Abstract
The present study was undertaken to determine the membrane-stabilizing effect of Bio-tea in the prevention of myocardial injury caused by isoproterenol in rats. The efficiency of Bio-tea pretreatment was compared against black tea pretreatment and the positive control (rats with isoproterenol-induced myocardial infarction) and negative control (normal control rats). For this purpose, biochemical analysis of the in vivo antioxidants (superoxide dismutase, catalase, and reduced glutathione), glycoprotein components (hexose, hexosamine, sialic acid, and fucose), lipids (total, ester and free cholesterol, triglycerides, free fatty acids, and phospholipids), and transmembrane protein activities (Na+/K+ ATPase, Ca2+ ATPase, and Mg2+ ATPase) was carried out along with the histological and ultrastructural study of the myocardial tissue. Induction of myocardial infarction using isoproterenol resulted in a significant decrease in tissue antioxidants and an increase in the levels of total, ester and free cholesterol, triglycerides, free fatty acids, and glycoprotein components in plasma and heart. The phospholipid content showed an increase in plasma and a simultaneous decrease in the heart tissue, while the Na+/K+ ATPase activity decreased and Ca2+ ATPase and Mg2+ ATPase activities increased, resulting in destabilization of the membranes. Pretreatment with Bio-tea was able to bring these components to near normal, indicating its reactive-oxygen-species-scavenging, lipid-lowering, membrane-stabilizing and glycoprotein-modulating effects and lending credibility to the regular use of Bio-tea.
Keywords: free fatty acids, hexose, Kombucha, Na+/K+ ATPase, phospholipids, superoxide dismutase, transmembrane proteins
1. Introduction
Cardiovascular disease is a term used for a heterogeneous group of disorders that affects the heart and blood vessels. Myocardial infarction (MI) is one of the main causes of cardiovascular death, which is estimated to reach 23.3 million by 2030 [1]. MI results from prolonged myocardial ischemia, which causes necrosis of the myocytes due to interruption of the blood supply to an area of the heart [2]. The period of ischemia is followed by reperfusion, where blood supply returns to the tissue, resulting in inflammation and oxidative stress. Isoproterenol (ISO) is a synthetic catecholamine and β-adrenergic agonist, which causes severe stress in the myocardium due to the generation of free radicals, and stimulates lipid peroxidation, which is a causative factor of irreversible damage to the myocardial membrane [3].
The study of the cardioprotective effects of natural products has shown an upsurge in recent years as they have fewer side effects than synthetic drugs. Kombucha, known as Bio-tea in Karnataka, India, is one such natural product, produced by the fermentation of sugared black tea using a Kombucha pellicle, which is a combination of acetic acid bacteria and yeast. It is a traditional drink that is said to regulate gastric, intestinal, and glandular activities, and relieve joint rheumatism, gout, and hemorrhoids. It has a positive influence on the cholesterol level, arteriosclerosis, diabetes, and aging problems [4]. Efficacy of Bio-tea against hypoglycemic and hypolipidemic effects in diabetes condition, and its hepatoprotective properties have been well studied [5,6].
Although there is a paucity of data regarding the cardioprotective effect of Bio-tea, a previous study in our laboratory has shown that its consumption protects the heart against myocardial injury, probably by preventing membrane destabilization [7]. Thus, the present study was designed to investigate the membrane-stabilizing action of Bio-tea by analyzing the levels of antioxidants, lipids, lipid peroxidation products, glycoprotein components, transmembrane protein activity, and the histopathological analysis of the myocardial tissue.
2. Materials and Methods
2.1. Experimental animals and diet
All the experiments were carried out as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India after approval by the Institutional Animal Ethics Committee (IAEC) of Mangalore University (MU/AZ/99/2013-14/IAEC, dated 2 April 2013). The study was carried out using 24 male albino Wistar rats (Rattus norvegicus) aged 4 months, which were maintained in the Animal House of the Department of Biosciences, Mangalore University, Karnataka, India. The rats were housed in polypropylene cages, lined with husk, renewed every 24 hours under a 12: 12 hour light: dark cycle at around 22°C, food and water was supplied ad libitum, and they were fed a standard pellet diet (Pranav Agro Industries Limited, Maharashtra, India).
2.2. Drugs and chemicals
Isoproterenol hydrochloride, 1,1’,3,3’-tetra methoxy propane, cholesterol, palmitic acid, galactose, mannose, galactosamine hydrochloride, N-acetyl neuraminic acid, adenosine triphosphate (ATP), and cysteine hydrochloride were purchased from Sigma Chemical Company (St. Louis, MO, USA), while all the other chemicals used were of analytical grade.
2.3. Preparation of ISO
ISO was prepared at a dose of 85 mg/kg body weight in cold saline.
2.4. Preparation of Bio-tea
Tea was prepared using 10% (w/v) commercial sucrose and 7.5 g/L (w/v) tea leaves (Brooke Bond Red Label). The tea decoction was cooled to 30°C and filtered into clean glass bottles. Kombucha pellicle from previous culture was placed on it and incubation was carried out at room temperature under aerobic conditions for 7 days. After 7 days, the Bio-tea obtained was filtered and sterilized before refrigeration.
2.5. Experimental protocol
The present study was carried out using four groups of six rats each in the following treatment groups: Group 1: normal control rats; Group 2: ISO-induced control rats; Group 3: tea pretreated + ISO-induced rats; and Group 4: Bio-tea pretreated + ISO-induced rats. Tea and Bio-tea were administered daily to the respective groups orally at a concentration of 1.71 mL/kg [8] for a period of 30 days. MI was induced by subcutaneous administration of ISO (85 mg/kg) on Days 29 and 30 at a 24-hour interval [9]. At the end of the study period, the animals were anesthetized using ketamine (22–24 mg/kg intramuscularly) and blood collected by heart puncture for biochemical estimations. The animals were sacrificed by cervical dislocation and the heart excised.
