Abstract
To determine the extent of physiological variation of uptake of 18F-flurodeoxyglucose (FDG) within palatine tonsils. To define normal limits for side-to-side variation and characterize factors affecting tonsillar uptake of FDG.
Over a period of 16 weeks 299 adult patients at low risk for head and neck pathology, attending our center for FDG positron emission tomography/computed tomography (PET/CT) scans were identified. The maximum standardized uptake value (SUVmax) was recorded for each palatine tonsil. For each patient age, gender, smoking status, scan indication and prior tonsillectomy status as well as weather conditions were noted.
There was a wide variation in palatine tonsil FDG uptake with SUVmax values between 1.3 and 11.4 recorded. There was a strong left to right correlation for tonsillar FDG uptake within each patient (P < .01). The right palatine tonsil showed increased FDG uptake (4.63) compared to the left (4.47) (P < .01). In multivariate analysis, gender, scan indication, and prevailing weather had no significant impact of tonsillar FDG uptake. Lower tonsillar uptake was seen in patients with a prior history of tonsillectomy (4.13) than those without this history (4.64) (P < .01). Decreasing tonsillar FDG uptake was seen with advancing age (P < .01). Significantly lower uptake was seen in current smokers (SUVmax 4.2) than nonsmokers (SUV 4.9) (P = .03).
Uptake of FDG in palatine tonsils is variable but shows a strong side-to-side correlation. We suggest the left/ right SUVmax ratio as a guide to normality with a first to 99th percentiles of (0.70–1.36) for use in patients not suspected to have tonsillar pathology.
Keywords: FDG, fluorodeoxyglucose, laterality, normal variation, palatine tonsil, smoking, weather conditions
1. Introduction
18F-Fluorodeoxyglucose (18F-FDG) is widely used throughout the world as a tracer in positron emission tomography/computed tomography (PET/CT) imaging to assess malignant and nonmalignant diseases.[1] Many tissues have variable physiological uptake of FDG and understanding the range of normal variation and the factors contributing to that variation is helpful in identifying pathology, as well as distinguishing this from normal physiological variation. The palatine tonsils are paired aggregations of lymphoid tissue between the palatoglossal and palatopharygeal arches.[2,3]
Given the normal defence function of lymphoid tissue of Waldeyer's ring, tonsillar tissue is normally in a variable state of physiological inflammation. As increased uptake of FDG has been described in a wide variety of infectious and inflammatory disorders,[4] increased tracer uptake is to be expected, as is wide variation between individuals.
Anatomic studies from tonsillectomies and postmortem studies have shown the palatine tonsils are most prominent in childhood and adolescence, undergoing atrophy throughout later adult life.[5] The immune function and balance of B cell types also changes with age.[6]
A number of studies have evaluated uptake of FDG within palatine tonsils, mostly in the context of evaluating many other structures. Such studies have shown most patients have identifiable increased uptake of FDG within the palatine tonsils and a wide variation between individuals.[7–11] Many of these studies did not specifically exclude patients with head and neck pathology.
A retrospective review of 78 patients has been undertaken studying patients at low risk of focal head and neck pathology.[8] Patients were routinely excluded if their blood glucose levels were more than 11.1 mmol/L. Within this population there was no correlation between blood glucose and FDG uptake of the palatine tonsils. The mean SUVmax was reported at 3.48 and a negative correlation between advancing age and FDG uptake within the palatine tonsils was described. A similar inverse relationship between age and FDG uptake in palatine tonsils was seen amongst patients known or suspected to have malignancy of the head and neck.[12]
A case–control type study has explored the potential utility of asymmetry of tonsillar FDG uptake in the diagnosis of tonsillar carcinoma. This study examined 43 control subjects with no evidence of focal head and neck pathology, and 10 cases of proven tonsillar carcinoma. An absolute 0.83 SUV difference between the 2 sides was proposed as a possible predictor for tonsillar malignancy.[13]
To our knowledge no previous study has evaluated the impact on FDG uptake in palatine tonsils of prior tonsillectomy for benign disease.
Lymphoma is a common indication for FDG PET/CT imaging. Differentiating physiological from pathological tonsillar uptake may be problematic in these patients. In post-therapy and response to treatment scans, the 5-point scale or Deauville scale[14] has been suggested and is widely used in both research and clinical settings.[15] We are not aware of any study that has systematically graded tonsillar uptake according to the 5-point scale in “normal” subjects.
Upper respiratory tract infections are known to be more common in periods of cold and wet weather.[16] In our center, as in many others, consideration is given to delaying PET imaging during episodes of acute infection. It is however possible that higher tonsillar FDG uptake might be seen in colder, wetter weather conditions. To our knowledge there has been no report exploring correlations between weather conditions and tonsillar FDG uptake.
The aim of this study was to evaluate the range of palatine tonsil 18F-FDG uptake in a large cohort of patients in whom there was no clinical or imaging evidence of focal upper respiratory tract pathology. The hope was to give further guidance for a reporting radiologist or physician in terms of a “cut off” beyond which absolute uptake and in particular asymmetry should be considered potentially abnormal and worthy of further investigation. In terms of asymmetry, we sought to explore whether a ratio or absolute side-to-side difference was more meaningful. We felt it was important to document the frequency of various grades of uptake as defined by the 5-point scale (Deauville criteria) within the tonsils as this is of value in the context of interpreting post-treatment scans in lymphoma. We aimed to systematically evaluate the effect of age, gender, smoking status, a prior history of tonsillectomy, scan indication, and prevailing weather conditions on tonsillar uptake of FDG.
