Abstract
The differentiation of Leishmania parasites from the insect stage, the promastigote, toward the pathogenic mammalian stage, the amastigote, is triggered primarily by the rise in ambient temperature encountered during the insect-to-mammal transmission. We show here that inactivation of heat shock protein (Hsp) 90, with the use of the drugs geldanamycin or radicicol, mimics transmission and induces the differentiation from the promastigote to the amastigote stage. Geldanamycin also induces a growth arrest of cultured promastigotes that can be forestalled by overexpression of the cytoplasmic Hsp90. Moreover, we demonstrate that Hsp90 serves as a feedback inhibitor of the cellular heat shock response in Leishmania. Our results are consistent with Hsp90 homeostasis serving as cellular thermometer for these primitive eukaryotes, controlling both the heat shock response and morphological differentiation.
INTRODUCTION
Protozoan parasites of the genus Leishmania are transmitted by blood-feeding sand flies to mammalian hosts, including humans, whereby they encounter a rise in ambient temperature. Subjecting cultured insect stages, the promastigotes, of various Leishmania species to a similar temperature upshift in vitro results in the transient posttranscriptional up-regulation of both heat shock protein (Hsp) 90 (also known as Hsp83) and Hsp70 synthesis and a persistent induction of Hsp100 expression (Argaman et al., 1994; Brandau et al., 1995; Krobitsch et al., 1998). Moreover, species such as L. mexicana, L. pifanoi, and L. donovani will, during heat stress and acidification of the medium, show a morphological transition toward the mammalian stage, the amastigote (Zilberstein and Shapira, 1994; Saar et al., 1998). Such axenic amastigotes are indistinguishable from true host tissue-derived intracellular amastigotes (Bates, 1993; Pan et al., 1993; Charest and Matlashewski, 1994; Charest et al., 1996; Gupta et al., 1996; Saar et al., 1998). Thus, the rise of temperature encountered during transmission can be viewed not as stress but rather as signal for cellular differentiation.
We have shown previously that Hsp100 is critical for the expression of some amastigote stage-specific proteins and for overall virulence of the parasites (Hubel et al., 1997; Krobitsch et al., 1998); however, gene replacement mutants lacking Hsp100 still show, induced by elevated temperature, morphological differentiation to viable amastigote-like culture stages, indicating that the induction of morphological differentiation occurs independently or upstream of Hsp100 (Krobitsch et al., 1998; Krobitsch and Clos, 1999).
Another chaperone, Hsp90 (Hsp83), is one of the most abundant proteins in Leishmania, constituting 2.8% of the cellular protein (Brandau et al., 1995). Hsp90 (Hsp83) is encoded by multiple gene copies and is found in the soluble fraction of the cytoplasm (Shapira and Pinelli, 1989; Brandau et al., 1995; Hubel and Clos, 1996). Its sequence is distinct from the Grp94 homologue of L. infantum, which was characterized recently (Larreta et al., 2000). In higher eukaryotes and in yeast, cytosolic Hsp90 has been known to interact directly with ligand-dependent transcription factors and cell cycle regulators (Rutherford and Zuker, 1994; Scheibel and Buchner, 1998; Buchner, 1999). Its interaction with regulatory factors is a prerequisite for such factors to interact properly with ligands and target molecules. In addition to the typically dimeric Hsp90, the functional complexes usually include other heat shock proteins and associated factors (Chang et al., 1997; Scheibel and Buchner, 1998). The impact of Hsp90 on the morphological differentiation of multicellular organisms was demonstrated recently, and a role as molecular capacitor of morphological evolution has been proposed (Rutherford and Lindquist, 1998; Lele et al., 1999). In addition, Hsp90 can also play a more general chaperoning role maintaining the structure of heat-labile proteins (Nathan et al., 1997; Scheibel et al., 1998). Although viable Hsp90 null mutants could not be obtained so far, inactivation of Hsp90 by highly specific inhibitors such as geldanamycin (GA) or radicicol (RAD) (Whitesell et al., 1994, 1998; Schulte et al., 1998) has been helpful in assessing the impact of this Hsp family on various cellular processes (Uma et al., 1997; Ali et al., 1998; Galigniana et al., 1998; Rutherford and Lindquist, 1998; Zou et al., 1998; Lele et al., 1999; Morano and Thiele, 1999; Nathan et al., 1999; Yorgin et al., 2000).
In this article we present evidence that Hsp90 (Hsp83) of L. donovani plays a key role in the progression of this parasite from its insect stage toward the pathogenic mammalian stage. We also show an involvement of Hsp90 in cell cycle control and cellular stress response.
MATERIALS AND METHODS
Parasite Strains and Culture
In our analyses we used the Lo8 strain of L. donovani, a gift from D. Zilberstein (Techniow, Haifa, Israel).
Promastigotes frozen directly after a passage through BALB/c mice were thawed and cultivated for up to 2 mo at 25°C in M199 medium supplemented with 25% fetal calf serum and 20 μg/ml gentamicin. For in vitro differentiation into the amastigote stage, cells were treated as described (Krobitsch et al., 1998). Briefly, cells were heat shocked for 24 h at 37°C and then cultivated for 5 d at 37°C in mildly acidic medium (pH 5.5).
Cell density was determined with the use of a CASY cell counter (Schärfe Systems).
To select for GA escape mutants, promastigotes were cultivated for several weeks under 50 ng/ml GA (Sigma, St. Louis, MO; dissolved in 100% DMSO). When the culture resumed growth, the GA concentration was increased in 50 ng/ml steps up to 150 and 200 ng/ml.
Electrotransfection
Electrotransfection was performed essentially as described (Laban and Wirth, 1989; Kapler et al., 1990; Krobitsch et al., 1998).
Indirect Immunofluorescence Microscopy
Leishmania cells (1 × 107) were harvested by centrifugation, resuspended in PBS, and applied to glass slides coated with poly-l-lysine. After fixing the cells for 5 min in methanol, the slides were incubated with blocking buffer (0.2% iBlock, 0.02% Tween 20, 0.1% Triton X-100 in PBS). Slides were incubated for 1 h at 35°C with a monoclonal anti–alpha-tubulin antibody (clone B-5–2-1; Sigma) 1:2000 in blocking buffer, followed by incubation with goat anti-mouse antibody, coupled with dichlorotriazin amino fluorescein (DTAF) (1:50; Dianova, Hamburg, Germany).
The samples were analyzed on a Leica DM RB microscope with a confocal TCS NT system. Fluorescence was measured in the FITC channel.
Scanning Electron Microscopy
Leishmania cells were washed twice in PBS, fixed in 2% glutaraldehyde in sodium cacodylate buffer, and post-fixed with 1% osmium. Samples were dehydrated at increasing ethanol concentrations (30–100%). After critical point drying, samples were treated with gold and analyzed on a Philips SEM 500 electron microscope.