2.6. Estimation of lipid peroxidation products and antioxidants in heart
The heart thiobarbituric acid reactive substances (TBARS) were estimated as per the protocol of Fraga et al [10] against 1,1’,3,3’-tetra-methoxy propane as standard. The values were expressed as mmol/100 g wet tissue weight. Superoxide dismutase (SOD) in the heart samples was assayed according to Kakkar et al [11] by using phenazine methosulfate and nitroblue tetrazolium along with reduced nicotinamide adenine dinucleotide. The enzyme activity was expressed as units/mg protein, where, one unit of enzyme activity was defined as the enzyme concentration required to inhibit the optical density at 560 nm of chromogen production by 50% in 1 minute. The catalase enzyme activity was estimated by the method of Sinha [12], with dichromate–acetic acid reagent and hydrogen peroxide as standard. The activity of catalase was expressed as μmol hydrogen peroxide consumed/min/mg protein. The estimation of reduced glutathione (GSH) was carried out by the method of Ellman [13] using 5,5’-dithiobis-(2-nitrobenzoic acid) in 1% sodium citrate. The results were expressed as mmol/g wet tissue weight of heart.
2.7. Extraction of lipids and glycoprotein components
Lipids were extracted from plasma and tissues by the method of Folch et al [14]. The tissues were rinsed thoroughly in ice-cold physiological saline and dried using filter paper. The samples were homogenized in cold chloroform–methanol (2:1 v/v) and the contents were extracted after 24 hours. The extraction protocol was repeated until all the lipids were extracted. The filtrates were combined and washed with 0.7% potassium chloride in a separating funnel. The lower lipid layer was made up to a known volume with chloroform and used for various estimations. The defatted samples were suspended in 3 mL 2N hydrochloric acid and digested by heating at 90°C for 4 hours. The digested samples were cooled and neutralized with 3 mL 2N sodium hydroxide. Aliquots from this were used for the estimation of hexose, hexosamine, sialic acid, and fucose.
2.8. Estimation of lipids
The levels of total cholesterol were estimated by the method of Zlatkis et al [15] by using ferric chloride–acetic acid reagent, while the ester and free cholesterol concentrations were estimated by the method of Varley et al [16] against a cholesterol standard. Triglyceride estimation was carried out according to Fossati and Lorenzo [17] and the levels of free fatty acids were estimated as per Falholt et al [18] against palmitic acid standard. Phospholipid estimation was carried out according to Zilversmit and Davis [19] where the samples were digested in Kjeldahl flasks and the inorganic phosphate obtained was estimated. All the results were expressed as mg/dL for plasma and mg/g wet tissue weight for heart.
2.9. Estimation of glycoprotein components
Protein-bound hexose was estimated by the method of DuBois and Gilles [20] by using orcinol–sulfuric acid reagent and galactose–mannose standard, while protein-bound hexosamine was estimated by the method of Wagner [21] using galactosamine hydrochloride as standard. Sialic acid estimation was carried out by the method of Warren [22] using N-acetyl neuraminic acid as standard and fucose was estimated by the method of Dische and Shettles [23], where cysteine reagent was used for the analysis and fucose content was calculated from the differences in absorbance at 393 nm and 430 nm. All the values were expressed as mg/dL for plasma and mg/g defatted tissue for heart.
2.10. Estimation of transmembrane protein activity
The Na+/K+ ATPase activity was assayed according to the protocol of Bonting [24] using 40 mM ATP. The activity of Ca2+ ATPase was estimated as per the method of Hjertén and Pan [25] with 10 mM ATP. Mg2+ ATPase activity was analyzed with 10 mM ATP following the protocol of Ohnishi et al [26]. The above estimations were followed by estimation of the liberated phosphorous by Fiske and Subbarow method [27]. All the results are expressed as μmol Pi liberated/min/mg protein.
2.11. Light microscopy and transmission electron microscopy
Heart tissues from all experimental groups were washed and fixed in 10% buffered neutral formaldehyde solution. After fixation, the tissues were processed by dehydrating in alcohol, followed by xylene prior to embedding in paraffin wax. Sections of 5–7 μm thickness were subjected to hematoxylin and eosin staining and observed under a light microscope.
For the ultrastructural study, 2.5% buffered gluteraldehyde and post fixation was carried out in 1% osmium tetroxide. Dehydration was carried out using varying grades of alcohol followed by propylene oxide prior to embedding in resin. Tissue sectioning was carried out using a Leica EM UC6 Ultramicrotome, Germany, stained with uranyl acetate and lead citrate, observed under a transmission electron microscope (Tecnai G2 Spirit Bio-twin, USA), and representative areas were photographed using a Megaview III CCD camera.
2.12. Statistical analysis
The data were statistically analyzed using one-way analysis of variance followed by Duncan's multiple range test (IBM SPSS Statistics 20.0). A value of p < 0.05 was considered statistically significant. The values were expressed as mean ± standard error of the mean.
3. Results
3.1. Lipid peroxidation products and antioxidants in heart
The initial cellular defense against any oxidative stress is via GSH and the antioxidant enzymes SOD and catalase [28]. Our study showed a significant drop in the levels of these endogenous antioxidants in the heart during ISO-induced MI, along with an increase in the products of lipid peroxidation, TBARS, when compared to normal control rats (Table 1). Oral pretreatment with tea decreased the levels of TBARS and significantly protected the antioxidants, while, results of higher significance were observed upon pretreatment of rats with Bio-tea.