2. Materials and methods
2.1. Patient selection
The study was based at the Wales Research and Diagnostic PET Imaging Centre (PETIC) in Cardiff, UK. This tertiary referral center serves a population of approximately 2.3 million. Since December 2015, all adult patients attending our center for PET/CT imaging for clinical purposes are invited to give written informed consent for the use of their scan and clinical data for the purposes of research. The scheme has been granted ethical and local research and development permissions and this allows us to perform multiple observational studies without the need for further individual permissions (PET tracer variations in health and disease 15/WA/0018). A study period between February 15, 2016 and June 3, 2016 was selected (16 weeks). Patients having undergone clinical FDG PET/CT imaging were identified from the hospital radiology database. From the radiology database, the number of patients giving and withholding consent was noted. Only data from patients having given consent were further analyzed.
Patients aged 16 or over were considered for inclusion if they had undergone a clinical FDG PET scan at our center during the study period and had consented for the use of their data for research. Patients were excluded if the scan was performed for the assessment of known or suspected head and neck carcinoma or if it became apparent that there was a previous history of head and neck malignancy or previous radiotherapy to the head and neck (34 patients). All patients with known or suspected lymphoma were excluded (123 patients). Patients were excluded if the scan data were incomplete or unavailable (3 patients) or if on viewing the scan data it was felt that movement or misregistration made accurate measurement of tonsillar FDG uptake impossible (5 patients). The exclusions left a total of 436 eligible patients. From this group, a sample of 299 patient data sets was randomly selected. This further selection was based on manual random selection from the list without reference to any of the clinical or scan data. Patients were selected evenly throughout the study period with no particular bias towards time of day or date within the study period. The selection was made blinded to all clinical and demographic factors.
2.2. Clinical data collection
A radiographer administered preimaging questionnaire was reviewed for each included patient. From this, the following data was extracted for each included patient; history of prior tonsillectomy for benign disease, smoking history: This was the patient's self-reported smoking status either as a current smoker, an ex-smoker, or a nonsmoker. From the radiology database, the following data were ascertained: patient gender, age, scan date, and indication. The imaging request form and report were also scrutinized to look for any exclusion criteria.
2.3. Meteorological data
Meteorological data (daily mean temperature and rainfall) were provided by the UK Met Office for the Bute Park Cardiff Weather Station for the time period between February 15, 2016 and June 3, 2016 as an Excel spreadsheet (Met Office, Fitzroy road, Exeter EX2 7NL). The weather center is within 1.5 miles of our PET/CT center, although many of our patients attended from a much wider geographic area.
2.4. PET/CT acquisition protocol
Patients were fasted for at least 6 hours prior to tracer administration. Serum glucose levels were routinely checked and confirmed to be less than 11.0 mmol/L prior to proceeding with imaging. Patients received a dose of 4 MBq of 18F-FDG per kilogram of body weight. Uptake time was 90 minutes. 18F-FDG PET/CT imaging was performed with a GE 690 PET/CT scanner (GE Healthcare, Pollards Wood, Buckinghamshire, UK). CT images were acquired in a helical acquisition with a pitch of 0.98 and a tube rotation speed of 0.5 seconds. Tube output was 120 kVp with output modulation between 20 and 200 mA. Matrix size for the CT acquisition was 512 × 512 pixels with a 50 cm field of view. No oral or intravenous contrast was administered. PET images were acquired at 3 minutes per field of view. The length of the axial field of view was 15.7 cm. Images were reconstructed with the ordered subset expectation maximization algorithm, with 24 subsets and 2 iterations. Matrix size was 256 × 256 pixels, using the VUE Point time of flight algorithm.
2.5. Image review and SUVmax recording
All FDG PET CT images were reviewed by a trained observer (EB) under direct supervision of three radiologists with at least 5 years of clinical PET/CT experience (PF, VSJ, and JR). Image analysis was undertaken on a GE Xeleris workstation using Volumetrics for PET/CT software version 2.1704. (GE healthcare, Pollards Wood, Buckinghamshire , UK.) A 2 cm diameter spherical region of interest was recorded for left and right palatine tonsil (one measurement each side), mediastinal blood pool and liver.
For measurement of palatine tonsils, the guidance was to identify tissue in the lateral aspect of the oropharynx immediately abutting the oropharyngeal cavity. Only laterally placed uptake between the level of the soft palate to the epiglottis was recorded and the 2 cm spherical region of interest was placed over the most avid part of the mucosal tissue here. Care was taken to avoid physiological muscular uptake. Uptake cranial to the level of the soft palate was not recorded. The PET and CT images were analyzed side by side with the region of interest being placed on the PET images.
For measurements of the mediastinal blood pool uptake, the SUVmax was taken as the maximal uptake region acquired within the lumen of the arch of the aorta. Care was taken to avoid any physiological uptake from structures above and below the aorta or metastases in the anterior mediastinum. For uptake measurements in the liver, the SUVmax value was taken as a region centrally in the right lobe of the liver, which did not overlap with any liver metastases or avid physiological uptake by bowel contents below the liver.
Uptake in the palatine tonsils was also categorized according to the 5-point scale (14). For the purposes of this study, we have defined the categories as follows: (the 5-point scale itself has only descriptive criteria for defining grade 4 and grade 5 uptake) Grade 1: no uptake, Grade 2: uptake but less than or equal to mediastinal blood pool, Grade 3: uptake more than mediastinal blood pool but less than or equal to liver, grade 4: uptake greater than liver but not over twice liver and Grade 5: uptake more than twice liver.
2.6. Reproducibility sub study
To provide further assurance of the accuracy of the data collection, a second observer (KM) independently made blinded measurements of SUVmax for the left- and right-palatine tonsils, mediastinal blood pool and liver for 20 randomly selected cases. Interobserver reproducibility was assessed as set out in statistical analysis.