Immunoblot Analysis
SDS-PAGE and Western transfer were performed as described (Brandau et al., 1995; Hubel et al., 1995). Briefly, membranes were treated with blocking buffer (5% milk powder and 0.1% Tween 20 in Tris-buffered saline), with antibodies (1:2500–1:5000 in blocking buffer) against Leishmania heat shock proteins (Hubel et al., 1995), and with anti-chicken IgG–alkaline phosphatase conjugate (Dianova; 1:2500 in blocking buffer). Anti-A2 mAb, a gift from Greg Matlashewski (McGill University, Montreal, Quebec), was used at a 1:50 dilution; the secondary AP-conjugated goat anti-mouse IgG was used at 1:2500 dilution. Blots were stained with nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate.
Pulsed Field Gel Electrophoresis
Leishmania cells were harvested by centrifugation and washed twice in PBS. After centrifugation, parasites were resuspended in PBS and mixed with an equal volume of prewarmed 1% InCert agarose (FMC BioProducts, Rockford, ME). The mixture was aliquoted in block formers (Bio-Rad, Munich, Germany) at 2.5 × 107 parasites per block. For cell lysis, the agarose blocks were incubated for 2 d at 37°C in 0.5 M EDTA, pH 9.0, and 1% Laurylsarcosin containing 2 mg/ml Proteinase K.
Pulsed field gel electrophoresis was performed at 13°C in 0.25 × TBE electrophoresis buffer in a Rotaphor unit (Biometra, Goettingen, Germany) under the following conditions: interval in sec 100 → 10 log, switching angle 120 → 110 lin, voltage 200 → 150 log. Chromosomes from Saccharomyces cerevisiae strain YPH80 (New England Biolabs, Beverly, MA) served as size standards.
After staining with ethidium bromide (1 μg/ml), the gel was blotted onto a positively charged nylon membrane (Qiagen, Hilden, Germany) by alkaline transfer (Sambrook et al., 1989). The membrane was hybridized with a digoxigenin-labeled Hsp90 gene probe (DIG DNA Labeling and Detection Kit, Roche Molecular Biochemicals, Mannheim, Germany). The probe was detected with the use of anti-digoxigenin–AP Fab fragments (1:2000; Roche) in PBS with 0.2% iBlock (Tropix, Bedford, MA) and 0.02% Tween 20. Blots were stained as described above.
Flow Cytometric Analysis
Leishmania parasites were cultivated for 24 h at 37°C or at 25°C with or without 100 ng/ml GA. Log phase parasites had a cell density of ∼5 × 106 cells/ml; stationary phase cells had a cell density of ∼7 × 107 cells/ml.
For analysis, 4 × 106 parasites were harvested by mild centrifugation, washed twice in PBS, and fixed for 1 h in 1 ml PBS containing 70% methanol, at 4°C. After the methanol was removed and the cells were resuspended in PBS containing 20 μg/ml RNase A (20 min, 37°C), the cells were harvested, resuspended in citrate buffer (45 mM MgCl2, 30 mM sodium citrate, 20 mM MOPS, pH 7.0, 0.1% Triton X-100), and labeled for 20 min with 1 μM SYTOX Green nucleic acid stain (Molecular Probes, Eugene, OR). After staining, cells were washed two times in PBS supplemented with 5% fetal calf serum and 0.01% sodium azide. Samples were stored in the dark at 4°C until analysis. Fluorescence was monitored by flow cytometry on a fluorescence-activated cell sorter (Becton Dickinson, Heidelberg, Germany) counting 10,000 cells per sample.
Imaging
Halftone images were digitalized on a flatbed scanner (Agfa Snapscan 600, Agfa-Gevaert, Leverkusen, Germany) or a Nikon Coolscan (Duesseldorf, Germany) 35 mm film scanner. Images were cropped and optimized for color saturation with the use of Adobe Photoshop software, version 5.0. Images were combined with line drawings and text with the use of Claris Draw (Claris Corp., Santa Clara, CA) software, version 1.0d.
RESULTS
Geldanamycin Treatment Induces Growth Arrest in G2 Phase of the Cell Cycle
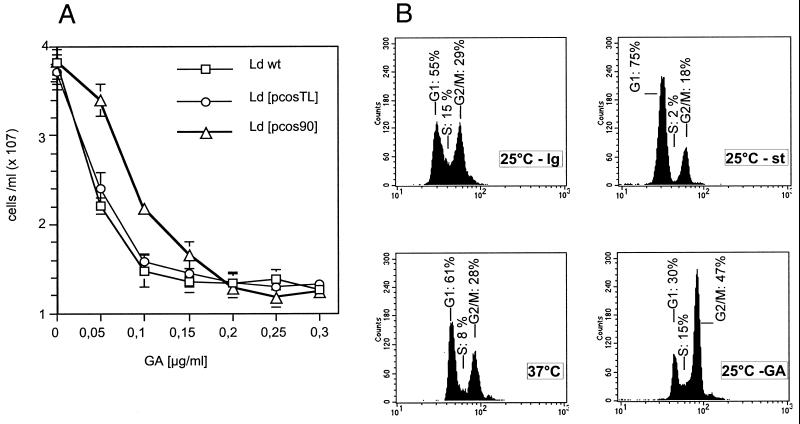
Treatment of L. donovani promastigotes (Ld wt) with geldanamycin results in a dose-dependent growth arrest (Figure 1A). To determine whether this effect is due to the inhibition of the cytoplasmic Hsp 90 species, we transfected L. donovani cells (Ld wt) stably with a cosmid, pcos90, derived from an L. donovani genomic DNA library. This cosmid includes a part of the large multicopy Hsp90 gene cluster that encodes the cytoplasmic Hsp90. Cells transfected with the cosmid vector, Ld [pcosTL] (Kelly et al., 1994), served as control. Quantification of Hsp90 expression in the recombinant strains revealed a 35% overexpression of Hsp90 in L. donovani Ld [pcos90] compared with both Ld wt and the vector control strain, Ld [pcosTL] (Wiesgigl, unpublished data). This modest increase of Hsp90 expression is due to the high number of Hsp90 genes, which form large tandem clusters (Hubel and Clos, 1996). Wild-type promastigotes and both recombinant strains were then tested for growth under various GA concentrations. As shown in Figure 1A, we observed a reduced growth rate for Ld wt and Ld [pcosTL] cells at a concentration of 50 ng/ml GA, whereas 100 ng/ml leads to a growth arrest. The monitored increase of cell density from 5 × 106 to ∼1–1.5 × 107 cells/ml indicates that cells are arrested after one complete cell cycle. In contrast, the Ld [pcos90] cells that overexpress Hsp90 show a half-maximal growth rate at 100 ng/ml GA, whereas a total arrest requires 150 ng/ml, in good correlation with the 35% increase of cytoplasmic Hsp90. This indicates that GA acts specifically on Hsp90, because overexpression of this protein can forestall the effect of the drug.
