. The numerical computations have been carried out using the statistical software STATA (version 14).
4. Results
Data on 4016 patients were available from 4067 who were surveyed in the study period. Forty patients did not meet the inclusion criteria and were erroneously screened, or had incomplete information. Patients’ characteristics are described in Table 1. The prevalent care settings were oncology and pain therapy. Patients were mainly seen as hospital inpatients. Most patients had a metastatic disease and were receiving disease-oriented anticancer treatments. Five-hundred-seventy patients (14.2%) presented with different grades of oral mucositis: 379 patients (9.4%) grade 1; 127 patients (3.2%) grade 2; and 64 patients (1.6%) grade 3–4. Candidiasis and dry mouth were detected in 224 (5.6%) and 589 (14.7%) patients, respectively. The percentage of older patients was higher in the home care setting, with a mean age of 72.4 years (SD 12.0, p = 0.00). Women were more represented in hospice, whereas men were more represented in home care, day hospital, outpatient clinics, and hospital wards (p = 0.04). Karnofsky status was lower in the home care setting (39.7, SD 11.2), and higher in day-hospital units (73.2, SD 14.5) (p = 0.00).
Table 1.
Characteristics of patients.
| Age (Years) Mean (SD) | 64.6 (12.24), Range 18–97 Years | |
| Gender (M/F) | 2202 (54.8%)/1814 (45.2%) | |
| Karnofsky mean (SD) | 61.8 (18.73), range 10–100 | |
| Primary tumor | Lung | 1089 (27.1%) |
| Gastrointestinal | 647 (16.1%) | |
| Breast | 480 (11.9%) | |
| Pancreas | 349 (8.7%) | |
| Urological | 242 (6.0%) | |
| Prostate | 224 (5.6%) | |
| Head-neck | 205 (5.1%) | |
| Gynecologic | 185 (4.6%) | |
| Liver | 135 (3.4%) | |
| Haematological | 98 (2.4%) | |
| Others | 511 (12.7%) | |
| Disease | Loco-regional | 642 (16.0%) |
| Metastatic | 3374 (84.0%) | |
| Anticancer treatment | Disease-oriented | 3030 (78.0%) |
| Palliative Care | 854 (22.0%) | |
| Place of Visit | Outpatients | 1378 (34.3%) |
| Day hospital | 462 (11.5%) | |
| Home care | 577 (14.4%) | |
| Hospice | 101 (2.5%) | |
| Hospital inpatient | 1498 (37.3%) | |
| Setting | Palliative care | 720 (17.9%) |
| Oncology | 2087 (52.0%) | |
| Pain therapy | 1184 (29.5%) | |
| Radiotherapy | 25 (0.6%) | |
| Mean background pain intensity at T0 | 3.0 (SD 1.83) | |
| Mean opioid doses (expressed as oral morphine equivalents) | 69.4 mg/day (SD 88.7) mg/day |
4.1. Background Pain and Analgesic Regimen
The average background pain intensity in the previous week was 2.98 (SD 1.7) and pain intensity on the day of assessment was 3.0 (SD 1.83). The prevalent mechanism of background pain was mixed (n = 2512, 62.5%), nociceptive (n = 1174, 29.2%), and neuropathic (n = 330, 8.2%). Background pain sites were: vertebral (n = 1557, 38.8%), abdomen (n = 1239, 30.8%), extremities (n = 816, 20.3%); pelvis (n = 455, 11.3%), head and neck (n = 301, 7.5%). The mean dosage of opioids used for background pain was 69.4 mg/day of OME (SD 88.7).
Drugs given for background pain were: anti-inflammatory drugs (n = 365, 9.1%), paracetamol (n = 1077, 26.8%); weak opioids (n = 389, 9.7%: codeine n = 129, tramadol n = 260); oral morphine (n = 329, 8.2%), oral hydromorphone (n = 128, 3.2%), oxycodone (n = 664, 16.5%); oxycodone/naloxone (n = 1152, 28.7%), tapentadol (n = 195, 4.9%), parenteral morphine (n = 192, 4.8%), methadone (n = 43, 1.1%) transdermal fentanyl (n = 1102, 27.5%), transdermal buprenorphine (n = 121, 3.0%); other drugs (n = 72, 1.8%). 2749 (68.45%) patients were receiving adjuvant drugs, including benzodiazepines (n = 427, 15.5%), antiepileptics (n = 1230, 44.7%), antidepressants (n = 377, 13.7%), antiemetics (n = 362, 13.2%), laxatives (n = 666, 24.2%) corticosteroids (n = 1503, 54.7%). One-hundred-thirty-nine (3.96%) patients reported some adverse effects from the background analgesic regimen. They included constipation (n = 93, 66.9%), confusion (n = 34, 24.5%), nausea (n = 29, 20.9%), pruritus (n = 15, 10.8%), gastralgia (n = 12, 8.6%), vomiting (n = 10, 7.2%), and headache (n = 8, 5.8%).