Table 1.
Effect of Bio-tea on lipid peroxidation products and antioxidants in heart tissue.
| Variable | Control | ISO control | Tea + ISO | BT + ISO |
|---|---|---|---|---|
| TBARS | 0.632 ± 0.03a | 1.098 ± 0.03d | 0.897 ± 0.03c | 0.821 ± 0.02b |
| SOD | 11.236 ± 0.29c | 7.880 ± 0.45a | 9.805 ± 0.41b | 10.579 ± 0.37c |
| CAT | 7.040 ± 0.38c | 3.419 ± 0.24a | 5.562 ± 0.67b | 6.399 ± 0.47c |
| GSH | 7.533 ± 0.34d | 4.224 ± 0.43a | 6.018 ± 0.24b | 6.995 ± 0.32c |
Values are mean ± standard error of the mean for six rats in each group. Values not sharing a common superscript differ significantly from each other (p < 0.05, Duncan's multiple range test). Values expressed as mmol/100 g wet tissue weight for TBARS; U/mg protein for SOD; μmol hydrogen peroxide consumed/min/mg protein for CAT; and mmol/g wet tissue weight for GSH. BT = Bio-tea; CAT = catalase; GSH = reduced glutathione; ISO = Isoproterenol;SOD = superoxide dismutase; TBARS = thiobarbituric acid reactive substances.
3.2. Lipid profile
The alteration of lipid profile in the plasma and heart of the control and pretreated rats is shown in Tables 2 and 3, respectively. The levels of free fatty acids, triglycerides, phospholipids, and total cholesterol, increased in the plasma upon administration of ISO. Pretreatment with Bio-tea was able to decrease the concentrations of triglycerides, while maintaining the levels of free fatty acids, phospholipids, and total cholesterol at near normality. The tissue concentrations of free fatty acids, triglycerides, total cholesterol, cholesterol ester, and free cholesterol increased during MI, while the tissue phospholipids content decreased drastically. Pretreatment with Bio-tea was able to protect the heart tissue during MI by maintaining all these levels at near normality.
Table 2.
Effect of Bio-tea on plasma lipids in MI rats.
| Variable | Control | ISO control | Tea + ISO | BT + ISO |
|---|---|---|---|---|
| Free fatty acids | 28.64 ± 1.98a | 44.74 ± 3.40c | 31.68 ± 2.30b | 25.56 ± 1.16a |
| Triglycerides | 40.75 ± 1.93a | 52.25 ± 1.49c | 46.50 ± 1.88c | 43.75 ± 1.42b |
| Phospholipids | 36.50 ± 2.37a | 53.40 ± 3.61b | 48.13 ± 1.60b | 42.06 ± 2.86a |
| Total cholesterol | 64.50 ± 2.21a | 87.00 ± 1.41c | 71.75 ± 1.93b | 66.75 ± 1.49a |
Values are mean ± standard error of the mean for six rats in each group. Values not sharing a common superscript differ significantly from each other (p < 0.05, Duncan's multiple range test). All the results are expressed as mg/dL. BT = Bio-tea; ISO = isoproterenol induced.
Table 3.
Effect of Bio-tea on myocardial lipids in MI rats.
| Variable | Control | ISO Control | Tea + ISO | BT + ISO |
|---|---|---|---|---|
| Free fatty acids | 3.37 ± 0.33a | 4.87 ± 0.40c | 3.70 ± 0.26b | 3.54 ± 0.15a |
| Triglycerides | 3.30 ± 0.28a | 6.87 ± 0.41c | 5.12 ± 0.38b | 3.85 ± 0.27a |
| Phospholipids | 14.07 ± 0.56b | 10.82 ± 0.37a | 11.83 ± 0.47a | 13.60 ± 0.35b |
| Total cholesterol | 4.13 ± 0.33a | 7.75 ± 0.39c | 5.79 ± 0.38b | 4.52 ± 0.29a |
| Ester cholesterol | 2.99 ± 0.20a | 5.48 ± 0.25c | 4.23 ± 0.28b | 3.05 ± 0.23a |
| Free cholesterol | 1.13 ± 0.21a | 2.24 ± 0.19c | 1.56 ± 0.20b | 1.19 ± 0.10a |
Values are mean ± standard error of the mean for six rats in each group. Values not sharing a common superscript differ significantly from each other (p< 0.05, Duncan's multiple range test). All the results are expressed as mg/g wet tissue weight. BT = Bio-tea; ISO = isoproterenol.
3.3. Glycoprotein components
Tables 4 and 5 show the concentrations of glycoproteins components (hexose, hexosamine, sialic acid, and fucose) in the plasma and heart of normal and ISO-induced MI rats. The levels of all the glycoprotein components increase drastically in both plasma and heart tissue of ISO-treated control rats when compared to the normal control rats. Pretreatment with tea is able to control the rise in glycoprotein component levels, while pretreatment with Bio-tea is able to maintain their levels at near normality.
Table 4.
Effect of Bio-tea on plasma glycoprotein components in MI rats.
| Variable | Control | ISO Control | Tea + ISO | BT + ISO |
|---|---|---|---|---|
| Hexose | 175.31 ± 6.21a | 214.16 ± 6.89c | 190.59 ± 7.22b | 171.72 ± 6.40a |
| Hexosamine | 44.83 ± 3.69a | 65.33 ± 4.06c | 49.49 ± 1.64b | 42.34 ± 2.35a |
| Sialic acid | 58.72 ± 4.0a | 75.34 ± 2.53c | 64.42 ± 2.48b | 59.01 ± 1.90a |
| Fucose | 48.83 ± 3.96a | 65.29 ± 2.60c | 54.88 ± 2.48b | 49.50 ± 1.55a |
Values are mean ± standard error of the mean for six rats in each group. Values not sharing a common superscript differ significantly from each other (p < 0.05, Duncan's multiple range test). All the results are expressed as mg/dL. BT = Bio-tea; ISO = isoproterenol.