Figure 1.
Geldanamycin-induced growth arrest. (A) Cells of three strains, wild-type (Ld wt), vector control (Ld [pcosTL]), and Hsp90-overexpressing cells (Ld [pcos90]) were seeded at 5 × 106 cells/ml and incubated for 24 h at the indicated concentrations of geldanamycin (GA). Cell density was measured, and the mean values from six independent experiments were calculated and plotted against drug concentration. The error bars represent the SD. (B) Leishmania promastigotes from logarithmically growing (25°C - lg) or from stationary (25°C - st) culture as well as cells heat shocked at 37°C or treated with GA (200 μg/ml) were analyzed by flow cytometry. Culture aliquots were removed after 24 h and stained with SYTOX. G1, S, and G2/M refer to the corresponding cell cycle stages. Percentages of cells in respective cell cycle stages are given.
Given the known number of Hsp90 molecules per cell (1.5 × 106) (Brandau et al., 1995) and the cell density (5 × 106/ml), we calculate Hsp90 concentration as 12.5 pmol/ml. Opposed to that, 50 ng/ml of GA, 89 pmol/ml, are required to obtain a half-maximal growth arrest. The nominal sevenfold molar excess of GA over Hsp90 that is sufficient for Hsp90 inhibition is probably even lower in reality given that GA is unstable in aqueous solution. This would indicate a free diffusion of GA through the cell membranes of Leishmania promastigotes and a near-stoichiometrical mode of interaction with Hsp90.
Withdrawal of geldanamycin will, over time, reverse the growth arrest; however, up to 1 wk is required before cell division resumes.
To compare the GA-induced growth arrest with heat shock-induced or stationary phase growth arrest, we performed a flow cytometric analysis with SYTOX-stained cells (Figure 1B). Cells growing at logarithmic rate (25°C - lg) show an even distribution between G1-phase and G2/M-phase and a considerable S-phase signal. Promastigotes in stationary phase (25°C - st) are arrested primarily in the G1 phase of the cell cycle. Heat-shocked cells (37°C) are distinguished by a reduced S-phase signal. In contrast, geldanamycin-treated cells (25°C - GA) undergo an arrest that appears to occur primarily in the G2/M-phase of the cell cycle. The effect of the drug, therefore, differs from that of heat shock or high cell density in stationary phase. Under heat shock and under GA treatment, there is a general shift of the populations toward an increased fluorescence. This shift is observed consistently and may reflect an increased autofluorescence that we observe under these conditions (Wiesgigl, unpublished observations).
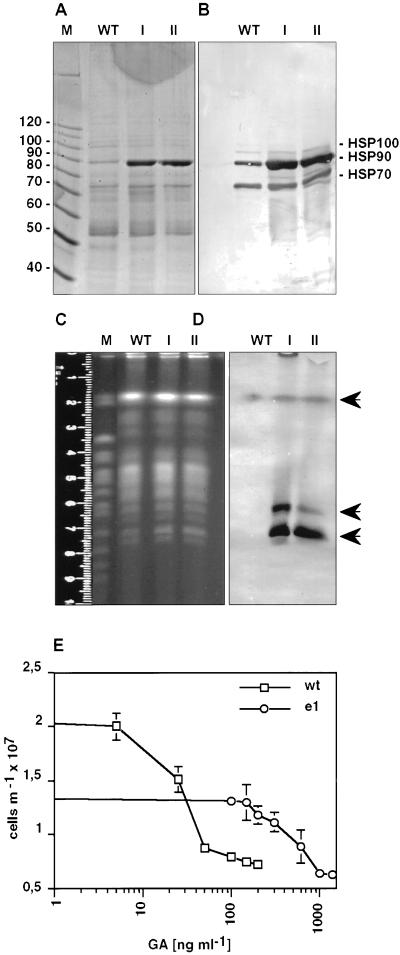
Continuous Culture of L. donovani Cells under GA Results in Spontaneous Amplification of the Hsp90 Gene Locus
The previous experiment showed that cells which overexpress Hsp90 are partially resistant to GA. We wanted to know whether Leishmania cells cultivated at threshold concentration are able to develop spontaneous escape mechanisms. To this end we started a prolonged cultivation of promastigotes beginning at 50 ng/ml of GA. As soon as these cells resumed cellular growth, the concentration was increased gradually up to 150 and 200 ng/ml (corresponding to escape populations I and II), respectively. SDS-PAGE analysis of wild-type cells showed a normal protein pattern after Coomassie blue staining (Figure 2A). In contrast, cell lysates of escape strain cells revealed a single strongly induced protein band in the 90-kDa range, whereas the rest of the protein pattern remained unchanged. Immunoblot analysis with the use of specific anti-HSP antibodies revealed the prominent band to represent cytoplasmic Hsp90 (Figure 2B). Scanning densitometric analyses revealed an 8- to 16-fold increase of Hsp90 levels for the escape strains compared with wild type. At 270 pmol/ml of GA and 200 pmol/ml of Hsp90 in the escape mutants, the nominal molar ratio (Hsp90:GA) is only 1:1.35. Given that GA has only limited stability, Hsp90 is probably in molar excess over geldanamycin.
Figure 2.
Amplification of Hsp90 gene copies attributable to geldanamycin selection. (A) SDS-PAGE analysis of wild-type promastigotes (WT) and of two escape strains, I and II, selected at 150 and 200 ng/ml GA, respectively. An equivalent of 106 cells per lane was dissolved in sample buffer and run on a 7.5% gel. The gel was stained with Coomassie blue. The sizes of selected marker proteins (10-kDa ladder; Life Technologies, Gaithersburg, MD) are shown on the left. (B) Immunoblot analysis of the samples described in A. After Western blot, the membrane was probed with a mixture of antibodies directed against Hsp70, Hsp90 and Hsp100. (C) Karyotyping. Chromosomes of L. donovani wild-type and escape strains I and II were separated by pulsed-field gel electrophoresis and stained with ethidium bromide. Saccharomyces cerevisiae chromosomes (strain YPH80) were used as size markers (M). (D) The gel shown in C was subjected to Southern blot, and the membrane was hybridized with a digoxigenin-labeled hsp90 gene probe. The arrows point at bands positive for Hsp90 hybridization. (E) L. donovani wild-type (wt) or escape mutant (e1) cells were seeded at 2 × 106 and incubated for 24 h at increasing GA concentrations. Cell density was measured and plotted against GA concentration.