4.2. Characteristics of BTP
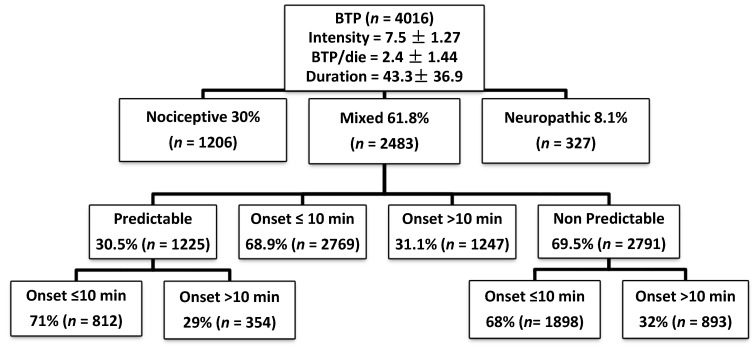
The characteristics of BTP summarized in Figure 1. The mean number of BTP episodes/day was 2.4 (SD 1.4, range 1–10); 64.4% of patients had 1–2 episodes/day, 29.4% had 3–4 episodes/day, and 6.2% had ≥5 episodes of BTP/day. The mean intensity of BTP was 7.5 (SD 1.3). The majority of patients (n = 2971, 73.9%) had an intensity of ≥7. BTP was unpredictable in 69.5% of patients. BTP sites were: vertebral (n = 1476, 36.7%), abdomen (n = 1183, 29.5%) extremities (n = 784, 19.5%), chest wall (n = 751, 18.7%), and pelvis (n = 401, 10%). BTP mechanism was mixed (n = 2483, 61.8%), nociceptive (n = 1206, 30%), and neuropathic (n = 327, 8.1%) type.
Figure 1.
Characteristics of breakthrough cancer pain (BTP).
The main triggering factors for predictable BTP were activity-movement (67.4%) and swallowing (16.6%). BTP was predominantly of mixed (61.8%) or nociceptive (30%) type. Time to maximum pain intensity was ≤10 min (fast-onset BTP) in 68.9% of patients and >10 min (slow-onset BTP) in 31.1% of patients. The mean duration of untreated BTP episodes was 43.3 min (SD 36.9). 85% of patients reported that BTP limited systematically daily life. BTP interfered with daily activity: much (n = 2276, 56%): very much (n = 1127, 28.1%), a little (n = 542, 13.5%), nothing (n = 71, 1.77%), Medications for BTP were: OTCF (oral transmucosal fentanyl citrate, n = 130, 3.2%), FBT (fentanyl buccal tablet, n = 435, 10.8%), FBST (sublingual fentanyl, n = 570, 14.2%), FPNS (fentanyl pectin nasal spray, n = 807, 20.1%), INFS (intranasal fentanyl spray, n = 40, 1%), oral morphine (n = 563, 14.0%), intravenous and subcutaneous morphine (n = 129, 3.21%). Five-hundred-twenty-nine (13.2%) patients were not receiving BTP medications. The mean doses of FPNS, OM, FBST, FBT, OTFC, SC-M, IV-M, and INFS, were 167.7 μg (SD 125.7), 11.8 mg (SD 8.2), 231.4 μg (SD 171.1), 234.6 μg (SD 183.1), 395.4 μg (SD 280.5), 10.1 mg (SD 4.6), 8.2 mg (SD 6.1), and 100 μg (SD 50.7), respectively.
4.3. Adverse Effects of BTP Medications
Adverse reactions attributed to BTP medications were reported in 53 out of 2139 (2.5%) patients with available data, and were: constipation (n = 21), confusion (n = 20), nausea (n = 7), headache (n = 2), vomiting (n = 4) and other unspecified adverse effects (n = 12). The intensity was mild, moderate, and severe in 44 patients (83.0%), five patients (9.4%), and two patients (3.8%), respectively. In 33 of these patients (62.3%) no specific therapeutic changes were required, while in 11 cases (20.7%) it was deemed necessary to treat the adverse effects or discontinue the BTP medication. (OM and FBT p = 0.01). No association was found between adverse reactions and dosage of opioids used for BTP (p = 0.78).
5. Factors Influencing BTP Clinical Presentation
5.1. Number of BTP Episodes/Day
Males, old patients, and patients with higher Karnofsky levels, had a higher number of episodes/day (p = 0.00, p = 0.04, and p = 0.00, respectively). Distribution of the number of episodes of BTP/day among the different setting was irregular: patients admitted in hospice or home care had more episodes in comparison with inpatients or outpatients (p = 0.00), Patients with nociceptive pain and predictable BTP had a higher number of BTP episodes/day (p = 0.00). Patients with colon-rectal cancer and esophageal cancer, had a lower number of BTP episodes (p = 0.01 and p = 0.00 respectively), while patients with head and neck cancer and pancreatic cancer had a higher number of BTP episodes/day (p = 0.00 and p = 0.01, respectively). Patients with fast onset BTP had a higher number of BTP episodes/day (p = 0.00). There was a weak correlation with background pain intensity (Pearson correlation coefficient: 0.3, p = 0.00).
Data of multivariate analysis is presented in Table 2. Age, head and neck cancer, higher Karnofsky levels, background pain intensity, predictable BTP, and fast onset were independently associated with a higher number of BTP episodes.
Table 2.
Multivariate analysis for the number of BTP episodes/day.
| Factors | β | p | (95% CI) | |
|---|---|---|---|---|
| Age | 0.05 | 0.03 | 0.00 | 0.87 |
| Gender | −0.00 | 0.90 | −0.01 | 0.00 |
| Karnofsky | 0.01 | 0.05 | −0.01 | 0.00 |
| Setting | −0.00 | 1.00 | −0.02 | 0.02 |
| Colon-rectal cancer | −0.03 | 0.38 | −0.09 | 0.04 |
| Head and neck | 0.13 | 0.00 | 0.05 | 0.23 |
| Pancreatic cancer | 0.02 | 0.54 | −0.05 | 0.09 |
| Esophageal cancer | 0.17 | 0.07 | −0.01 | 0.34 |
| Background pain intensity | 0.15 | 0.00 | 0.12 | 0.16 |
| Type of BTP (predictable) | −0.21 | 0.00 | −0.24 | −0.16 |
| Onset | −0.16 | 0.00 | −0.20 | −0.11 |
5.2. Intensity of BTP
At the univariate analysis, variables associated with a higher BTP intensity were younger age (p = 0.00), in-hospital place of visit, outpatient, and day-hospital visit (p = 0.00), neuropathic and mixed pain (p = 0.00), lung cancer and urological cancer (p = 0.00), grade of oral mucositis (p = 0.02), and background pain intensity (p = 0.01), BTP intensity was lower in colon-rectal cancer (p = 0.05), liver (p = 0.04, breast cancer (p = 0.02) and fast onset BTP (p = 0.00). Table 3 shows the results of multivariate analysis for BTP intensity.