2.7. Statistical analysis
Patient demographics and pathology were summarized through descriptive statistics. Left and right tonsil uptake were assessed by Pearson's correlation and compared by paired t-test and also Wilcoxon signed rank test as sensitivity checking. Regression models of average tonsillar uptake with forward/backward/stepwise selection approaches were used to select significant factors from age, gender, smoking status and pathology indication. Effect sizes of final selected factors in the model, with their associated 95% confidence intervals and P-values were reported. We also described the normal ranges of the left/right tonsil uptake ratio via percentiles. The effect of weather conditions on tonsillar uptake of FDG were tested by regression models. The reproducibility of data collection are assessed though inter-rater reliability analysis with calculated Intraclass Correlation Coefficient (ICC). IBM SPSS version 20 was used for all analysis.
3. Results and discussion
3.1. Patient characteristics
During the study period, 644 adult patients underwent clinical body FDG PET-CT scans at our center. Of these 17 patients had uncertain capacity or lacked capacity to consent and 26 patients refused consent. This left 601 patients eligible for inclusion in the study. This is an uptake/agreement rate of 93% suggesting that most patients are happy for the use of anonymized data for research purposes. Figure 1 outlines the patient selection and exclusion process leading to a final study population of 299.
Figure 1.
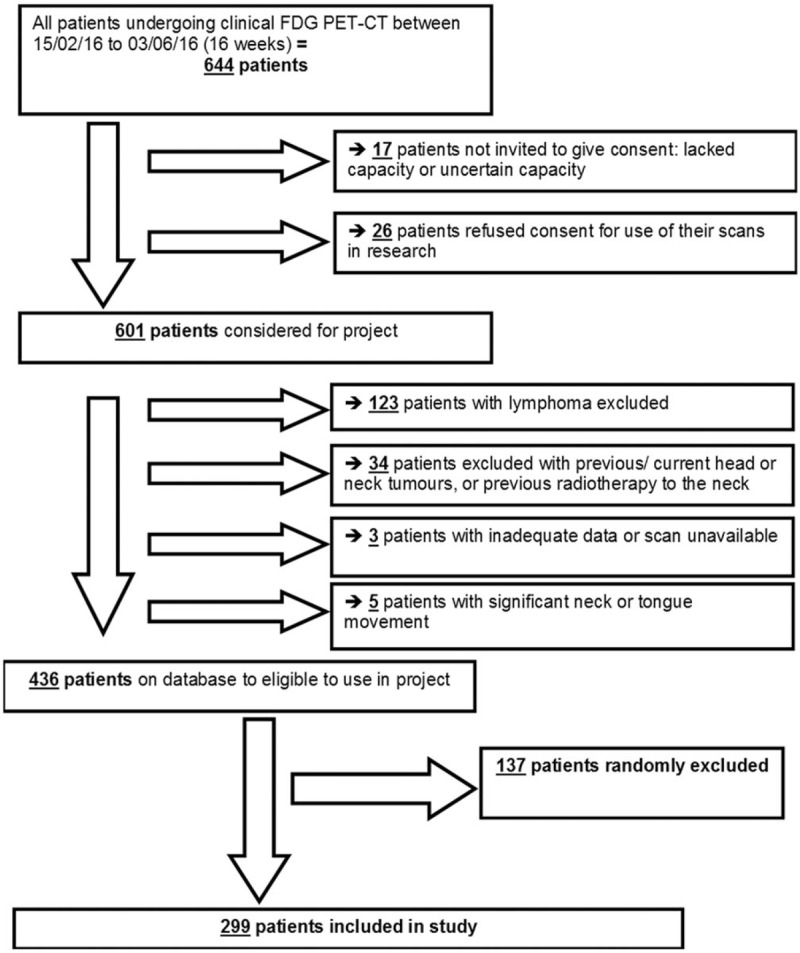
Patient selection flowchart.
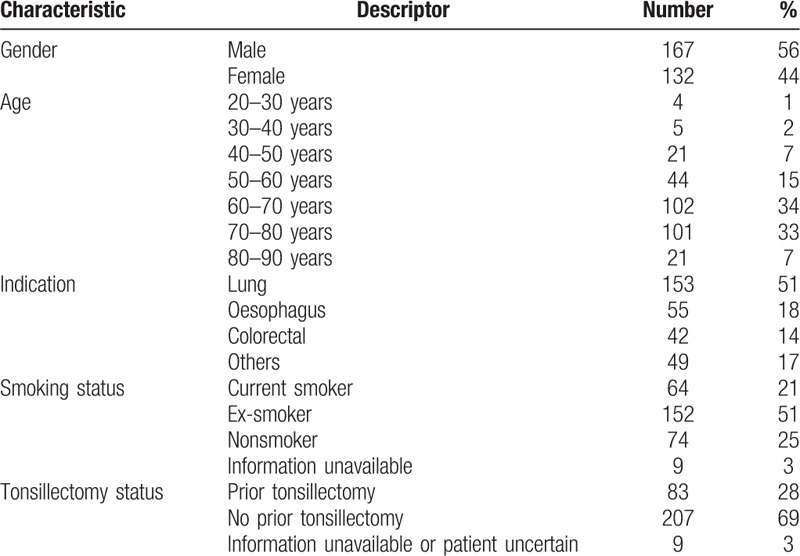
Table 1 outlines the demographic characteristics of the 299 patients enrolled in the study. Of the 299 subjects, 167 were male. The mean age was 66 with a range of 20 to 91 years. Just over half of the patients (153, 51%) included were being imaged for known or suspected lung malignancy. Colorectal carcinoma and oesophageal malignancy were the other main indications for imaging amongst our study population.
Table 1.
Baseline characteristics of patients included in analysis.
3.2. Interobserver reliability of SUV measurements
Using the Intraclass Correlation Coefficient, comparisons were drawn between the two observers for 20 randomly selected patients. The ICC values (left tonsil 0.98, right tonsil 1.0, MBP 0.85, liver 0.93) indicate high levels of inter-observer agreement for all measurements. We have showed that measurements of tonsillar FDG uptake are highly reproducible between blinded observers.
