Abstract
Overlapping morphological characteristics pose some difficulties in making a proper diagnosis of clear cell renal cell carcinoma (CCRCC), chromophobe renal cell carcinoma (ChRCC), and oncocytoma, on the basis of hematoxylin–eosin-stained tissue sections. Our objective was to find out a fast, reliable panel of immunohistochemical markers for differentiation between them. The study was carried out on 55 selected renal tumor specimens: 36 cases of CCRCC, seven cases of ChRCC, and 12 cases of oncocytoma. The specimens were stained immunohistochemically for vimentin, CD117, cytokeratin (CK)7, and caveolin (Cav)-1. Sensitivity and specificity for each marker were calculated. Vimentin expression was exclusively observed in CCRCC (100%) and negative in ChRCC and oncocytoma. CD117 was absent in CCRCC, but it was strongly expressed in ChRCC (85.5%) and oncocytoma (91.7%), with high sensitivity and specificity. Most CCRCCs and oncocytomas were negative for CK7 (91.7% and 83.3%, respectively), in contrast to ChRCCs, which showed positivity in nearly 86% of the cases. Good sensitivity and specificity were calculated for CK7 in differentiating studied oncocytic tumors. Cav-1 was positive in ~78% of the CCRCCs and in all ChRCCs, whereas the vast majority of oncocytomas were negative. So the immunoprofile of CCRCC was vimentin+/CD117−/CK7−/Cav-1±, ChRCC was vimentin−/CD117+/CK7+/Cav-1+, and oncocytoma was vimentin−/CD117+/CK7±/Cav-1−. So, by using combination of four markers (vimentin, CD117, CK7, and Cav-1), we achieved excellent sensitivity and specificity for differential diagnosis of CCRCC, ChRCC and oncocytoma.
Keywords: caveolin-1, CD117, chromophobe renal cell carcinoma, clear cell renal cell carcinoma, cytokeratin-7
1. Introduction
Renal cell carcinoma (RCC) is a common urological tumor and accounts for ~3% of all human malignancies. It has been shown to have a significantly greater increase in incidence and mortality than other tumors of the genitourinary tract [1,2].
Tumor characteristics such as tumor stage and grade seem to have limited value in predicting the clinical outcome of individual patients, as ~50% of patients who undergo surgery with curative intent for less-advanced disease can be expected to develop a distant recurrence [3].
Moreover, RCC is a heterogeneous disease, comprised of different histological variants with a distinct clinical course, genetics, and response to treatment [4].
Clear cell renal cell carcinoma (CCRCC) is overwhelmingly the most common type of renal cancer, accounting for 70% of cases [5,6]. The World Health Organization defines CCRCC as “a malignant neoplasm composed of clear or eosinophilic cytoplasmic cells arranged within a delicate distinct vascular network” [6].
Chromophobe renal cell carcinoma (ChRCC) is a type of malignant renal epithelial tumor, accounting for ~5% of renal cancer, including classic, eosinophilic, and mixed variants. Classic type of ChRCC is composed mainly of pale cells with “soap bubble” appearance of the cytoplasm. The eosinophilic variant (containing > 80% eosinophilic cells) has nested, alveolar, or sheet-like architecture with eosinophilic granularity, perinuclear clearing, and peripheral accentuation of cytoplasm, which may mimic renal oncocytoma in many circumstances. The mixed variant ChRCC contains a mixture of pale and eosinophilic cells [7].
Oncocytoma is a benign renal tumor. It can frequently be confused with ChRCC, because it consists of nests of neoplastic oncocytic cells. These are rounded, well-differentiated, neoplastic cells with intensely eosinophilic granular cytoplasm as a result of a large number of mitochondria and have round regular nuclei [8].
Accurate classification of renal neoplasms is important not only for its correlation with the cytogenetic findings, but more importantly for its prognostic significance. The outcome for patients with ChRCC generally is better than for those with CCRCC [9], but worse than for renal oncocytoma, of which the overwhelming majority are benign and do not metastasize [10,11].
One of the diagnostic criteria of ChRCC is Hale colloidal iron [12], and another is intracytoplasmic microvesicles observed by electron microscopy [13], but the eosinophilic variant of ChRCC is particularly difficult to distinguish from renal oncocytoma and CCRCC [14].