Table 3.
Multivariate analysis for BTP intensity.
| Factors | β | p | (95% CI) | |
|---|---|---|---|---|
| Age | −0.01 | 0.09 | −0.02 | 0.00 |
| Place of visit | 0.00 | 0.84 | −0.01 | 0.01 |
| Karnofsky | −0.01 | 0.00 | −0.02 | 0.00 |
| Grade of mucositis | 0.02 | 0.30 | −0.01 | 0.03 |
| Colon-rectal cancer | −0.01 | 0.70 | −0.04 | 0.03 |
| Liver | −0.03 | 0.36 | −0.10 | 0.04 |
| Breast | −0.01 | 0.50 | −0.05 | 0.03 |
| Lung | 0.00 | 0.51 | −0.02 | 0.04 |
| Urological | 0.03 | 0.36 | −0.03 | 0.07 |
| Disease-oriented anticancer treatment | −0.01 | 0.57 | −0.04 | 0.02 |
| Background pain intensity | 0.04 | 0.00 | 0.03 | 0.51 |
| Type of BTP (predictable) | 0.01 | 0.10 | −0.01 | 0.03 |
| Time to maximum BTP intensity | −0.04 | 0.00 | −0.06 | −0.01 |
In the multivariate analysis, BTP pain intensity was independently associated with higher background pain intensity, fast onset BTP, and lower level of Karnofsky.
5.3. Predictability of BTP
At the univariate analysis, variables associated with predictable BTP were older age (p = 0.00), outpatient and in-hospital places of visit (both p = 0.00), pain mechanism (p = 0.00, mixed and nociceptive) head and neck cancer (p = 0.00), breast cancer (p = 0.05), grade or oral mucositis (p = 0.00), low intensity of BTP and fast onset of BTP (p = 0.00 and 0.05, respectively), and loco-regional disease (p = 0.03). Pancreatic cancer and gastric cancer were associated with unpredictable BTP (p = 0.02 and 0.03, respectively). Table 4 shows the results of multivariate analysis for BTP predictability. Pain mechanism (neuropathic pain) was independently associated with unpredictable BTP (p = 0.00).
Table 4.
Multivariate analysis for predictable BTP.
| Factors | β | p | (95% CI) | |
|---|---|---|---|---|
| Age | −0.01 | 0.37 | −0.02 | 0.00 |
| Place of visit | 0.00 | 0.63 | −0.03 | 0.02 |
| Karnofsky | 0.00 | 0.82 | −0.01 | 0.00 |
| Head and neck cancer | −0.05 | 0.43 | −0.17 | 0.07 |
| Breast | −0.04 | 0.39 | −0.11 | 0.04 |
| Pancreas | 0.04 | 0.38 | −0.05 | 0.12 |
| Gastric | 0.06 | 0.39 | −0.07 | 0.18 |
| Metastatic disease | 0.03 | 0.49 | −0.04 | 0.09 |
| Grade of mucositis | −0.02 | 0.25 | −0.07 | 0.02 |
| Background pain intensity | 0.01 | 0.34 | −0.01 | 0.03 |
| Mechanism of Background pain | 0.05 | 0.00 | 0.01 | 0.07 |
| Time to maximum BTP intensity | 0.02 | 0.36 | −0.03 | 0.07 |
5.4. Time to Maximum BTP Intensity (BTP Onset)
At the univariate analysis, variables associated with fast-onset BTP were day-hospital and in-hospital places of visit (p = 0.00), pain mechanism (mixed and nociceptive) (p = 0.00), predictable BTP (p = 0.05), colon-rectal cancer (p = 0.05), disease-oriented anticancer treatment (p = 0.00), radiotherapy, targeted therapy and chemotherapy, targeted therapy (p = 0.00), grade of mucositis (G2–G4) (p = 0.00). The slow onset was associated with gynecological cancer and breast cancer (p = 0.02 and p = 0.01, respectively). Variables independently associated with slow onset were lower Karnofsky Performance Scale (KPS), gynecological and breast cancer, no anticancer treatment, nociceptive background pain, and unpredictable BTP (Table 5).
Table 5.
Multivariate analysis for BTP slow onset.
| Factors | OR | p | (95% CI) | |
|---|---|---|---|---|
| Place of visit | 0.02 | 0.47 | −0.03 | 0.07 |
| Karnofsky | −0.01 | 0.00 | −0.01 | 0.00 |
| Colon-rectal cancer | −0.16 | 0.15 | −0.38 | 0.06 |
| Gynecological | 0.38 | 0.01 | 0.07 | 0.69 |
| Breast | 0.32 | 0.00 | 0.12 | 0.53 |
| Anticancer treatment | −0.31 | 0.00 | −0.47 | −0.14 |
| Grade of mucositis | 0.08 | 0.18 | −0.04 | 0.20 |
| Mechanism of Background pain | −0.11 | 0.00 | −0.19 | −0.03 |
| Type of BTP | 0.23 | 0.00 | 0.08 | 0.39 |
5.5. Duration of Untreated BTP Episodes
A long duration of BTP was associated with old age (p = 0.00), hospice or home setting (p = 0.00), nociceptive background pain (p = 0.00), predictable BTP (p = 0.00), pancreatic cancer (p = 0.00), prostate cancer (p = 0.00), and disease-oriented anticancer treatment (p = 0.00). BTP duration was inversely related to the background pain intensity, the higher background pain intensity, the shorter BTP duration (p = 0.00). Head and neck and gastric cancer were associated with a short duration of BTP (p = 0.003 and 0.003, respectively). Table 6 shows the results of multivariate analysis for BTP duration. Variables independently associated with a longer duration of BTP were: age, place of visit (home > hospice > day-hospital > outpatients, inpatients) (p = 0.00), and some type of cancers, disease-oriented therapy, background pain intensity and mechanism, and unpredictable BTP.