3.3. Normal ranges for SUVmax palatine tonsils
In our large cohort of patients, we have found a wide range of uptake in the palatine tonsils. Figure 2 shows a representative example of uptake in the palatine tonsils and a typical patient with rather high symmetrical FDG uptake in the palatine tonsils. The mean and (range) for uptake in the left palatine tonsil was 4.47 (1.3–10.7) and for the right 4.63 (1.4–11.4). The 5th and 95th percentiles for average tonsil SUVmax is 2.45 to 7.70.
Figure 2.
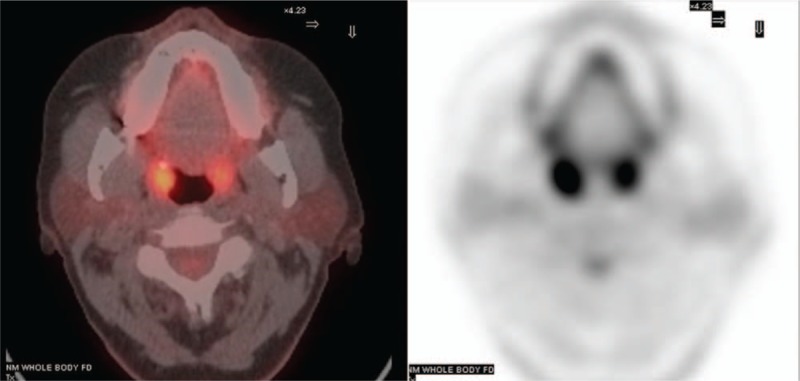
Uptake of FDG in palatine tonsils. Axial FDG PET and fused PET/CT images at the level of the palatine tonsils showing symmetrical increased FDG uptake. FDG = flurodeoxyglucose, PET/CT = positron emission tomography/computed tomography.
3.4. Tonsillar symmetry and asymmetry
Figure 3 shows a scatter plot for the SUVmax of palatine tonsil for all patients. The right and left tonsils show a close correlation within individuals (Pearson's correlation 0.92, P < .01). Figure 4 shows the left- and right-tonsil uptake to be roughly normal distributed (with slight left skewness). On average, the right palatine tonsil shows higher FDG uptake than the left, this is confirmed by the paired T test and The Wilcoxon Signed Ranks Test (R>L = 0.17 P < .01). This difference persists when patients with a stated history of tonsillectomy are excluded (difference for nontonsillectomy patients R>L 0.18 P < .01).
Figure 3.
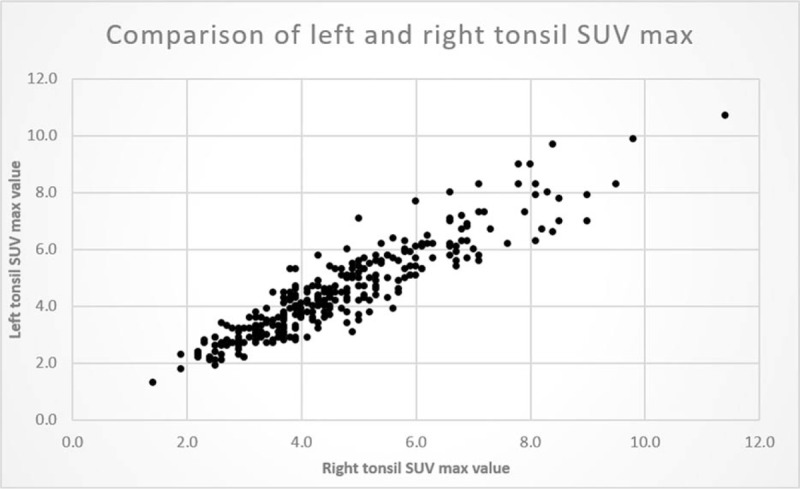
Symmetry and correlation between FDG uptake in tonsils. Scatter plot for SUV max of left and right palatine tonsils for all patients. There is a wide variety of FDG uptake between different patients but a close side-to-side correlation within each patient. FDG = flurodeoxyglucose, SUVmax = maximum standardized uptake value.
Figure 4.
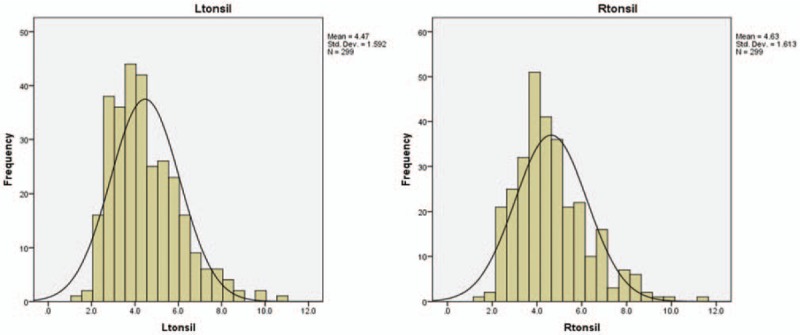
Distribution of SUVmax for palatine tonsils. It displays the frequency distribution of SUVmax for the left and right palatine tonsils. Both distributions are nearly normally distributed with slight left skewness. SUVmax = maximum standardized uptake value.
Interestingly, in the relatively smaller population of only 43 patients analyzed by Wong et al,[13] a higher SUVmax was recorded for the right palatine tonsil compared to the left (R>L 0.14). The magnitude and direction of asymmetry whilst not statistically significant in the previous study is nonetheless nearly exactly the same as seen here.
Whilst it is a common assumption that paired organs are symmetrical, this is often not the case. The right lung has greater volume and mass than the left[17] and right sided weight dominance has been described for the right testis[18] and right lobe of the thyroid gland.[19] There are also laterality differences in incidence of cancer between paired organs.[20] The absolute volume or mass of the palatine tonsils are very difficult to measure in any reliable way either on imaging or surgically. It is nonetheless entirely plausible that the small SUVmax difference seen here (R>L) simply reflects a greater average volume of the right palatine tonsil.