Owing to the overlapping morphological characteristics, separation of CCRCC and ChRCC from oncocytoma based on conventional hematoxylin and eosin (H&E) staining is often challenging, even in the hands of experienced pathologists [15,16]. Immunohistochemistry is available in most pathology laboratories and is technically easier to perform and interpret than Hale colloidal iron and electron microscopy [14].
Vimentin, a major constituent of the intermediate filament family of proteins, is expressed in normal mesenchymal cells and is known to maintain cellular integrity [17].
CD117 is a transmembrane tyrosine kinase receptor protein that plays a role in intracellular signal transduction in several cell types. It regulates apoptosis and cell differentiation and proliferation. It is well known that KIT pathological activation through mutations may lead to neoplasia [18].
Cytokeratin (CK)7 belongs to the neutral basic type II subfamily of cytokeratins of low molecular weight [19]. CK7 has been reported in many adenocarcinomas, such as breast, endometrium, ovaries, and thyroid, as well as in carcinoma of the bladder [20].
Caveolin (Cav)-1, a scaffolding protein, has important roles in many cellular functions, including transporting cholesterol and glycosphingolipids, sequestrating and organizing many signaling molecules, and regulating some kinase activities [21]. Also, it participates in the adjustment of endothelial cell differentiation and angiopoiesis, and plays an important role in tumor advancement [22].
Vimentin and CK7 are useful in differentiating ChRCC from oncocytoma according to most investigators, but conflicting results have been reported [23].
In recent years, other studies have suggested that CD117 [24,25] and Cav-1 [21] can be also valuable for differentiation. However, no single marker appears to be sufficiently accurate by itself [14].
Accordingly, we examined the specific staining patterns of four markers (vimentin, CD117, CK7, and Cav-1) using immunohistochemistry for distinguishing oncocytic renal neoplasms including CCRCC, ChRCC, and oncocytomas.
2. Materials and methods
2.1. Case selection and tissue specimens
This work was approved by the Ethical Committee of the Faculty of Medicine, Tanta University, Tanta, Egypt. The present study included 55 selected renal tumor cases: 36 cases of CCRCC, seven cases of eosinophilic variant of ChRCC, and 12 cases of oncocytoma, from the pathology records archives of Tanta University. All specimens were obtained by radical or partial nephrectomy. Cases with needle biopsies were excluded. Paraffin sections of 4–5 μm thickness were stained with H&E to confirm their histological diagnosis.
2.2. Immunohistochemical staining of the studied cases
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded 4-mm-thick sections mounted on positively charged slides. Tissue sections were deparaffinized and rehydrated in graded alcohols to distilled water, then they were incubated in 3% hydrogen peroxide for 10 minutes to block the endogenous peroxidase. Slides were immersed in acetic acid and heated in a microwave at 95°C for 30 minutes for antigen retrieval; left to cool down at room temperature and rinsed with phosphate-buffered saline; and incubated overnight at room temperature with the following primary antibodies: vimentin (V9 monoclonal, 1:500; Dako, Carpinteria, CA, USA); CD117 (clone: A4502, 1:200; Dako); CK7 (K72.7 monoclonal, 1:50; Biocare, California, USA) and Cav-1 (rabbit monoclonal E249, 1:250, Novus Biologicals, Littleton, CO, USA).
The staining was completed using the streptavidin–biotin complex (Dako Envision System). 3,3′-Diaminobenzidine was applied for 10–15 minutes and the reaction was stopped using distilled water. Counter staining with hematoxylin was applied for 2 minutes and washed with distilled water. Appropriate positive and negative controls were included in each run. The slides of negative controls were prepared by excluding the primary antibody and replacing it with phosphate-buffered saline.
As regards vimentin, CD117, and CK7, tumor cells were considered positive only when the appropriate staining pattern was noted (vimentin gives cytoplasmic staining, CD117 gives membranous staining, and CK7 gives cytoplasmic staining with membranous accentuation). The extent of immunoreactivity was performed by analyzing the extent of the staining positivity among tumor cells. The score of these markers was as follows: 0 or negative ≤ 5% tumor cell positivity; +1 < 10% tumor cell positivity; +2 (focal) = 10–50% tumor cell positivity; and +3 (diffuse) > 50% tumor cell positivity. Scores 0 and 1 were considered negative, and cytoplasmic CK7 staining without membranous accentuation was considered negative [14].