Table 5.
Effect of Bio-tea on myocardial glycoprotein components in MI rats.
| Variable | Control | ISO Control | Tea + ISO | BT + ISO |
|---|---|---|---|---|
| Hexose | 159.31 ± 3.70a | 201.0 ± 4.63c | 173.42 ± 5.21b | 166.88 ± 4.10a |
| Hexosamine | 5.94 ± 0.26a | 10.83 ± 0.40d | 9.55 ± 0.23c | 7.31 ±0.34b |
| Sialic acid | 45.61 ± 0.61a | 58.49 ± 1.09c | 53.55 ± 1.13b | 48.14 ±0.95a |
| Fucose | 35.67 ± 0.63a | 48.59 ± 1.13c | 43.54 ± 1.13b | 38.12 ±0.84a |
Values are mean ± standard error of the mean for six rats in each group. Values not sharing a common superscript differ significantly from each other (p < 0.05, Duncan's multiple range test). All the results are expressed as mg/g defatted tissue weight. BT = Bio-tea; ISO = isoproterenol.
3.4. Transmembrane protein activity
A significant decrease in the activity of Na+/K+ ATPase and a corresponding increase in the activities of Ca2+ ATPase and Mg2+ ATPase were observed in MI rats when compared to normal control rats (Table 6). Pretreatment with Bio-tea was able to efficiently prevent the increase in activity of Mg2+ ATPase and maintain the activities of Na+/K+ ATPase and Ca2+ ATPase at near normality. However, pretreatment with tea did not cause any significant alterations in their activities when compared ISO control rats.
Table 6.
Effect of Bio-tea on transmembrane proteins in the heart in MI rats.
| Variable | Control | ISO Control | Tea + ISO | BT + ISO |
|---|---|---|---|---|
| Na+/K+ ATPase | 0.538 ± 0.02b | 0.297 ± 0.03a | 0.333 ± 0.02a | 0.477 ± 0.03b |
| Ca2+ ATPase | 0.805 ± 0.04a | 1.795 ±0.08b | 1.426 ±0.07b | 0.929 ± 0.09a |
| Mg2+ ATPase | 5.438 ± 0.24a | 8.447 ± 0.36c | 7.233 ± 0.34c | 6.383 ± 0.34b |
Values are mean ± standard error of the mean for six rats in each group. Values not sharing a common superscript differ significantly from each other (p < 0.05, Duncan's multiple range test). All the results are expressed as μmol Pi liberated/min/mg protein. BT = Bio-tea; ISO = isoproterenol.
3.5. Light microscopy and transmission electron microscopy
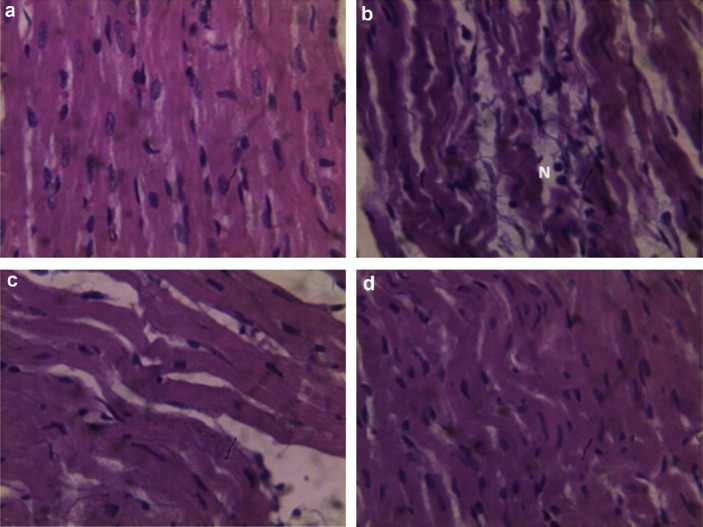
Figures 1A–1D show the effect of Bio-tea on the extent of histopathological changes in the myocardial tissues of normal and ISO-treated control rats. Figure 1A is a light micrograph of rat heart in the negative control group, showing normal architecture without edema and lack of neutrophil infiltration. The histological study of the ISO-induced MI (positive control group) shows edema leading to mucopolysaccharide deposition, wavy myocardial fibers due to slippage of myofibrillar alignment, infarcted zones, necrosis, and separation of cardiac muscle fibers and neutrophil infiltration, which are characteristic during myocardial ischemia (Figure 1B). High levels of plasma cardiac injury markers observed in a previous study carried out in our laboratory [7] clearly support the destruction of cardiomyocytes. In rats pretreated with tea, there was less edema and decreased waviness of the muscle fibers along with less neutrophil infiltration (Figure 1C). Oral pretreatment with Bio-tea showed protective effects in ISO-induced MI as demonstrated by lack of edema and the absence of characteristic neutrophil invasion compared to ISO-induced MI and near normalization of the myocardial fibers (Figure 1D).
Fig. 1.
Histological study of heart tissue using light microscopy. (A) Structure of normal heart tissue in rat in control group. (B) Structure of ISO-induced MI heart tissue with wavy muscle fibers, edema, and neutrophil infiltration. (C) Structure of heart tissue from tea-pretreated rat, showing reduced edema, wavy muscle fibers, and decreased neutrophil infiltration. (D) Structure of heart tissue from Bio-tea-pretreated rats, showing mildly wavy muscle fibers, no edema, and absence of neutrophil infiltration (40×). MI = myocardial infarction; N = neutrophils.