Table 6.
Multivariate analysis for duration of untreated BTP episodes.
| Factors | β | p | (95% CI) | |
|---|---|---|---|---|
| Age | 0.01 | 0.00 | 0.00 | 0.02 |
| Place of visit | 0.09 | 0.00 | 0.08 | 0.10 |
| Karnofsky | 0.00 | 0.51 | −0.01 | 0.00 |
| Head & neck cancer | −0.41 | 0.00 | −0.46 | −0.36 |
| Pancreas | 0.38 | 0.00 | 0.36 | 0.41 |
| Gastric | −0.39 | 0.00 | −0.45 | −0.33 |
| Prostate | 0.25 | 0.00 | 0.22 | 0.29 |
| Disease-oriented anticancer treatment | 0.38 | 0.00 | 0.35 | 0.40 |
| Background pain intensity | −0.11 | 0.00 | −0.12 | −0.10 |
| Background pain mechanism (nociceptive) | −0.08 | 0.00 | −0.09 | −0.07 |
| Unpredictable BTP | 0.55 | 0.00 | 0.53 | 0.56 |
5.6. Interference of BTP with Daily Activities
Variables associated with interference of BTP with daily activities were: younger age (less interference, p = 0.00), Karnofsky (less interference, p = 0.00), gender (male, more interference, p = 0.04), places of visit (day-hospital and in-hospital had higher level of interference in comparison with hospice, (p = 0.00), pain mechanism (mixed and nociceptive, higher level of interference, p = 0.00), unpredictable BTP (higher level of interference, p = 0.00). Brain, breast cancer, and sarcoma were associated with little or no interference (p = 0.02, p = 0.00 and p = 0.02, respectively), Lung cancer was associated with higher level of interference (p = 0.01). Much or very much interference with life activities were associated with fast onset BTP (p = 0.00), metastatic disease (p = 0.04), disease-oriented anticancer treatment (p = 0.01), and oral mucositis (p = 0.00).
The level of interference was directly proportional to background pain intensity (p = 0.00), and duration of BTP (p = 0.00). Table 7 shows the results of multivariate analysis of factors interfering with daily activities. Age, Karnofsky, background pain intensity, fast onset and long duration of BTP were independently associated with interference with daily activity.
Table 7.
Multivariate analysis of factors interfering with daily activities.
| Factors | β | p | (95% CI) | |
|---|---|---|---|---|
| Age | −0.01 | 0.03 | −0.02 | 0.00 |
| Karnofsky | −0.01 | 0.00 | −0.02 | −0.01 |
| Gender | 0.03 | 0.20 | −0.02 | 0.08 |
| Place of visits | −0.02 | 0.21 | −0.03 | 0.01 |
| Brain | −0.33 | 0.17 | −0.79 | 0.14 |
| Breast | −0.04 | 0.37 | −0.11 | 0.04 |
| Lung | 0.01 | 0.96 | −0.05 | 0.05 |
| Sarcoma | −0.01 | 0.92 | −0.18 | 0.16 |
| Disease-oriented anticancer treatment | 0.02 | 0.48 | −0.03 | 0.04 |
| Disease | 0.01 | 0.81 | −0.05 | 0.06 |
| Grade of mucositis | 0.02 | 0.54 | −0.03 | 0.05 |
| Background pain intensity | 0.05 | 0.00 | 0.02 | 0.07 |
| Mechanism of Background pain | 0.01 | 0.28 | −0.01 | 0.04 |
| Type of BTP | 0.01 | 0.89 | −0.04 | 0.05 |
| Time to maximum BTP intensity | −0.05 | 0.03 | −0.10 | 0.00 |
| Duration of untreated BTP | 0.01 | 0.00 | 0.00 | 0.02 |
6. Discussion
To our knowledge, this is the largest survey currently available of BTP in cancer patients. The only existing study of BTP in such a large sample was performed in a US population of commercially insured community-dwelling patients, predominantly non-cancer, with opioid-treated chronic pain [18]. Information gathered from this study provided a new step in the knowledge of the phenomenon of BTP in cancer patients, underlining the complex interactions among a series of factors influencing the clinical presentation.
This study indicates that the BTP may have different characteristics and that treatment should be set on the individual patient’s condition. A diagnostic algorithm [10,11] based on a clear distinction between background pain and BTP, pointed out that the difference between background pain intensity and BTP intensity was more than 3 points on a 0 to 10 numerical scale, thus ensuring a clear distinction between background pain and BTP for most patients. This information also helps preventing possible misinterpretations of data, as occurred in previous studies where background pain intensity and BTP intensity were not clearly distinguished [5]. Of interest, adverse reactions attributed to BTP medications were reported in a small number of patients and were mostly mild in intensity, and not related to BTP medication doses.