Cav-1 expression was based on the presence of cytoplasmic and/or membranous staining and positive staining of the endothelium provided an internal positive control for Cav-1 immunostaining. Cav-1 was scored into three categories (0 = negative, +1 (focal) < 20%, and +2 (diffuse) ≥ 20%) [21].
2.3. Statistical analysis
The sensitivity and specificity were calculated for each marker [26].
Sensitivity (also called the true-positive rate) measured the proportion of positives that were correctly identified as such.
Sensitivity
=, Number of true positives-Number of true positives + number of false negatives (1)
Specificity (also called the true-negative rate) measured the proportion of negatives that were correctly identified as such.
Sensitivity
=, Number of true negatives-Number of true negatives + number of false positives (2)
3. Results
3.1. Histopathological and immunohistochemical results of the studied cases
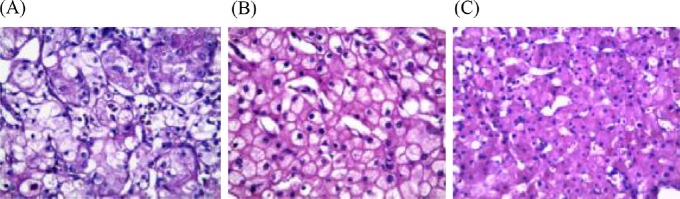
The present study included 55 selected oncocytic renal tumor cases: 36 were CCRCC, seven were eosinophilic variant of ChRCC, and 12 were oncocytoma (Table 1). CCRCC showed a solid, nesting, and tubular pattern of growth with eosinophilic cytoplasm embedded in a delicate vascular network (Figure 1A). ChRCC comprised large vegetable-like polygonal cells with eosinophilic cytoplasm, irregular nuclei, perinuclear clear halo, and prominent cell membrane (Figure 1B). Renal oncocytomas were composed exclusively of oncocytes that were arranged in nests or alveoli separated from each other by loose fibrous stroma (Figure 1C).
Table 1.
Immunohistochemical results of oncocytic renal cell tumors.
| Studied markers | Immunoexpression | CCRCC No. (%) | ChRCC No. (%) | Oncocytoma No. (%) | Sensitivity (%) | Speciflty(%) |
|---|---|---|---|---|---|---|
| Vimentin | 0 | — | 7 (100) | 12 (100) | ||
| 1+ | — | — | — | 100 | 100 | |
| 2+ | — | — | — | |||
| 3+ | 36 (100) | — | — | |||
| CD117 | 0 | 36 (100) | 1 (14.2) | 1 (8.3) | ||
| 1+ | — | — | — | 89.5 | 100 | |
| 2+ | — | — | 1 (8.3) | |||
| 3+ | — | 6 (85.8) | 10 (83.4) | |||
| CK7 | 0 | 31 (86.1) | 1 (14.2) | 8 (66.6) | ||
| 1+ | 2 (5.6) | — | 2 (16.7) | 85.7 | 89.6 | |
| 2+ | 3 (8.3) | — | 2 (16.7) | |||
| 3+ | — | 6 (85.8) | — | |||
| Cav-1 | 0 | 8 (22.3) | — | 11 (91.7) | 100 | 91.7 |
| 1+ | — | — | 1 (8.3) | |||
| 2+ | 28 (77.7) | 7 (100) | — | |||
| Total (N = 55) | 36 (65.5) | 7 (12.7) | 12 (21.8) |
Cav-1=caveolin-1; CCRCC=clear cell renal cell carcinoma; ChRCC=chromophobe renal cell carcinoma; CK7=cytokeratin-7.
Fig. 1.
(A) Clear cell renal cell carcinoma showing solid, nesting, and tubular pattern of growth with eosinophilic cytoplasm in delicate vascular network. (B) Chromophobe renal cell carcinoma composed of large vegetable-like polygonal cells with eosinophilic cytoplasm, irregular nuclei, perinuclear clear halo, and prominent cell membrane. (C) Renal oncocytomas composed of oncocytes that are arranged in nests or alveoli separated by loose fibrous stroma (hematoxylin and eosin, 400×).