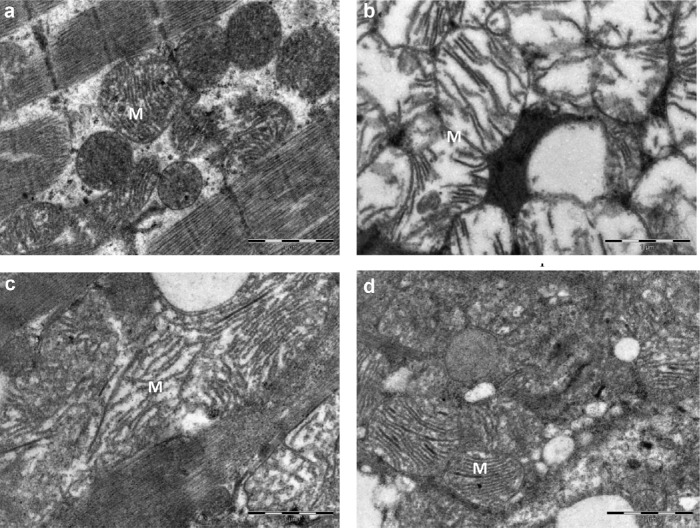
Figures 2A–2D show the ultrastructural study of the effect of Bio-tea on the subcellular membranes in the myocardial tissues of normal and ISO-treated control rats. The ISO-treated heart cells showed swollen mitochondria with disrupted cristae (Figure 2B) when compared to normal control rats (Figure 2A). In Figure 2C, the ultrastructure of the heart muscle of tea-pretreated rats showed less swollen mitochondria than in the ISO control, with reduced separation of cristae. Pretreatment with Bio-tea considerably reduced the damage due to ISO, as indicated by the near normal architecture of the mitochondria (Figure 2D).
Fig. 2.
Effect of Bio-tea on the ultrastructure of the heart in MI rats. (A) Heart of normal control rat. Note the compact mitochondria and muscle fibers. (B) Heart of rat with ISO-induced MI. Note the swollen mitochondria and disrupted cristae. (C) Heart of black tea pretreated rat with mildly swollen mitochondria and reduced separation of cristae. (D) Heart of Bio-tea pretreated rat with near normal architecture of mitochondria. Scale bar 1 μm. ISO = isoprenaline; M = mitochondria.
4. Discussion
Ischemia followed by reperfusion causes an increase in the levels of the reactive oxygen species leading to oxidative stress [29], which plays a major role in lipid peroxidation and the degenerative changes that follow. Under normal physiological conditions, endogenous antioxidants keep the levels of oxidative stress under control. During ISO-induced myocardial injury, there is a drastic decrease in the levels of the in vivo antioxidants along with an increase in TBARS levels. Pretreatment with Bio-tea was able to prevent the increase in the levels of lipid peroxidation and curtailed the loss of antioxidants, indicating its ability to relieve oxidative stress. Previous studies in our laboratory have shown that Bio-tea is a rich source of polyphenols and flavonoids such as gallic acid, quercetin, and various catechins [30]. Quercetin has been shown to prevent the development of atherosclerosis by reducing lipid peroxidation [31]. Quercetin, gallic acid, and polyphenols also act as antioxidants by chelating iron and preventing free radical damage [32], thereby adding to its in vivo antioxidant potential. In addition to this, TBARS, which is a marker of oxidative stress, has also been shown to decrease after intake of mixed catechins [33], indicating that, catechins have a direct (antioxidant) as well as indirect (increase of activity or expression) effect. Quercetin also increases the concentration of GSH, which is essential for membrane stability [31], while catechins increase the levels of SOD and catalase [34].
ISO-induced MI is associated with increased levels of circulatory lipids, which cause hypercholesterolemia and atherogenesis due to unregulated accumulation of lipids in tissues. Increase in cholesterol levels leads to increased membrane fluidity and permeability, which in turn alters the cytoplasmic viscosity and its chemical composition [35]. Increased levels of triglycerides are associated with cardiovascular disturbances as ISO promotes lipolysis in the myocardium [36]. The altered levels of phospholipids in ISO-induced MI rats might be due to enhanced membrane degradation and the increased peroxidation of membrane phospholipids and subsequent release of free fatty acids by the action of phospholipase A2 [37]. Bio-tea catechins successfully maintain the plasma and tissue lipid levels at near normality. This is because catechins not only decrease the absorption of cholesterol and triglycerides and increases fat excretion [38] but also suppress the activities of phospholipase A2 thereby protecting the membrane [39].
Glycoproteins are important components of intracellular matrix, cell membrane and membranes of the subcellular organelles, and are also involved in myocardial necrosis and repair [40]. Hexose, hexosamine, sialic acid, and fucose are the basic components of glycoproteins and are reported to be significantly increased in cardiovascular diseases [41]. The accumulation of free fatty acids (mentioned above) inhibits pyruvate oxidation, which in turn causes the accumulation of glucose in the heart and plasma. The accumulated hexose is converted to hexosamine, causing an increase in its level [42]. The observed changes in myocardial hexosamine are also a reflection of accumulation of plasma glycoproteins, which form mucopolysaccharides, during early myocardial necrosis and subsequent repair of the myocardium [43]. Thus, the degree of myocardial hexosamine is a good index of the severity of myocardial damage. Sialic acid is present as terminal sugars on oligosaccharides attached to protein or lipid moieties and appear in the coatings of cell surfaces and in secretions. It has been reported that sialic acid concentration is increased in the plasma of patients with MI [44] and hypertriglyceridemia [45]. The increase in plasma sialic acid concentration may be a result of the shedding or secretion of sialic acid and fucose from the cell membrane surface or due to the increased synthesis of acute-phase proteins involved in chronic inflammatory process [46]. The components of Bio-tea are able to indirectly modulate the levels of plasma and tissue glycoprotein by upregulating antioxidant activity and minimizing lipid peroxidation.