Spontaneous gene amplification is known to occur in Leishmania and was identified as a mechanism for developing drug resistance (Beverley, 1991; Segovia, 1994; Haimeur and Ouellette, 1998). To test whether Hsp90 genes were amplified in the escape populations, we separated chromosomes of wild-type and escape mutant cells by pulsed-field gel electrophoresis. Figure 2C displays the chromosome pattern of L. donovani and of the two escape populations after ethidium bromide staining. The gel was then subjected to a Southern blot, and the membrane was probed with a 750-bp fragment derived from the ORF of Hsp90 (Figure 2D). The uppermost arrow points at the signal obtained from the chromosome that harbors the Hsp90 gene cluster (Hubel and Clos, 1996). No increase is observed with the escape populations; however, additional bands (arrows) are observed in the karyotype of the escape mutant populations that are absent from the wild-type pattern. We conclude that inhibition of Hsp90 by GA is bypassed by a spontaneous episomal amplification of the Hsp90 gene cluster encoding the cytosolic Hsp90 or of parts thereof. Indeed, the escape population shows a remarkably different dose response (Figure 2E). Growth of escape mutants is reduced by 50% compared with wild-type control in the absence of GA. The IC50, however, was determined as 525 ng/ml of GA, as opposed to 32 ng/ml of GA determined as IC50 for wild-type L. donovani. Thus, the tremendous overexpression of Hsp90 slows growth by 50% but increases GA tolerance by a factor of 16.
Because Hsp90 is usually organized in a multiprotein complex (Scheibel and Buchner, 1998), we tested whether overexpression of other proteins could also bypass the GA-induced growth arrest. Such proteins could be client proteins, chaperone complex partners, or, for that matter, other Hsp90 family members such as Grp94. To test this possibility, we performed a genetic complementation screen. L. donovani promastigotes were transfected with a L. donovani genomic DNA cosmid library and placed under selection with GA or under double selection with G418 and GA. Cosmid DNA from proliferating transfectants was isolated and used to transform Escherichia coli. Cosmid clones were then analyzed by PCR or Southern Blot for the presence of Hsp90 gene copies. All 181 clones that conferred GA resistance to L. donovani harbored Hsp90 gene copies that encode the cytosolic form of Hsp90. This is further indication of the specificity of GA for Hsp90 and the importance of this chaperone for proliferation of promastigote Leishmania cells. It also excludes the possibility that the growth arrest induced by geldanamycin is due to an interaction with other Hsp90 family members.
Hsp90 as Regulator of the Stress Response in Leishmania
In higher eukaryotes, activation of heat shock genes in response to stress is mediated by heat shock transcription factors of the HSF-1 type (Morimoto et al., 1994; Morimoto and Santoro, 1998). Recent results show that components of the Hsp90 chaperone complex may be involved in regulation of HSF-1 activity (Ali et al., 1998; Zou et al., 1998). In Leishmania, there is no evidence for the existence of either heat shock promotor elements or heat shock transcription factor(s). Elevated temperature alone will induce Hsp synthesis, which is regulated at a posttranscriptional level. Common chemical inducers of the cellular stress response do not induce heat shock protein synthesis in L. donovani, probably because of the lack of transcription regulation (Brandau et al., 1995; Clos et al., 1998).
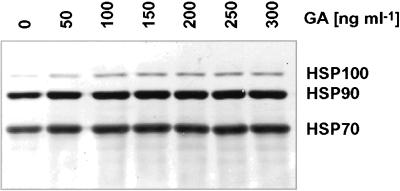
Surprisingly, the inhibition of Hsp90 in Leishmania parasites with the use of GA still induces overall Hsp synthesis that, in turn, leads to increased Hsp100 concentrations. Figure 3 shows an immunoblot of cell lysates, derived from promastigotes treated with various GA concentrations. The filter was probed with antibodies directed against Leishmania Hsp70, Hsp90, and Hsp100. Although Hsp100 is barely detectable in control cells, GA induces a dose-dependent increase of Hsp100 levels. We observe no significant increase of either Hsp70 or Hsp90. Both heat shock proteins are highly abundant in unstressed cells and do not increase under heat stress either (Brandau et al., 1995). Nevertheless, metabolic labeling experiments with the use of 35S-methionine revealed both Hsp70 and Hsp90 to be synthesized at increased rates under GA treatment (Wiesgigl, unpublished data). Hsp90 homeostasis obviously plays some role in the posttranscriptional control of heat shock protein synthesis.
Figure 3.
Effect of geldanamycin on Hsp levels in Leishmania. L. donovani promastigotes were incubated at increasing geldanamycin concentrations. After 24 h, cells were lysed, and the equivalent of 106 cells per lane was separated by SDS-PAGE. Proteins were blotted and probed with a mixture of antibodies directed against Hsp70, Hsp90, and Hsp100. Marker proteins (10-kDa ladder; Life Technologies) are shown on the left.
Geldanamycin Treatment Induces Amastigote-specific Protein Synthesis
The similarity of the effects of heat shock and GA treatment prompted us to compare the two stimuli with regard to amastigote-specific gene expression. Promastigote-to-amastigote differentiation can be induced in vitro by the application of a heat shock for 24 h and subsequent acidification of the culture medium. The synthesis of a set of closely related proteins, the A2 protein family (Charest and Matlashewski, 1994; Charest et al., 1996), is a hallmark for this stage differentiation process. These proteins with so far unknown functions are important for the virulence of the parasites and can be detected both in axenically cultured and in tissue-derived amastigotes, but not in promastigotes.
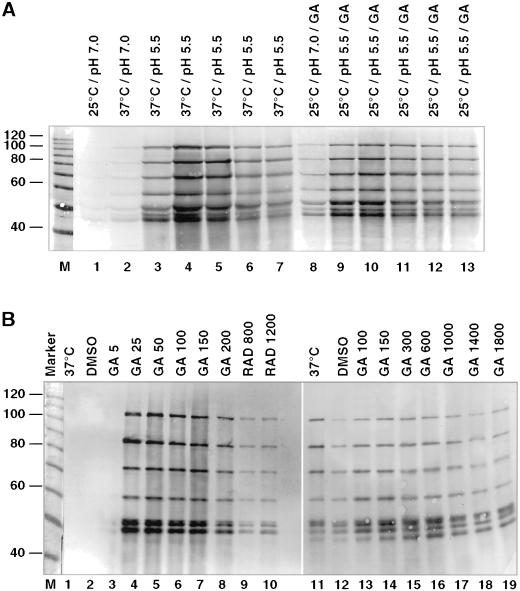
We first tested whether GA treatment can substitute for heat stress in the axenic amastigote development. As shown by immunoblot analysis (Figure 4A), promastigotes lack A2 proteins (lane 1). After 24 h treatment at 37°C, faint bands that represent the various A2 family members become visible (lane 2), the intensity of which increases considerably once the medium is acidified for 1–5 d (lane 3–7). Surprisingly, we detected A2 protein after cultivating Leishmania parasites for 24 h with geldanamycin at neutral pH and 25°C (lane 8). Further cultivation of the cells with geldanamycin in acidic milieu was sufficient to achieve A2 protein levels equivalent to standard stage differentiation conditions (lane 9–13).