6.1. Factors Influencing BTP Presentation
6.1.1. Number of BTP Episodes/Day
A mean number of four BTP episodes/day is commonly considered acceptable [3]. In this study, only a minority of patients presented ≥5 episodes per day. Some independent variables were identified to be associated with more episodes/day of BTP. Patients with higher levels of activity are more likely to develop episodes of BTP, particularly with an incident component (predictable pain) with a rapid onset. Interestingly, patients who had ≥5 episodes of BTP/day were more likely to have predictable and fast-onset BTP, possibly induced by movement. These relationships are commonly observed in a clinical setting. On the other hand, higher background pain intensity, despite being within the range of so-called controlled pain (mild pain, 0–4 on a numerical scale) may favor the development of BTP episodes, confirming previous observations. It has been reported that severe background pain intensity was shown to be a powerful predictor of BTP scores [19,20]. In many epidemiological studies of BTP, background analgesia was not optimal and was based on non-opioid or weak opioids medications [20,21,22,23,24]. This finding confirms that a better background analgesia may decrease the number of BTP episodes [9], and allow a better patient mobilization [5]. This suggests the need to optimize background analgesia to limit the number of BTP episodes [25], particularly in patients with bone metastases, and their intensity (see paragraph below).
6.1.2. Intensity of BTP
Younger patients with higher background pain, a fast onset and predictable BTP, and a lower Karnofsky level develop higher BTP intensities. A rapid development of BTP is likely to produce a high intensity of pain. Pain expression is more important in younger patients, possibly because of their psychological distress [26], as well in patients with head and neck cancer. Similarly, patients with worse clinical conditions may have a greater disease burden.
In this study, BTP intensity was well distinguished from background pain intensity (about three points difference on average), suggesting that patients with BTP were well selected, according to a predefined algorithm. In previous European studies, the percentages of patients with severe BTP were 57–61% only, and often a distinction between BTP and background pain intensities was unclear [10,16,27].
6.1.3. Predictable BTP
Predictable BTP has obvious implications in terms of prevention and therapeutic interventions. Physical activity and swallowing were the most frequent factors triggering BTP episodes. The percentage of patients with predictable BTP was consistent with previous epidemiological studies [11,16]. In a previous analysis, with a limited number of patients, predictable BTP was more likely observed in certain disease-related conditions (primary tumor, setting, anticancer treatments) and in patients with lower background pain and BTP intensities [15]. Patients with bone metastases, who are more often observed in radiotherapy setting, may have lower pain levels at rest. The lower BTP intensity in patients with predictable BTP might be explained, for example, by stopping a movement that triggers BTP in case of bone metastases [5]. In fact, patients with predictable BTP were more likely to report a non-pharmacological relieving factor [10,16]. The BTP predictability in the presence of oral damage could be easier to explain, as in the presence of mucositis, swallowing typically evokes BTP. However, in this study, the only relevant finding was that patients with a neuropathic pain component were more likely to have unpredictable BTP. Consequently, patients with a prevalent nociceptive component, for example bone metastases, tend to develop predictable episodes.
6.1.4. Time to Maximum BTP Intensity (Onset)
Time to maximum BTP intensity has obvious clinical implications for a timely therapeutic intervention and possible psychological input in asking for a medication [27]. A dichotomous measure was chosen for distinguishing fast-onset from slow-onset BTP (≤10 or >10 min, respectively). In multicenter European surveys [10,11,16], time to maximum BTP intensity onset was 10–15 min, but no further analyses were conducted to identify risk factors. In a preliminary analysis of a limited number of patients, a fast-onset BTP was associated with serious mucositis or radiotherapy setting [15]. This can be explained by the immediate pain input by a damaged mucosa, for example on swallowing. Moreover, a fast-onset BTP is typical in patients with pain on movement due to bone metastases [10], who are often referred to radiotherapy. Indeed, BTP developed more slowly when BTP intensity was lower or in patients with a lower Karnofsky status. Thus, the risk of fast or slow-onset BTP could be characterized according to these conditions. In this study, representing the pattern of a large number of patients, a lower Karnofsky level, gynecological and breast cancer, no anticancer treatment, nociceptive background pain, and unpredictable BTP were independently associated with a slow onset of BTP. One could argue that more advanced patients, no longer receiving anticancer treatment, develop BTP slowly and in an unpredictable way, possibly because their limited activity, that is they are more likely to have an idiopathic BTP, not triggered by known causes. Other features regarding certain types of cancer deserve further analysis and should be better explored.
6.1.5. Duration of Untreated BTP Episodes
The mean duration of untreated BTP episodes was similar to that described in previous surveys, reporting a variable duration of 30–60 min [10,11,15,16,19,20,21,22]. Indeed, duration of untreated episodes is based on patients’ recalling and difficult to be properly assessed by patients. Thus, interpretation of this data should be cautious.
Some factors were independently associated with the duration of BTP. A longer duration of BTP episodes was found in hospice and home care patients, as well as in older patients, in previous studies, patients who had a low background pain intensity, or who were admitted to hospice or were seen at home, had a longer BTP duration [11,15]. Of interest, predictable BTP was less likely to be associated with a longer BTP duration. As reported before, one could argue that patients with predictable pain, due to an incident component for example, could stop their precipitating activity and pain can spontaneously disappear [5], differently from patients with idiopathic BTP, not triggered by any known precipitating factor [16]. The finding that nociceptive was independently associated with a longer BTP duration should deserve further investigation, as in this category somatic and visceral pain could be differently represented. Finally, some cancers and disease-oriented treatments were more likely to be associated with a different BTP duration. These aspects deserve further analyses.