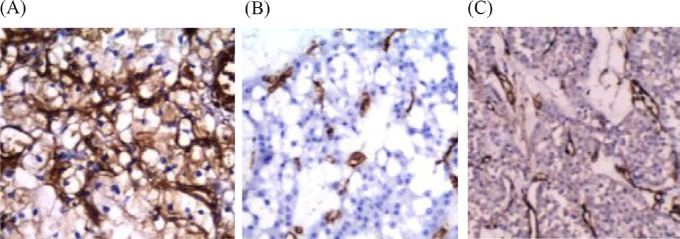
Vimentin showed diffuse cytoplasmic expression in all 36 CCRCCs, with 80–100% positivity in tumor cells (Figure 2A). It was absent in all seven ChRCCs (Figure 2B) and all 12 oncocytomas (Figure 2C).
Fig. 2.
(A) Clear cell renal cell carcinoma showing diffuse cytoplasmic vimentin expression; (B) chromophobe renal cell carcinoma showing negative expression of vimentin; and (C) oncocytoma showing negative vimentin expression (Avidin Biotin Complex, 400×).
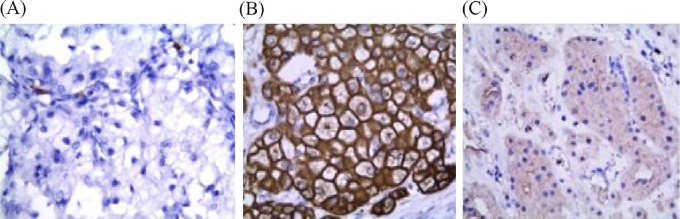
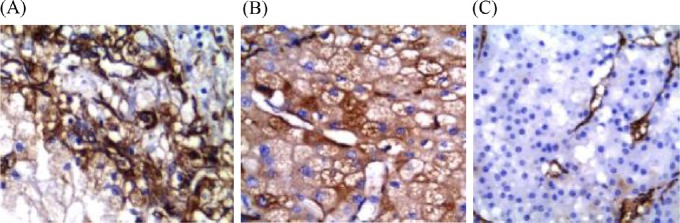
CD117 was absent in CCRCCs (Figure 3A). It was strongly expressed in six of seven (85.8%) ChRCCs (Figure 3B) and 11 of 12 (91.7%) oncocytomas, with diffuse staining in almost all positive cases (Figure 3C). The staining was complete–membranous (Figure 3).
Fig. 3.
(A) Negative expression of CD117 in clear cell renal cell carcinoma. (B) CD117 was strongly diffusely positive in chromophobe renal cell carcinoma and (C) oncocytoma. The staining was complete–membranous (Avidin Biotin Complex, 400×).
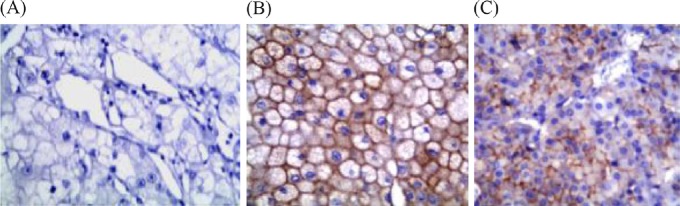
Most CCRCCs (33/36) were negative for CK7 (Figure 4A), of which, three showed only cytoplasmic staining without distinct membrane accentuation and were considered negative. CK7 was positive in six of seven ChRCCs (Figure 4B), whereas 10 of 12 (83.3%) renal oncocytomas were negative (Figure 4C), of which, two (16.7%) showed few scattered immunoreactivity in < 10% of tumor cells and were considered negative. The two CK7-positive oncocytomas showed focal reactivity in < 50% of tumor cells.
Fig. 4.
(A) Negative expression of cytokeratin-7 (CK7) in clear cell renal cell carcinoma. (B) CK7 was positive in chromophobe renal cell carcinoma, and (C) negative in renal oncocytoma (Avidin Biotin Complex, 400×).
Cav-1 was expressed in 28/36 (78%) CCRCCs (Figure 5A), and in all seven ChRCCs (Figure 5B), whereas the vast majority of oncocytomas were negative (Figure 5C). Only one case of oncocytoma out of 12 showed focal positivity for Cav-1 in < 20% of tumor cells (1+) (Figure 5).
Fig. 5.
(A) Caveolin-1 was positively expressed in clear cell renal cell carcinoma with score 2 and (B) in chromophobe renal cell carcinoma with score 2, whereas (C) oncocytoma was negative (Avidin Biotin Complex, 400×).