There have been some suggestions that a complex relationship between handedness, asymmetry in peripheral immunity, and lateralization of infections and malignancy may exist.[21] The slight asymmetry in metabolic activity of the palatine tonsils may be an indirect sign of these complex interactions. Finally, anatomic studies have shown systematic asymmetries in the orientation of the laryngeal cartilaginous framework in adults,[22] perhaps contributing to asymmetric measurements. Whilst these issues might be interesting points for speculation, the key finding for PET reporters is that on average the right palatine tonsil has a slightly higher SUVmax than the left, and this impact on the normal ranges.
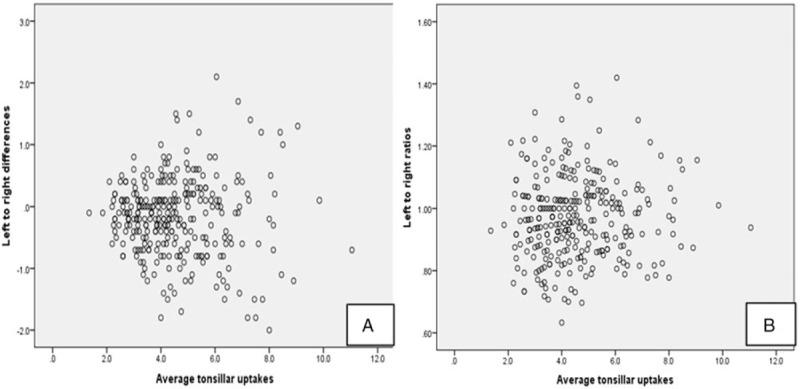
As shown in Figure 5A, higher absolute side-to-side variability occurs at higher average tonsillar uptake values. Figure 5B shows the ratio of L/R palatine tonsil SUVmax which is more evenly distributed over the range of uptake values observed. The 1st to 99th percentiles for L/R SUVmax are 0.70 to 1.36, the 5th to 95th percentiles 0.76–1.20 and 10th to 90th percentiles 0.81–1.14.
Figure 6.
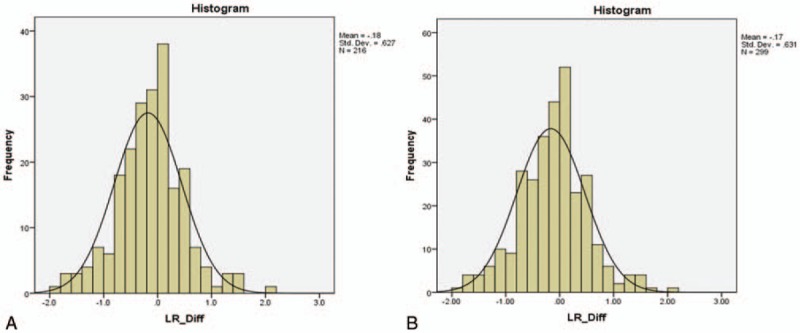
Evaluation of the effect of tonsillectomy on tonsillar symmetry. Left–right tonsillar SUVmax difference plotted against frequency for the whole group (n = 299) (A) and for 209 patients with no prior history of tonsillectomy (B). SUVmax = maximum standardized uptake value.
A previous study proposed an absolute side-to-side difference of 0.83 SUV as a cut off for the range of side-to-side variation.[13] Whilst this is a helpful suggestion, we suggest that it needs to be nuanced in two respects: Firstly, any normal range must reflect physiological tonsillar asymmetry (R>L). Secondly, we have shown that over the range of tonsillar SUVmax, the side-to-side ratio is better preserved than the absolute value difference between the 2 sides. We recommend the side-to-side ratio as a guide to identifying pathology.
In clinical practice, there are 2 distinct clinical scenarios: The first is the situation where the patient is not known or suspected to have tonsillar pathology but asymmetric uptake is incidentally noted on a scan performed for other reasons. In this circumstance, we would suggest that only exceptional asymmetry should be reported and a L/R SUVmax ratio between 0.70 and 1.36 should be considered normal (1st–99th percentiles). On the other hand, if tonsillar pathology is being actively sought, the 10th to 90th percentiles range might be considered (0.81–1.14). Many factors will affect the judgment regarding whether to report tonsillar asymmetry, including the findings on clinical evaluation, other imaging studies and the side of the increased tonsillar uptake in relation to involved lymph nodes. The ranges of variation should however serve as some guide to the extent to which any asymmetry is considered unusual or abnormal.
3.5. Grade of uptake according to “5-point scale”
According to our interpretation of the 5-point scale the numbers of patients with each of the Deauville grades of uptake were: grade 1: 0, grade 2: 13 (4%), grade 3: 45 (15%), grade 4: 202 (68%), grade 5: 39 (13%). Using our definitions 81% of patients either have grade 4 or grade 5 tonsillar FDG uptake. Great caution should therefore be taken in suggesting the presence of active tonsillar lymphoma on interim or end of treatment FDG PET/CT scans. This point has already been stated in imaging recommendations[15] and the current study acts as a reminder that grade 4 or even grade 5 uptake in palatine tonsils should be considered an entirely normal finding.
3.6. Effect of tonsillectomy for benign disease
Around 83 patients gave a prior history of tonsillectomy, 207 patients denied tonsillectomy, (9 patients status unknown). The mean SUVmax for the tonsillectomy patients was 4.13 and for patients denying tonsillectomy was 4.64 (P < .01).