6.1.6. Interference of BTP with Daily Activities
The presence of BTP limited patients’ daily life, particularly in younger patients and in patients with a lower Karnofsky status and higher background pain intensity. These findings are clinically understandable, although never reported in literature. Younger patients may have more psychological distress [26], and patients with worse clinical conditions may have a greater disease burden. Of course, higher background pain, even within the level of so-called controlled pain (≤4/10) is commonly associated with larger interference with daily life activities. More importantly, a fast-onset BTP and a longer duration were also associated with notable interference with daily activities. This means that the faster and the longer BTP event, the worse interference exists.
7. Conclusions
The data gathered from the large sample of patients included in this study revealed that BTP is a multifaceted phenomenon. This study suggests that physicians should investigate the presence of BTP in every patient with cancer pain. In addition, BTP therapy should be calibrated on the clinical condition and characteristics of BTP. The identification of risk factors for the development of specific subtypes of BTP may allow better personalizing treatments according to individual clinical features. Further studies have been planned to assess some variables associated with certain care settings, disease types, and anticancer therapies.
Acknowledgments
The paper is dedicated to Alessandro Sabato and Antonio Gatti who died during the project. The following are members of the IOPS-MS study group:
Massimiliano ALÙ 11, Silvia ANGELINI 2, Andrea ANTONUZZO 32, Carmela ARCURI 11, Grazia ARTIOLI 9, Paola BALLARINO 14, Giovanna BALLERINI 5, Daniele BARILLARI 20, Elisa BELLINI 8, Roberto BERARDO 8, Giovanni BERTOLDERO 9, Elena BOLZACCHINI 10, Silvia BOLZONELLO 28, Paolo BONOME 2, Marta BONOTTO 28, Lucia BORGATO 9, Cristina BORGHESI 29, Giorgia BOSCOLO 9, Samuela BOZZONI 16, Paola BRACCHI 4, Emanuela BRUNO 25, Alberto BUCCERI 13, Paola BUDEL 14, Pietro BUONAVOLONTÀ 31, Clarissa CALDARULO 7, Tiziana CAMPA 4, Chiara CAPARELLO 32, Anna CARAMELLINO 14, Maura CARBONI 20, Roberta CARELLA 26, Alessia CARNEVALE 2, Amanda CARUSELLI 1, Alfredo CELANO 8, Antonio COCCHIRELLA 36, Angela COCQUIO 16, Viviana CONTU 8, Rosalba CORTINOVIS 18, Andrea COSTANZI 2, Giovanni CUCCU 36, Maria Concetta CURSANO 35, Chiara DE DIVITIIS 17, Edoardo DE RUVO 30, Paola DEMARTINI 8, Maria Teresa DI DATO 31, Sara DIACCIATI 23, Marco DIVIZA 7, Lucia DODARO 5, Emanuela DONELLI 15, Silvia ELLERO 28, Gennaro ESPOSITO 3, Azzurra FARNESI 32, Gianpiero FASOLA 28, Maurizio FERRARA 31, Patrizia FERRERA 1, Simona FINOCCHI 7, Iacopo FIORONI 35, Gianluca FORA 8, Cira Antonietta FORTE 3, Michele FORTIS 18, Gloria GALLO 36, Davide GERBONI 25, Lucia GERMANI 30, Silvia GHEDINI 24, Silvia GHIDONI 18, Valentina GIANFELICE 7, Laura GINOCCHI 32, Raffaele GIUSTI 2, Silvia GRECCHI 4, Gianni GRILLI 22, Daniela IACONO 2,Massimiliano ICARDI 8, Luca IMPERATORI 22, Alma KASA 14, Gianluca LAICI 22, Agata LAUDANI 11, Vito LORUSSO 19, Maurizio LUCCHESI 32, Miriam MANFREDA 24, Roberta MARCHESI 18, Federica MARCHETTI 30, Federica MARSIGLI 24, Lucia MASU 30, Federica MAZZUCA 2, Francesca MELONI 20, Gabriella Maria MONASTEROLO 36, Adelaide MONTALTO 2, Gianna MUSETTINI 32, Anna NAPPI 17, Luca NICOSIA 2, Elena ONGARO 28, Oliviero OSTELLINO 8, Elisa PALOMBO 7, Milena PASQUALE 30, Fulvia PEDANI 8, Alessio PEPE 11, Paolo PETRENI 23, Alessandra PIGNI 4, Graziella PINOTTI 10, Andrea PIRONTI 31, Giovanni Maria PISANU 20, Manuela PISCIOTTA 34, Cinzia POTESTÀ 35, Giovanna PRESTIA 1, Massimo RAIMONDI 13, Raffaele RATTA 35, Carmela ROMANO 17, Francesco ROSETTI 9, Gennaro RUSSO 3, Benhaz SABER 36, Emanuele SALVINI 25, Annunziata SAMMARO 4, Boaz SAMOLSKY DEKEL 24, Daniele SANTINI 35, Elisabetta SARACCO 31, Donata SARTORI 9, Katya SARTORI 8, Serena SAULLE 26, Sarah SCAGLIARINI 12, Claudia SCARINGI 2, Raffaella SCROCCARO 9, Massimiliano Savio Maria SGARLATA 13, Claudia SILVESTRI 7, Lucrezia SILVESTRO 17, Stefania SILVESTRO 15, Dario TAMMARO 31, Rossella TONETTI 15, Alessandro TUZI 10, Antonella USSET 11, Salvatore Maria Giovanni VALENTI 13, Ilaria VALLINI 10, Barbara VERONESE 10, Mari ZAMPIERI 34, Maria Rita ZAPPOLI 36, Ernesto ZECCA 4, and Carlo CHIARAMONTE 38.