3.2. Sensitivity and specificity of studied markers in diagnosis of selected cases
Evaluation of the sensitivity and specificity of vimentin in diagnosis of CCRCC showed that vimentin was sensitive and specific for diagnosis of CCRCC (both 100%; Table 1). CD117 showed 89.5% sensitivity and 100% specificity in diagnosis of both ChRCC and oncocytoma and exclusion of CCRCC. CK7 showed 85.5% sensitivity and specificity for ChRCC, and lastly, Cav-1 showed 100% sensitivity and 91.7% specificity for diagnosis of ChRCC when compared to oncocytoma.
4. Discussion
RCC accounts for 2–3% of all new cancers diagnosed and 85% of all primary renal neoplasms in adults [2]. Adult renal epithelial neoplasms are a heterogeneous group comprised of subtypes that have distinct gross, histological, ultrastructural, and immunohistochemical features. Thus, classification of renal cell neoplasms is important from the treatment and prognosis point of view as well as for understanding their histogenesis, and molecular and cytogenetic behavior that allow further improvement in their management [27].
The most common subtype in the present study was CCRCC, accounting for 84% of malignant cases of RCC, similar to previous findings of Vera-Badillo et al [28]. The other studied subtype of RCC was ChRCC, which accounts for 16% of malignant cases, as previously reported by Pan et al [24]. Similarly, Vera-Badillo et al [28] showed that ChRCC comprised 5–10% of their total cases of RCC. We found that renal oncocytoma comprised 21.8% (n = 12) of total studied cases in contrast to the finding of Pradhan et al [27], who showed that, among the total cases they examined, CCRCC was the most common tumor with 74.8%, ChRCC was 7.9%, and oncocytoma was only 1.8%.
The separation of different subtypes of oncocytic renal neoplasms (CCRCC, ChRCC, and oncocytoma) using ordinary H&E remains problematic in some cases. This prompted us to explore a set of immunohistochemical markers that would improve the accuracy of diagnosis, as reliance on a single marker in differential diagnosis of tumors with overlapping morphology may be insufficient or even misleading, especially when the interpretation of the stain is not straightforward or the tissue sample is small.
Accordingly, the objective of this study was to identify the specific staining patterns of four studied markers (vimentin, CD117, CK7, and Cav-1) in three different types of oncocytic renal tumor (CCRCC, ChRCC, and oncocytoma), to determine an optimal diagnostic strategy for differentiation.
Our results showed that vimentin was only positive in CCRCC, whereas all ChRCCs and oncocytomas were negative with 100% sensitivity and specificity. Such findings coincided with those of Bazille et al [29], who found that vimentin was only positive in CCRCC, whereas all cases of ChRCC and oncocytoma were negative. However, other reports showed vimentin positivity varied from 54.5% to 85% in CCRCC [15,27]. A few studies have documented vimentin positivity in 20% of ChRCCs [14,27] and 9.7% of oncocytomas [30]. These discrepancies could be related to the variance in pathological diagnosis, use of different antibodies and reagents for the studies, and different laboratory staining procedures, as previously reported by Liu et al [14]. The diagnosis of ChRCC or oncocytoma should be rendered with caution if diffuse vimentin staining is detected.
The present study showed CD117 expression in nearly 86% of ChRCCs and 92% of oncocytomas, in contrast to negative staining in CCRCCs, with 89.5% sensitivity and 100% specificity. Such findings coincided with those of Liu et al [14], who showed that vimentin and CD117 each showed 100% specificity and high sensitivity (100% and 90%, respectively), allowing separation of CCRCC from both ChRCC and oncocytoma. Also, Pan et al [24] found that 83% (24/29) of ChRCCs and 71% (5/7) of oncocytomas had membranous immunoreactivity for CD117, whereas all CCRCCs were negative; similar to another study with 88% positivity in ChRCC, 71% in oncocytoma, and 0% in CCRCC [31]. Wang and Mills [32] observed 100% immunoreactivity with CD117 in both ChRCC and oncocytoma. Yamazaki et al [33] also detected overexpression of CD117 in membranes of cells of ChRCC, which ranged from 88% to 100% of tumor cells. Most cases of ChRCC and oncocytoma in the current study were positive for CD117 (85.8% and 91.7%, respectively), thus, it could not be used in the differentiation between them, but it could be used as a good marker for exclusion of CCRCC.