Figure 4.
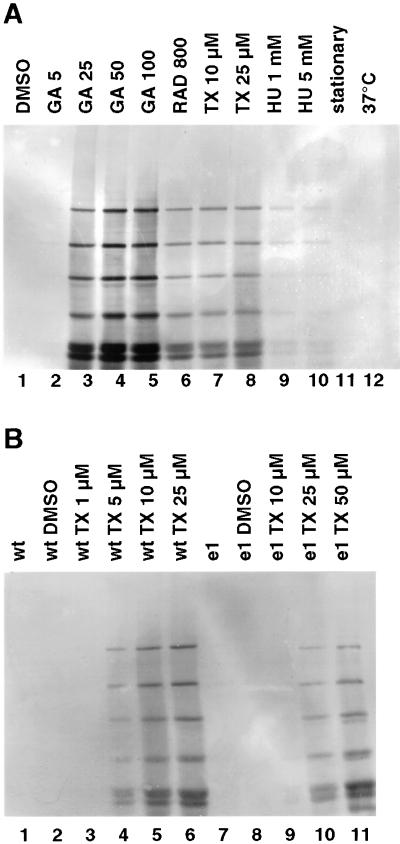
Geldanamycin-induced synthesis of amastigote-specific A2 proteins. (A) Immunoblot analysis. Samples were collected daily during an in vitro promastigote-to-amastigote differentiation of L. donovani and analyzed by SDS-PAGE and immunoblot with the use of anti-A2 mAb (lanes 1–7). Cells were treated with 200 ng/ml GA at 25°C (lane 8) for 24 h at neutral pH. The pH was shifted to 5.5, and incubation was continued at 25°C with 200 ng/ml GA (lanes 9–13). Note that a family of closely related proteins of different sizes is recognized by the antibody. (B) Immunoblot analysis of wild-type (lane 1–10) and escape strain I (lane 12–19) parasites, incubated for 48 h at 37°C (lanes 1 and 11), with the solvent DMSO (lanes 2 and 12), at the indicated GA concentrations (lanes 3–8 and 13–19), or at the indicated RAD concentrations (lanes 9 and 10). The equivalent of 106 cells per lane was subjected to SDS-PAGE and Western blot and probed with anti-A2 monoclonal antibodies. The positions of marker proteins (10-kDa ladder; Life Technologies) are shown on the left.
To test the dosage effect, we exposed promastigotes to increasing GA concentrations. We also tested another specific Hsp90 inhibitor, RAD, and compared lysates of cells treated with these drugs for 2 d with lysates from heat-shocked and control cells, respectively (Figure 4B). Immunoblot analysis revealed that the induction of A2 protein synthesis started at the critical concentration of 25 ng/ml GA, whereas a lower dose of 5 ng/ml showed no effect. Hsp90 inhibition by RAD also induced A2 expression, albeit at a lower rate, whereas heat stress or the solvent DMSO had no such effect. Inhibition of Hsp90, therefore, is sufficient to mimic the environmental signals that induce the onset of A2 proteins synthesis.
In contrast, the escape populations that were kept cultivated under continuous GA exposure (100 ng/ml) showed only low levels of A2 proteins (lane 12) that barely increased when higher GA concentrations (lanes 13–19) or heat shock (lane 11) were applied. This is an indication that free Hsp90 is antagonistic to amastigote-specific gene expression.
To exclude the possibility that a growth arrest per se will induce A2 protein synthesis, we tested various growth inhibitors at concentrations that had been found to block proliferation of promastigotes. Heat stress or stationary culture conditions did not induce A2 synthesis (Figure 5A). Neither did growth inhibition by hydroxy urea. Interestingly, taxol (Tx), which blocks the promastigote cell cycle in G2/M-phase, also induces A2 synthesis similar to RAD, but less strongly than GA.
Figure 5.
Taxol-induced synthesis of A2 protein family. (A) Leishmania promastigotes were incubated for 48 h at various concentrations (nanograms per milliliter) of geldanamycin (GA; lanes 2–5) or radicicol (RAD; lane 6). Alternatively, taxol (TX; lanes 7–8) and hydroxy urea (HU; lanes 9–10) were added at the indicated concentrations. Control cells were cultivated with the solvent DMSO (lane 1), until they were stationary (lane 11) or at 37°C (lane 12). (B) Wild-type (wt; lanes 1–6) and escape strain I (e1; lanes 7–11) promastigotes were incubated at the indicated concentrations of taxol (TX; lanes 4–6 and 9–11) or with the solvent DMSO (lanes 2 and 8). To exclude effects attributable to DMSO, in lane 1 and 7, lysates of untreated cells are shown. After 48 h the cells were lysed, and an equivalent of 106 cells per lane was subjected to SDS-PAGE and Western blot and probed with anti-A2 monoclonal antibodies.
It has been reported (Byrd et al., 1999) that Hsp90 may interact directly with taxol. To test this possibility we compared A2 synthesis under taxol both in wild-type L. donovani and in the escape parasites that overexpress cytosolic Hsp90. As shown in Figure 5B, cells that overexpress Hsp90 require higher taxol concentrations to induce A2 protein synthesis. We therefore postulate that the induction by taxol is due at least in part to an interference of this drug with Hsp90 homeostasis.
Inactivation of Hsp90 Triggers Differentiation Toward the Amastigote
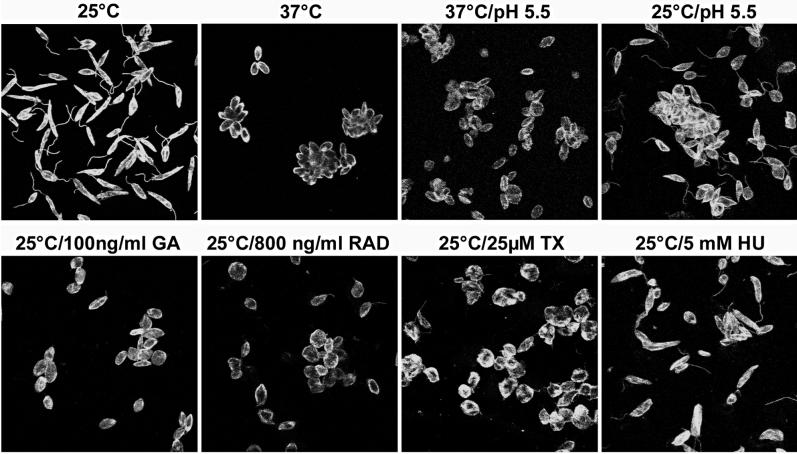
Typically, expression of the A2 proteins in L. donovani coincides with the morphological change associated with in vitro promastigote-to-amastigote differentiation. This change includes a length and size reduction and an almost complete loss of the flagellum that renders the amastigote nonmotile. To investigate whether inhibition of Hsp90 can induce a morphological differentiation, we analyzed, by indirect immunofluorescence, cells that had been cultivated under various growth conditions (Figure 6). Interestingly, a mere growth arrest by the G1 growth inhibitor hydroxy urea does not suffice to induce morphological change, and neither does acidification of the medium at 25°C. All three pharmocologicals that induce A2 protein synthesis and interact with Hsp90, GA, RAD, and Tx, induced a morphological differentiation similar to the developmental changes observed after exposure to heat stress and acidic pH.