The activities of the membrane-bound enzymes involved in the transportation of ions are directly dependent on the membrane lipid microenvironment and the stability of the membrane [35]. Therefore, the determination of membrane-bound enzyme activities will indicate any alteration to the membrane physiology under pathological conditions. Under resting conditions, Na+ diffuses in while K+ diffuses out of a cell [47]. This leads to an imbalance in the intracellular and extracellular concentrations of these ions, which is brought back to normal by the Na+/K+ ATPase. The decrease in the activity of Na+/K+ ATPase in MI can be attributed to its inactivation due to the lipid peroxidation of the membrane caused by the ROS generated by ISO or due to decrease in ATP levels due to mitochondrial damage. This leads to the accumulation of Na+ within the cell and decrease in intracellular K+ levels, causing depolarization of the membrane. The accumulation of Na+ inside the cells inactivates the Na+/Ca2+ translocator, thereby preventing the movement of Ca2+ out of the cell. The resulting calcium overload can damage mitochondria, alter cellular function, and increase the activity of phospholipase A2 [48]. Increase in intracellular Ca2+ levels results in an increase in the Ca2+ ATPase activity in ISO-treated rats. Ca2+ ATPases act by utilizing ATP for the translocation of Ca2+ out of the cell. This causes depletion in the ATP reserves, which further inhibit the activity of Na+/K+ ATPases [47]. Mg2+ ATPase functions by pumping magnesium ions into the cell. Magnesium ions are crucial in the regulation of Na+/K+ ATPases as low levels of Mg2+ activate Na+/K+ transport, while higher concentrations inhibit the same [49]. Magnesium ions also form calcium–magnesium interactions at the cellular level, preventing ischemic deposition of calcium in cardiac mitochondria in MI. However, the increase in intracellular Mg2+ leads to the inhibition of Na+/K+ ATPase, thus further destabilizing the membrane. The protective effects observed in rats pretreated with Bio-tea could be due to the blockage of ISO-induced calcium influx, thus providing protection to the mitochondria against ISO-induced damage. Pretreatment with Bio-tea also prevents the cascade of changes that cause the accumulation of Mg2+, thereby protecting the membranes.
Neutrophils are the primary source of ROS during reperfusion followed by endothelial cells, which also generate ROS. The increased ROS production is mainly due to activation of xanthine oxidase in endothelial cells, and nicotinamide adenine dinucleotide phosphate oxidase in the inflammatory cells [50]. ROS and inflammatory cytokines further activate the matrix metalloproteinases [51], which degrade collagens, causing slippage in myofibrillar alignment, resulting in left ventricular dilatation [52]. Increased ROS levels also modify phospholipids and proteins, leading to lipid peroxidation and thiol group oxidation, which leads to the alteration of membrane permeability and configuration [53]. Furthermore, neutrophils adhere to the endothelium and generate higher quantities of ROS which directly injure the cell membrane and cause cell death [54]. Pretreatment with Bio-tea decreases edema and neutrophil infiltration, as indicated by the histopathological studies, due to which there is a decrease in the synthesis of acute phase glycoproteins and their glycoconjugates, thus playing a protective role during ISO-induced MI. Catechins could also contribute towards inhibition of in vivo accumulation of ROS by inhibiting the activity of the enzyme xanthine oxidase which catabolizes purines to produce uric acid and ROS [55].
Transmission electron microscopic analysis of the myocardial tissue demonstrated the degenerative changes caused by the administration of ISO and the disruption of the membranes was apparent. Pretreatment with tea exhibits some protection to the cardiomyocytes, as shown by the biochemical tests and ultrastructure. However, the membrane-stabilizing effects of Bio-tea were clearly evident in the near normal architecture of the heart tissue of rats pretreated with Bio-tea.
5. Conclusion
The results of the present study indicate that Bio-tea pretreatment ameliorates ISO-induced MI due to its ROS-scavenging, lipid-lowering, glycoprotein-modulating, and membrane-stabilizing effects. Thus, regular supplementation with Bio-tea is beneficial to the body as it helps to cope with various stressful situations. Other natural products such as green tea, red wine, and blackberries are also rich in polyphenols. However, Bio-tea consumption is advantageous as it is easily available and can be economically produced.
Acknowledgements
The authors are grateful to Prof. B. K. Chandrasekhar Sagar, Additional professor and Mrs. Hema, Technical assistant, Electron Microscopy-Common Research Facility, Department of Neuropathology, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, Karnataka, India, for help with the transmission electron microscopic study.