-
38
Department of Statistics, University Hospital of Tor Vergata, 00133 Rome, Italy
Authors Contributions
Conceptualization, S.M. (Sebastiano Mercadante), P.M., A.C. (Arturo Cuomo), A.C. (Augusto Caraceni), R.D.M., R.V., M.M., S.N., M.L., M.D., C.A., M.A., G.A., M.B., L.B., B.C., B.V.P.C., D.D., F.F., V.G., V.I., S.L., L.P., S.M. (Sergio Mameli), F.M., R.M., T.M., R.M.M.,V.M., D.M., S.M. (Stefano Moroso), G.P., S.D.S., R.O., A.P., S.R., E.S., M.S., G.T., A.V., and F.A.; Data curation, S.M. (Sebastiano Mercadante), P.M., A.C. (Arturo Cuomo), A.C. (Augusto Caraceni), R.D.M., R.V., M.M., S.N., M.L., M.D., C.A., M.A., G.A., M.B., L.B., B.C., B.V.P.C., D.D., F.F., V.G., V.I., S.L., L.P., S.M. (Sergio Mameli), F.M., R.M., T.M., R.M.M.,V.M., D.M., S.M. (Stefano Moroso), G.P., S.D.S., R.O., A.P., S.R., E.S., M.S., G.T., A.V., and F.A.; Formal analysis, F.M. and F.A.; Investigation, S.M. (Sebastiano Mercadante), P.M., A.C. (Arturo Cuomo), A.C. (Augusto Caraceni), R.D.M., R.V., M.M., S.N., M.L., M.D., C.A., M.A., G.A., M.B., L.B., B.C., B.V.P.C., D.D., F.F., V.G., V.I., S.L., L.P., S.M. (Sergio Mameli), F.M., R.M., T.M., R.M.M.,V.M., D.M., S.M. (Stefano Moroso), G.P., S.D.S., R.O., A.P., S.R., E.S., M.S., G.T., A.V., and F.A.; Methodology, S.M., P.M., A.C.(Arturo Cuomo), A.C. (Augusto Caraceni), R.D.M., M.M., S.N. and F.M.; Supervision, .S.M. (Sebastiano Mercadante) Writing–original draft, S.M. (Sebastiano Mercadante), P.M., A.C. (Arturo Cuomo), A.C. (Augusto Caraceni), R.D.M., M.M., S.N. and F.M.; Writing–review and editing, all together.
Funding
The study was sponsored by Molteni, Italy. Data were independently analysed by the IOPS MS Scientific Committee.
Data Availability
The datasets generated during/and or analyzed during the current study are available from the corresponding author on request.
Conflicts of Interest
The authors declare on conflicts of interest.
References
- 1.Portenoy R.K. Treatment of cancer pain. Lancet. 2011;377:2236–2247. doi: 10.1016/S0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- 2.Mercadante S. The use of opioids for treatment of cancer pain. Expert Opin. Pharmacother. 2015;16:389–394. doi: 10.1517/14656566.2015.989213. [DOI] [PubMed] [Google Scholar]
- 3.Portenoy R.K., Hagen N.A. Breakthrough pain: Definition, prevalence and characteristics. Pain. 1990;41:273–281. doi: 10.1016/0304-3959(90)90004-W. [DOI] [PubMed] [Google Scholar]
- 4.Portenoy R.K., Payne D., Jacobson P. Breakthrough pain: Characteristics and impact in patients with cancer pain. Pain. 1999;81:129–134. doi: 10.1016/S0304-3959(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 5.Mercadante S., Portenoy R.K. Breakthrough cancer pain: Twenty-five years of study. Pain. 2016;157:2657–2663. doi: 10.1097/j.pain.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 6.Haugen D.F., Hjermstad M.J., Hagen N., Caraceni A., Kaasa S., European Palliative Care Research Collaborative (EPCRC) Assessment and classification of cancer breakthrough pain: A systematic literature review. Pain. 2010;149:476–482. doi: 10.1016/j.pain.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Løhre E.T., Klepstad P., Bennett M.I., Brunelli C., Caraceni A., Fainsinger R.L., Knudsen A.K., Mercadante S., Sjøgren P., Kaasa S., European Association for Palliative Care Research Network From “Breakthrough” to “Episodic” Cancer Pain? A European Association for Palliative Care Research Network Expert Delphi Survey toward a Common Terminology and Classification of Transient Cancer Pain Exacerbations. J. Pain Symptom Manag. 2016;51:1013–1019. doi: 10.1016/j.jpainsymman.2015.12.329. [DOI] [PubMed] [Google Scholar]
- 8.Mercadante S., Marchetti P., Cuomo A., Mammucari M., Caraceni A., IOPS MS study Group Breakthrough pain and its treatment: Critical review and recommendations of IOPS (Italian Oncologic Pain Survey) expert group. Support Care Cancer. 2016;24:961–968. doi: 10.1007/s00520-015-2951-y. [DOI] [PubMed] [Google Scholar]
- 9.Mercadante S., Valle A., Porzio G., Aielli F., Adile C., Ficorella C., Raineri M., Giarratano A., Casuccio A. Relationship between background cancer pain, breakthrough pain, and analgesic treatment: A preliminary study for a better interpretation of epidemiological and clinical studies. Curr. Med. Res. Opin. 2013;29:667–671. doi: 10.1185/03007995.2013.792247. [DOI] [PubMed] [Google Scholar]
- 10.Davies A., Zeppetella G., Andersen S., Damkier A., Vejlgaard T., Nauck F., Radbruch L., Sjolund K.F., Stenberg M., Buchanan A. Multi-centre European study of breakthrough cancer pain: Characteristics and patient perceptions of current and potential management strategies. Eur. J. Pain. 2011;15:756–763. doi: 10.1016/j.ejpain.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Mercadante S., Lazzari M., Reale C., Cuomo A., Fusco F., Marchetti P., Mediati R.D., Chiurazzi B., Ciuffedra L., Caraceni A., et al. Italian Oncological Pain Survey (IOPS): A multicentre Italian study of breakthrough pain performed in different settings. Clin. J. Pain. 2015;31:214–221. doi: 10.1097/AJP.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 12.Mercadante S., Adile C., Torta R., Varetto A., Fulfaro F., Giarratano A., Casuccio A. Meaningful cut-off pain intensity for breakthrough pain changes in advanced cancer patients. Curr. Med. Res. Opin. 2013;29:93–97. doi: 10.1185/03007995.2012.755120. [DOI] [PubMed] [Google Scholar]