Figure 6.
Morphological differentiation of Leishmania cells after Hsp90 inhibition. Promastigote parasites were cultivated for 48 h under the indicated conditions. Cells were then stained with anti–alpha-tubulin antibodies and anti-chicken IgG/DTAF and visualized by fluorescence microscopy.
To further analyze the changes induced by GA treatment, we compared promastigotes, axenic amastigotes, and GA-induced culture forms by scanning electron microscopy (Figure 7). The striking morphological changes during the differentiation from the promastigote (25°C, pH 7.0), during heat shock (37°C, pH 7.0), and after acidification (37°C, pH 5.5) are faithfully mimicked by an exposure to GA at neutral pH (25°C + GA, pH 7.0). We conclude that disturbances of Hsp90 homeostasis are a signal for the onset of stage differentiation.
Figure 7.
Morphological differentiation of GA-treated Leishmania cells. Promastigote L. donovani cells at 25°C, parasites incubated at 37°C for 24 h, axenic amastigotes after 5 d differentiation at 37°C and pH 5.5, and parasites treated at 25°C, 100 ng/ml GA for 24 h were imaged by scanning electron microscopy. Bar, 10 μm.
DISCUSSION
What Is the Target of Geldanamycin?
Several lines of indirect evidence support the idea that the effects observed under geldanamycin or radicicol treatment are indeed due to an inhibition of the cytosolic form of Hsp90. 1) Limited overexpression of Hsp90 from episomal gene copies will raise the threshold concentration of GA-induced growth arrest. 2) Selection under GA produces escape populations that overexpress the cytosolic Hsp90. 3) Escape strains that overexpress Hsp90 require higher doses of GA or taxol to induce synthesis of amastigote markers. 4) A genetic complementation screen with the use of a shuttle cosmid DNA library and GA selection selected only for cosmids that harbor Hsp90 gene copies. DNA and amino acid sequences of Hsp90 and Grp94 are too far diverged to allow for cross-hybridization of DNA sequences or for cross-specificity of Hsp90-specific antibodies. Therefore, in all likelihood, the effects observed under geldanamycin treatment reflect an inhibition of the cytosolic Hsp90 species.
Cell Cycle Control and Hsp90
The known role of Hsp90 as a chaperone for cellular growth factors such as cyclin-dependent kinases, etc., may be an indication for the presence of similar regulatory factors in the leishmaniae. Hsp90 seems to contribute to the fast growth rates (three to four replication cycles per 24 h) that one can observe with cultured promastigotes. The amastigote stage, by comparison, shows only slow growth rates of one replication cycle per 48 h (Wiesgigl, unpublished observations). The sequestration of Hsp90 at elevated temperatures may be one determinant for the different growth rates of the two life cycle stages.
Implications of Rational Drug Design
The speedy appearance of spontaneous escape mutants after prolonged GA exposure is indicative of the inherent ability of Leishmania spp. to bypass targeted deactivation of important cellular proteins by episomal amplification of the respective genes and concomitant overexpression. This finding further illustrates a problem for efforts to develop drugs designed to target certain key proteins of these parasites (“rational drug design”). The import rates for such drugs must exceed the ability of the parasite to synthesize the respective target proteins de novo, even from highly amplified genes.
A Role for Hsp90 in Posttranscriptional Stress Response
In several eukaryotic systems, Hsp90 has emerged as the chaperone for proteins that mediate regulated transcription, cellular differentiation, development, and cell cycle control (Rutherford and Zuker, 1994; Scheibel and Buchner, 1998; Buchner, 1999), and it is part of the feedback regulation of the HSF-1-dependent stress response (Ali et al., 1998; Zou et al., 1998). In contrast to the model organisms investigated so far in this context, the kinetoplastida do not belong to the Eukaryote Crown Group. Comparative sequence analyses group them with the Euglenozoa, which must have branched off very early in the evolution of the eukaryota (Schlueter et al., 2000). Nevertheless, as shown in this article, Hsp90 is used in a role very similar to its homologues in metazoan organisms. This implies that the function of Hsp90 is ancient. There is a remarkable difference, however, in that kinetoplastid protozoa do not use the major regulatory pathway common to all crown group eukaryotes, i.e., regulation of transcription mediated by transcription factors. Rather, transcription probably proceeds unidirectionally (Myler et al., 1999) and is mostly constitutive: gene expression is regulated at the levels of RNA processing, RNA stability, and/or translation control (Curotto de Lafaille et al., 1992; Aly et al., 1994; Brandau et al., 1995; Graham, 1995; Teixeira, 1998; Stiles et al., 1999). Nevertheless, Hsp90 can obviously chaperone the factors involved at these levels of regulation as well. It should be interesting in this context to investigate whether Hsp90 levels may play a role in the posttranscriptional regulation of Hsp synthesis in higher eukaryotes.
Antagonistic Roles of Hsp90 and Hsp100 in the Leishmania Life Cycle
The key outcome of our findings is the revelation of how a heat stress may be transduced into a developmental program, i.e., the differentiation from one life cycle stage to another. A sequestration of Hsp90 and Hsp70 by proteins that may denature at the elevated temperatures of a mammalian host can be envisioned. Indeed, Hsp90 has dual chaperone function. Although the Hsp90 multiprotein complex has specificity for cell cycle regulators and transcription factors, Hsp90 alone can act as a general chaperone stabilizing their structure of heat-labile proteins (Nathan et al., 1997; Scheibel et al., 1998). Under heat stress, Hsp90 complexes in Leishmania could therefore be in short supply to keep the cell cycle regulator proteins in a responsive state, thus abrogating their functions. In this, Leishmania may serve as a model for similar signaling pathways in other primitive eukaryotes.
So far, it is not clear whether GA-induced amastigote-like cells are identical or similar to axenic amastigotes cultivated under heat stress at acidic pH. In addition to morphological features and the expression of single marker genes, genome and proteome analysis should give more complete answers. The lack of transcription regulation in Leishmania and the incomplete genome project currently place severe limits on the use of genomics. The similarity of the proteome, on the other hand, should be a good indicator for comparing both culture forms, and this direction will be pursued. Differences, if any, should yield valuable information because they may allow a distinction between separate regulatory pathways, those including Hsp90 and others that may be independent of this chaperone.