References
- [1].World Health Organization. Global status report on noncommu-nicable diseases 2010. Geneva: WHO; 2011. [Google Scholar]
- [2].Reisner E, Reisner H. Crowley’s an introduction to human disease. Burlington, MA: Jones & Bartlett; 2016. pp. 277–9. [Google Scholar]
- [3].Meeran MFN, Selvaraj P. protective efficacy of thymol on glycoproteins in isoproterenol induced myocardial infarcted rats: an in vivo and in vitro study. Int J Pharm Biol Arch. 2014;5:168–73. [Google Scholar]
- [4].Jayabalan R, Malbasa RV, Loncar ES, Vitas JS, Sathishkumar M. A review on kombucha tea – microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Sci F. 2014;13:538–50. doi: 10.1111/1541-4337.12073. [DOI] [PubMed] [Google Scholar]
- [5].Aloulou A, Hamden K, Elloumi D, Ali MB, Hargafi K, Jaouadi B, et al. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Altern Med. 2012;12:63–71. doi: 10.1186/1472-6882-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Y, Ji B, Wu W, Wang R, Yang Z, Zhang D, et al. Hepato-protective effects of kombucha tea: identification of functional strains and quantification of functional components. J Sci Food Agric. 2014;94:265–72. doi: 10.1002/jsfa.6245. [DOI] [PubMed] [Google Scholar]
- [7].Lobo RO, Shenoy KC. Myocardial potency of Bio-tea against Iso-proterenol induced myocardial damage in rats. J Food Sci Tech. 2015;52:4491–8. doi: 10.1007/s13197-014-1492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shenoy C. Hypoglycemic activity of bio-tea in mice. Indian J Exp Biol. 2000;38:278–9. [PubMed] [Google Scholar]
- [9].Goyal S, Siddiqui MK, Siddiqui KM, Arora S, Mittal R, Joshi S, et al. Cardioprotective effect of ‘Khamira Abresham Hakim Arshad Wala’ a unani formulation in isoproterenol-induced myocardial necrosis in rats. Exp Toxicol Pathol. 2009;62:61–74. doi: 10.1016/j.etp.2009.02.115. [DOI] [PubMed] [Google Scholar]
- [10].Fraga CG, Leibovitz BE, Tappel AL. Lipid peroxidation measured as thiobarbituric acid reactive substances in tissue slices: characterization and comparison with homogenate and microsomes. Free Radic Biol Med. 1988;4:155–61. doi: 10.1016/0891-5849(88)90023-8. [DOI] [PubMed] [Google Scholar]
- [11].Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- [12].Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- [13].Ellman GC. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- [14].Folch J, Less M, Solane SGH. A simple method for isolation and purification of lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- [15].Zlatkis A, Zak B, Boyle GJ. A simple method for determination of serum cholesterol. J Clin Med. 1953;41:486–92. [PubMed] [Google Scholar]
- [16].Varley H, Gowenlock AH, Bell M. Determination of free and ester cholesterol. In: Varley H, editor. Practical clinical biochemistry. New Delhi: CBS Publishers; 1991. pp. 311–6. [Google Scholar]
- [17].Fossati P, Lorenzo P. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–80. [PubMed] [Google Scholar]
- [18].Falholt K, Falholt W, Lund B. An easy colorimetric method for routine determination of free fatty acids in plasma. Clin Chim Acta. 1973;46:105–11. doi: 10.1016/0009-8981(73)90016-8. [DOI] [PubMed] [Google Scholar]
- [19].Zilversmit BB, Davis AK. Microdetermination of plasma phos-pholipids by trichloroacetic acid precipitation. J Lab Clin Med. 1950;35:155–61. [PubMed] [Google Scholar]
- [20].DuBois M, Gilles KA. Methods in enzymology. New York: Academia Press; 1956. [Google Scholar]
- [21].Wagner WD. A more sensitive assay discriminating galactosamine and glucosamine in mixture. Anal Biochem. 1979;94:94–396. doi: 10.1016/0003-2697(79)90379-8. [DOI] [PubMed] [Google Scholar]
- [22].Warren L. Thiobarbituric acid assay of sialic acid. J. Biol. Chem. 1959;234:1974–5. [PubMed] [Google Scholar]
- [23].Dische L, Shettles LB. Specific colour reactions of methyl pentoses and spectrophotometric micromethod for their determination. J. Biol Chem. 1948;175:595–604. [PubMed] [Google Scholar]
- [24].Bonting SL. Sodium-potassium activated adenosinetriphosphatase and cation transport. In: Bittar EE, editor. Membranes and Ion Trans-port. New York: Wiley-Interscience; 1970. pp. 257–363. [Google Scholar]
- [25].Hjertén S, Pan H. Purification and characterization of two forms of low-affinity Ca2+-ATPase from erythrocyte membrane. Biochim. Biophys. Acta. 1983;728:281–8. doi: 10.1016/0005-2736(83)90480-7. [DOI] [PubMed] [Google Scholar]
- [26].Ohnishi T, Suzuki Y, Suzuki Y, Ozawa K. A comparative study of plasma membrane Mg2+ ATPase activities in normal, regenerating and malignant cells. Biochim. Biophys. Acta. 1982;684:67–74. doi: 10.1016/0005-2736(82)90050-5. [DOI] [PubMed] [Google Scholar]
- [27].Fiske CH, Subbarow Y. The colorimetric determination of phosphorous. J. Biol. Chem. 1925;66:375–400. [Google Scholar]
- [28].Rodrigo R, Prieto JC, Castillo R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n-3 fatty acids: molecular mechanisms and potential clinical applications. Clin. Sci. 2013;124:1–15. doi: 10.1042/CS20110663. [DOI] [PubMed] [Google Scholar]
- [29].Eaton PH, Clements-Jewery H. Peroxynitrite: in vivo cardioprotectant or arrhythmogen. Brit. J. Pharmacol. 2008;155:972–3. doi: 10.1038/bjp.2008.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lobo R.O, Dias F.O, Shenoy K.C. Kombucha for healthy living: eval-uation of antioxidant potential and bioactive compounds. Int. FoodRes. J. (in press) [Google Scholar]