Most CCRCCs and oncocytomas were negative for CK7 (91.7% and 83.3%, respectively), in contrast to ChRCCs, which showed positivity in nearly 86% of the cases. The only two positive cases of oncocytoma showed focal reactivity in < 50% of the tumor cells. Good sensitivity and specificity were calculated for CK7 in differentiating studied oncocytic tumors (85.7% and 89.6%, respectively). Our results coincided with those of Pradhan et al [27], who detected CK7 positivity in all cases of ChRCC, whereas all cases of CCRCCs and renal oncocytoma were negative. Also, Liu et al [14] found that CK7 expression yielded 100% specificity and 86% sensitivity in differentiation between oncocytoma and ChRCC. By contrast, Garcia and Li [21] detected CK7 positivity in all cases of ChRCC and in 96% of renal oncocytomas.
Cav-1 was immunohistochemically expressed in nearly 80% of cases of CCRCCs and in all ChRCCs cases, whereas the vast majority of oncocytomas were negative (91.7%). The single positive oncocytoma showed only focal reactivity for Cav-1. Such findings coincided with Garcia and Li [21], who detected 100% positivity in ChRCCs and 88% negativity in oncocytomas.
The results of the current study showed that Cav-1 is not useful in excluding CCRCC, as a large number of these cases were positive. By contrast, when Cav-1 was used for differentiation between ChRCC and oncocytoma, it gave excellent results with 100% sensitivity and 91.7% specificity.
Carrion et al [34] reported that oncocytoma had a higher labeling index for Cav-1, compared with several types of RCC, including four cases of ChRCC. These findings are in sharp contrast with ours and could be attributed to the method of immunohistochemical preparation. Tumors might contain endogenous biotin and generate false positivity if an avidin–biotin detecting system is used without blocking the endogenous biotin.
In conclusion, after the study of vimentin, CD117, CK7, and Cav-1 immunohistochemical expression in oncocytic renal tumors, we suggest the diagnostic strategy shown in Figure 6. The immunoprofile of CCRCC is vimentin+/CD117−/CK7−/Cav-1±; ChRCC is vimentin−/CD117+/CK7+/Cav-1+; and oncocytoma is vimentin−/CD117+/CK7±/Cav-1−. So, the use of the panel of these four markers is beneficial in differentiating problematic cases of oncocytic renal neoplasms (Figure 6).
Fig. 6.
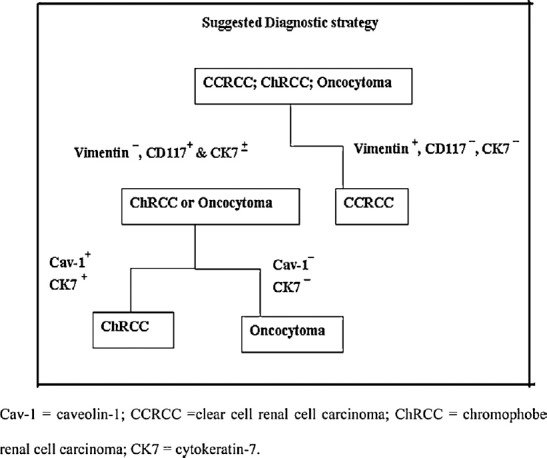
Suggested strategy for diagnosis of CCRCC, ChRCC, and oncocytoma
Cav-1 = caveolin-1; CCRCC =clear cell renal cell carcinoma; ChRCC = chromophobe renal cell carcinoma; CK7 = cytokeratin-7.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- [1].Schrader AJ, Sevinc S, Olbert PJ, Hegele A, Varga Z, Hofmann R. Gender specific characteristics and survival of renal cell carcinoma. Der Urologe. 2008;47:1182–6. doi: 10.1007/s00120-008-1832-0. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- [3].Steffens S, Schrader AJ, Blasig H, Vetter G, Eggers H, Tränkenschuh W, et al. Caveolin 1 protein expression in renal cell carcinoma predicts survival. BMC Urology. 2011;11:25. doi: 10.1186/1471-2490-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tamaskar I, Choueiri TK, Sercia L, Rini B, Bukowski R, Zhou M. Differential expression of caveolin-1 in renal neoplasms. Cancer. 2007;110:776–82. doi: 10.1002/cncr.22838. [DOI] [PubMed] [Google Scholar]
- [5].Eble JN, Togashi K, Pisani P. Renal cell carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. The WHO classification of tumours of the urinary system and male genital organs. Lyon: IARC; 2004. pp. 12–4. [Google Scholar]