If inactivation of Hsp90 is a signal for the induction of stress response, growth arrest, and differentiation toward the amastigote, how is the amastigote stage maintained? We know that induced synthesis of Hsp90 and Hsp70 ceases after a few hours of heat stress (Wiesgigl, unpublished observations). This would indicate that the system somehow approaches homeostasis; here is where a role of Hsp100 may lie. This chaperone is synthesized continuously under heat stress and reaches high concentrations in amastigotes of both L. donovani and L. major (Hubel et al., 1995, 1997; Krobitsch et al., 1998). We know that amastigote-to-promastigote differentiation and the concomitant resumption of rapid cell divisions is significantly accelerated in ΔclpB mutants of L. donovani that lack Hsp100 (Krobitsch and Clos, 1999). Once synthesized to relevant concentrations, Hsp100 may stabilize the amastigote stage by antagonizing promastigote development. The idea of antagonistic functions of Hsp90 and Hsp100 draws support from our finding that expression of the amastigote-specific A2 protein family, normally impaired by the lack of Hsp100 in ΔclpB mutants of L. donovani, can be restored by the pharmacological inactivation of Hsp90 (Wiesgigl, unpublished observations). It thus seems that heat shock proteins, by their balanced functions as chaperones, can establish regulatory pathways in primitive eukaryota.
CONCLUSION
In the case of the leishmaniae, the stress response appears to be used by the parasites to sense their respective environment, fly gut, or mammalian macrophage, and to trigger the appropriate developmental program. It will be of interest to test whether similar regulatory functions can be shown for the Hsp90 of other primitive eukaryotes, either parasitic or free-living.
ACKNOWLEDGMENTS
We express our thanks to Christl Schmetz and Anne Macdonald for performing scanning electron microscopy, and Michal Shapira and Dan Zilberstein for the communication of unpublished results. We thank Christine Queitsch, Sylvia Krobitsch, and Susan Lindquist for helpful advice, Uwe Speck for help with the FACS analysis, and our colleagues at the BNI for constructive criticism. M.W. was supported in part by the Hochschulförderprogramm of the Deutsche Forschungsgemeinschaft.
REFERENCES
- Ali A, Bharadwaj S, O'Carroll R, Ovsenek N. HSP90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol Cell Biol. 1998;18:4949–4960. doi: 10.1128/mcb.18.9.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly R, Argaman M, Halman S, Shapira M. A regulatory role for the 5′ and 3′ untranslated regions in differential expression of hsp83 in Leishmania. Nucleic Acids Res. 1994;22:2922–2929. doi: 10.1093/nar/22.15.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaman M, Aly R, Shapira M. Expression of heat shock protein 83 in Leishmania is regulated post-transcriptionally. Mol Biochem Parasitol. 1994;64:95–110. doi: 10.1016/0166-6851(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bates PA. Axenic amastigote culture of Leishmania amastigotes. Parasitol Today. 1993;9:143–146. doi: 10.1016/0169-4758(93)90181-e. [DOI] [PubMed] [Google Scholar]
- Beverley SM. Gene amplification in Leishmania. Annu Rev Microbiol. 1991;45:417–444. doi: 10.1146/annurev.mi.45.100191.002221. [DOI] [PubMed] [Google Scholar]
- Brandau S, Dresel A, Clos J. High constitutive levels of heat-shock proteins in human-pathogenic parasites of the genus Leishmania. Biochem J. 1995;310:225–232. doi: 10.1042/bj3100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J. Hsp90 & Co. – a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Bornmann W, Erdjument-Bromage H, Tempst P, Pavletich N, Rosen N, Nathan CF, Ding A. Heat shock protein 90 mediates macrophage activation by taxol and bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1999;96:5645–5650. doi: 10.1073/pnas.96.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Nathan DF, Lindquist S. In vivo analysis of the Hsp90 cochaperone Sti1 (p60) Mol Cell Biol. 1997;17:318–325. doi: 10.1128/mcb.17.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest H, Matlashewski G. Developmental gene expression in Leishmania donovani: differential cloning and analysis of an amastigote-stage-specific gene. Mol Cell Biol. 1994;14:2975–2984. doi: 10.1128/mcb.14.5.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest H, Zhang W-W, Matlashewski G. The developmental expression of Leishmania donovani A2 amastigote-specific genes is post-transcriptionally mediated and involves elements located in the 3′-untranslated region. J Biol Chem. 1996;271:17081–17090. doi: 10.1074/jbc.271.29.17081. [DOI] [PubMed] [Google Scholar]
- Clos J, Brandau S, Hoyer C. Chemical stress does not induce heat shock protein synthesis in Leishmania donovani. Protistologica. 1998;149:167–172. doi: 10.1016/S1434-4610(98)70021-5. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Laban A, Wirth DF. Gene expression in Leishmania: analysis of essential 5′ DNA sequences. Proc Natl Acad Sci USA. 1992;89:2703–2707. doi: 10.1073/pnas.89.7.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galigniana MD, Scruggs JL, Herrington J, Welsh MJ, Carter-Su C, Housley PR, Pratt WB. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol Endocrinol. 1998;12:1903–1913. doi: 10.1210/mend.12.12.0204. [DOI] [PubMed] [Google Scholar]
- Graham SV. Mechanisms of stage-regulated gene expression in kinetoplastida. Parasitol Today. 1995;11:217–223. doi: 10.1016/0169-4758(95)80081-6. [DOI] [PubMed] [Google Scholar]
- Gupta N, Goyal N, Kumar R, Agrawal AK, Seth PK, Rastogi AK. Membrane characterization of amastigote-like forms of Leishmania donovani. Trop Med Int Health. 1996;1:495–502. doi: 10.1046/j.1365-3156.1996.d01-90.x. [DOI] [PubMed] [Google Scholar]
- Haimeur A, Ouellette M. Gene amplification in Leishmania tarentolae selected for resistance to sodium stibogluconate. Antimicrob Agents Chemother. 1998;42:1689–1694. doi: 10.1128/aac.42.7.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel A, Brandau S, Dresel A, Clos J. A member of the ClpB family of stress proteins is expressed during heat shock in Leishmania spp. Mol Biochem Parasitol. 1995;70:107–118. doi: 10.1016/0166-6851(95)00012-p. [DOI] [PubMed] [Google Scholar]
- Hubel A, Clos J. The genomic organization of the HSP83 gene locus is conserved in three Leishmania species. Exp Parasitol. 1996;82:225–228. doi: 10.1006/expr.1996.0029. [DOI] [PubMed] [Google Scholar]