- [31].Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Aβ (1-42): relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009;20:269–75. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Bio-phys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- [33].Yokozawa T, Nakagawa T, Kitani K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J. Agri Food Chem. 2002;50:3549–52. doi: 10.1021/jf020029h. [DOI] [PubMed] [Google Scholar]
- [34].Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine. 2002;9:232–8. doi: 10.1078/0944-7113-00119. [DOI] [PubMed] [Google Scholar]
- [35].Subashini R. Pretreatment with Nelumbo nucifera leaf extract ameliorates on lipids, lipoproteins, marker enzymes of lipid metabolism and ECG pattern against isoproterenol induced cardiotoxicity. Int. J Pharm Pharmaceutical Sci. 2014;6:459–64. [Google Scholar]
- [36].Sushma Kumari S, Varghese A, Muraleedharan D, Menon VP. Protective action of aspirin in experimental myocardial infarction induced by isoproterenol in rats and its effect on lipid peroxidation. Indian J. Exp Biol. 1990;28:480–5. [PubMed] [Google Scholar]
- [37].Wei-hua L, Jun-yu H, Chang-qing S, Yong-jun G, Qiang X, Kai-min L, et al. Study on the relationship of cPLA2, CK-MB, and membrane phospholipid content in acute myocardial infarction. Heart Vessels. 2011;26:64–8. doi: 10.1007/s00380-010-0031-2. [DOI] [PubMed] [Google Scholar]
- [38].Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr Biochem. 2003;14:326–32. doi: 10.1016/s0955-2863(03)00054-8. [DOI] [PubMed] [Google Scholar]
- [39].Yang JA, Choi JH, Rhee SJ. Effects of green tea catechin on phospholipase A2 activity and antithrombus in streptozotocin diabetic rats. J. Nutr Sci Vitaminol. 1999;45:337–46. doi: 10.3177/jnsv.45.337. [DOI] [PubMed] [Google Scholar]
- [40].Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined-a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J. American Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- [41].Lindberg G, Eklund GA, Gullberg B, Råstam L. Serum sialic acid concentration and cardiovascular mortality. BMJ (Clinical research ed) 1991;302:143–6. doi: 10.1136/bmj.302.6769.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McNulty PH. Hexosamine biosynthetic pathway flux and cardiomyopathy in type 2 diabetes mellitus. Focus on “Impact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart”. Am. J Physio Cell Physio. 2007;292:C1243–4. doi: 10.1152/ajpcell.00521.2006. [DOI] [PubMed] [Google Scholar]
- [43].Judd JT, Wexler BC. Myocardial connective tissue metabolism in response to injury.histological and chemical studies of mucopolysaccharide and collagen in rat hearts after isoproterenol-induced infarction. Circulation Res. 1969;25:201–14. doi: 10.1161/01.res.25.2.201. [DOI] [PubMed] [Google Scholar]
- [44].Crook M, Haq M, Haq S, Tutt P. Plasma sialic acid and acute phase proteins in patients with myocardial infarction. Angiology. 1994;45:709–15. doi: 10.1177/000331979404500806. [DOI] [PubMed] [Google Scholar]
- [45].Lindberg G, Rastom L, Nilsson-Ehle P, Lundblad A, Ramstam J, Folsom AR, et al. Serum sialic acid and a sialoglycoproteins in asymptomatic carotid artery atherosclerosis, ARIC Investigators, Atherosclerosis Risk in Communities. Atherosclerosis. 1999;146:65–9. doi: 10.1016/s0021-9150(99)00130-6. [DOI] [PubMed] [Google Scholar]
- [46].Nandave M, Ojha SK, Kaur R. Changes in levels of serum glycoproteins in major depressive disorders. Indian J. Clin Biochem. 2005;20:154–7. doi: 10.1007/BF02867417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Klabunde RE. Cardiovascular physiology concepts. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 15–37. [Google Scholar]
- [48].Subashini R, Rajadurai M, Ponmurugan P. Nelumbo nucifera leaf extract ameliorates lipid peroxides and antioxidants in isoproterenol-induced myocardial infarction: biochemical and histological examination. J. Pharm Res. 2012;5:3878–82. [Google Scholar]
- [49].Mervaala EM, Pere AK, Lindgren L, Laakso J, Teräväinen TL, Karjala K, et al. Effects of dietary sodium and magnesium on cyclosporin A-induced hypertension and nephrotoxicity in spontaneously hypertensive rats. Hypertension. 1997;29:822–7. doi: 10.1161/01.hyp.29.3.822. [DOI] [PubMed] [Google Scholar]
- [50].Duilio C, Ambrosio G, Kuppusamy P, Dipaula A, Becker LC, Zweier JL. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am. J Physiol. 2001;280:H2649–57. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- [51].Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J Physiol. 2001;280:C53–60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- [52].Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, et al. Matrix metalloproteinase inhibition attenuates early left ventricular injury, enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–70. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- [53].Hool LC. Evidence for the regulation of L-type Ca2+ channels in the heart by reactive oxygen species: mechanism for mediating pathology. Clin. Exp. Pharmacol Physiol. 2008;35:229–34. doi: 10.1111/j.1440-1681.2007.04727.x. [DOI] [PubMed] [Google Scholar]
- [54].Dun Y, Zhi J-M, Sun H-Y, Zhao R-R, Zhao Z-Q. Activated polymor-phonuclear leukocytes induce cardiomyocytes apoptosis and the protective effects of carvedilol. Methods Finds Exp. Clin. Pharmacol. 2002;24:403–12. doi: 10.1358/mf.2002.24.7.696541. [DOI] [PubMed] [Google Scholar]
- [55].Sutherland BA, Rahman RM, Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J. Nutri. Biochem. 2006;17:291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]