- [6].Grignon DJ, Eble JN, Bonsib SM, Moch H. Clear cell renal cell carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. The WHO classification of tumors of the urinary system and male genital organs. Lyon: IARC; 2004. pp. 23–5. [Google Scholar]
- [7].Amin MB, Paner GP, Alvarado-Cabrero, Alvarado-Cabrero I, Young AN, Stricker HJ, et al. Chromophobe renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 145 cases. Am J Surg Pathol. 2008;32:1822–34. doi: 10.1097/PAS.0b013e3181831e68. [DOI] [PubMed] [Google Scholar]
- [8].Van der Kwast T, Perez-Ordonez B. Renal oncocytoma, yet another tumor that does not fit in the dualistic benign/malignant paradigm? J Clin Pathol. 2007;60:585–6. doi: 10.1136/jcp.2006.044438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–24. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- [10].Amin MB, Crotty TB, Tickoo SK, Farrow GM. Renal oncocytoma: a reappraisal of morphologic features with clinicopathologic findings in 80 cases. Am J Surg Pathol. 1997;21:1–12. doi: 10.1097/00000478-199701000-00001. [DOI] [PubMed] [Google Scholar]
- [11].Perez-Ordonez B, Hamed G, Campbell S, Erlandson RA, Russo P, Gaudin PB, et al. Renal oncocytoma: a clinicopathologic study of 70 cases. Am J Surg Pathol. 1997;21:871–83. doi: 10.1097/00000478-199708000-00001. [DOI] [PubMed] [Google Scholar]
- [12].Prasad SR, Humphrey PA, Catena JR, Narra VR, Srigley JR, Cortez AD, et al. Common and uncommon histologic subtype of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics. 2006;26:1795–810. doi: 10.1148/rg.266065010. [DOI] [PubMed] [Google Scholar]
- [13].Wu SL, Fishman IJ, Shanon RL. Chromophobe renal cell carcinoma with extensive calcification and ossification. Ann Diag Pathol. 2002;6:244–7. doi: 10.1053/adpa.2002.34731. [DOI] [PubMed] [Google Scholar]
- [14].Liu L, Qian J, Singh H, Meiers I, Zhou X, Bostwick DG. Immuno-histochemical analysis of chromophobe renal cell carcinoma, renal oncocytoma, and clear cell carcinoma an optimal and practical panel for differential diagnosis. Arch Pathol Lab Med. 2007;131:1290–7. doi: 10.5858/2007-131-1290-IAOCRC. [DOI] [PubMed] [Google Scholar]
- [15].Kim MK, Kim S. Immunohistochemical profile of common epithelial neoplasms arising in the kidney. Appl Immunohistochem Mol Morphol. 2002;10:332–8. doi: 10.1097/00129039-200212000-00008. [DOI] [PubMed] [Google Scholar]
- [16].Abrahams NA, MacLennan GT, Khoury JD, Ormsby AH, Tamboli P, Doglioni C, et al. Chromophobe renal cell carcinoma: a comparative study of histological, immunohistochemical and ultrastructural features using high throughput tissue microarray. Histopathology. 2004;45:593–602. doi: 10.1111/j.1365-2559.2004.02003.x. [DOI] [PubMed] [Google Scholar]
- [17].Sateffi A, Li S. Vimentin as a potential molecular target in cancer therapy Or Vimentin, an overview and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–46. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–20. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- [19].Barak V, Goike H, Panaretakis W, Einarsson M. Clinical utility of cytok-eratins as tumor markers. Clin Biochem. 2004;37:529–40. doi: 10.1016/j.clinbiochem.2004.05.009. [DOI] [PubMed] [Google Scholar]
- [20].Mathers ME, Pollock AM, Marsh C, O’Donnell M. Cytokeratin 7: a useful adjunct in the diagnosis of chromophobe renal cell carcinoma. Histopathology. 2002;40:563–7. doi: 10.1046/j.1365-2559.2002.01397.x. [DOI] [PubMed] [Google Scholar]