We suggest that the simplest explanation for this is that patients following tonsillectomy have a lower volume of remaining tonsillar tissue and this therefore leads to lower measured SUVmax values, presumably related to partial volume effects. A prior history of tonsillectomy does not however alter the slightly higher uptake in the right palatine tonsil nor the degree of asymmetry between the sides (Fig. 6A and B). Previous observational studies indicate a lower incidence of tonsillar carcinoma in patients with a prior history of tonsillectomy, presumably again due to a lower volume of remaining tonsillar tissue.[23]
Figure 5.
Scatter plots showing the relationship between average tonsillar SUVmax and (A) absolute measured left to right difference as well as (B) left: right ratio. The side-to-side ratio appears to be more robust across the range of tonsillar FDG uptake. FDG = flurodeoxyglucose, SUVmax = maximum standardized uptake value.
3.7. Effect of gender age and scan indication on tonsillar FDG uptake
For further analysis, the mean palatine tonsillar uptake for each patient ((left + right)/2) was assessed against the following variables: gender, age, smoking status, and scan indication.
Gender: The mean (SD) of tonsillar uptake of female patients and male patients are 4.6 (1.5) and 4.5 (1.6), respectively. Two sample T test suggests no difference across gender groups (P = .82), a finding also seen in other observational studies.[8,12,13]
Age: Pearson's correlation between age and average tonsillar uptake is −0.36, which is significant (P < .01). The negative sign indicates a decreasing trend in tonsillar uptake in older patients. This is displayed graphically in Figure 7. This finding is compatible with other observational studies[8,12,13] and is in keeping with the known reduction in size of the palatine tonsils and the declining immune importance of the tonsils with advancing age[24,25]
Scan indication: Amongst the 299 patients, 153 patients had known or suspected lung cancer and 55 patients had oesophageal cancer. Compared to other patients, neither those with suspected lung (4.36 vs 4.67, P = .10), nor oesophageal cancer (4.90 vs 4.67, P = .42) had significantly different levels of FDG uptake.
Figure 7.
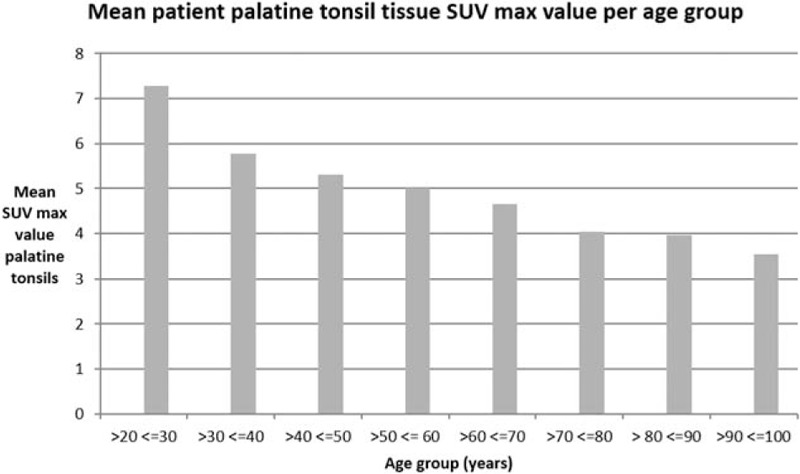
Relationship of age to tonsillar FDG uptake. Bar graph dividing patients into decade age groups, and displaying average SUVmax for each group. There were no patients under the age of 20 in the study. FDG = flurodeoxyglucose, SUVmax = maximum standardized uptake value.
Gender, age, smoking status, and pathology indication were considered initially for regression analysis on average tonsillar uptake. After applying variable selection approaches, in the final model, age, and current smoking status were both confirmed to be associated with lower uptake of FDG in the palatine tonsils (Table 2). In the final model gender and scan indication had no effect of palatine tonsil FDG uptake.
Table 2.
Final model indicating significant factors associated with tonsillar FDG uptake.
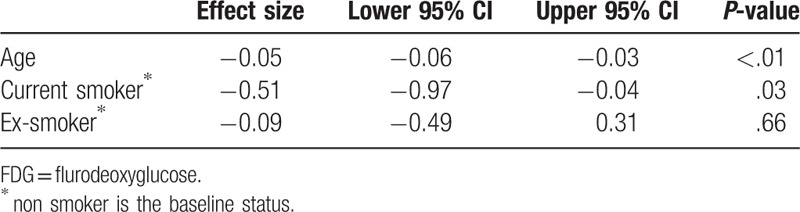
3.8. Smoking status and tonsil FDG uptake
ANOVA analysis shows that there are significant differences (P = .02) in average tonsillar FDG uptake between smokers and nonsmokers. The mean tonsillar SUVmax for nonsmokers was 4.9, whereas in current smokers the mean tonsillar SUVmax was 4.2. Patients identifying themselves as ex-smokers had intermediate SUVmax values of 4.4 (not significantly different from the other groups).
Smoking has a complex effect on the immune system. Whilst many inflammatory processes are triggered by tobacco smoke, some elements of defensive immunity are suppressed by both tobacco generally and nicotine specifically).[26,27] A pilot study of tonsillectomy specimens in smokers and nonsmokers revealed a variety of histological differences.[28] We propose that the reduced FDG uptake demonstrated in smokers reflects overall suppression of tonsillar immune function.
3.9. Weather conditions and tonsillar FDG uptake
For this analysis, mean temperature and rainfall on the scan date, adjusted with age and smoking status were assessed. The results indicate no effect of temperature or rainfall on tonsillar FDG uptake. These results are displayed graphically in Figure 8. The weekly average mean temperature at the beginning of the period was only 4°C, rising steadily to 16°C by the end of the study period indicating a significant variation over the study period. It seems unlikely that there is any major seasonal variation in tonsillar FDG uptake.
Figure 8.