- Hubel A, Krobitsch S, Horauf A, Clos J. Leishmania major Hsp100 is required chiefly in the mammalian stage of the parasite. Mol Cell Biol. 1997;17:5987–5995. doi: 10.1128/mcb.17.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler GM, Coburn CM, Beverley SM. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990;10:1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JM, Das P, Tomás AM. An approach to functional complementation by introduction of large DNA fragments into Trypanosoma cruzi and Leishmania donovani using a cosmid shuttle vector. Mol Biochem Parasitol. 1994;65:51–62. doi: 10.1016/0166-6851(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Krobitsch S, Brandau S, Hoyer C, Schmetz C, Hübel A, Clos J. Leishmania donovani heat shock protein 100: characterization and function in amastigote stage differentiation. J Biol Chem. 1998;273:6488–6494. doi: 10.1074/jbc.273.11.6488. [DOI] [PubMed] [Google Scholar]
- Krobitsch S, Clos J. A novel role for 100 kD heat shock proteins in the parasite Leishmania donovani. Cell Stress Chaperones. 1999;4:191–198. doi: 10.1379/1466-1268(1999)004<0191:anrfkh>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laban A, Wirth DF. Transfection of Leishmania enriettii and expression of chloramphenicol acetyltransferase gene. Proc Natl Acad Sci USA. 1989;86:9119–9123. doi: 10.1073/pnas.86.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larreta R, Soto M, Alonso C, Requena JM. Leishmania infantum: gene cloning of the GRP94 homologue, its expression as recombinant protein, and analysis of antigenicity. Exp Parasitol. 2000;96:108–115. doi: 10.1006/expr.2000.4553. [DOI] [PubMed] [Google Scholar]
- Lele Z, Hartson SD, Martin CC, Whitesell L, Matts RL, Krone PH. Disruption of zebrafish somite development by pharmacologic inhibition of Hsp90. Dev Biol. 1999;210:56–70. doi: 10.1006/dbio.1999.9262. [DOI] [PubMed] [Google Scholar]
- Morano KA, Thiele DJ. The Sch9 protein kinase regulates Hsp90 chaperone complex signal transduction activity in vivo. EMBO J. 1999;18:5953–5962. doi: 10.1093/emboj/18.21.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Jurivich DA, Kroeger PE, Mathur SK, Murphy SP, Nakai A, Sarge K, Abravaya K, Sistonen LT. Morimoto, R.I., Tissières, A., and Georgopoulos, C. Plainview, New York: Cold Spring Harbor Laboratory Press; 1994. Regulation of heat shock gene transcription by a family of heat shock factors; pp. 417–456. [Google Scholar]
- Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- Myler PJ, et al. Leishmania major Friedlin chromosome 1 has an unusual distribution of protein-coding genes. Proc Natl Acad Sci USA. 1999;96:2902–2906. doi: 10.1073/pnas.96.6.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci USA. 1997;94:12949–12956. doi: 10.1073/pnas.94.24.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S. Identification of SSF1, CNS1, and HCH1 as multicopy suppressors of a Saccharomyces cerevisiae Hsp90 loss-of-function mutation. Proc Natl Acad Sci USA. 1999;96:1409–1414. doi: 10.1073/pnas.96.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan AA, Duboise SM, Eperon S, Rivas L, Hodgkinson V, Traub-Cseko Y, McMahon-Pratt D. Developmental life cycle of Leishmania—cultivation and characterization of cultured extracellular amastigotes. J Euk Microbiol. 1993;40:213–223. doi: 10.1111/j.1550-7408.1993.tb04906.x. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Zuker CS. Protein folding and the regulation of signaling pathways. Cell. 1994;79:1129–1132. doi: 10.1016/0092-8674(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Saar Y, Ransford A, Waldman E, Mazareb S, Amin-Spector S, Plumblee J, Turco SJ, Zilberstein D. Characterization of developmentally-regulated activities in axenic amastigotes of Leishmania donovani. Mol Biochem Parasitol. 1998;95:9–20. doi: 10.1016/s0166-6851(98)00062-0. [DOI] [PubMed] [Google Scholar]
- Scheibel T, Buchner J. The Hsp90 complex—a super-chaperone machine as a novel drug target. Biochem Pharmacol. 1998;56:675–682. doi: 10.1016/s0006-2952(98)00120-8. [DOI] [PubMed] [Google Scholar]
- Scheibel T, Weikl T, Buchner J. Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence. Proc Natl Acad Sci USA. 1998;95:1495–1499. doi: 10.1073/pnas.95.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlueter A, Wiesgigl M, Hoyer C, Fleischer S, Klaholz L, Schmetz C, Clos J. Expression and subcellular localization of Cpn60 protein family members in Leishmania donovani. Biochim Biophys Acta. 2000;1491:65–74. doi: 10.1016/s0167-4781(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–108. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia M. Leishmania gene amplification: a mechanism of drug resistance. Ann Trop Med Parasitol. 1994;88:123–130. doi: 10.1080/00034983.1994.11812849. [DOI] [PubMed] [Google Scholar]
- Shapira M, Pinelli E. Heat-shock protein 83 of Leishmania mexicana amazonensis is an abundant cytoplasmic protein with a tandemly repeated genomic arrangement. Eur J Biochem. 1989;185:231–236. doi: 10.1111/j.1432-1033.1989.tb15107.x. [DOI] [PubMed] [Google Scholar]
- Stiles JK, Hicock PI, Shah PH, Meade JC. Genomic organization, transcription, splicing and gene regulation in Leishmania. Ann Trop Med Parasitol. 1999;93:781–807. doi: 10.1080/00034989957781. [DOI] [PubMed] [Google Scholar]
- Teixeira SM. Control of gene expression in Trypanosomatidae. Braz J Med Biol Res. 1998;31:1503–1516. doi: 10.1590/s0100-879x1998001200001. [DOI] [PubMed] [Google Scholar]
- Uma S, Hartson SD, Chen JJ, Matts RL. Hsp90 is obligatory for the heme-regulated eIF-2alpha kinase to acquire and maintain an activable conformation. J Biol Chem. 1997;272:11648–11656. doi: 10.1074/jbc.272.17.11648. [correction published in J. Biol. Chem. (1997) 272, 16068]. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Sutphin PD, Pulcini EJ, Martinez JD, Cook PH. The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1998;18:1517–1524. doi: 10.1128/mcb.18.3.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgin PD, Hartson SD, Fellah AM, Scroggins BT, Huang W, Katsanis E, Couchman JM, Matts RL, Whitesell L. Effects of geldanamycin, a heat-shock protein 90-binding agent, on T cell function and T cell nonreceptor protein tyrosine kinases. J Immunol. 2000;164:2915–2923. doi: 10.4049/jimmunol.164.6.2915. [DOI] [PubMed] [Google Scholar]
- Zilberstein D, Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]