- [21].Garcia E, Li M. Caveolin-1 immunohistochemical analysis in differentiating chromophobe renal cell carcinoma from renal oncocytoma. Am J Clin Pathol. 2006;125:392–8. [PubMed] [Google Scholar]
- [22].Quest AF, Gutierrez-Pajares JL, Torres VA. Caveolin-1: an ambiguous partner in cell signalling and cancer. J Cell Mol Med. 2008;12:1130–50. doi: 10.1111/j.1582-4934.2008.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu SL, Kothari P, Wheeler TM, Reese T, Connelly JH. Cytokeratins 7 and 20 immunoreactivity in chromophobe renal cell carcinomas and renal oncocytomas. Mod Pathol. 2002;15:712–7. doi: 10.1097/01.MP.0000017566.29755.8A. [DOI] [PubMed] [Google Scholar]
- [24].Pan CC, Chen PC, Chiang H. Overexpression of KIT (CD117) in chromophobe renal cell carcinoma and renal oncocytoma. Am J Clin Pathol. 2004;121:878–83. doi: 10.1309/A7M2-XTMJ-QK0K-PQER. [DOI] [PubMed] [Google Scholar]
- [25].Miliaras D, Karasavvidou F, Papanikolaou A, Sioutopoulou D. KIT expression in fetal, normal adult, and neoplastic renal tissues. J Clin Pathol. 2004;57:463–6. doi: 10.1136/jcp.2003.013532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Loong T. Understanding sensitivity and specificity with the right side of the brain. BMJ. 2003;327:716–9. doi: 10.1136/bmj.327.7417.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pradhan D, Kakkar N, Bal A, Singh SK, Joshi K. Sub-typing of renal cell tumours; contribution of ancillary techniques. Diagn Pathol. 2009;4:21. doi: 10.1186/1746-1596-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vera-Badillo FE, Conde E, Duran I. Chromophobe renal cell carcinoma: a review of an uncommon entity. Int J Urol. 2012;19:894–900. doi: 10.1111/j.1442-2042.2012.03079.x. [DOI] [PubMed] [Google Scholar]
- [29].Bazille C, Allory Y, Molinie V, Vieillefond A, Cochand-Priollet B, Cussenot O, et al. Immunohistochemical characterisation of the main histologic subtypes of epithelial renal tumors on tissue-microarrays: study of 310 cases [in French] Ann Pathol. 2004;24:395–406. doi: 10.1016/s0242-6498(04)93995-8. [DOI] [PubMed] [Google Scholar]
- [30].Mazal PR, Exner M, Haitel A, Krieger S, Thomson RB, Aronson PS, et al. Expression of kidney-specific cadherin distinguishes chromo-phobe renal cell carcinoma from renal oncocytoma. Hum Pathol. 2005;36:22–8. doi: 10.1016/j.humpath.2004.09.011. [DOI] [PubMed] [Google Scholar]
- [31].Petit A, Castillo M, Santos M, Mellado B, Alcover JB, Mallofre C. KIT expression in chromophobe renal cell carcinoma: comparative immunohistochemical analysis of KIT expression in different renal cell neoplasms. Am J Surg Pathol. 2004;28:676–8. doi: 10.1097/00000478-200405000-00017. [DOI] [PubMed] [Google Scholar]
- [32].Wang HY, Mills SE. KIT and RCC are useful in distinguishing chromophobe renal cell carcinoma from the granular variant of clear cell renal cell carcinoma. Am J Surg Pathol. 2005;29:640–6. doi: 10.1097/01.pas.0000157943.33903.92. [DOI] [PubMed] [Google Scholar]
- [33].Yamazaki K, Sakamoto M, Ohta T, Kanai Y, Ohki M, Hirohashi S. Over-expression of KIT in chromophobe renal cell carcinoma. Oncogene. 2003;22:847–52. doi: 10.1038/sj.onc.1206153. [DOI] [PubMed] [Google Scholar]
- [34].Carrion R, Morgan BE, Tannenbaum M, Salup R, Morgan MB. Caveolin expression in adult renal tumors. Urol Oncol. 2003;21:191–66. doi: 10.1016/s1078-1439(02)00235-1. [DOI] [PubMed] [Google Scholar]