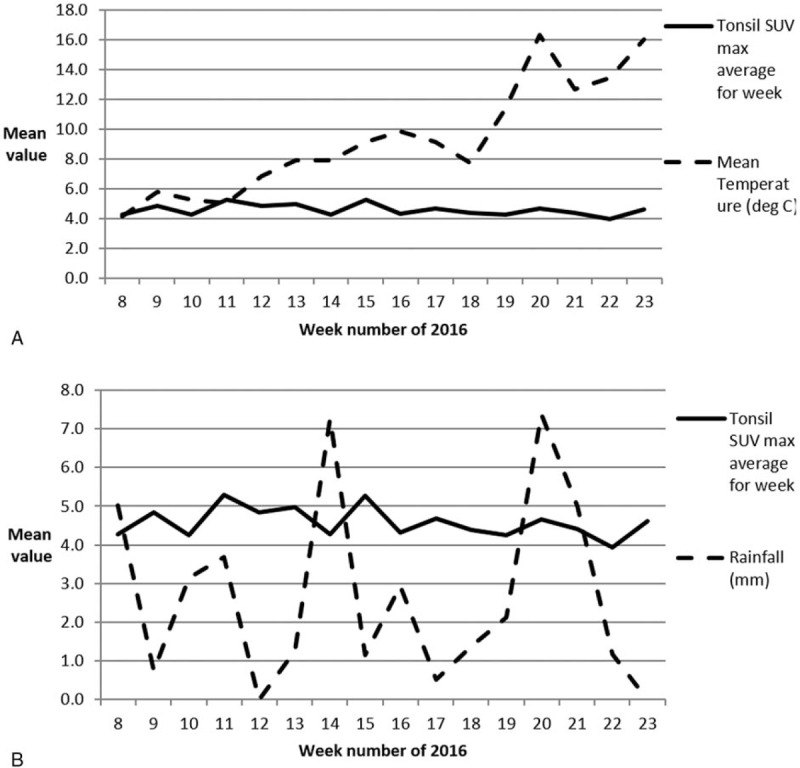
Weather parameters and tonsillar FDG uptake grouped on a weekly basis. (A) Displays average tonsillar SUVmax values for each of 16 weeks plotted against mean temperature throughout the study period. Whilst the temperature rises during the period from February to June there is no obvious change in tonsillar FDG uptake. (B) It shows rainfall averaged over a weekly basis of the 16-week study period. FDG = flurodeoxyglucose, SUVmax = maximum standardized uptake value.
3.10. Patient selection: how “normal” is our population?
In this study, we have attempted to describe the range of normal variation of FDG in palatine tonsils. Our assumption is that none of our patients had focal tonsillar pathology. This is, of course, not necessarily the case and to prove this beyond doubt all of the patients would have to have a comprehensive clinical ENT examination which would be impractical and unethical. The most fundamental challenge with this type of study is that of patient selection. Nearly all of our clinical FDG PET/CT scans were performed either in people with cancer or persons suspected to have cancer. None of our patients are therefore “normal.” The question then arises as to how many patients to exclude and the choice of exclusion criteria. We feel however that we have taken reasonable steps to exclude patients at high risk of focal tonsillar pathology. We did not specifically exclude patients with oesophageal malignancy or lung cancer however; within our population these groups did not differ from the remainder in terms of the level of tonsillar FDG uptake. Our mean values for pharyngeal tonsil uptake are very similar to those reported previously using more rigorous exclusions[13] (left 4.68 and right 4.82). It therefore seems unlikely that we are systematically including significant numbers of people with focal tonsil pathology in our population.
4. Conclusion
There is a wide variation in FDG uptake in the palatine tonsils but a striking side-to-side correlation within each patient. Uptake time and scanner configuration may have effects on FDG uptake and given these and the wide range of variation in our population we suggest that the left/right tonsillar SUVmax ratio is a much more robust and widely applicable parameter than absolute levels of FDG uptake or absolute differences in FDG uptake.
We propose left/right SUVmax ratios as a guide to identify pathology rather than any absolute value for SUVmax. Advancing age, current smoking status, and previous tonsillectomy are all correlated with lower levels of FDG uptake
Author contributions
Conceptualization: Emily Birkin, Patrick A Fielding.
Data curation: Chao Huang, Marshall Christopher, Vetrisudar Jayaprakasam, Patrick A Fielding.
Formal analysis: Emily Birkin, Katherine S Moore, Chao Huang, Patrick A Fielding.
Funding acquisition: Patrick A Fielding.
Investigation: Emily Birkin, Katherine S Moore, Chao Huang, Vetrisudar Jayaprakasam, Patrick A Fielding.
Methodology: Emily Birkin, Chao Huang, John I Rees, Patrick A Fielding.
Project administration: Emily Birkin, Katherine S Moore, Patrick A Fielding.
Resources: Chao Huang, Patrick A Fielding.
Software: Katherine S Moore, Chao Huang, Patrick A Fielding.
Supervision: Marshall Christopher, Patrick A Fielding.
Validation: Emily Birkin, Marshall Christopher, John I Rees, Vetrisudar Jayaprakasam, Patrick A Fielding.
Visualization: Emily Birkin, Patrick A Fielding.
Writing – original draft: Emily Birkin, Katherine S Moore, Chao Huang, Patrick A Fielding.
Writing – review & editing: Emily Birkin, Katherine S Moore, Chao Huang, Marshall Christopher, John I Rees, Vetrisudar Jayaprakasam, Patrick A Fielding.
Footnotes
Abbreviations: CT = computed tomography, FDG = flurodeoxyglucose, kVp = kilovolts peak, mA = milliamperes, MBq = megabequerels, PET = positron emission tomography, PET/CT = positron emission tomography/computed tomography, SUV = standardized uptake value, SUVmax = maximum standardized uptake value.
The authors have no conflicts of interest to disclose.