- 13.Mercadante S., Costanzo B.V., Fusco F., Buttà V., Vitrano V., Casuccio A. Breakthrough pain in advanced cancer patients followed at home: A longitudinal study. J. Pain Symptom Manag. 2009;38:554–560. doi: 10.1016/j.jpainsymman.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Mercadante S., Zagonel V., Breda E., Arcara C., Gebbia V., Porzio G., Aielli F., David F., Gammucci T., Narducci F., et al. Breakthrough pain in oncology: A longitudinal study. J. Pain Symptom Manag. 2010;40:183–190. doi: 10.1016/j.jpainsymman.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Mercadante S., Marchetti P., Cuomo A., Caraceni A., Mediati R.D., Mammucari M., Natoli S., Lazzari M., Dauri M., Airoldi M., et al. Breakthrough cancer pain: Preliminary data of the Italian Oncologic Pain Multisetting Multicentric Survey (IOPS-MS) Adv. Ther. 2017;34:120–135. doi: 10.1007/s12325-016-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies A., Buchanan A., Zeppetella G., Porta-Sales J., Likar R., Weismayr W., Slama O., Korhonen T., Filbet M., Poulain P., et al. Breakthrough cancer pain: An observational study of 1000 European oncology patients. J. Pain Symptom Manag. 2013;46:619–628. doi: 10.1016/j.jpainsymman.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . WHO Handbook for Reporting Results of Cancer Treatment. WHO; Geneva, Switzerland: 1979. pp. 15–22. [Google Scholar]
- 18.Narayana A., Katz N., Shillington A.C., Stephenson J.J., Harshaw Q., Frye C.B., Portenoy R.K. National Breakthrough Pain Study: Prevalence, characteristics, and associations with health outcomes. Pain. 2015;156:252–259. doi: 10.1097/01.j.pain.0000460305.41078.7d. [DOI] [PubMed] [Google Scholar]
- 19.Caraceni A., Martini C., Zecca E., Portenoy R.K., Ashby M.A., Hawson G., Jackson K.A., Lickiss N., Muirden N., Pisasale M., et al. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat. Med. 2004;18:177–183. doi: 10.1191/0269216304pm890oa. [DOI] [PubMed] [Google Scholar]
- 20.Hjermstad M.J., Kaasa S., Caraceni A., Loge J.H., Pedersen T., Haugen D.F., Aass N., European Palliative Care Research Collaborative (EPCRC) Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Support Palliat. Care. 2016;6:344–352. doi: 10.1136/bmjspcare-2015-000887. [DOI] [PubMed] [Google Scholar]
- 21.Zeppetella G., O’Doherty C.A., Collins S. Prevalence and characteristics of breakthrough pain in cancer patients admitted to a hospice. J. Pain Symptom Manag. 2000;20:87–92. doi: 10.1016/S0885-3924(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Batiste X., Madrid F., Moreno F., Gracia A., Trelis J., Nabal M., Alcalde R., Planas J., Camell H. Breakthrough cancer pain: Prevalence and characteristics in patients in Catalonia, Spain. J. Pain Symptom Manag. 2002;24:45–52. doi: 10.1016/S0885-3924(02)00421-9. [DOI] [PubMed] [Google Scholar]
- 23.Greco M.T., Corli O., Montanari M., Deandrea S., Zagonel V., Apolone G., Writing Protocol Committee. Cancer Pain Outcome Research Study Group (CPOR SG) Investigators Epidemiology and pattern of care of breakthrough cancer pain in a longitudinal sample of cancer patients. Results from the Cancer Pain Outcome Research Study Group. Clin. J. Pain. 2011;27:9–18. doi: 10.1097/AJP.0b013e3181edc250. [DOI] [PubMed] [Google Scholar]
- 24.Caraceni A., Bertetto O., Labianca R., Maltoni M., Mercadante S., Varrassi G., Zaninetta G., Zucco F., Bagnasco M., Lanata L., et al. Episodic (breakthrough) pain prevalence in a population of cancer pain patients. Comparison of clinical diagnoses with the QUDEI—Italian questionnaire for intense episodic pain. J. Pain Symptom Manag. 2012;43:833–841. doi: 10.1016/j.jpainsymman.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Mercadante S., Villari P., Ferrera P., Casuccio A. Optimization of opioid therapy for preventing incident pain associated with bone metastases. J. Pain Symptom Manag. 2004;28:505–510. doi: 10.1016/j.jpainsymman.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Arthur J., Yennurajalingam S., Nguyen L., Tanco K., Chisholm G., Hui D., Bruera E. The routine use of the Edmonton Classification System for Cancer Pain in an outpatient supportive care center. Palliat. Support Care. 2015;13:1185–1192. doi: 10.1017/S1478951514001205. [DOI] [PubMed] [Google Scholar]
- 27.Davies A.N., Vriens J., Kennett A., McTaggart M. An observational study of oncology patients’ utilization of breakthrough pain medication. J. Pain Symptom Manag. 2008;35:406–411. doi: 10.1016/j.jpainsymman.2007.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during/and or analyzed during the current study are available from the corresponding author on request.