References
- [1].Scarsbrook AF, Barrington SF. PET-CT in the UK: current status and future directions. Clin Radiol 2016;71:673–90. [DOI] [PubMed] [Google Scholar]
- [2].Agur MR, Ming JL. Neck. Grant's Atlas of Anatomy 10th ed.199;Philadelphia, USA: Lippincott Williams and Wilkins, 686–687. [Google Scholar]
- [3].Brandtzaeg P. Immune functions of nasopharyngeal lymphoid tissue. Adv Otorhinolaryngol 2011;72:20–4. [DOI] [PubMed] [Google Scholar]
- [4].Vaidyanathan S, Patel CN, Scarsbrook AF, et al. FDG PET/CT in infection and inflammation - current and emerging clinical applications. Clin Radiol 2015;70:787–800. [DOI] [PubMed] [Google Scholar]
- [5].Nave H, Gebert A, Pabst R. Morphology and immunology of the human palatine tonsil. Anat Embryol (Berl) 2001;204:367–73. [DOI] [PubMed] [Google Scholar]
- [6].Lee J, Chang DY, Kim SW, et al. Age-related differences in human palatine tonsillar B cell subsets and immunoglobulin isotypes. Clin Exp Med 2016;16:81–7. [DOI] [PubMed] [Google Scholar]
- [7].Tan LT, Ong KL. Semi-quantitative measurements of normal organs with variable metabolic activity on FDG PET imaging. Ann Acad Med Singapore 2004;33:183–5. [PubMed] [Google Scholar]
- [8].Nakamoto Y, Tatsumi M, Hammoud D, et al. Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology 2005;234:879–85. [DOI] [PubMed] [Google Scholar]
- [9].Wang Y, Chiu E, Rosenberg J, et al. Standardized uptake value atlas: characterization of physiological 2-deoxy-2-[18F]fluoro-D-glucose uptake in normal tissues. Mol Imaging Biol 2007;9:83–90. [DOI] [PubMed] [Google Scholar]
- [10].Zincirkeser S, Sahin E, Halac M, et al. Standardized uptake values of normal organs on 18F-fluorodeoxyglucose positron emission tomography and computed tomography imaging. J Int Med Res 2007;35:231–6. [DOI] [PubMed] [Google Scholar]
- [11].Kawabe J, Okamura T, Shakudo M, et al. Physiological FDG uptake in the palatine tonsils. Ann Nucl Med 2001;15:297–300. [DOI] [PubMed] [Google Scholar]
- [12].Sturkenboom MG, Franssen EJ, Berkhof J, et al. Physiological uptake of [18F]fluorodeoxyglucose in the neck and upper chest region: are there predictive characteristics? Nucl Med Commun 2004;25:1109–11. [DOI] [PubMed] [Google Scholar]
- [13].Wong WL, Gibson D, Sanghera B, et al. Evaluation of normal FDG uptake in palatine tonsil and its potential value for detecting occult head and neck cancers: a PET CT study. Nucl Med Commun 2007;28:675–80. [DOI] [PubMed] [Google Scholar]
- [14].Barrington SF, Qian W, Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 2010;37:1824–33. [DOI] [PubMed] [Google Scholar]
- [15].Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014;32:3048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].du Prel JB, Puppe W, Gröndahl B, et al. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis 2009;49:861–8. [DOI] [PubMed] [Google Scholar]
- [17].de la Grandmaison GL, Clairand I, Durigon M. Organ weight in 684 adult autopsies: new tables for a Caucasoid population. Forensic Sci Int 2001;119:149–54. [DOI] [PubMed] [Google Scholar]
- [18].Spyropoulos E, Borousas D, Mavrikos S, et al. Size of external genital organs and somatometric parameters among physically normal men younger than 40 years old. Urology 2002;60:485–9. [DOI] [PubMed] [Google Scholar]
- [19].Yildirim M, Dane S, Seven B. Morphological asymmetry in thyroid lobes, and sex and handedness differences in healthy young subjects. Int J Neurosci 2006;116:1173–9. [DOI] [PubMed] [Google Scholar]
- [20].Roychoudhuri R, Putcha V, Møller H. Cancer and laterality: a study of the five major paired organs (UK). Cancer Causes Control 2006;17:655–62. [DOI] [PubMed] [Google Scholar]
- [21].Sumner RC, Parton A, Nowicky AV, et al. Hemispheric lateralisation and immune function: a systematic review of human research. J Neuroimmunol 2011;240–241:1–2. [DOI] [PubMed] [Google Scholar]
- [22].Hirano M, Kurita S, Yukizane K, et al. Asymmetry of the laryngeal framework: a morphologic study of cadaver larynges. Ann Otol Rhinol Laryngol 1989;98:135–40. [DOI] [PubMed] [Google Scholar]
- [23].Fakhry C, Andersen KK, Christensen J, et al. The impact of tonsillectomy upon the risk of oropharyngeal carcinoma diagnosis and prognosis in the danish cancer registry. Cancer Prev Res (Phila) 2015;8:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mahne A, El-Haddad G, Alavi A, et al. Assessment of age-related morphological and functional changes of selected structures of the head and neck by computed tomography, magnetic resonance imaging, and positron emission tomography. Semin Nucl Med 2007;37:88–102. [DOI] [PubMed] [Google Scholar]
- [25].Yamanaka N, Matsuyama H, Harabuchi Y, et al. Distribution of lymphoid cells in tonsillar compartments in relation to infection and age. A quantitative study using image analysis. Acta Otolaryngol 1992;112:128–37. [DOI] [PubMed] [Google Scholar]
- [26].Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002;2:372–7. [DOI] [PubMed] [Google Scholar]
- [27].Forsslund H, Mikko M, Karimi R, et al. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest 2014;145:711–22. [DOI] [PubMed] [Google Scholar]
- [28].Torre V, Bucolo S, Giordano C, et al. Palatine tonsils in smoker and non-smoker patients: a pilot clinicopathological and ultrastructural study. J Oral Pathol Med 2005;34:390–6. [DOI] [PubMed] [Google Scholar]


